Key Points
Initiating rituximab in the inpatient setting for all TTP admissions produces $30 000+ in cost savings per patient to the hospital.
Cost to the hospital should not be a barrier to initiating rituximab in the inpatient setting.
Abstract
Patients with severe autoimmune thrombotic thrombocytopenic purpura (TTP) experience acute hematologic emergencies during disease flares and a lifelong threat for relapse. Rituximab, in addition to steroids and therapeutic plasma exchange (TPE), has been shown to mitigate relapse risk. A barrier to care in initiating rituximab in the inpatient setting has been the presumed excessive cost of medication to the hospital. Retrospectively reviewing TTP admissions from 2004 to 2018 at our academic center, we calculated the actual inpatient cost of care. We then calculated the theoretical cost to the hospital of initiating rituximab in the inpatient setting for both initial TTP and relapse TTP cohorts, with the hypothesis that preventing sufficient future TTP admissions offsets the cost of initiating rituximab in all patients with TTP. At a median follow-up of 55 months in the initial TTP cohort, rituximab use produced a projected cost savings of $905 906 and would have prevented 185 inpatient admission days and saved 137 TPE procedures. In the relapse TTP setting, rituximab use produced a projected cost savings of $425 736 and would have prevented 86 inpatient admission days and saved 64 TPE procedures. From a hospital cost standpoint, cost of rituximab should no longer be a barrier to initiating inpatient rituximab in both initial and relapse TTP settings.
Visual Abstract
Introduction
Autoimmune acquired thrombotic thrombocytopenic purpura (TTP) is a hematologic emergency comprising thrombocytopenia and hemolytic anemia, often characterized by end-organ damage in the setting of thrombotic microangiopathy. It can present with a variety of chief concerns that are important to recognize to promptly diagnose patients in both the initial and relapse setting.1 Each hospitalization for a patient with TTP is associated with a significant inpatient length of stay (LOS) and associated cost of care.2,3 The standard of care for treatment of an initial TTP episode is a combination of steroids and therapeutic plasma exchange (TPE). The addition of rituximab at standard dosing (375 mg/m2 weekly for 4 weeks) during an acute episode of TTP has been shown to reduce relapse rates and may be associated with reduced hospital stay.4-7 As such, rituximab is recommended along with TPE and steroids for patients with relapsed TTP (rTTP) in expert-based guidelines from the United Kingdom.8 The UK guidelines also recommend consideration of upfront rituximab in patients with an initial presentation of TTP, although a 2009 randomized clinical trial to evaluate the addition of upfront rituximab plus TPE and steroids was stopped due to low enrollment.9 Hence, adjuvant, full-dose rituximab is not yet viewed as the standard of care in treatment of an initial episode of TTP but continues to gain favor over time, with more recent data supporting a potential role for low-dose rituximab as well.1,10-12
A major barrier to incorporating rituximab into treatment paradigms for TTP is hospital cost.13 A common practice in the United States is to administer the first dose of rituximab within the first few days of hospitalization for TTP, with subsequent doses given in either the outpatient or inpatient setting depending on other indications for continued admission. In the United States, hospitals are reimbursed according to a bundled payment system designed to cover expected in-hospital costs without additional reimbursement for extra services or medications. This creates a financial incentive, from the hospital’s perspective, to defer administration of expensive medications to the outpatient setting where cost can be in part covered by the patient’s insurance company without cost to the hospital. To date, there is no analysis of the financial implication to the hospital of initiating rituximab in the inpatient setting. We hypothesized that the costs to the hospital of initiating rituximab in the inpatient setting during an admission for TTP would be offset by savings in preventing future hospitalizations. With this in mind, we conducted a retrospective chart review to examine hospital costs of initiating rituximab therapy in the inpatient setting in addition to TPE and steroids compared with TPE and steroids alone, both in patients with initial TTP and rTTP.
