Abstract
This work presents spatially compounded plane wave imaging using a laser-induced ultrasound source. The plane wave source consisted of a 30 μm thick film of carbon black-doped PDMS cured on a 100 μm thick polyester substrate and presented a rectangular aperture of 40 × 3 mm. It was placed in front of a linear ultrasound array, passing through the imaging plane allowing overlap of the detection plane and the insonification plane. Illumination was provided by an array of optical fibre bundles placed above the imaging plane, at an angle. We will first present the general imaging set up and instrumentation used, after which details are given on the fabrication of the transmitter itself and on the objects that were imaged. Comparing laser-induced and conventional ultrasound images of wire phantoms shows the point-spread-function to be, in general, slightly better laterally in the conventional case but more homogeneous throughout the imaging plane with the laser-induced source. Imaging of a tissue-mimicking phantom shows a 55% improvement in contrast between a tumour and the background when using laser-induced ultrasound, as compared to the conventional case.
Keywords: Laser-induced ultrasound, Plane wave imaging, Spatial compounding, PDMS, Carbon black, Phantom
1. Introduction
Laser-induced ultrasound (LIUS) sources, which exploit the photoacoustic effect in a designed optical absorber as an ultrasound source for imaging, are a relatively new subject of study in the field of biomedical imaging, often used in combination with photoacoustic tomography [1], [2], [3]. Most effective LIUS transmitters for biomedical imaging purposes are based on some combination of an elastomeric host material seeded with an optically absorbing agent [4], [5], [6], [7], [8], [9], [10], [11]. The host material ideally provides a high thermal expansion coefficient, allowing for efficient photoacoustic pressure generation, as well as simple fabrication techniques and low cost. The added optical absorber is often a carbon compound such as carbon black, as this is highly optically absorbing. The easy and flexible fabrication methods offered by elastomeric materials such as polydimethylsiloxane (PDMS) [12] allow for a wide range of transmitter designs and field geometries adaptable to an array of biomedical applications.
Several groups have demonstrated purpose-built systems for combined photoacoustic and LIUS tomography of small animals. Wurzinger et al. [13] built an all-optical system based on a free-space Mach–Zehnder interferometer and a plane wave LIUS source, where a tomographic image is obtained by rotating the sample, in this case a zebrafish. Similarly Ermilov et al. [2] built a small animal imaging device with a cylindrical geometry based on rotation of detectors and emitters around the sample, using piezoelectric detectors.
Alles et al. performed imaging using a fibre-optic LIUS pencil beam source and a fibre-optic US detector based on a Fabry–Pérot etalon [14]. Linear scanning of the colocated source and detector produced images of ex vivo swine aorta. In a different paper [15] they also performed video-rate LIUS imaging, using a single fibre-optic detector and LIUS sources at multiple locations along a linear aperture to image a zebrafish and pulsating swine carotid artery, both ex vivo.
Kruizinga et al. [16] presented LIUS imaging with an off-the-shelf linear array US detector, in a biomedically relevant hydrogel phantom containing a hyperechoic mass and several point scatterers. Their LIUS plane wave source was based on a black polyethylene film placed in direct contact with the top surface of the phantom, and detected by a clinical ultrasound array placed at 10 mm distance from the film. This allowed transmission of plane waves at a single insonification angle, although the resulting images exhibited a relatively poor resolution and signal-to-noise ratio. The quality of the images may be improved substantially by the usage of multiple insonification angles and spatial compounding, as is already done routinely in conventional plane wave imaging [17].
This work presents a set up which enables multiple plane wave insonification angles by using a mechanically rotated LIUS source suspended in front of a clinical US array. Images are taken of a crossed-wire phantom and a breast-mimicking phantom to assess the performance of spatially compounded LIUS plane wave imaging (PWI), while also comparing the results with conventionally acquired spatially compounded plane wave images. Comparable values of axial and lateral resolution are found between the two methods and higher contrast from a hyperechoic tumour in the breast phantom is seen for the LIUS case.
2. Materials and methods
2.1. Concept
The LIUS plane wave imaging set up, installed in a water tank, is presented in Fig. 1. It consists of a linear ultrasound array as a receiver and a carbon black-doped PDMS film on an acoustically transparent polyester substrate as LIUS transmitter. The LIUS transmitter is placed in front of the receiver at a distance of 11 mm, leaving space for it to rotate freely over a range of 30°, allowing acquisition of signals over multiple insonification angles as indicated in the top view in Fig. 1(a). LIUS pulses are generated by laser illumination of the transmitter through four fibre bundle outputs connected to a single input, into which the laser light is coupled. The generated LIUS plane wave will back-scatter from the sample (a wire phantom or tissue mimicking breast phantom) and propagate through the transmitter to the receiver, where it is detected as illustrated by the red arrow in the side view in Fig. 1(c). For a series of different transmit angles the detected signals can be reconstructed into individual images, which are summed to create a spatially compounded image.
