Key Points
β-Adrenergic signaling downregulates multiple molecular pathways that contribute to cancer progression.
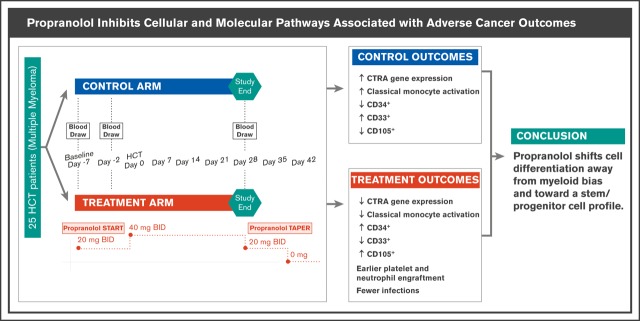
Propranolol shifts cell differentiation away from myeloid bias and toward a CD34+ stem/progenitor cell profile.
Abstract
Preclinical research shows that stress-induced activation of the sympathetic nervous system can promote hematopoietic malignancies via β-adrenoreceptor–mediated molecular pathways. Hematopoietic cell transplant (HCT) recipients exposed to conditions of chronic stress show activation of a conserved transcriptional response to adversity (CTRA) gene expression profile, which in turn is associated with increased relapse and decreased disease-free survival. We conducted a randomized controlled phase 2 biomarker trial testing the impact of the nonselective β-antagonist propranolol on CTRA-related gene expression of 25 individuals receiving an autologous HCT for multiple myeloma. Propranolol was administered for 1 week prior to and 4 weeks following HCT. Blood was collected at baseline, day −2, and day +28. Intention-to-treat analyses controlling for demographic characteristics, high-risk disease (International Myeloma Working Group risk score), and tumor stage tested effects on a 53-gene CTRA indicator profile and measures of CTRA-related cellular processes in peripheral blood mononuclear cells. Twelve participants were randomized to the intervention and 13 to the control. Relative to the control group, propranolol-treated patients showed greater decreases from baseline to HCT day −2 and day +28 for both CTRA gene expression (P = .017) and bioinformatic measures of CD16− classical monocyte activation (P = .005). Propranolol-treated patients also showed relative upregulation of CD34+ cell–associated gene transcripts (P = .011) and relative downregulation of myeloid progenitor–containing CD33+ cell–associated gene transcripts (P = .001). Ancillary analyses identified nonsignificant trends toward accelerated engraftment and reduced posttransplant infections in propranolol-treated patients. Peri-HCT propranolol inhibits cellular and molecular pathways associated with adverse outcomes. Changes in these pathways make propranolol a potential candidate for adjunctive therapy in cancer-related HCT.
Visual Abstract
Introduction
Preclinical research has found that the sympathetic nervous system (SNS) innervates the bone marrow microenvironment and regulates hematopoiesis, stem cell trafficking, and engraftment while upregulating hematopoietic stem/progenitor cell (HSPC) myeloid lineage commitment at the expense of lymphoid differentiation.1,2 Early studies initially demonstrated that the SNS regulates HSPC egress from bone marrow.3 Subsequent research suggests this physiologic trafficking is specifically regulated by circadian signaling4 and through cooperation between several β-adrenoceptor subtypes.5 SNS activity also regulates multiple molecular processes that contribute to the initiation and progression of cancer, including inflammation, angiogenesis, epithelial-mesenchymal transition, macrophage and neutrophil recruitment, cell motility and invasion, and resistance to programmed cell death and chemotherapy.6 Experimental cancer models find that SNS effects are mediated in large part via β-adrenergic receptors (particularly the β2 subtype).7-9 Consistent with these findings, retrospective pharmacoepidemiologic studies have found reduced progression of incident cancers among individuals exposed to β-adrenergic antagonists in general and nonselective (ie, β2-inhibiting) agents in particular.10-12
Given the joint involvement of both hematopoietic processes and tumor biology, SNS/β-adrenergic signaling may be particularly relevant in the context of hematologic cancers, especially when they are treated using hematopoietic cell transplantation (HCT). For example, retrospective epidemiologic data link the use of β-blockers to better prognosis in multiple myeloma, including progression-free and overall survival.13 Together, these data suggest that inhibiting β-adrenergic signaling systemically, and particularly in the bone marrow environment, could potentially improve treatment outcomes in the context of HCT.
As a consequence of altered HSPC function in the bone marrow hematopoietic environment, the circulating leukocyte pool also shows a systematic shift in basal gene expression profiles under conditions of increased SNS activity.14-16 This shift, termed the conserved transcriptional response to adversity (CTRA), is β-adrenergically mediated and characterized by increased expression of genes involved in inflammation (eg, proinflammatory cytokines such as IL1B, IL6, IL8/CXCL8, and TNF) and decreased expression of genes involved in type I interferon (IFN) antiviral responses (eg, IFI, OAS, and MX family genes) and antibody synthesis (eg, IGJ) in the peripheral blood mononuclear cell (PBMC) pool.15,17,18 This CTRA gene expression pattern is driven in part by increased hematopoietic output of immature myeloid lineage cells (particularly CD16− classical monocytes and neutrophils)14,19 and predicts poor HCT outcomes, including increased relapse and decreased disease-free survival in acute myelogenous leukemia.18
HCT is increasingly used to treat hematologic malignancies, but its efficacy remains limited by significant morbidity and mortality.20 SNS activity and CTRA effects on HSPC biology may be particularly detrimental in the peritransplantation period,21,22 and blocking SNS activity during this period could potentially enhance HCT response by promoting hematopoietic stem cell (HSC) niche homing and engraftment, enhancing immune reconstitution, and reducing posttransplant myeloid lineage bias due to changes in myelopoiesis. Here, we report on the first translational phase 2 randomized controlled trial (RCT) evaluating the efficacy of propranolol, a nonselective β-blocker, to mitigate expression of the prespecified CTRA gene profile and myeloid lineage bias in bulk PBMCs from patients undergoing their first autologous HCT for multiple myeloma. This is the first RCT to test the efficacy of a β-blocker in HCT patients.
