Abstract
Cardiovascular diseases (CVD) have been the leading causes of death in the U.S. for nearly a century. Numerous studies have linked eicosanoids to cardiometabolic disease. Objectives and Methods: This review summaries recent advances and innovative research in eicosanoids and CVD. Numerous review articles and their original human or animal studies were assessed in the relevant and recent studies.
Outcome
We identified and discussed recent trends in eicosanoids known for their roles in CVD. Their subsequent relationships were assessed for any possible implications associated with consumption of different dietary lipids, essentially omega fatty acids. Eicosanoids have been heavily sought after over recent decades for their direct role in mediating the enhancement and resolution of acute immune responses. Given the short half-life of these oxidized lipid metabolites, studies on atherosclerosis have had to rely on the metabolites that are actively involved in eicosanoid production, signaling or redox reactions as markers for atherosclerosis-related molecular behaviors.
Conclusion
Further investigations expending current knowledge, should be applied to narrow the specific class and species of eicosanoids responsible for inciting inflammation especially in the context of recent clinical studies assessing the role of dietary lipid in cardiovascular diseases.
Keywords: Atherosclerosis, Cardiovascular diseases (CVD), Prostaglandins, Leukotrienes, Arachidonic acid (AA), Eicosapentaenoic acid (EPA), Inflammation, Oxidative stress
Introduction
According to the CDC’s National Vital Statistics Reports in the United States, heart disease has been the leading cause of death for approximately 90 years [1]. The onset of cardiovascular event is often sudden and deadly, unless treated immediately. Cardiovascular events, such as a myocardial infarction, and angina pectoris, are often the result of atherosclerosis, a coronary artery disease (CAD). Notable risk factors, based on the Framingham Heart Study and other studies by the CDC and WHO, include: smoking, diabetes, physical inactivity, body mass index (BMI), systolic blood pressure and total blood cholesterol/lipid levels [2].
The formation of atherosclerotic fibrous plaque is the result of an acute phase response of the innate immune system, involving monocytes, macrophages, neutrophils and platelets. Studies involving an acute phase response have identified extracellular molecules, cytokines and eicosanoids, as mediators of inflammation [3], [4], [5], [6], [7].
Eicosanoids are a group of lipid mediators derived from eicosapolyenoic acid. They represent the oxidized lipid products of an acute innate response and have been widely explored since the genesis of non-steroidal anti-inflammatory drugs (NSAIDs) [8].
Precursors to eicosanoids are polyunsaturated, long fatty acid chains derived from ω-3 (n-3) and ω-6 (n-6) fatty acids (Table 1). Eicosanoids contain 20 carbons. Eicosanoid synthesis may only begin if the precursor PUFA has been cleaved from the membrane bound phospholipids by cytosolic phospholipase A2 (cPLA1) [9], [10], [11]. cPLA2 activation relies on Ca2+, which rises as a result of inositol triphosphate receptor (IP3) activation. Eicosanoids have been shown to mediate receptors involved in Ca2+ influx and transcription factors that potentiate the propagation of an acute immune response [12], [13], [14], [15].
Table 1.
ω-3 and ω-6 PUFAs involved in the eicosanoid family.