Fig. 1.
(a) Top view schematic of the set up used, including laser-induced ultrasound source with illumination fibres, ultrasound array and sample. The maximum and minimum insonification angles are indicated. (b) Photograph of the set up in side view, including the probe, custom-built LIUS mount and breast phantom. (c) Schematic side view of the set up, indicating the location of the array, LIUS source, illumination and sample. Yellow arrows point to the corresponding structures in the photograph, the red arrow indicates the path the LIUS pulse takes from the transmitter to the sample and back through the transmitter to the detector.
Conventional spatially compounded plane wave imaging is also performed, using the receiver as a phased-array transmitter to generate angled plane waves, allowing for comparison to LIUS imaging. To ensure a fair comparison, the PDMS film is left in place during conventional imaging and rotated to match the insonification angle. The in-plane placement of the LIUS transmitter has the advantage that it enables a one-to-one comparison of LIUS to conventional images, however it does lead to some reflection artefacts, which have to be removed in post-processing.
2.2. Instrumentation
The excitation light, 10-ns pulses at 532 nm from a frequency-doubled Nd:YAG laser (Quanta Ray pro-250, Spectra Physics, USA), is coupled directly into a multi-output fibre bundle (CeramOptec GmbH, Germany) with a 12 mm input diameter. Four 4.3 mm diameter outputs of the fibre bundle illuminate the LIUS transmitter with the pulse energy distributed equally between them. The pulse energy per fibre output for LIUS generation is 10 mJ. The fibre bundles are rigidly connected to the LIUS transmitter in a custom-designed, 3D-printed mount and illuminate the transmitter from outside the imaging plane at a 30° angle as shown in Fig. 1(b) and (c). The mount is connected to a rotation stage such that the lateral centre of the transmitter is aligned with the rotation axis, at a distance of 11 mm from the receiver to allow for a 30-degree range of rotation, from −15° to +15° in 1° steps. The US receiver is a 5–11 MHz 128-element linear array (L3-12, Alpinion Medical Systems, South Korea), with an inter-element pitch of 0.3 mm and elevational size of 4.5 mm, providing a transmit and receive aperture of 38 mm width. The elevational focus of the receiver is located at 20 mm depth, meaning that the samples are always placed beyond the focal region due to the geometry of the experiment. This will have some effect on the quality of the images. Signals from the receiver are read and stored by a research ultrasound system (EC12-R, Alpinion Medical Systems, South Korea), based on a clinical model by the same manufacturer.
Characterisation of the ultrasound fields transmitted by both the linear array and the LIUS transmitter is performed with a hydrophone system consisting of a 1 mm diameter PVDF needle hydrophone, a submersible preamplifier and a DC coupler (Precision Acoustics, UK). The signals from this system are detected on a digitiser (DP105, Agilent Technologies, USA) built into a PC.
2.3. LIUS transmitter fabrication and characterisation
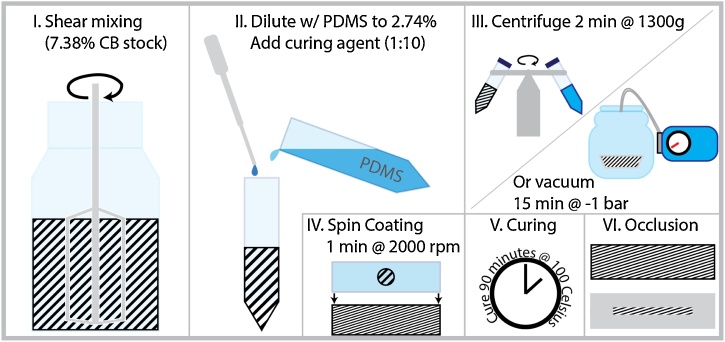
The LIUS transmitters consist of a layer of 2.74% (w/w) carbon black-doped (Printex 60, Palmer Holland, USA) PDMS (Sylgard 184, Dow Corning, USA) cured on a 100 μm thick polyester substrate. The 80 × 20 mm2 substrate is cut out of a transparency film (CG3460, 3M, USA). A schematic of the production of a transmitter is shown in Fig. 2. The first step in the process is to create a high-concentration stock mix of CB-PDMS, in this case having a 7.38% concentration of CB. Having a large amount of stock solution premixed allows more efficient fabrication of multiple transmitters with different properties. To create a homogeneous suspension of small (≈20 nm) carbon black particles shear mixing with a mounted hand mixer is applied for 2 h to break up the agglomerates [18]. Some of the stock mix is diluted to the desired concentration by adding more PDMS, and the corresponding amount of curing agent to achieve a 10:1 ratio of PDMS to curing agent. After mixing in the curing agent the tube of CB-PDMS mixture is degassed, either by centrifuging at 1300g for 2 min or by placing it in a vacuum chamber at −1 bar, after which it is ready to be coated onto the substrate. A 200 μL volume of mixture is pipetted onto the substrate, which is attached to a microscope slide for stability, then spin coated at 2000 rpm for 1 min. After coating the CB-PDMS is cured in an oven at 100 °C for 90 min.