Materials and methods
Patient population
The target patient population for this study was patients with multiple myeloma undergoing their first autologous HCT, between 18 and 75 years of age, and not on a contraindicated medication (see supplemental Tables 1 and 2 for lists of medications) or β-blocker within 3 weeks of study commencement. Multiple myeloma was chosen as the HCT disease group of interest for several reasons. First, it is the most common disease indication for HCT in the United States, with most patients receiving autologous HCT.20 Although it is not curative, HCT prolongs progression-free survival and overall survival. Thus, myeloma patients face a chronic, recurrent condition with ongoing high risk of active disease relapse, which is associated with both physiological and psychological stress.23 Finally, individuals with multiple myeloma comprise an HCT treatment group that may benefit from adjunctive propranolol administration. Akin to the perioperative period previously evaluated in the setting of β-blocker administration for antineoplastic purposes,24,25 the first 30 days following autologous HCT generally constitute the time period of greatest psychological and physiological stress and inflammatory response21,22 and subsequently also likely β-adrenergic activation.
Additional eligibility criteria included ≤1 year of initiation of systemic antimyeloma therapy; no prior progression or relapse of myeloma prior to HCT; complete response, very good partial response, partial response, or stable disease at the time of HCT, according to the International Uniform Response Criteria; able to receive melphalan 200 mg/m2 as a conditioning regimen; and available peripheral stem cell graft with >2.0 × 106 CD34+ cells/kg available for transplant. Additional exclusion criteria included patients who had a previous intolerance to β-blocker therapy or contraindications to β-blocker therapy or who had active, untreated depression. Differences in patient-, disease-, and transplant-related variables between the intervention and control groups were assessed using the Wilcoxon rank sum and Student t tests for continuous outcomes and the χ2 statistic for categorical variables. The study protocol (www.clinicatrials.gov identifier NCT02420223) was approved by the institutional review board of the Medical College of Wisconsin. Written informed consent was obtained from patients before performing any study-related procedures.
Study design and drug treatment
This was a single-site, phase 2 RCT of β-blocker administration with propranolol to individuals undergoing first autologous HCT for multiple myeloma (Figure 1). The primary objective of this study was to assess whether β-blocker administration to individuals undergoing HCT reduces (1) CTRA gene expression (an a priori–defined gene set and known risk factor for poor HCT outcomes18) and (2) myeloid lineage bias in the recovering PBMC pool (which is also prognostic of poor outcomes26). Secondary objectives included assessment of safety (adverse event [AE] rates) and quantification of differences in hematopoietic engraftment and infection rates.
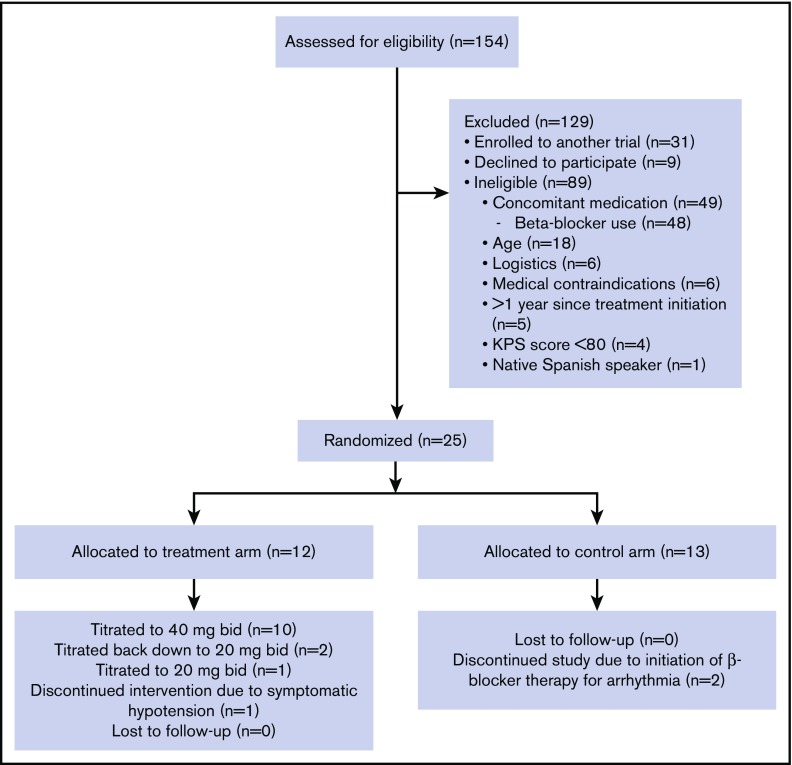
Figure 1.
CONSORT diagram of clinical trial enrollment and treatment. KPS, Karnofsky Performance Status.
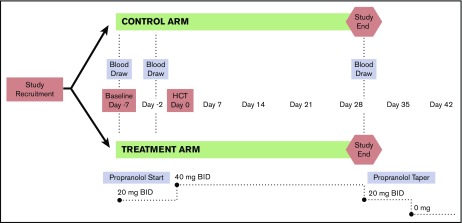
Propranolol was our chosen β-blocker for several reasons; it is the most studied nonselective β-blocker,27 has a safe side effect profile, is cost-effective, and demonstrates efficacy in vitro and in preclinical models in blocking SNS-induced alterations in HSPC biology and myeloid lineage bias15,16 and preventing tumor progression as compared with selective β-antagonists.6,28 Further, propranolol does not have any significant drug interactions with the more common antineoplastic (melphalan) and infection-related drugs used during HCT. Propranolol dosing was established by applying human dose-findings studies29 to mouse data reporting serum concentrations of propranolol needed to mitigate adverse β-adrenergic effects on tumor progression30 while also taking into account its unique pharmacokinetic profile.31 Participants began propranolol 20 mg orally twice daily at the time of study commencement (day −7 ±2 days). Data from mouse models have demonstrated that β-blockade with propranolol 8 days prior to exogenous stress exposure is effective in blocking β-adrenergic signaling at the tumor level7 and inhibiting tumor progression.9 Propranolol was taken twice daily until day +28 posttransplant. The principal investigator (PI), treating HCT physician, and study coordinator assessed drug tolerability to adjust dosing after 1 week. If participants were tolerating propranolol without any side effects, the dose was increased to 40 mg twice daily after 1 week. If participants had noticeable but less severe side effects and were able to tolerate staying on propranolol, their dose was maintained at 20 mg twice daily. Study participants who remained on ≥20 mg orally twice daily continued on as study subjects. Patients were weaned off propranolol for 1 of 3 reasons: (1) study completion, (2) intolerance secondary to side effects, or (3) onset of new medical symptoms rendering β-blocker therapy contraindicated. For patients who were at 40 mg twice daily at the time of weaning, the dose was reduced to 20 mg twice daily for 1 week before being discontinued entirely. Patients who were treated with 20 mg twice daily at the time of weaning were discontinued immediately. See Figure 2 for full treatment and assessment schema.