| PUFA | Acronym | ω | C:Δ | Δ | IUPAC |
|---|---|---|---|---|---|
| α – Linolenic Acid | ALA (αLA) | 3 | 18:3 | 9, 12, 15 | (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid |
| Arachidonic Acid | AA | 6 | 20:4 | 5, 8, 11, 14 | (5Z,8Z,11Z,14Z)-eicosa-5,8,11,14-tetraenoic acid |
| dihomo – γ – linolenic acid | DGLA | 6 | 20:3 | 8, 11, 14 | (8Z,11Z,14Z)-eicosa-8,11,14-trienoic acid |
| Docosahexaenoic acid | DHA | 3 | 22:6 | 4, 7, 10, 13, 16, 19 | (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoic acid |
| Eicosapentaenoic acid | EPA | 3 | 20:5 | 5, 8, 11, 14, 17 | (5Z,8Z,11Z,14Z,17Z)-eicosa-5,8,11,14,17-pentaenoic acid |
| Linoleic Acid | LA | 6 | 18:2 | 9, 12 | (9Z,12Z)-octadeca-9,12-dienoic acid |
Specific eicosanoids that have been identified for having a role in atherosclerosis pathogenesis have been found to derive mainly from endothelial cells, epithelial cells and myeloid-derived granulocytes [15], [16]. The three pathways responsible for the production of eicosanoids are recognized by the enzymes involved, such as cyclooxygenase-1 (COX-1), cyclooxygenase-2 (COX-2), lipoxygenases 5, 12 or 15 (5-LO, 12-LO, 15-LO) and cytochrome P450 (cyP450) [14], [17]. This review will discuss the biomarkers, enzymatic activities and precursors involved in the production of notable eicosanoids, such as prostaglandin H1 (PGH1), prostaglandin D2 (PGD2), prostaglandin I2 (PGI2) and its precursor, prostaglandin E2 (PGE2), thromboxane A2 (TXA2), leukotriene B4 (LTB4) and its products, Lipoxin A4 (LXA4), 12(S)-hydroxy-eicosatetraenoic acid (12(S)-HETE), 15(S)-hydroxy-eicosatetraenoic acid (15(S)-HETE), 13-hydroxyoctadeca-dienoic acid (13-HODE), resolvin E (RvE), protectin D1 (PD1) and 15-epi-lipoxain A4 (15-epi-LXA4) (Table 2, Table 3). These specific eicosanoids have been identified in past and recent studies for their observable relationship with atherogenesis and relevant nutritional studies [12], [13], [14], [15], [18], [19], [20], [21]. Oxidative states are known to be highly involved in the exogenous production of eicosanoids and oxidized low density lipoproteins (oxLDL), which are generally pro-atherogenic [21]. The reactive oxygen species (ROS) released by necrotic foam cells only further drives this oxidative state.
Table 2.
Actions of selected prostaglandins, and thromboxane involved in cell signaling.
| Molecule | Molecular Formula | Action |
|---|---|---|
| PGH2 | 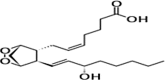 |
Precursor to mediators of inflammation |
| PGD2 | 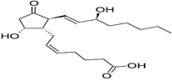 |
Cell adhesion molecule expression activation, enabling leukocytic chemotaxis |
| PGE2 | 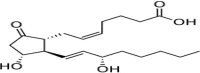 |
15-ketoPGE2 precursor, induces hypertrophy |
| PGF2α | 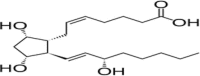 |
Increases intracellular Ca2+ levels, stress on heart enhances production, biomarker of heart failure |
| PGI2 | 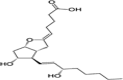 |
Vasodilation, platelet aggregation inhibitor |
| TxA2 | 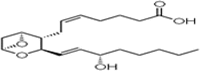 |
Induces vasoconstriction, platelet aggregation |
| 15-keto-PGE2 | 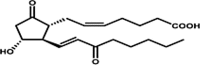 |
PPAR-γ agonist: cell growth regulation (adipogenesis, tumor suppression) |
Table 3.
Actions of selected Leukotrienes and other precursors involved in cell signaling.
| Molecule | Molecular Formula | Action |
|---|---|---|
| LTA4 | 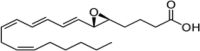 |
Precursor to mediators of inflammation (e.g., smooth muscle contraction; leukocyte recruitment) |
| LTB4 | 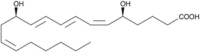 |
Activates lipid metabolism, leukocyte recruitment, catabolized by PPARα, inhibition of apoptosis |
| LTE4 | 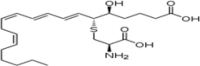 |
Major product of PMN leukocytes and lymphocytes (murine) |
| 5(S)-HETE | 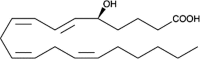 |
Leukotriene precursor, regulates LDL oxidation in monocytes and Mφ’s |
| 12(S)-HETE | 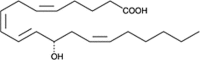 |
Activates lipid oxidation (stimulate oxLDL accumulation), leukocyte recruitment & proliferation, regulates monocyte-endothelial cell adhesion interactions |
| 15(S)-HETE | 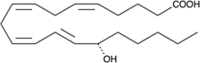 |
Activates lipid oxidation (stimulate oxLDL accumulation), leukocyte recruitment and differentiation, PPAR-γ-specific activation, regulates monocyte-endothelial cell adhesion interactions, activate LDL oxidation in monocytes & Mφ’s, Mφ cholesterol efflux regulatory factor |