Methods
Patients, definitions, and inclusion and exclusion criteria
Chart records for patients hospitalized at a major academic center between 1 January 2004 and 31 December 2018, with an International Classification of Diseases diagnosis code corresponding to TTP were identified by the hospital’s Joint Data Analytics Team and reviewed. Institutional review board waiver was obtained for retrospective chart review. A diagnosis of severe autoimmune TTP was defined as laboratory and smear evidence of microangiopathy with an ADAMTS13 level of ≤10% and a concomitant elevated ADAMTS13 inhibitor titer or evidence of microangiopathy in a patient with a prior established diagnosis of TTP. ADAMTS13 levels were measured via a FRET platform-based assay, which was a send-out test before 2010, then performed in-house from 2010 onward at this institution. Patients not meeting the diagnosis of severe autoimmune TTP were excluded from analysis in the study. TTP remission was defined as no evidence of TTP over 30 days following the last TPE. rTTP was defined as recrudescence of thrombocytopenia following remission, with or without clinical symptoms, necessitating reinitiation of TTP-directed therapy. These definitions are consistent with the latest consensus of terminology in the field.14
On analysis by the Joint Data Analytics Team, 29 patients were identified as having a diagnosis of TTP. Using the aforementioned criteria of severe autoimmune TTP, 2 patients were excluded from the study, 1 of whom had familial TTP with a negative ADAMTS13 inhibitor titer, and 1 who had an ADAMTS13 level of 16%, above the cutoff for severe autoimmune TTP. The final study population consisted of 27 patients with severe autoimmune TTP. The clinical course of both initial TTP and rTTP presentations of all of these patients was examined for presenting symptoms, blood abnormalities, treatments received, hospitalizations, and LOS. Although surveillance monitoring of ADAMTS13 levels in previously treated TTP patients began at this institution in 2017, none of this cohort received additional prophylactic rituximab during the study period based on ADAMTS13 levels.
Establishing hospital costs of TTP treatments, hospitalizations, and rituximab use
To estimate the average cost of the hospital stay for both initial TTP and rTTP hospitalizations, the cost of an inpatient medicine bed ($490) as well as the professional and technical cost of TPE ($6000/TPE) at our medical center were obtained. These costs were combined with the average number of TPE procedures and the average LOS to calculate hospitalization cost. In the case of first TTP hospitalization, the average number of TPE procedures was 10.1 (standard deviation [SD], 4.8), and the average LOS was 14.6 days (SD, 8.0). In the case of rTTP hospitalization, the average number of TPE procedures was 7.1 (SD, 3.1), and the average LOS was 9.6 days (SD, 3.3). These amounted to average initial TTP and rTTP hospitalization costs of $67 754 and $47 304, respectively. The average wholesale price of rituximab ($1084 per 100-mg vial) was used to calculate the total cost of treatment with 1 rituximab dose (375 mg/m2) administered in the inpatient setting for a 170 cm, 70 kg individual, amounting to $7724. One rituximab cycle was defined as 4 rituximab doses. All costs are listed in US dollars.
Cost savings for first TTP admission treated with rituximab
We compared the clinical outcomes of patients with an initial presentation of TTP who did or did not receive rituximab during their first TTP admission. In general, the patients with TTP who received rituximab during their initial TTP presentation had more refractory disease than those who did not receive rituximab upfront, as evidenced by an increase in LOS and in TPE procedures (by 10 days and 6 procedures, respectively). The clinical efficacy of rituximab in preventing further relapse was calculated at a median follow-up of 58 months. Assuming that patients with a first presentation of TTP who did not receive rituximab would have responded to rituximab at the same rate as those who received it upfront, we calculated the theoretical cost savings to the hospital in this setting using the hospital cost of 1 dose of inpatient rituximab ($7724) and the average cost of hospitalization for an initial presentation of TTP ($67 754).
Cost savings for rTTP treated with rituximab
Many patients described in this cohort relapsed multiple times before rituximab was given. We considered a theoretical model to calculate the savings to the hospital if rituximab had been given to this cohort during the first episode of rTTP (ie, their second episode of TTP). The clinical efficacy of rituximab in the rTTP patient population was calculated at a median follow-up of 44 months. Assuming these patients would have responded to rituximab given at first relapse (second TTP hospitalization) at the same rate they did upon later relapse, we calculated the theoretical cost savings to the hospital in initiating rituximab as an inpatient during the patients’ first relapse. For these theoretical calculations, the hospital cost of 1 dose of inpatient rituximab ($7724) and the average cost of relapse hospitalization ($47 304) were used.