Figure 2.
Study design, intervention, and assessment schema. A phase 2 RCT was conducted to evaluate the impact of β-blocker treatment on the HSC niche among a cohort of multiple myeloma patients undergoing a first autologous HCT. Patients were treated starting 1 week prior to transplant and continuing for 4 weeks following HCT. Blood samples were collected at baseline/before drug initiation (T1), 2 days prior to HCT and prior to conditioning treatment with melphalan (T2), and at 28 days posttransplant (T3).
The study drug was dispensed by the investigational drug services pharmacy, and compliance was monitored by clinical research coordinators (CRCs). Tolerability was assessed clinically in both arms by the study CRC and PI on a weekly basis and once at the 1-week postpropranolol time point. Blood pressure and heart rate were assessed during weekly appointments. AEs were recorded in compliance with the National Cancer Institute’s Common Terminology Criteria for Adverse Event (CTCAE) v 4.0. Assessments at weeks 5 and 6 were required in the treatment arm only. Additional details regarding the follow-up study visit schedule and assessment time points have been previously published.32
Data and specimen collection
A study CRC drew blood at 3 study time points: day −7 (baseline, time 1 [T1]), day −2 (T2), and day +28 (T3). Blood specimens were stored in PAXgene RNA tubes at −80°C and shipped in batch overnight to the University of California, Los Angeles (UCLA) Social Genomics Core Laboratory for gene expression analysis. The Hospital Anxiety and Depression Scale (HADS) was collected weekly in coordination with β-blocker assessments to monitor for the possible AE of depression in the setting of propranolol administration. Scores of ≥8 on HADS-A (7 items) and HADS-D (7 items) connoted significant anxiety or depression, respectively33; patients scoring in these ranges were contacted by the study PI and offered a referral for further mental health care.
Transcriptome analysis
The sample size estimate of 25 was based on previous studies with similar sample sizes that have evaluated gene expression as a function of SNS activation and yielded hundreds of differentially expressed genes that generate statistically significant results in higher-order bioinformatics analyses of CTRA gene expression and related transcription factor (TF) activity (ie, the primary outcomes assessed in this trial).16,24,34 Primary analyses tested for a systematic effect of propranolol on change from baseline to follow-up in a previously defined composite score measuring the CTRA gene expression profile14-16,18 (repeated measures contrast: average of follow-up T2 and T3 values − T1 baseline value35), with contrast values tested for difference across treatment groups using a standard linear statistical analysis (ie, regression model) that controlled for a prespecified set of covariates that were either found to affect CTRA gene expression levels in previous research (ie, sex, race [white vs nonwhite], body mass index [BMI], smoking history [present/absent]) or represented major clinical variables that might be expected to significantly affect blood gene expression profiles in HCT patients (ie, high-risk cytogenetics vs other cytogenetic profiles, according to International Myeloma Working Group criteria, and disease stage represented as a 3-level categorical variable). Multicollinearity was minimal (variance inflation factor for experimental condition indicator = 1.52, well below the material threshold of 10). Two cases of missing data on disease stage were absent from primary analyses. However, these cases were included in ancillary sensitivity analyses that excluded covariates, and results continued to yield substantively similar results.
Genome-wide messenger RNA profiling was performed on isolated PBMCs in a single batch, with total RNA extracted (RNeasy; Qiagen), tested for suitable mass (Nanodrop ND1000) and integrity (Bioanalyzer; Agilent), converted to fluorescent complementary DNA (QuantSeq 3′ FWD; Lexogen), and sequenced on an Illumina HiSeq 4000 instrument in the UCLA Neuroscience Genomics Core Laboratory, following the manufacturers’ standard protocols. Data acquisition targeted >2 million 65-nt single-stranded sequence reads for each sample (achieved mean, 3.6 million; range, 2.8-4.4 million), each of which were mapped to the reference human transcriptome and quantified as gene transcript counts per million (TPM). All samples passed end-point quality control criteria, including >2 million reads, >90% aligning to the consensus human transcriptome, and a profile average correlation with other samples >0.80. TPM values were log2-transformed, floored at 0 (1 TPM), and transformed to intraindividual (repeated measures) contrast values for analysis of group differences in change over time using standard linear statistical models.
Primary analyses tested for change in an a priori–defined composite score representing the CTRA profile of upregulated expression of proinflammatory genes (19 indicator transcripts positively weighted: IL1A, IL1B, IL6, IL8/CXCL8, TNF, PTGS1, PTGS2, FOS, FOSB, FOSL1, FOSL2, JUN, JUNB, JUND, NFKB1, NFKB2, REL, RELA, and RELB) and downregulated expression of genes involved in type I IFN and antibody responses (32 indicator transcripts negatively weighted: GBP1, IFI27, IFI27L1-2, IFI30, IFI35, IFI44, IFI44L, IFI6, IFIH1, IFIT1-3, IFIT5, IFIT1B, IFITM1-3, IFITM4P, IFITM5, IFNB1, IRF2, IRF7-8, MX1-2, OAS1-3, OASL, JCHAIN, and IGLL1), with a specific focus on the myeloid cell lineage, as consistent with our hypothesis.15,17,36 Composite scores were computed for each individual at each time point, change over time in composite score values was quantified by intraindividual (repeated measures) contrasts as described above, and group differences in change contrast values were tested for statistical significance by comparison with standard errors (SEs) derived from bootstrap resampling of linear model residual vectors from the primary linear model analysis (which controls for correlation among residuals across genes).37
To cross-validate analyses of the primary end point using a different analytic approach, we reanalyzed the transcriptome profiling data using Transcript Origin Analysis (TOA) to identify the specific leukocyte subsets mediating the empirically observed differences in gene expression, as previously described.18,38 Analyses used reference data on isolated samples of (1) major leukocyte subsets, including total monocytes, B cells, CD4+ and CD8+ T cells, natural killer cells, and plasmacytoid dendritic cells (Gene Expression Omnibus series GSE1133); (2) major bone marrow mononuclear cell populations, including HSC-containing CD34+ cells, myeloid lineage CD33+ cells, erythroid lineage CD71+ cells, and endothelial CD105+ cells (also from GSE1133); and (3) classical (CD16−) and nonclassical (CD16+) monocyte subsets (GSE25913). Reference data yield a cell-type diagnosticity score for each gene, and the average cell-type diagnosticity score for the subset of genes identified as differentially expressed in this study was compared with the null hypothesis value of all gene transcripts in the assay sampling frame, using bootstrap-derived SEs to assess statistical significance as described above. These analyses tested the hypothesis that propranolol would reduce the myeloid-lineage (monocyte) bias in PBMC transcriptome changes from baseline to follow-up.