Eicosanoids
The three major pathways involved in eicosanoid production are referred to as the COX pathways, the LOX pathways or the cyP450 pathway [12]. The COX pathway is mediated by COX-1/2 activities within nearly every cell of the body and is responsible for the production of prostaglandins, prostanoids and thromboxanes. The LOX pathway refers to the actions of 5/12/15-LO within leukocytes to produce leukotrienes, lipoxins and some hydroxyeicosatetraenoic acids (HETEs). The cyP450 pathway involves cytochrome P450’s production of the remaining HETE’s and epoxyeicosatrienoic acids (EET’s). While cyP450 is present within all cells, the majority of its activity involves the metabolism of drugs occurring within the liver, large and small intestines, lungs, kidneys and plasma [18], [22], [23]. The first major studies conducted to track disease-related differences in eicosanoid levels were limited to the analysis of urine samples containing mainly cyP450 metabolites, due to the extremely short half-life and predisposition for oxygenation of eicosanoids and their metabolites [23], [24]. CYP450 can produces several other lipid mediators with vasoactive such as the epoxyeicosatrienoic acids (EETs) which are mainly vasodilators, and the 20-hydroxyeicosatetraenoic acid (20 HETE) which is a known vasoconstrictor. Arachidonic acid metabolized by CYP enzymes results in epoxygenase and hydroxylase metabolites. Epoxygenase metabolites are generated by CYP2C or CYP2J enzymes and hydroxylasemetabolites are generated by CYP4A or CYP4F enzymes. Several CYP450 single nucleotide polymorphisms (SNPs) genes involved in arachidonic acid metabolism via have been investigated for possible association with cardiovascular disease (CVD). The development of fatty acid epoxy drugs has advanced dramatically in the recent years. Large body of literature on arachidonic acid CYP pathway is currently available on its chemical structure, evaluation and potential clinical utilizations. The recent identification of identified G-protein receptor 75 (GPR75) as a specific target of 20-HETE has provided the molecular basis for the signaling and pathophysiological functions of its modulation mediated by 20-HETE in cardiovascular diseases. This also in line with the recent identification of the soluble epoxide hydrolase inhibitors and the new epoxyeicosatrinenoic acid analogs EET-A which are shown to reduced renal TNF-α, IL-6, IL-1β, and IFN-γ expression and prevents renal injury in a mouse model of SLE by reducing inflammation and EET-B treatment reported to markedly increases heme oxygenase-1 (HO-1) immunopositivity in cardiomyocytes and reduced cardiac inflammation and has an overall beneficial therapeutic actions to reduce cardiac remodeling in after myocardial infarction [25], [26], [27], [28], [29], [30], [31], [32]
The metabolic events mediated by COX-1/2 and 5/12/15-LO are heavily focused on the oxidation of arachidonic acid (AA) although these metabolic pathways are assumed to be universal for all precursors of eicosanoids, especially for EPA. However, there is evidence that the minor molecular differences between products could be the difference that dictates a pro-inflammatory role or an anti-inflammatory role (e.g. EPA and γ-linolenic acid (GLA) products are categorized as anti-inflammatory while the majority of AA products are pro-inflammatory) [25].
Cyclooxygenase-derived eicosanoids
The COX-1/2 pathways are widely known for their role as modulators of inflammation and are the major target of non-steroidal anti-inflammatory drugs (NSAID’s). COX-1/2 are also referred to as prostaglandin H-synthase 1 (PGH-S1) and prostaglandin H-synthase 2 (PGH-S2), respectively. The most abundant products of the COX pathways are prostaglandins, prostanoids and thromboxanes. Prostaglandins were the first eicosanoids discovered in the early 1930’s, making them the oldest group within the eicosanoid family [25]. Of those, the COX products most involved with atherosclerosis and their roles are PGD2, PGE2, PGI2, TXA2 and 15-keto-PGE2.
COX-1/2 can produce any prostaglandins, their activities rely on the cleavage of AA from its membrane bound phosphoglycerates by cytosolic phospholipase A2 (cPLA2). cPLA2 requires activation by Ca2+ levels. cPLA2 has a unique mechanism for the removal of AA, as it resides within the bilayer of the plasma membrane and converts AA to prostaglandin G2 (PGG2) within the cytosol [12], [13], [33], [34].