Fig. 2.
Schematic representation of the LIUS transmitter fabrication process. (I.) Shear mixing to break up any agglomerates in high-concentration stock suspension of CB-PDMS. (II.) Dilution to desired concentration of 2.78% CB by adding PDMS and curing agent, final solution contains PDMS and curing agent in a 10:1 ratio. (III.) Degassing by centrifuge, use of a vacuum pump is also an option. (IV.) Spin coating onto polyester substrate. (V.) Curing for 90 min in an oven at 100 °C. (VI.) Occlusion (selective blocking of the excitation light) with sticky aluminium foil to produce the desired aperture shape and size.
The optical absorbance of the film is determined in a spectrophotometer (UV1600, Shimadzu, Japan), while the CB-PDMS layer thickness is measured using an in-house optical coherence tomography (OCT) apparatus [19]. Because the absorbance of the 2.74% CB-PDMS mix is outside the linear range of the spectrophotometer, the optical absorption coefficient (μa) is instead determined by extrapolating from the linear concentration-dependence of four further diluted mixtures (0.13–0.58%), placed between two microscope slides with a 140 μm spacer.
Hydrophone scans of the acoustic field are also taken using the hydrophone system described in the previous section. A 2-D scan is taken on a 10 × 50 mm grid with a 0.5 mm resolution, at a distance of 39 mm from the conventional array and 28 mm from the LIUS transmitter corresponding to the location of the objects to be imaged. The distances differ by 11 mm to account for the distance between conventional array and LIUS transmitter, so that the hydrophone measurements are taken at the same location in the imaging plane.
2.4. Signal processing and image reconstruction
The hydrophone signals stored in the field scans were used to visualise the temporal behaviour and frequency content of the generated signals, as well as producing pressure maps and height maps to determine the field flatness. The pressure maps were obtained by taking the peak-to-peak value of the time signal in mV and dividing by the hydrophone needle sensitivity in mV/MPa. By determining the arrival time of the maximum of the signal for each point in the scan and taking the speed of sound in water into account, a height map is generated. To find the spectral characteristics of the detected signals, a representative signal at the centre of the field was taken and Fourier transformed in a square window around the main pulse. This means taking a 0.2 μs window for the conventional signal and a 0.1 μs window for the LIUS signal.
To obtain a spatially compounded plane wave image from the RF data collected by the detector array, individual plane wave images were taken for insonification angles of −15° to 15°, in 1° steps, giving a total of 31 images. The imaging depth was 10 cm to ensure complete inclusion of the phantoms to be imaged. The image is reconstructed from the RF data by a delay-and-sum algorithm [17], implemented in MATLAB®. The data gathered for each plane wave angle is stored as 128 time signals, one for each individual element in the detector array. Per angle, each of these time signals is processed with a band pass Butterworth filter, with a 4–8 MHz passband for both conventional and laser-induced sources, taking the opening angle of the elements into account, for an image for a single steering angle. The presence of the LIUS transmitter in the imaging plane leads to a series of reverberations between transmitter and probe in each individual image. In the compounded image this would lead to a series of washed-out regions at multiples of the receiver–transmitter distance, possibly obscuring features if the imaged objects. To overcome this, a background image without a sample is taken at each angle, which is then subtracted from the sample image, post-beamforming. Repeating this process for all steering angles and summing the absolute value of the signals leads to an incoherently compounded image. As a final step the envelope of the image in the axial direction is taken by performing a Hilbert transform.