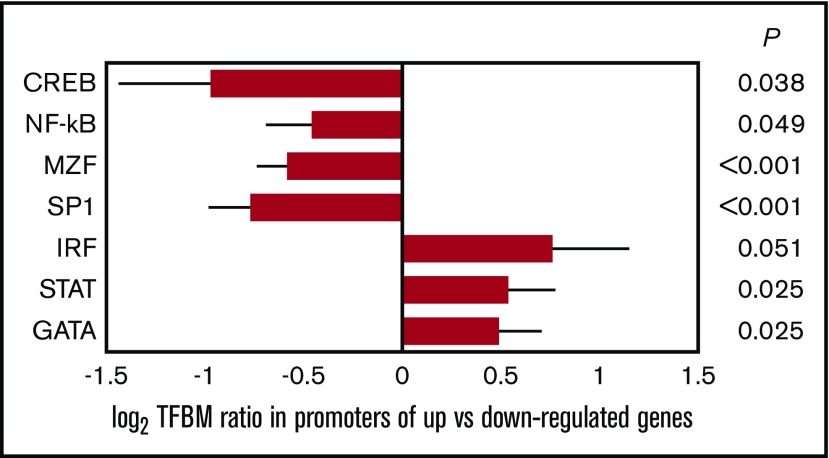
To further cross-validate the primary CTRA results, we also conducted secondary bioinformatics analyses of transcription control pathway activity using the Transcription Element Listening System (TELiS) promoter-based bioinformatic analysis. This analysis tested the hypothesis that propranolol would (1) reduce activity of a priori–defined CTRA-relevant TFs, including the cyclic adenosine monophosphate response element–binding protein (CREB) factors involved in mediating β-adrenergic signals, the myeloid zinc finger (MZF) and specificity protein 1 (Sp1) family factors involved in myeloid differentiation, and the proinflammatory NF-κB/Rel factors; and (2) increase activity of the IFN-mediating signal transducer/activator of transcription 1 (STAT1) and IFN response factors (IRF), which are inverse components of the CTRA, and the GATA family involved in lymphoid differentiation. Promoters of all genes showing more than twofold differential change in expression over time for the propranolol vs control group were scanned for TF-binding motifs (TFBM) using TRANSFAC position-specific weight matrices V$CREB_Q4, V$GATA1_03, V$MZF1_01, V$SP1_01, V$CREL_01, V$STAT1_01, V$IRF_01.39 Transcription factor activity was inferred from the ratio of TFBM prevalence in up- vs downregulated gene sets,39 with log2-transformed TFBM prevalence ratios averaged over 9 parametric variations of TRANSFAC MatInspector scan stringency and promoter length as previously described.39 Mean TFBM ratios for differentially expressed genes were tested for statistical significance using SEs derived from bootstrap resampling of linear model residual vectors as described above.
This study was not designed or powered for de novo discovery of statistically significant effects of propranolol on expression of individual gene transcripts, and we did not conduct any analysis of statistical significance at the level of individual genes; we analyzed bulk PBMCs focusing on the a priori defined hypotheses regarding the myeloid cell lineage within the PBMC pool. To avoid capitalization on chance in any single analytic approach, we conducted 2 convergent validation analyses to confirm the results of the primary hypothesis tested by CTRA indicator score, using TELiS analyses of TF regulation and TOA analyses of cellular origin to verify indications of myeloid lineage gene regulation as hypothesized above based on previous preclinical research. This general statistical approach is appropriate for studies that test a single conceptual hypothesis using multiple convergent measures.40,41 Maximum likelihood point estimates of individual transcripts’ differential change in expression between study groups served solely as inputs into high-level TELiS and TOA gene set expression analyses, which maintained their own control over statistical error rates as described above and serve as the only genomics outcomes analyzed in this study.
Clinical outcomes
General linear model analyses were used to assess the relationship between treatment group assignment and (log-transformed) days to neutrophil engraftment (first day of absolute neutrophil count >0.5 × 109/L sustained for 3 consecutive assessments at least 1 day apart) and platelet engraftment (first day of platelet count of >20 × 109/L independent of platelet transfusions for 3 consecutive assessments at least 1 day apart). Fisher’s exact test assessed group differences in prevalence of infection (occurrence of ≥1 documented culture-positive infection or neutropenic fever episodes >100.4°F). For original data inquiries, please contact the corresponding author.
Results
Demographics, AEs, and drug adherence
Between July 2015 and March 2017, 154 patients were identified as meeting initial criteria of having a planned first autologous transplant for multiple myeloma. Comprehensive screening, eligibility, randomization, and exclusion criteria are contained in Figure 1, with a final enrollment of 25 participants (12 in the propranolol group and 13 in the control arm). There were no significant baseline differences on patient-, disease-, and transplant-related characteristics (Table 1). No patients in either study arm experienced any serious AEs. Of the 13 patients in the control arm, 2 patients came off study due to starting a β-blocker posttransplant, 1 for tachycardia (day +18) and 1 for atrial fibrillation with rapid ventricular response (day +0). Of the 12 patients in the propranolol group, 11 (92%) were able to remain on the study drug, with 1 coming off study due to persistent hypotension despite dose reduction. Of the 10 patients who returned their pill bottles, there was a 94% drug adherence rate. Please see Knight et al32 for additional details on AEs and adherence.