COX-1 is expressed within nearly every cell of the body, as is COX-2, however COX-1 is constitutively active while COX-2 is only activated by inflammation. Studies have shown COX-2 to be constitutively active within kidney, brain, tracheal, epithelial and endothelial cells. 19 The reasoning for this is believed to be due to the sensitivity of these tissues or their constant interactions with toxins, similar to cyP450. Thus, the mechanisms of AA metabolism into series 2 prostaglandins are generalized in the majority of literature as COX-1/2, where the only factors telling whether COX-1 and COX-2 are acting simultaneously or not is the type of cell being examined and the presence of inflammation. This difference between COX-1 and COX-2 is that in the face of inflammation, COX-2 activation adds to COX-1’s constitutive metabolism of AA, resulting in a significantly enhanced robust inflammatory response. Just as COX-1/2 mechanisms are generalized as one, the same mechanisms that are known to happen to AA, are also believed to occur with EPA, save for a few molecular differences in COX-1/2 products, as EPA has one more double bond than AA does. EPA derivatives from the COX pathways are referred to as series 3 prostaglandins, thromboxane and prostanoids.
Upon Ca2+ activated cPLA2 metabolism of AA into PGG2, PGG2 is converted to PGH2 intracellularly along the plasma membrane via heme dependent COX-1/2 (FADS1/2), present in nearly all cells of the body [12], [13]. PGH2 is the primary precursor of all prostanoids, prostacyclins and thromboxanes. Leukocytes are unique in that they possess hematopoietic PGD synthase (H-PGDS), which is glutathione (GSH) and Ca2+ dependent and has Mg2+ as a co-factor that reacts with PGH2 to produce PGD2 [35]. Given this characteristic of H-PGDS, PGD2 is a leukocyte-specific product that is only produced in response to inflammation. PGE2 is derived from PGH2 within myeloid-lineage derived cells as well, with an enzyme containing GSH as a co-factor. PGE2 has a demonstrated capacity to sustain cytokine signaling and regulate hormone production (i.e. estrogen and progesterone). However, PGE2 associated cytokines relationship with atherogenesis is not well understood [36].
PGE2 is indirectly correlated with atherosclerosis via its influence on estrogen and progesterone production, due to its products: PGF2α and PGI2. PGF2α has been shown to prevent corpus luteum formation in vivo in microminipigs in Japan, providing novel insights for contraceptive methods that could decrease risk for CVD in females currently using contraceptive pills [37]. Higher estrogen levels exhibit lower LDL and higher HDL levels in whole blood while progesterone exhibits the opposite effect. As the precursor for PGI2, a prostacyclin known to induce vasodilation and prevent platelet aggregation, PGE2 is necessary for the subsequent resolution of inflammation, despite the abundance of studies over the last 30 years depicting PGE2 as pro-atherogenic. In addition to the findings of these studies, a novel contraceptive mechanism has been discovered [36], [38].
Prostaglandin I synthase (PGIS) is a heme dependent, ferrous enzyme that is responsible for converting PGH2 into the anti-atherogenic prostacyclin, PGI2 [10]. PGI2 receptors (IP1 and IP2) are isomers of IP3 and are present on endothelial cells, VSMC’s, platelets and mesangial cells [39]. Unlike IP3’s known mechanism for protein mediated Ca2+ influx leading to cPLA2 activation, IP1/2 have been shown to have overall cardio-protective actions, suggesting they have an antagonist mechanism from IP3 [40]. Alternatively, PGH2 interactions with the heme dependent thromboxane A synthase (TXAS) result in the production of pro-atherogenic TXA2 [13], [41]. Expression of TXA2 receptor isoforms, thromboxane receptor’s α and β (TPα and TPβ) is abundant on platelets, VSMC’s, macrophages and mesangial cells [42].
PGE2 is also a precursor for 15-keto-PGE2, which has specific anti-atherogenic roles in relation to cell cycle regulation and tissue growth, nearly opposite of TXA2. This conversion is facilitated by 15-hydroxyprostaglandin dehydrogenase (15-HPGD) in association with NAD+ or NADP+ for COX-1 or COX-2, respectively [13], [42]. 15-keto-PGE2 is anti-atherogenic due to its agonist relationship with peroxisome proliferator receptor-γ (PPAR-γ), which is a ligand-activated transcription factor that has been shown to regulate lipid metabolism, glucose homeostasis, proliferation, specifically adipogenesis and inflammation [42]; by binding PPAR-γ and blocking its activation. One other ligand of PPAR-γ, 15-deoxy-Δ12,14-prostaglandin J2 (15ΔPGJ2), has also been shown to have anti-inflammatory effects that decrease mPGES expression levels, lowering PGE2 production. Lastly, one more atherogenic product of PGE2 is PGF2α, which has been shown to increase intracellular Ca2+ levels regulate estrogen and progesterone and is considered a marker of atherogenesis within endothelial cells [35], [43]. Although PGE2 has been viewed as one of the major mediators of inflammation, however recent studies have demonstrated that PGE2 can serve both pro- and anti-inflammatory functions [44]. Frolov et al. have demonstrated PGE2 as important negative regulators of neutrophil-mediated Inflammation [44]. In another study Qian et al. reported that PGE2 negatively regulate inflammation by inhibiting CCL5 expression in activated macrophages [45]. Work by Loynes et al. illustrated that PGE2 production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo [46]. Similarly, a study by Thompson et al. on myeloid-PTP1B knockout mice on atherogenic background (ApoE-/-/LysM-PTP1B) revealed a decreased atherosclerotic plaque lesions associated with increased secretion of circulating anti-inflammatory cytokines and PGE2 [47], these reports clearly indicate that PGE2 indeed retain two-edged properties.