Results
Study population
A total of 27 patients with severe autoimmune TTP were identified from 2004 to 2018. During a median follow-up of 50 months from the time of diagnosis, these 27 patients had 54 total hospitalizations. A summary of patient characteristics and laboratory studies is shown in Table 1. For the entire cohort, the median age of initial presentation with TTP was 43 years. Of the 27 patients, 21 were female, 15 black, 8 Hispanic, and 4 white; 13 had non-O blood type. Autoimmune comorbidities included 2 patients with systemic lupus erythematosus and 1 patient with multiple sclerosis. Twenty-two of 27 patients were treated with rituximab. Of these, 10 received rituximab upon initial presentation with TTP and 12 received rituximab at first relapse or afterward. In total, 4 of 22 severe autoimmune rituximab-naive TTP patients (initial TTP and rTTP) relapsed after treatment with 1 cycle of rituximab at a median follow-up of 47 months, corresponding to a relapse rate of 18.2%.
Table 1.
Median and range of parameters in the initial and rTTP cohorts
| Condition | Median | Range |
|---|---|---|
| Initial TTP | ||
| Age, y | 43 | 20-70 |
| ADAMTS13, % | 5 | 0-10 |
| Platelet count, ×109/L | 12 | 4-39 |
| Hematocrit, % | 25.5 | 16-47 |
| Reticulocyte, % | 6.4 | 2.0-31.0 |
| LDH, U/L | 1834 | 574-21 060 |
| Creatinine, mg/dL | 1.2 | 1.0-1.4 |
| Total bilirubin, mg/dL | 3.8 | 1.7-7.6 |
| Relapsed TTP | ||
| Age, y | 39 | 21-61 |
| ADAMTS13, % | 3 | 0-10 |
| Platelet count, ×109/L | 20 | 8-82 |
| Hematocrit, % | 31 | 14-46 |
| Reticulocyte, % | 4.5 | 1.5-11.6 |
| LDH, U/L | 713 | 236-4175 |
| Creatinine, mg/dL | 1.1 | 0.9-2.5 |
| Total bilirubin, mg/dL | 2.0 | 0.3-11 |
LDH, lactate dehydrogenase.
Initial TTP cohort
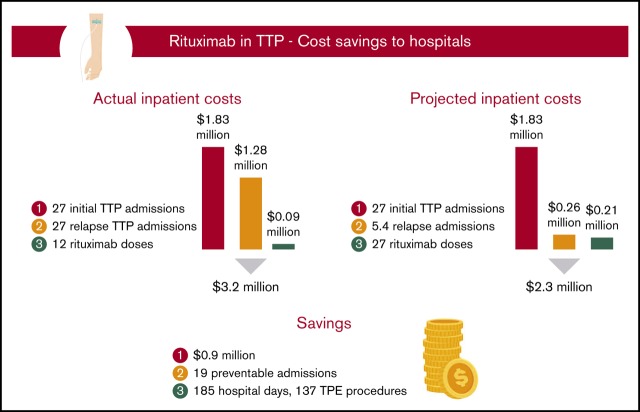
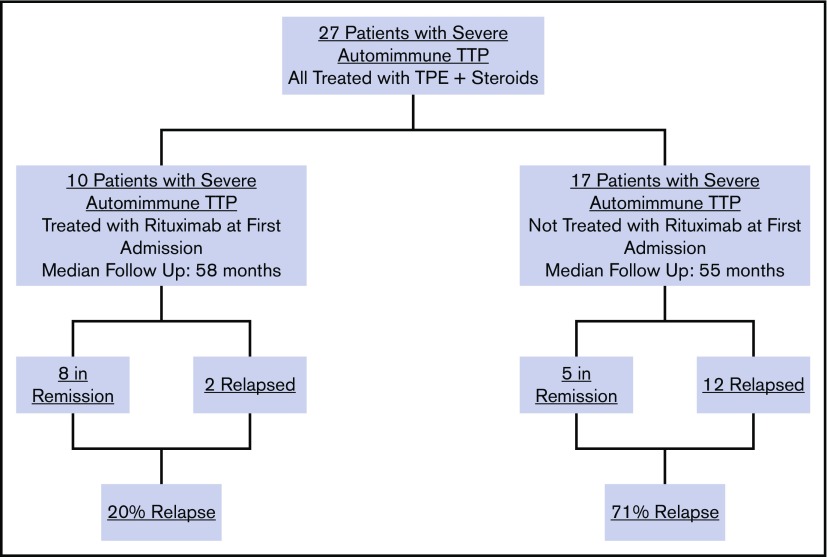
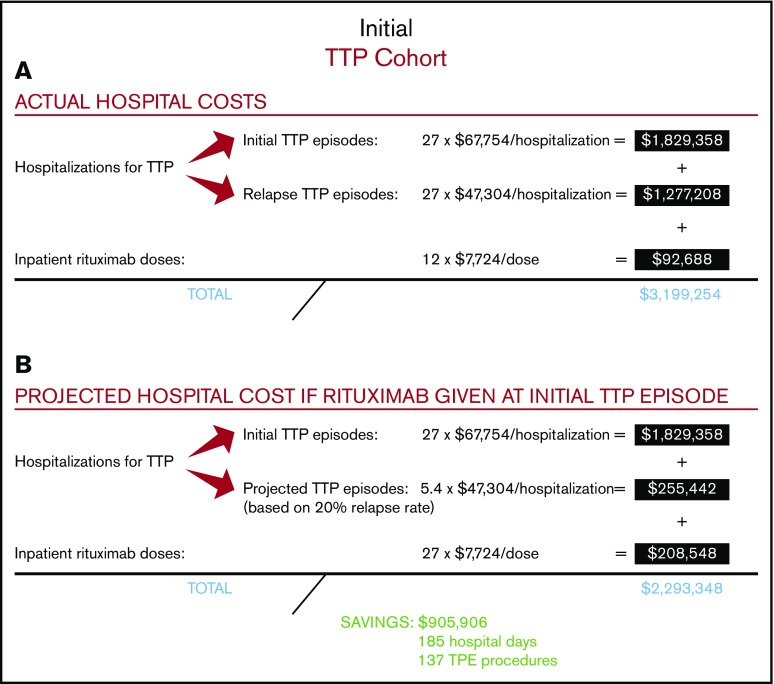
Among the total study population of 27 patients with severe autoimmune TTP, 10 were treated with rituximab upfront, which was started during their initial TTP admission, whereas 17 did not receive rituximab during their initial TTP admission (Figure 1). The relapse rate after initial TTP was 20% for patients treated with rituximab at a median follow-up of 58 months and 71% for those who did not receive rituximab at a median follow-up of 55 months. The total number of hospitalizations for all 27 patients who did or did not receive upfront rituximab during initial TTP was 54, with a total cost of $3 106 566 (Figure 2A). The 12 doses of rituximab administered for initial TTP in the inpatient setting yielded a cost to the hospital of $92 688 for rituximab administration. The total cost to the hospital was therefore $3 199 254.