Table 1.
Baseline patient and disease characteristics by treatment group
| Propranolol | Control | |
|---|---|---|
| Age, mean (range), y | 59 (36-72) | 63 (54-74) |
| Sex, % | ||
| Male | 58 | 62 |
| Female | 42 | 38 |
| Race | ||
| White, % | 67 | 85 |
| Black or African American, % | 33 | 15 |
| BMI, mean (range) | 30 (25.9-33.6) | 33 (30.1-35.9) |
| KPS | 88 | 90 |
| ≥90 | 7 | 11 |
| <90 | 5 | 2 |
| IMWG risk score, % | ||
| High-risk disease | 25 | 46 |
| Standard-risk disease | 75 | 54 |
| T staging | ||
| Stage 1 | 3 | 5 |
| Stage 2 | 7 | 5 |
| Stage 3 | 1 | 2 |
| Smoking history, % | 33 | 54 |
| Income status, $ | ||
| <23 000 | 1 | 2 |
| 23 000-34 999 | 4 | 1 |
| 35 000-44 999 | 1 | 1 |
| 45 000-54 999 | 2 | 2 |
| 55 000-64 999 | 0 | 1 |
| >75 000 | 4 | 3 |
| Prefer not to answer | 0 | 3 |
| Education | ||
| 8th grade education or less | 0 | 1 |
| Some high school, no diploma | 0 | 2 |
| High school diploma, GED, or equivalent | 5 | 2 |
| Postsecondary work (vocational, technical, some college) | 5 | 5 |
| 4-y degree | 0 | 1 |
| Master’s degree | 1 | 1 |
| Postgraduate/professional degree (eg, PhD, MD, JD) | 0 | 1 |
| Prefer not to answer | 1 | 0 |
| Outpatient transplant, % | 25 | 23 |
| CD34+ dose, 106/kg | 5.1 | 4.6 |
| Clinical response pretransplant | ||
| Stable disease | 1 | 0 |
| Partial response | 5 | 5 |
| Very good partial response | 5 | 5 |
| Complete response | 1 | 3 |
Values are number of patients, unless otherwise indicated.
IMWG, International Myeloma Working Group.
CTRA
In analyses controlling for sex, race, BMI, smoking history, high-risk cytogenetics, and disease stage, propranolol reduced the magnitude of increase in CTRA composite scores from baseline to the average of T2 and T3 follow-ups by an average of 25% relative to the control group (Figure 3A; differential change in log2 RNA abundance = −0.407 ± SE 0.165, P = .017). CTRA gene expression showed a nonsignificant 7% increase from pre- to posttransplant periods in placebo-treated controls (+0.099 ± SE 0.103, P = .337), whereas propranolol-treated patients showed a statistically significant 18% reduction in CTRA gene expression from pre- to posttransplant (−0.285 ± 0.102, P = .007). Effects of the covariates on CTRA expression are reported in supplemental Table 3. Similar results emerged from unadjusted analyses that omitted all covariates (change in CTRA gene expression for placebo-treated patients = −0.046 ± 0.098, P = 0.644; for propranolol-treated patients: −0.211 ± 0.094, P = .028).
Figure 3.
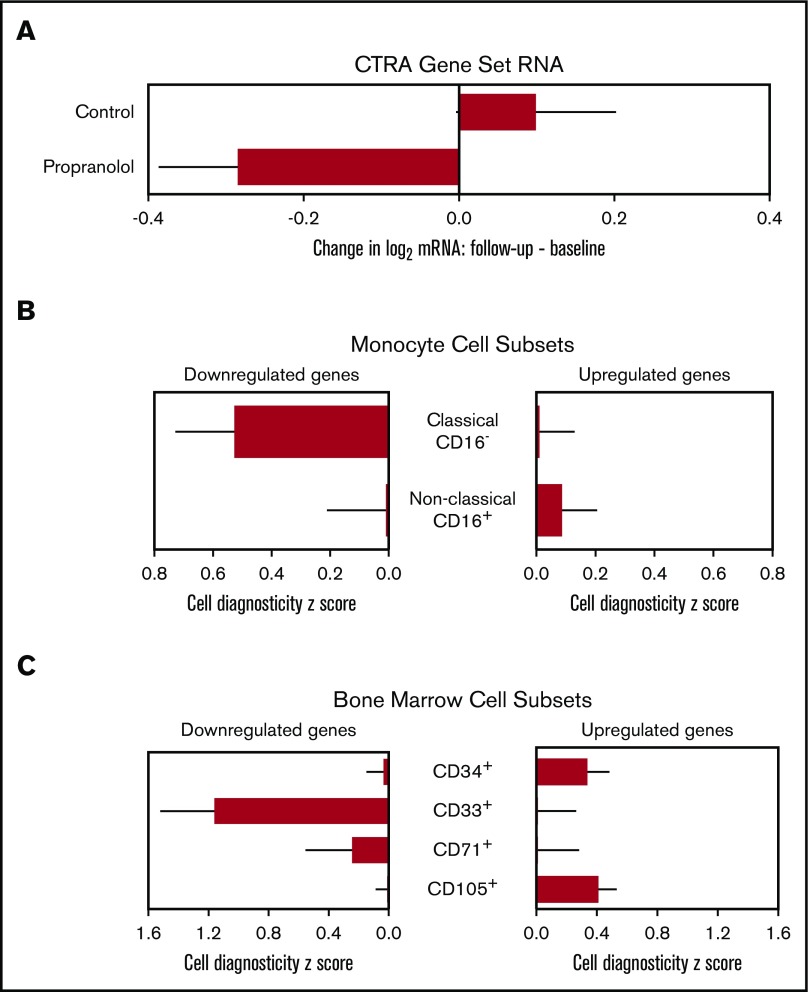
Expression of the CTRA gene set, cellular origin, and HSC lineage. (A) Linear model–based estimates of fold-difference from the mean in expression in a 51-gene CTRA contrast score in PBMCs from propranolol-treated patients relative to controls from baseline to T2 and T3 (P = .017) (adjusting for age, sex, race, BMI, smoking history, cytogenetics, and stage) (P = .017). (B) Bioinformatic measures of CD16− classical monocyte activation within PBMC population as indicated by TOA cell-type diagnosticity z scores (P = .005).38 (C) Propranolol-treated patients showed relative upregulation of HSC-containing CD34+ cells (P = .011) and relative downregulation of myeloid progenitor–containing CD33+ cells (P = .001). Error bars indicate SE.