2.5. Test objects and phantoms
To quantify the performance of both conventional and LIUS PWI, a crossed-wire phantom (Fig. 3(a)) was used to determine the point-spread function (PSF) throughout the imaging field. A total of 6 lengths of 60 μm thick, transparent fishing wire (Extreme Nylon, JVS, The Netherlands) were passed through holes in a pair of PMMA plates, spaced 10 cm apart. By passing a wire diagonally through opposing holes in the two plates, and crossing them in the middle, the wires will appear as point scatterers in different locations laterally depending on the height of the imaging system relative to the phantom. By crossing wires at several depths any variation in the PSF throughout the imaging region can be taken into account.
Fig. 3.
(a) Crossed-wire phantom, in this case threaded with black wire for visibility. Wires are identified by being in column 1 or 2, and row A, B or C increasing in distance from the US source and detector. (b) Top view of the CIRS breast phantom, dashed circle indicates the location of a tumour that was imaged, the solid arrow indicates the imaging direction. (c) Side view of the breast phantom, dashed line indicates the imaging plane.
To test the imaging system on a more complex structure, a tissue mimicking breast phantom (Model 073, CIRS Inc., USA) including several hyperechoic tumour-like structures and hypoechoic cyst-like structures and speed-of-sound values typical of human tissue (centred around 1540 m/s) was imaged. Analysis of contrast values in the obtained images will enable a comparison of image quality between LIUS and conventional excitation. As the phantom images acquired featured a tumour embedded in the background glandular material, contrast was determined by taking the ratio of the mean pixel value within the tumour to that in the surrounding background material. Figure 3(b) and (c) show top and side-view photographs of the breast phantom, the location of the tumour and imaging plane are indicated with dotted lines and arrows.
3. Results and discussion
3.1. LIUS transmitter properties
Based on a linear least-squares fit to the optical transmission measurement series of low-concentration CB-PDMS mixtures (0.13–0.58%), an extrapolated value of μa = 244 ± 10 mm−1 at 532 nm wavelength is found for the 2.74% concentration transmitter. The OCT measurements give a PDMS-film thickness of 35 ± 2 μm, making the total transmitter thickness, including the substrate, around 135 μm.
Figure 4(a) shows the temporal signals of both the conventional US array (green) and the LIUS transmitter (blue) as measured in the centre of the field at a distance of 38 mm from the receiver and 28 mm from the LIUS transmitter, the upper time axis corresponds to the LIUS signal, the lower one to the conventional signal. The conventional array generates a 0.2 μs burst with a maximum pressure of 1200 kPa, and a minimum of 950 kPa, after passing through the LIUS transmitter. The LIUS transmitter produces a 0.07 μs positive pressure pulse with an amplitude of 360 kPa, while some minor negative pressure components follow it, presenting pressures of around 100 kPa. The frequency spectra of both signals a presented in Fig. 4(b). The conventional array has a spectrum centred at 7.9 MHz, with a −6 dB bandwidth of 5.6 MHz. The LIUS transmitter spectrum peaks at 8.0 MHz, but has a much larger bandwidth, 15.2 MHz at −6 dB. While not perfectly matched, the degree of overlap of the two spectra ensures efficient detection of the LIUS pulses by the receiver array. The lower amplitude presented by the LIUS pulse may cause a slight decrease in signal-to-noise ratio in the LIUS images, though it is still well within the range of detectability for the detector array, and should thus form no impediment to the experiments.
Fig. 4.
(a) Time signals of the LIUS source and the conventional array, showing a peak positive pressure of 1200 kPa for the conventional source, 360 kPa for the LIUS and (b) the frequency spectra of both sources, showing the overlap between the two spectra.