Figure 1.
Relapse rates of TTP patients with and without rituximab administration after the initial TTP admission.
Figure 2.
Actual and projected hospital costs for the initial TTP cohort. (A) Actual hospital costs. (B) Projected hospital costs.
Cost savings for initial TTP treated with rituximab
We then calculated the projected total cost to the hospital if all 27 patients had been treated with rituximab upfront at the time of their initial TTP presentation, with the first rituximab dose administered in the inpatient setting (Figure 2B). Utilizing the 20% relapse rate following rituximab use in the initial TTP admission, we estimated that upfront rituximab treatment would have reduced the total number of relapse hospitalizations in the study cohort to 5.4, leading to a hospitalization cost of $2 084 800 (ie, 27 hospitalizations for initial TTP plus 5.4 hospitalizations for rTTP). The total number of inpatient rituximab doses would have increased to 27, leading to an inpatient rituximab cost of $208 548. The projected total cost to the hospital of treating the entire study cohort with upfront rituximab, including all hospitalizations for initial TTP, projected hospitalizations for rTTP, and all upfront rituximab doses administered during the first TTP hospitalization, would be $2 293 348. Therefore, the projected total cost savings to the hospital of upfront rituximab initiated in the inpatient setting would be $905 906, corresponding to a hospital cost savings of $33 552 per patient with TTP treated upfront with rituximab. In our hospital, this would have prevented ∼185 inpatient days and 137 TPE procedures.
rTTP cohort
Of 27 autoimmune TTP patients with 54 total hospitalizations, 14 had rTTP, accounting for 41 relapse hospitalizations. The median age of rTTP patients was 39 years at the time of their first relapse of TTP, with a median ADAMTS13 level of 3% at relapse. Ten (71%) of the 14 patients with rTTP were female. Other laboratory abnormalities at the time of presentation are shown in Table 1. The 3 leading rTTP presentations were either asymptomatic with the laboratory finding of thrombocytopenia (27%), symptomatic with abdominal pain (18%), or petechiae (16%).