Myeloid lineage bias
To cross-validate the primary results involving the a priori CTRA composite score, we conducted an independent analysis of the cellular mechanisms involved in propranolol’s empirical effects on PBMC gene expression (ie, not based on an a priori–specified gene set but testing an a priori hypothesis about cellular influences on the empirical transcriptome differences). Because the CTRA is mediated by β-adrenergic upregulation of classical monocyte gene expression via myelopoiesis,15,17,18 and upregulation of classical monocyte gene expression is prognostic of poor outcomes in HCT,26 we performed TOA to quantify monocyte activation within the PBMC pool. In analyses of all 407 genes showing a maximum likelihood point estimate of more than twofold differential change in expression from baseline to the average of follow-up time points 2 and 3 in propranolol-treated patients relative to controls (131 relatively upregulated by propranolol and 276 relatively downregulated), results indicated that gene transcripts downregulated by propranolol derived predominately from monocytes in general (mean cell diagnosticity score = 0.768 ± SE 0.193, P < .001), and from CD16− classical monocyte transcripts in particular (Figure 3B; 0.527 ± 0.201, P = .005). Parallel TOA analyses using reference profiles from isolated bone marrow cell populations also found propranolol-upregulated gene transcripts to derive preferentially from CD34+ stem/early progenitor cells (Figure 3C; 0.338 ± 0.145, P = .011) and CD105+ endothelial cells (Figure 3C; 0.409 ± 0.122, P < .001), whereas propranolol-downregulated genes derived from more differentiated CD33+ myeloid progenitors (Figure 3C; 1.161 ± 0.361, P = .001).
TFBMs
To further cross-validate primary CTRA analyses, we conducted TELIS TF analyses of empirical transcriptome differences to determine whether they involved decreased activity of CTRA-related TFs (eg, β-adrenergic–responsive CREB, myeloid-linked MZF, and proinflammatory NF-κB) and/or increased activity of antiviral (eg, IRF and STAT) and lymphoid lineage–associated TFs (eg, GATA). In TF analyses of the same 407 genes showing more than twofold differential change over time for propranolol-treated patients, results showed the expected reduction in CREB activity (consistent with β-blockade), MZF and Sp1 activity (reduced myeloid bias), and NF-κB activity (reduced inflammation), as well as increased activity for IRF, STAT, and GATA family TFs (Figure 4).
Figure 4.
Bioinformatic measures of TF activity. Data represent log2 ratio TFBM prevalence within the promoters of genes showing relative upregulation vs downregulation in PBMC samples from propranolol-treated patients relative to controls. Results are provided for TF pathways positively associated with CTRA biology (β-adrenergic–responsive CREB, myeloid-linked MZF, and proinflammatory NF-κB) and negatively associated with CTRA biology (IFN-related IRF and STAT and lymphoid-linked GATA). Error bars indicate SE.
Clinical and hematological outcomes
This study was not designed or powered to assess propranolol effects on clinical outcomes (eg, progression-free survival or overall survival). However, for descriptive and safety purposes, we monitored hematopoietic recovery times and infection rates. Average time to neutrophil engraftment was 10.5 days in the propranolol group vs 11.9 days in the control group (difference P = .14 by Student t test). Average time to platelet engraftment was 16.6 days in the propranolol group vs 19.6 days in the control group (P = .41). Among those in the control group, there were 6 episodes of either documented culture positive infection or neutropenic fever episodes >100.4°F (46%) compared with 1 in the propranolol-treated group (8%; difference P = .06 by likelihood ratio χ2 test).
Discussion
In this randomized controlled phase 2 biomarker trial of peritransplant propranolol administration in 25 multiple myeloma patients undergoing autologous HCT, propranolol-treated patients showed significant reductions in expression of CTRA indicator genes (primary outcome), as well as significant reductions in myeloid lineage bias and proinflammatory TF activity, and significant increases in innate antiviral and lymphoid lineage-related TF activity (secondary outcomes) during hematologic recovery. Propranolol administration was found to be safe, well tolerated, and associated with a nonsignificant trend toward reduced infection rates and accelerated hematopoietic engraftment. All results emerged from analyses that controlled for sex, race, BMI, smoking history, high-risk cytogenetics, and disease stage (and similar effects also emerged in unadjusted analyses). These data provide the first clinical test of the concept that pharmacologic inhibition of β-adrenergic signaling during HCT may facilitate hematopoietic recovery and reduce the myeloid lineage bias that has been found to be prognostic of poor long-term outcomes.42 These data also demonstrate the ability of propranolol to downregulate, in a human clinical setting, the expression of the CTRA gene expression profile, a molecular marker of SNS regulation of stem cell biology derived from extensive preclinical analyses of neural regulation of hematopoiesis43 and a profile previously linked to poor HCT outcomes in clinical transplantation.18 Overall, these findings provide translational biomarker evidence that peritransplant propranolol could potentially provide a safe, inexpensive, and accessible treatment option to supplement traditional cancer therapies and improve cancer outcomes through regulation of the bone marrow niche.