Figure 5 shows a comparison of the (peak-to-peak) pressure fields and height maps at a distance of 38 mm from the receiver, and 28 mm from the LIUS transmitter. For the pressure maps, the LIUS pressure field is more homogeneous, whereas a series of hot spots (±10%) can be seen in the conventional field, local maxima and minima of pressure. The height maps show the conventional field to be flat to within 20 μm laterally, while the LIUS field has a 100 μm bulge in the middle, caused by a slight deformation of the substrate. Aside from the bulge, fluctuations in the LIUS height map are also within 20 μm. Mounting the transmitter in a different way, e.g. adding some lateral strain may be a good approach to flatten out this bulge. However, as will become clear based on the wire phantom images (Section 3.2.2), the 100 μm bulge in the LIUS field is still well within the point-spread function of the system, meaning it should have no adverse effect on the image quality. The results of more detailed scans, addressing the overlap between the LIUS and conventional fields as well as the accuracy of the transmit delays used in the reconstruction are shown in the supplemental materials. These scans show that the LIUS field at a 15° angle still has an overlap of 56% with the receive field at a depth of 80 mm. At that same depth and angle the lateral shift of the LIUS field relative to the transmitted conventional field, due to the placement of the LIUS source in front of the detector array, is no more than 3 mm. The elevational opening angle of the conventionally transmitted field is 7.56° at −20 dB, while that of the LIUS field is only 3.79°. For both sources the difference in transmit delay for all angles and positions between hydrophone measurements and estimates used in the reconstruction do not differ more than 100 ns, which would correspond to a miscalculation in position of 150 μm in the hydrophone scan.
Fig. 5.
Hydrophone measurements of ultrasound fields emitted by the conventional probe (left) and the LIUS transmitter (right) at the same location in the imaging plane. The top two figures compare the pressure distributions in the fields, and the bottom figures show height maps, providing an indication of the flatness of the fields. The conventional field is flat to within 20 μm, whereas the LIUS field exhibits a 100 μm bulge in the middle due to a slight flexing of the substrate.
3.2. Imaging results
3.2.1. Removal of reflection artefacts
Figure 6(a) is a background LIUS image taken by the system without the presence of a sample in the imaging tank. Several reverberations of the back-propagating LIUS pulse between receiver and transmitter combine to form the washed out regions at regular depth intervals, corresponding to the transmitter-receiver distance. Figure 6(b) shows the LIUS image of a wire phantom before removal of the reflection artefacts caused by the in-plane presence of the transmitter, Fig. 6(c) shows the effect of subtracting the reference sub-images for each insonification angle from the original sub-images and then compounding. It can be seen that most reflections are suppressed to below −50 dB, the most intense parts to −30 dB. The signal intensity from the wires is maintained after background subtraction, the lowest value being 95% of the value in the original image. Assessing the axial and lateral extent of the wires at −6 dB shows that they remain of an identical shape, the only difference being an improved SNR in those parts of the image affected by the artefacts as shown in Table 1 for each wire.
Fig. 6.
(a) A background image, taken without presence of a sample in the water tank, (b) LIUS wire phantom image with reflection artefacts from transmitter and (c) the same image after angle-by-angle subtraction of the reference images.
Table 1.
Comparison of SNR values for the 6-wire image shown in Fig. 6, column and row indications correspond to those indicated in Fig. 3(a).
| Row | Pre-compensation | Post-compensation | ||
| A | 19.4 | 16.0 | 31.0 | 33.3 |
| B | 15 | 15.6 | 14 | 16 |
| C | 13.5 | 11.5 | 13.5 | 11.5 |
| Column | 1 | 2 | 1 | 2 |
3.2.2. Wire phantom images
The crossed-wire phantom was imaged at ten elevational positions, allowing for a sampling of the PSF at a total of 60 different positions, there being six wires. Images from two positions are shown in Fig. 7. Cross sections of the wires in the images are shown in the plots and compared between LIUS and conventional imaging. The axial PSF shows little change in relation to lateral position, as well as between imaging techniques. Some differences can be seen in the lateral cross sections as a function of axial position for LIUS and conventional images, as illustrated in Fig. 7a and c.
Fig. 7.
Representative examples of fishing wire phantom images. (a) Lateral PSF comparison for LIUS and conventional images with wires at a large distance, the lateral spread of the point scatterer is relatively large in the LIUS image, close to the probe. (b) Comparison of axial PSF for the same case, it is difficult to discern a difference between the LIUS and conventional quality here. (c) Lateral PSF comparison with the wires at a smaller distance. (d) Axial PSF comparison with wires at a close distance, again there are no discernible differences between the two techniques apart from the different relative amplitudes.