Cost savings for rTTP treated with rituximab
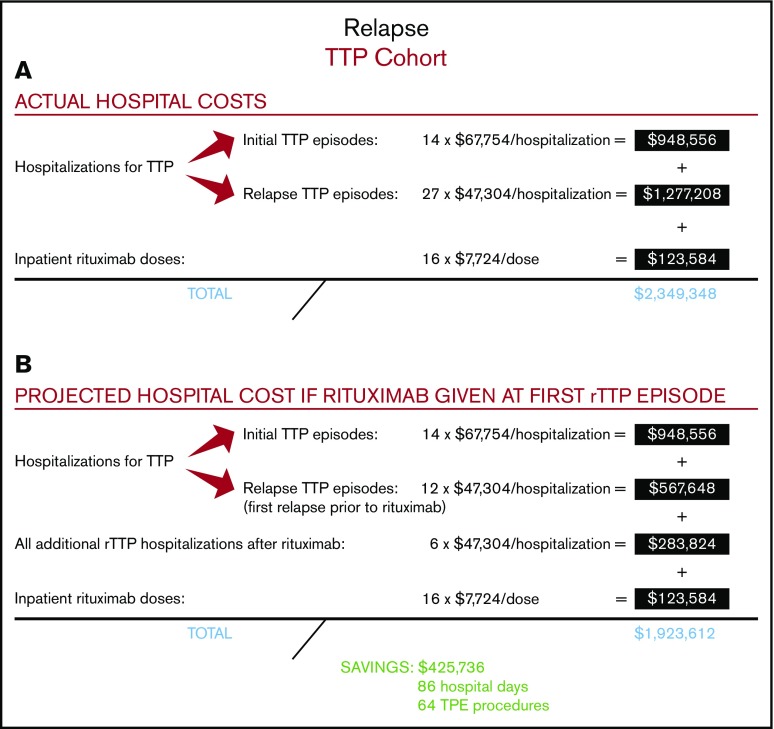
Among the 14 patients with rTTP, 9 received rituximab at the time of their first relapse (ie, their second TTP episode), and 5 received it during a subsequent rTTP episode (supplemental Figure 1). A total of 16 cycles of rituximab were administered to the 14 patients with rTTP over the course of the follow-up period, with the first dose given in the inpatient setting. Four of these patients had a cumulative total of 9 repeat relapse hospitalizations before ever receiving rituximab. The relapse rate following administration of rituximab in rTTP was 29% at a median follow-up of 44 months. The total number of hospitalizations for all patients with rTTP was 41, including all 14 initial TTP presentations and 27 additional hospitalizations for rTTP, yielding a total cost of $2 225 764 for all 41 hospitalizations (Figure 3A). A total of 16 doses of rituximab were administered in the inpatient setting, yielding a cost to the hospital of $123 584 for rituximab administration. The total cost to the hospital of treating the 14 patients with rTTP in our cohort, including all hospitalizations for initial TTP and rTTP and all rituximab doses administered in the inpatient setting, was therefore $2 349 348.
Figure 3.
Actual and projected hospital costs for the rTTP cohort. (A) Actual hospital costs. (B) Projected hospital costs.
We then calculated the total cost to the hospital if all 14 patients with rTTP had been treated with rituximab earlier, at the time of their first episode of rTTP (ie, their second episode of TTP) (Figure 3B). This approach would have saved 9 preventable hospitalizations in the 4 treatment-naive patients with rTTP and led to a hospitalization cost of $1 800 028 (ie, 14 hospitalizations for initial TTP plus 18 hospitalizations for rTTP). Adding in the constant of 16 inpatient doses of rituximab (as the savings come in treating frequent relapsers at first relapse), the total cost to the hospital would be $1 923 612. Therefore, the projected total cost savings to the hospital of rituximab in treatment of the first rTTP episode, with the first dose given in the inpatient setting, would be $425 736, or $30 410 per patient with rTTP. In our hospital, this would have prevented roughly 86 inpatient days and 64 TPE procedures.