Dysregulation of the bone marrow hematopoietic niche by SNS activation is associated with worse engraftment in preclinical models.42,43 The current data are consistent with those findings and suggest that pharmacologic blockade of SNS-induced β-adrenergic signaling during human HCT may promote engraftment, with greater levels of CD34+ progenitor cells and fewer days to neutrophil and platelet engraftment observed among patients exposed vs unexposed to propranolol. While evidence suggests an important role for the β2 adrenergic subtype in cancer processes and HSC trafficking,7-9 a key role for β3 has also been described in mouse models,4,5,44 and other research suggests a prominent role of β1-adrenoreceptors in SNS-mediated inflammatory biology.45 These divergencies highlight the importance of continued investigation regarding potential off-target propranolol interactions with β3 as well as further delineation of the β-adrenergic subset lineage(s) responsible for HSC niche and malignant processes. TOA analyses link propranolol to reduced myeloid lineage bias during hematopoietic recovery, an effect associated with improved overall and disease-free survival and reduced transplant-related mortality and relapse following HCT.46
While the current study was not powered to evaluate clinical outcomes and they did not reach statistical significance, it is notable that the propranolol group experienced clinically meaningful improvement in average days to engraftment for both neutrophils (1.4 days sooner) and platelets (3 days sooner). In conjunction with favorable effects on CTRA expression and myeloid lineage bias, these results suggest that propranolol in the peritransplant period could potentially impact clinical outcomes. Upregulated expression of CD34+ cell–associated gene transcripts and earlier engraftment in the propranolol cohort is consistent with the concurrent upregulation of CD105+ cell–associated gene transcripts, as CD105 marks immature, long-term repopulating HSCs.47 CD105 can also be found on mesenchymal stem cells, but those are not expected to be present at any significant level in the circulating PBMC samples analyzed here. An appropriately powered clinical trial assessing both hematologic recovery and other relevant clinical outcomes is justified. Finally, based on prior work indicating an association between low socioeconomic status, increased CTRA biology, and worse clinical outcomes following HCT,18 propranolol may have the capacity to diminish cancer outcome disparities in combination with traditional treatments.
Despite advances in targeted cancer immunotherapies, these agents have had modest impact on event-free and overall survival.48 Emerging evidence suggests a biologically heterogeneous cancer milieu, necessitating the need for combination treatments.48-50 Further, with cancer being largely a disease of aging and accumulating evidence that cancer treatments promote advanced physiologic age,51 it should be noted that recent evidence suggests a role for β2 signaling in contributing to advanced HSC niche aging and myeloid expansion.52 Finally, many of the newer cancer drugs remain inaccessible to a large portion of the global population due to cost, while existing drugs such as propranolol are significantly less expensive,53 providing not only an efficacious concomitant therapy choice but also an attainable one.
The study’s findings are limited in several respects. The study was designed as a randomized controlled phase 2 biomarker trial of propranolol to inhibit cellular and molecular pathways previously associated with adverse HCT outcomes. It was not powered on clinical outcomes; however, early indication that propranolol-exposed individuals experienced clinically significant improvements in posttransplant hematopoietic recovery is congruent with prior preclinical data describing neural regulation of the bone marrow niche42 and supports future studies powered to assess these and other long-term outcomes. Relapse and reduced disease-free survival are associated with heightened β-adrenergic signaling and CTRA biology18,26 and may have promise as future clinical study biomarker end points. Larger sample sizes would be needed for genome-wide discovery studies aimed at detecting associations at the level of specific individual genes. If possible, future studies should note percentage of potential participants who were on a β-blocker but enrolled into another trial or declined to participate. Finally, the current study examined a single disease subtype of autologous HCT recipients in a circumscribed peritransplant timeframe of 5 weeks of propranolol exposure. Future work is needed to assess additional disease groups, allogeneic recipients, differential drug timing and duration, and drug combinations.
This study demonstrates the capacity of the nonselective β-antagonist propranolol to inhibit an SNS-regulated, bone marrow–derived adverse gene expression profile previously associated with poor HCT outcomes,18,26 shift cell differentiation away from a myeloid bias, and promote engraftment. As such, propranolol may be effective in supplementing current cancer therapies to improve HCT outcomes through regulation of the bone marrow niche.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was funded in part by the National Cancer Institute, National Institutes of Health contract HHSN261200800001E; the National Cancer Institute Network on Biobehavioral Pathways in Cancer, the National Center for Advancing Translational Sciences, National Institutes of Health grants UL1TR001436 and KL2TR001438; Medical College of Wisconsin institutional research grant 86-004-26 from the American Cancer Society; National Institute on Aging, National Institutes of Health grant P30 AG017265; the USC/UCLA Center on Biodemography and Population Health; and the Laura Gralton Philanthropic Fund.
Footnotes
For data sharing, e-mail the corresponding author, Jennifer M. Knight (e-mail: jmknight@mcw.edu).
Authorship
Contribution: J.M.K., J.D.R., M.C.P., B.R.L., M.M.H., and S.W.C. designed research; J.M.K., J.D.R., P.H., M.C.P., K.E.G., A.D., B.R.L., M.H., S.C., B.D., N.S., D.S., M.M.H., and S.W.C. performed research; B.R.L. and S.W.C. contributed vital new reagents or analytical tools; J.M.K., P.H., M.C.P., K.E.G., A.D., M.H., S.C., B.D., N.S., and D.S. collected data; J.M.K., J.D.R., K.E.G., B.R.L., and S.W.C. analyzed and interpreted data; B.R.L. and S.W.C. performed statistical analysis; and J.M.K., J.D.R., P.H., M.C.P., K.E.G., A.D., B.R.L., M.H., S.C., B.D., N.S., D.S., M.M.H., and S.W.C. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jennifer M. Knight, Medical College of Wisconsin, 9200 W Wisconsin Ave, 4th Floor Cancer Center, Suite 4100, Milwaukee, WI 53226; e-mail: jmknight@mcw.edu.