To compare the performance of the LIUS system to the conventional imaging method, an analysis of PSF behaviour is performed for both. Out of the 60 wire positions measured, throughout the imaging region, the axial PSF values are stable, having a −6 dB value of 0.35 ± 0.03 mm (mean ± standard deviation) in both the LIUS and conventional images. The lateral PSF deteriorates with imaging depth, which is to be expected as both the LIUS (3.79° full opening angle at −20 dB) and conventional (7.56°) fields diverge elevationally with depth. This divergence leads to smearing of the deeper-lying wires as more signals from out of the imaging plane, where the wire is in a different position, are received. Because of this depth-dependence, the lateral PSF is evaluated per depth, per imaging method. The most shallowly placed wires (at the top of the image) exhibit -6dB PSF values of 0.96 ± 0.06 and 0.86 ± 0.04 mm in the LIUS and conventional images respectively. The middle wire pair has the same mean size of 1.37 mm accross both techniques, the standard deviation being 0.07 mm in the LIUS case, and 0.08 mm in the conventional one. Finally, the deepest-lying wires have a LIUS PSF of 1.74 ± 0.19 mm and a conventional PSF of 1.77 ± 0.30 mm.
These results indicate that the axial PSF is limited by the detection bandwidth, as the axial resolution of LIUS would be between 100 and 200 μm based solely on the spatial extent of the pulse. The mean size of the lateral PSF is consistently larger by an average of 100 μm in the LIUS images for the shallowest pair of wires, while it is identical to the conventional results for the deeper wires, with the exception of the standard deviation values. The larger value of the standard deviation indicates a larger variability with lateral position for the conventional source than the LIUS source, especially for the deepest-lying wires. This can be explained in terms of the laterally inhomogeneous conventional pressure map shown in Fig. 5, and the accompanying variations in the elevational extent of the field, and thus the amount of blurring due to out-of-plane signals. While the 11% increase of the mean lateral PSF size for the shallow wires in the LIUS image is striking, the difference amounts to a third of the inter-element spacing of the detector array, and thus a third of the lateral pixel size used in the reconstruction.
3.2.3. Tissue-mimicking phantom
Figure 8 shows a side-by-side comparison of part of the CIRS phantom, imaged using both LIUS and conventional plane wave imaging. The illustration in Fig. 3(b) presents the various components of the phantom that can be seen in the images. A spherical tumour is clearly distinguishable beneath the fat layer (top) as a circular, hyperechoic area in both images. In both images the fat layer has a thickness of 11 mm, and the tumour presents as a solid circle with a diameter of 6.5 mm. Some strong scatterers can be seen on the outside surface of the fat layer, at the top of both images, as well as on the interface between the fat layer and the glandular layer. These scatterers are residual air bubbles. The bubbles on the inside are due to an incipient separation between the outer fat layer and the inside of the phantom. While the presence of the bubbles in the imaging plane could be minimised manually, it was not possible to clear them out altogether. Several differences can already be seen by eye in the images, such as the higher tumour contrast in the LIUS image, as well as a difference in the speckle density and size in the fatty layer. The lateral extent of the conventional image also appears larger than that of the LIUS image. The tumour contrast values were determined as 2.33 and 1.50 in the LIUS and conventional images respectively, a difference of 0.83. It is most likely that the cause of the improved contrast in the LIUS images is an effect of the positioning of the phantom outside of the elevational focus of the detection array. The field emitted to take the conventional images will have spread out of the imaging plane at these depths, leading to out-of-plane artefacts which decrease the contrast in the final images. The LIUS field, on the other hand, stays confined within the imaging plane at larger depths, due to its less focused nature. A way to verify this would be to bend the substrate of the LIUS transmitters to have a more similar elevational opening angle as the conventional transducer, and check image quality again.
Fig. 8.
Comparison of LIUS and conventional images of commercial breast phantom. The images both show a fat layer and embedded hyperechoic, spherical tumour. Some minor differences in speckle behaviour can be seen, as well as the tumour contrast appearing to be higher in the LIUS image. The transducer is located at the top of the image, at a distance of 30 mm from the phantom/water boundary. The inset shows a schematic view of the various layers of the phantom, showing water, fat and glandular material with an embedded tumour from top to bottom. The small, bright discs indicate scatterers on the outer surface and the interface between fat and glandular components.
3.3. Further discussion
Overall, a major limit is imposed on the quality of the presented images by the fact that the elevational focus of the employed US probe lies at 20 mm. The presence of the LIUS transmitter, and the need to rotate it to an angle of 15° means that the phantoms could only realistically be placed at a distance of 30 mm and beyond, meaning the bulk of the image is being taken well out of the focal area. The main consequence of this is a decreasing lateral resolution with depth, as can be observed in Figs. 6 and 7 . More may be learned by repeating these experiments with the addition of a probe with a deeper and larger focal area. While the current work was achieved with a flat LIUS transmitter, the fact that it is coated on a flexible substrate means it could be bent into a range of shapes, depending on the dimensions of the film. This offers possibilities for future work, allowing an elevationally focused LIUS source to match the field of a traditional US probe more closely. Additionally, it might be worth exploring different imaging geometries or compounding techniques that may allow a closer positioning of the samples to the probe.