Discussion
In this study, we show that the addition of rituximab to standard treatment (TPE and steroids) in patients either with initial TTP or at the time of their first episode of rTTP has the capacity to yield significant cost savings to the hospital ($33 552 per patient for initial TTP, or $30 410 per patient for rTTP). We estimated that among patients in our cohort, the administration of rituximab upon initial TTP admission would have prevented 185 admission days and 137 TPE procedures, and that the use of rituximab at the time of first rTTP would have prevented 86 admission days and 64 TPE procedures. Our cost projection models are based on our observed efficacy rates of rituximab in our own TTP cohort, which are in keeping with those reported in other studies in the literature.7,11,15-17 If more recent data examining the use of lower doses of rituximab are further supported, this would conceivably further reduce hospital costs if rituximab were incorporated into standard treatment paradigms for all patients with TTP.12,18
In most patients in our cohort, the first dose of rituximab was administered in the hospital several days after admission, but due to incomplete records, the exact timing of rituximab administration in the hospital in relation to day of admission was uncertain for many patients. With a LOS of >14 days for initial TTP presentation, it is conceivable that some patients may have received up to 2 doses of inpatient rituximab if the first dose was administered within the first few days of presentation, which would incur more cost to the hospital. However, even if every patient in our cohort were to receive 2 doses of inpatient rituximab, the projected cost savings would still be substantial ($25 828 per patient).
In the literature, relapse rates following adjunctive treatment of TTP with rituximab, either upon initial presentation or at the time of relapse, are in the range of 10% to 20%, with some loss of efficacy over time. A UK phase 2 observational study reported relapse rates of 10% with adjunctive rituximab use compared with 57% in matched historical control subjects.7 A French observational study showed no relapses in the first year after rituximab treatment vs a relapse rate of 15.8% 3 years later.19 Data from the Oklahoma TTP Registry reported relapse rates of 12.5% when rituximab was given during a first TTP episode compared with 43% when rituximab was not given.11 In a cohort study of patients with TTP from 3 different US academic centers, relapses were rare during the first year after rituximab treatment but at 5 years were comparable to non-rituximab–treated patients.17 Our relapse rate of 18.2% at 47.2 months after rituximab treatment of our overall TTP and rTTP cohort fits well with these numbers. By comparison, the observed rates of TTP relapse in our cohort for patients who did not receive rituximab were on the higher side (71%), comparable to those reported in a different French registry study of patients with persistent, severe ADAMTS13 deficiency (74%).20 Because posttreatment surveillance ADAMTS13 levels were not routinely measured at our institution until 2017, it is possible that the patients in our study cohort may have represented a similarly higher risk population or that other factors such as ethnicity or blood type may have affected some of our numbers.11,20
Currently, the literature on rituximab favors its use during a TTP flare for relapsed patients, whereas use of rituximab during an initial TTP hospitalization as adjunctive front-line therapy with steroids and TPE to help prevent future relapses remains in question.8,21 Taken together, all studies show a consistent tendency toward decreased relapse with adjunctive rituximab use during first and relapse TTP hospitalizations. From a cost savings standpoint, we estimate that the break-even relapse rate with rituximab use in our model is ∼37%. Seeing that all observed relapse rates with rituximab in the literature are well below this, from the standpoint of potential cost savings to the hospital, our data would favor the routine incorporation of rituximab into TTP treatment paradigms, even if the first dose of rituximab were to be routinely given in the inpatient setting.
Ultimately, it is the incorporation of rituximab into standard treatment regimens for TTP, not whether it is administered on an inpatient or outpatient basis, or how many doses are administered inpatient, that is the primary cost savings measure. Logically, savings to the hospital would be maximized further by deferring rituximab administration to the outpatient setting altogether; there are potential benefits to earlier administration of rituximab, however, including fairly rapid B-cell depletion and hematologic recovery beginning within days after the first rituximab infusion, and logistical delays in initiating rituximab (eg, insurance approval) that might arise from deferring its administration entirely to the outpatient setting.22,23 Taken together, our sense is not that rituximab in the treatment of initial TTP or rTTP has to be started in the inpatient setting but, rather, that inpatient administration can be undertaken if deemed clinically necessary without adversely affecting hospital costs.