References
- 1.Hanoun M, Zhang D, Mizoguchi T, et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell. 2014;15(3):365-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arranz L, Sánchez-Aguilera A, Martín-Pérez D, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512(7512):78-81. [DOI] [PubMed] [Google Scholar]
- 3.Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407-421. [DOI] [PubMed] [Google Scholar]
- 4.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442-447. [DOI] [PubMed] [Google Scholar]
- 5.Méndez-Ferrer S, Battista M, Frenette PS. Cooperation of beta(2)- and beta(3)-adrenergic receptors in hematopoietic progenitor cell mobilization. Ann N Y Acad Sci. 2010;1192(1):139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18(5):1201-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamkin DM, Sloan EK, Patel AJ, et al. Chronic stress enhances progression of acute lymphoblastic leukemia via β-adrenergic signaling. Brain Behav Immun. 2012;26(4):635-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neeman E, Zmora O, Ben-Eliyahu S. A new approach to reducing postsurgical cancer recurrence: perioperative targeting of catecholamines and prostaglandins. Clin Cancer Res. 2012;18(18):4895-4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sloan EK, Priceman SJ, Cox BF, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70(18):7042-7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powe DG, Voss MJ, Zänker KS, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1(7):628-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol. 2011;29(19):2635-2644. [DOI] [PubMed] [Google Scholar]
- 12.De Giorgi V, Grazzini M, Gandini S, et al. Treatment with β-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med. 2011;171(8):779-781. [DOI] [PubMed] [Google Scholar]
- 13.Hwa YL, Shi Q, Kumar SK, et al. Beta-blockers improve survival outcomes in patients with multiple myeloma: a retrospective evaluation. Am J Hematol. 2017;92(1):50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell ND, Sloan EK, Bailey MT, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA. 2013;110(41):16574-16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11(9):625-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8(9):R189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole SW, Conti G, Arevalo JMG, Ruggiero AM, Heckman JJ, Suomi SJ. Transcriptional modulation of the developing immune system by early life social adversity. Proc Natl Acad Sci USA. 2012;109(50):20578-20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knight JM, Rizzo JD, Logan BR, et al. Low socioeconomic status, adverse gene expression profiles, and clinical outcomes in hematopoietic stem cell transplant recipients. Clin Can Res. 2016;22(1):69-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidt T, Sager HB, Courties G, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20(7):754-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Souza A, Lee S, Zhu X, Pasquini M.. Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2017;23(9):1417-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McQuellon RP, Russell GB, Rambo TD, et al. Quality of life and psychological distress of bone marrow transplant recipients: the “time trajectory” to recovery over the first year. Bone Marrow Transplant. 1998;21(5):477-486. [DOI] [PubMed] [Google Scholar]
- 22.Wang XS, Shi Q, Shah ND, et al. Inflammatory markers and development of symptom burden in patients with multiple myeloma during autologous stem cell transplantation. Clin Cancer Res. 2014;20(5):1366-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih M, Simon PA. Health-related quality of life among adults with serious psychological distress and chronic medical conditions. Qual Life Res. 2008;17(4):521-528. [DOI] [PubMed] [Google Scholar]
- 24.Shaashua L, Shabat-Simon M, Haldar R, et al. Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin Cancer Res. 2017;23(16):4651-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang HI, Lim SH, Lee YY, et al. Perioperative administration of propranolol to women undergoing ovarian cancer surgery: A pilot study. Obstet Gynecol Sci. 2017;60(2):170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight J, Rizzo JD, Wang T, et al. Genomic mechanisms of SES-related outcome disparities in hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2018;24(3):S26. [Google Scholar]
- 27.Wong GWK, Wright JM. Blood pressure lowering efficacy of nonselective beta‐blockers for primary hypertension. Cochrane Database Syst Rev. 2014;(2):CD007452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin X, Luo K, Lv Z, Huang J. Beta-adrenoceptor action on pancreatic cancer cell proliferation and tumor growth in mice. Hepatogastroenterology. 2012;59(114):584-588. [DOI] [PubMed] [Google Scholar]
- 29.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659-661. [DOI] [PubMed] [Google Scholar]
- 30.Kim-Fuchs C, Le CP, Pimentel MA, et al. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun. 2014;40:40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shargel L, Yu AB Applied Biopharmaceutics and Pharmacokinetics. New York: McGraw-Hill/Appleton & Lange; 1999. [Google Scholar]
- 32.Knight JM, Kerswill SA, Hari P, et al. Repurposing existing medications as cancer therapy: design and feasibility of a randomized pilot investigating propranolol administration in patients receiving hematopoietic cell transplantation. BMC Cancer. 2018;18(1):593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69-77. [DOI] [PubMed] [Google Scholar]
- 34.Miller GE, Chen E, Sze J, et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64(4):266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenthal R, Rosnow RL Contrast Analysis: Focused Comparisons in the Analysis of Variance. New York: Cambridge University Press; 1985. [Google Scholar]
- 36.Fredrickson BL, Grewen KM, Coffey KA, et al. A functional genomic perspective on human well-being. Proc Natl Acad Sci USA. 2013;110(33):13684-13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Efron B, Tibshirani RJ An Introduction to the Bootstrap. Boca Raton, FL: CRC Press; 1994. [Google Scholar]
- 38.Cole SW, Hawkley LC, Arevalo JMG, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci USA. 2011;108(7):3080-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole SW, Yan W, Galic Z, Arevalo J, Zack JA. Expression-based monitoring of transcription factor activity: the TELiS database. Bioinformatics. 2005;21(6):803-810. [DOI] [PubMed] [Google Scholar]
- 40.Cao J, Zhang SJJ. Multiple comparison procedures. JAMA. 2014;312(5):543-544. [DOI] [PubMed] [Google Scholar]
- 41.Bender R. Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol. 2001;54(4):343-349. [DOI] [PubMed] [Google Scholar]
- 42.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20(8):833-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.del Toro R, Méndez-Ferrer S. Autonomic regulation of hematopoiesis and cancer. Haematologica. 2013;98(11):1663-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maryanovich M, Zahalka AH, Pierce H, et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat Med. 2018;24(6):782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.García-Prieto J, Villena-Gutiérrez R, Gómez M, et al. Neutrophil stunning by metoprolol reduces infarct size. Nat Commun. 2017;8(1):14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turcotte LM, Cao Q, Cooley SA, et al. Monocyte subpopulation recovery as predictors of hematopoietic cell transplantation outcomes. Biol Blood Marrow Transplant. 2019;25(5):883-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kays SK, Kaufmann KB, Abel T, et al. CD105 is a surface marker for receptor-targeted gene transfer into human long-term repopulating hematopoietic stem cells. Stem Cells Dev. 2015;24(6):714-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fojo T, Parkinson DR. Biologically targeted cancer therapy and marginal benefits: are we making too much of too little or are we achieving too little by giving too much? Clin Cancer Res. 2010;16(24):5972-5980. [DOI] [PubMed] [Google Scholar]
- 49.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6(3):240-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15(9):563-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106(4):dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ho YH, Del Toro R, Rivera-Torres J, et al. Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiological aging. Cell Stem Cell. 2019;25(3):407-418.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bertolini F, Sukhatme VP, Bouche G. Drug repurposing in oncology—patient and health systems opportunities. Nat Rev Clin Oncol. 2015;12(12):732-742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.