4. Conclusions
The results presented in this work show the possibility to perform multi-angled plane wave imaging using a laser-induced ultrasound source and a separately placed US probe. In addition, the use of a thin, acoustically transparent polyester film for a substrate allowed for co-planar imaging, meaning a relevant comparison of the LIUS and conventional images could be achieved. While the axial PSF was quite stable and similar for both techniques throughout the imaging area, this was not so for the lateral PSF. LIUS imaging appears to generate a slightly worse PSF than the conventional variant at shallower depths, though the exact cause of this is not known. While the mean lateral resolution is identical to the conventional values at larger depths, it is more homogeneous throughout the imaging area, perhaps due to the more even pressure distribution as seen in Fig. 5. In the presented geometry the images of a breast-mimicking phantom look to be of a similar quality, although LIUS wins out in terms of contrast at depth, while the field of view appears larger in the conventionally obtained image.
Conflict of interest
None declared.
Acknowledgements
The authors would like to thank Dr. Libertario Demi of the University of Trento for many fruitful discussions, and assistance in the realisation of the image reconstruction algorithms used. Thanks are also due to Marieke Olsman, for her exploratory work on this subject, to Wilma Petersen for assistance in the fabrication of the LIUS transmitters and to Johan van Hespen and Tom Knop for their contributions to the development of the imaging apparatus. This work was funded as a part of the European Horizon 2020 PAMMOTHproject under grant agreement no. 732411, an initiative of the Photonics Public-Private Partnership.
Biographies

David Thompson obtained his M.Sc. in Applied Physics from the University of Twente in 2016, and is currently pursuing a Ph.D. in the Biomedical Photonic Imaging group at the University of Twente. His M.Sc. research focussed on wavelength-modulated Raman spectroscopy to suppress fluorescent backgrounds in Raman spectra. He also spent 3 months on an internship at APE GmbH in Berlin, working on difference-frequency generation for mid-infrared spectroscopy. His current research interests include laser-induced ultrasound imaging, photoacoustic tomography and ultrasound computed tomography.

Damien Gasteau (Ph.D.) received his Master's degree in acoustics at Université du Maine in Le Mans, France. He obtained his Ph.D. degree on optoacoustic characterisation of polycrystalline steels elastic properties with the Laboratoire d’Acoustique de l’Université du Maine (LAUM) and Commissariat à l’Énergie Atomique (CEA) in 2016. He worked in 2017 at Institut Fresnel in Marseille, France on photoacoustic tomography and the development of a photoacoustic microscope for small animal application. Since 2018 he is working at the Biomedical Photonic Imaging (BMPI) group at the University of Twente, The Netherlands focusing on photoacoustic and laser-induced ultrasound imaging modalities for breast cancer detection.

Srirang Manohar (Ph.D.) is Full Professor and Chair of the newly formed Multi-modality Medical Imaging (M3I) Group at the Technical Medical Centre, University of Twente, The Netherlands. He received his Ph.D. degree from the Indian Institute of Science, Bangalore, India. Prof. Manohar's research expertise is in photoacoustic imaging and spans technology development to early clinical assessment. The intended applications of the technologies span the range of ex vivo tissue imaging, minimally invasive imaging to non-invasive imaging. Prof. Manohar is the coordinator of the Domain Imaging & Diagnostics at the University of Twente. He also coordinates several international projects and is member of the Organizing Committee of the conference “Photons plus ultrasound: imaging and sensing held annually under auspices of SPIE”.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.pacs.2019.100154.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Bychkov A., Simonova V., Zarubin V., Cherepetskaya E., Karabutov A. The progress in photoacoustic and laser ultrasonic tomographic imaging for biomedicine and industry: a review. Appl. Sci. 2018;8(10):1931. [Google Scholar]