This study has several limitations. It is a retrospective, single-institution study with a relatively small sample size given the rarity of TTP. Because of our small sample size, it will be important for other institutions to perform similar analyses of rituximab use to see if comparable hospital cost savings are incurred. Also, we did not calculate a comprehensive hospitalization cost and instead used only costs of TPE and inpatient LOS as proxies. Incomplete records as to the exact transfers of patients from different levels of care did not allow us to include intensive care unit level costs; instead, all patients were assigned the cost of a general medicine bed, which has a lower cost than an intensive care unit bed. Because these factors underestimated the complete cost of a TTP hospitalization, it is likely that the net cost benefit of initiating inpatient rituximab is greater than the numbers stated here.
In conclusion, the addition of initial inpatient rituximab to TPE and steroids in the treatment of TTP produces cost savings for the hospital compared with TPE and steroids alone. This is owed to the sufficient efficacy of rituximab in preventing relapse and the cumulative costs of TPE and hospital LOS. Because analysis of cost savings of rituximab in patients with TTP has not been previously reported, we have shown for the first time that cost savings to the hospital are had in treating with rituximab both in the initial TTP and rTTP settings. Taken together, the reduction in relapse risk with rituximab and its consequent hospital cost savings would support the incorporation of rituximab into standard treatment paradigms for both initial and relapsed TTP. Although not within the scope of this study, it should be noted that prophylactic use of rituximab based on surveillance ADAMTS13 levels in the outpatient setting is emerging as an effective intervention to decrease TTP relapse rates, which would benefit from a similar cost savings analysis in the future.11 Considering the devastating effects of repeated bouts of TTP, including impairments in quality of life, mental health, and cardiovascular disease, the benefits of early rituximab treatment in TTP are expected to reverberate far beyond any initial hospital cost.24
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors acknowledge all of the patients whose data made this analysis possible. They also acknowledge the Yale Harvey Cushing/John Hay Medical Library and, in particular, Katherine Stemmer Frumento for help in conducting an exhaustive literature database search.
Footnotes
Data-sharing requests may be submitted to the corresponding author, George Goshua (e-mail: george.goshua@yale.edu).
Authorship
Contribution: G.G. and A.I.L. conceived of and designed the study; G.G. conducted chart review and collected data; and G.G., A.G., J.E.H., C.T., and A.I.L. analyzed the data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: George Goshua, Yale University School of Medicine, 333 Cedar St, Room WWW201, New Haven, CT 06520; e-mail: george.goshua@yale.edu.
References
- 1.George JN. The remarkable diversity of thrombotic thrombocytopenic purpura: a perspective. Blood Adv. 2018;2(12):1510-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westwood JP, Webster H, McGuckin S, McDonald V, Machin SJ, Scully M. Rituximab for thrombotic thrombocytopenic purpura: benefit of early administration during acute episodes and use of prophylaxis to prevent relapse. J Thromb Haemost. 2013;11(3):481-490. [DOI] [PubMed] [Google Scholar]
- 3.Butterly SJ, Pillans P, Horn B, Miles R, Sturtevant J. Off-label use of rituximab in a tertiary Queensland hospital. Intern Med J. 2010;40(6):443-452. [DOI] [PubMed] [Google Scholar]
- 4.de la Rubia J, Moscardó F, Gómez MJ, et al. ; Grupo Español de Aféresis . Efficacy and safety of rituximab in adult patients with idiopathic relapsing or refractory thrombotic thrombocytopenic purpura: results of a Spanish multicenter study. Transfus Apheresis Sci. 2010;43(3):299-303. [DOI] [PubMed] [Google Scholar]
- 5.Iioka F, Shimomura D, Ishii T, et al. Short- and long-term effects of rituximab for the treatment of thrombotic thrombocytopenic purpura: four case reports. Int J Hematol. 2012;96(4):506-512. [DOI] [PubMed] [Google Scholar]