- 2.Ermilov S.A., Su R., Conjusteau A., Anis F., Nadvoretskiy V., Anastasio M.A., Oraevsky A.A. Three-dimensional optoacoustic and laser-induced ultrasound tomography system for preclinical research in mice. Ultrason. Imaging. 2016;38(1):77–95. doi: 10.1177/0161734615591163. [DOI] [PubMed] [Google Scholar]
- 3.Jose J., Willemink R.G., Steenbergen W., Slump C.H., van Leeuwen T.G., Manohar S. Speed-of-sound compensated photoacoustic tomography for accurate imaging. Med. Phys. 2012;39(12):7262–7271. doi: 10.1118/1.4764911. [DOI] [PubMed] [Google Scholar]
- 4.Baac H.W., Ok J.G., Park H.J., Ling T., Chen S.-L., Hart A.J., Guo L.J. Carbon nanotube composite optoacoustic transmitters for strong and high frequency ultrasound generation. Appl. Phys. Lett. 2010;97(23):234104. doi: 10.1063/1.3522833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou Y., Kim J.-S., Ashkenazi S., O’Donnell M., Guo L.J. Optical generation of high frequency ultrasound using two-dimensional gold nanostructure. Appl. Phys. Lett. 2006;89(9):93901. [Google Scholar]
- 6.Hsieh B.-Y., Kim J., Zhu J., Li S., Zhang X., Jiang X. A laser ultrasound transducer using carbon nanofibers-polydimethylsiloxane composite thin film. Appl. Phys. Lett. 2015;106(2):21902. [Google Scholar]
- 7.Hsieh B.-Y., Chen S.-L., Ling T., Guo L.J., Li P.-C. All-optical scanhead for ultrasound and photoacoustic dual-modality imaging. Opt. Express. 2012;20(2):1588–1596. doi: 10.1364/OE.20.001588. [DOI] [PubMed] [Google Scholar]
- 8.Lee S.H., Lee Y., Yoh J.J. Reduced graphene oxide coated polydimethylsiloxane film as an optoacoustic transmitter for high pressure and high frequency ultrasound generation. Appl. Phys. Lett. 2015;106(8):81911. [Google Scholar]
- 9.Biagi E., Margheri F., Menichelli D. Efficient laser-ultrasound generation by using heavily absorbing films as targets. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2001;48(6):1669–1680. doi: 10.1109/58.971720. [DOI] [PubMed] [Google Scholar]
- 10.Biagi E., Cerbai S., Gambacciani P., Masotti L. Fiber optic broadband ultrasonic probe. Sensors, 2008 IEEE. 2008:363–366. [Google Scholar]
- 11.Buma T., Spisar M., O’Donnell M. High-frequency ultrasound array element using thermoelastic expansion in an elastomeric film. Appl. Phys. Lett. 2001;79(4):548–550. [Google Scholar]
- 12.Kim E., Xia Y., Whitesides G.M. Polymer microstructures formed by moulding in capillaries. Nature. 1995;376(6541):581. [Google Scholar]
- 13.Wurzinger G., Nuster R., Schmitner N., Gratt S., Meyer D., Paltauf G. Simultaneous three-dimensional photoacoustic and laser-ultrasound tomography. Biomed. Opt. Express. 2013;4(8):1380–1389. doi: 10.1364/BOE.4.001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alles E.J., Noimark S., Zhang E., Beard P.C., Desjardins A.E. Pencil beam all-optical ultrasound imaging. Biomed. Opt. Express. 2016;7(9):3696–3704. doi: 10.1364/BOE.7.003696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alles E.J., Noimark S., Maneas E., Zhang E.Z., Parkin I.P., Beard P.C., Desjardins A.E. Video-rate all-optical ultrasound imaging. Biomed. Opt. Express. 2018;9(8):3481–3494. doi: 10.1364/BOE.9.003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruizinga P., Cox B., de Jong N., Beard P., van der Steen A.F., van Soest G. Plane wave ultrasound imaging with a broadband photoacoustic source. 2012 IEEE International Ultrasonics Symposium (IUS) 2012:1414–1416. [Google Scholar]
- 17.Montaldo G., Tanter M., Bercoff J., Benech N., Fink M. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2009;56(3):489–506. doi: 10.1109/TUFFC.2009.1067. [DOI] [PubMed] [Google Scholar]
- 18.Rwei S.-P., Manas-Zloczower I., Feke D. Observation of carbon black agglomerate dispersion in simple shear flows. Polym. Eng. Sci. 1990;30(12):701–706. [Google Scholar]
- 19.Veenstra C., Petersen W., Vellekoop I.M., Steenbergen W., Bosschaart N. Spatially confined quantification of bilirubin concentrations by spectroscopic visible-light optical coherence tomography. Biomed. Opt. Express. 2018;9(8):3581–3589. doi: 10.1364/BOE.9.003581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.