- 6.Rüfer A, Brodmann D, Gregor M, Kremer Hovinga JA, Lämmle B, Wuillemin WA. Rituximab for acute plasma-refractory thrombotic thrombocytopenic purpura. A case report and concise review of the literature. Swiss Med Wkly. 2007;137(37-38):518-524. [DOI] [PubMed] [Google Scholar]
- 7.Scully M, McDonald V, Cavenagh J, et al. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood. 2011;118(7):1746-1753. [DOI] [PubMed] [Google Scholar]
- 8.Scully M, Hunt BJ, Benjamin S, et al. ; British Committee for Standards in Haematology . Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158(3):323-335. [DOI] [PubMed] [Google Scholar]
- 9.Uhl L, Kiss JE, Malynn E, Terrell DR, Vesely SK, George JN. Rituximab for thrombotic thrombocytopenic purpura: lessons from the STAR trial. Transfusion. 2017;57(10):2532-2538. [DOI] [PubMed] [Google Scholar]
- 10.Cuker A. Adjuvant rituximab to prevent TTP relapse. Blood. 2016;127(24):2952-2953. [DOI] [PubMed] [Google Scholar]
- 11.Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Rituximab reduces risk for relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2016;127(24):3092-3094. [DOI] [PubMed] [Google Scholar]
- 12.Zwicker JI, Muia J, Dolatshahi L, et al. Adjuvant low-dose rituximab and plasma exchange for acquired TTP. Blood. 2019;134(13):1106-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westwood JP, Thomas M, Alwan F, et al. Rituximab prophylaxis to prevent thrombotic thrombocytopenic purpura relapse: outcome and evaluation of dosing regimens. Blood Adv. 2017;1(15):1159-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scully M, Cataland S, Coppo P, et al. ; International Working Group for Thrombotic Thrombocytopenic Purpura . Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15(2):312-322. [DOI] [PubMed] [Google Scholar]
- 15.Falter T, Herold S, Weyer-Elberich V, et al. Relapse rate in survivors of acute autoimmune thrombotic thrombocytopenic purpura treated with or without rituximab. Thromb Haemost. 2018;118(10):1743-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chemnitz JM, Uener J, Hallek M, Scheid C. Long-term follow-up of idiopathic thrombotic thrombocytopenic purpura treated with rituximab. Ann Hematol. 2010;89(10):1029-1033. [DOI] [PubMed] [Google Scholar]
- 17.Sun L, Mack J, Li A, et al. Predictors of relapse and efficacy of rituximab in immune thrombotic thrombocytopenic purpura. Blood Adv. 2019;3(9):1512-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vazquez-Mellado A, Pequeño-Luévano M, Cantu-Rodriguez OG, et al. More about low-dose rituximab and plasma exchange as front-line therapy for patients with thrombotic thrombocytopenic purpura. Hematology. 2016;21(5):311-316. [DOI] [PubMed] [Google Scholar]
- 19.Froissart A, Buffet M, Veyradier A, et al. ; Experience of the French Thrombotic Microangiopathies Reference Center . Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Crit Care Med. 2012;40(1):104-111. [DOI] [PubMed] [Google Scholar]
- 20.Jestin M, Benhamou Y, Schelpe AS, et al. ; French Thrombotic Microangiopathies Reference Center . Preemptive rituximab prevents long-term relapses in immune-mediated thrombotic thrombocytopenic purpura. Blood. 2018;132(20):2143-2153. [DOI] [PubMed] [Google Scholar]
- 21.Froissart A, Veyradier A, Hié M, Benhamou Y, Coppo P; French Reference Center for Thrombotic Microangiopathies . Rituximab in autoimmune thrombotic thrombocytopenic purpura: a success story. Eur J Intern Med. 2015;26(9):659-665. [DOI] [PubMed] [Google Scholar]
- 22.Scully M, Cohen H, Cavenagh J, et al. Remission in acute refractory and relapsing thrombotic thrombocytopenic purpura following rituximab is associated with a reduction in IgG antibodies to ADAMTS-13. Br J Haematol. 2007;136(3):451-461. [DOI] [PubMed] [Google Scholar]
- 23.McDonald V, Manns K, Mackie IJ, Machin SJ, Scully MA. Rituximab pharmacokinetics during the management of acute idiopathic thrombotic thrombocytopenic purpura. J Thromb Haemost. 2010;8(6):1201-1208. [DOI] [PubMed] [Google Scholar]
- 24.Chaturvedi S, Abbas H, McCrae KR. Increased morbidity during long-term follow-up of survivors of thrombotic thrombocytopenic purpura. Am J Hematol. 2015;90(10):E208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.