Metabolisms of COX-1/2 pathways have been shown to occur within the cytosol, along the plasma membrane, as the enzymes are mainly membrane bound with local receptors. The subsequent implications of where prostaglandin synthesis and signal transductions occur could be explanatory with the short amount of time that is required to produce eicosanoids. Recent findings involving γ-linolenic acid (GLA) have shown that its molecular behavior favors its metabolism into Dihomo-gamma linolenic acid (DGLA, 20:3, n-6) (DGLA), PGG1 then PGH1 and the remainder of series 1 prostaglandins accordingly to the aforementioned COX-1/2 mechanisms modeling AA metabolism. Series 1 prostaglandins resulting from GLA are highly cited for their anti-inflammatory benefits and rare plant-derived oils that are high in GLA have recently grown in popularity due to this knowledge [25].
As NSAIDs target the COX enzymes to prevent inflammation and pain, numerous studies have shown the necessary role of pro-inflammatory eicosanoids in order to produce anti-inflammatory eicosanoids with specific NSAIDs being more correlated to developing CAD risk than others, this been reported in numerous studies. The loss of COX-2 in an in vivo murine model was reported to be detrimental to the resolution of inflammation, potentiating damage, resulting in higher rates of atherogenesis [48], [49]. In addition to these findings, a very recent study has displayed that two different bacterial pathogens can elicit very different products and behaviors from the COX enzymes within macrophages [50]. Meanwhile, the most beneficial NSAID currently is aspirin, due to its numerous glycosylation events with enzymes belonging to both the COX and LOX pathways. Studies have shown that specific COX enzyme inhibition allows for LOX enzyme compensation to occur, by metabolizing HETEs and other essential fatty acids, such as DHA or EPA instead of AA, into anti-inflammatory oxidized fatty acids.
Lipoxygenase-derived eicosanoids
Lipoxygenase expression was previously debated as being solely unique to leukocytes or unique to parenchymal cells and their associated mast cells, however there is evidence for both now [51]. Most evidence identifies leukotrienes (LTs), however, that are derived from leukocytes and before settling this debate, LTs were named based on their source and structure, as leuko- is the Greek root word, meaning white in relation to the white blood cells that produced them, and triene refers to the three conjugated double bonds in a given oxidized PUFA chain produced via lipoxygenase activities [52]. The most abundant products of the LOX pathway include leukotrienes and HETEs, which are precursors for lipoxins, protectins, resolvins and hydroxyoctadeca-dienoic acids (HODEs), which are most often noted due to their apparent role in resolving inflammation and protecting tissue from further damage (i.e. fibrosis or oxLDL induced necrosis). Mast cells, in response to allergy-triggered asthma or platelet activation, and PMN’s are the focal point of most LOX studies as they are the most abundant source of LTs and are easier to culture than parenchymal cells [15], [53], [54], [55].
Nearly all LTs are derived from leukotriene A4 (LTA4), the primary product of AA through conditional lipoxygenase activation. Synthesis of LTA4 relies on a complex mechanism that includes the dimerization of 5-LO activating protein (FLAP) leading to its translocation from the cytosol to the perinuclear membrane to activate 5-LO cPLA2 with AA [56]. Methods to inhibit 5-LO target FLAP (e.g. MK886) to prevent its phosphorylation-induced dimerization and therefore, its ability to activate 5-LO. By developing these agonists, the mechanisms of FLAP and 5-LO perinuclear translocation could be better under as these agonists did not appear to interrupt the translocation event of 5-LO, suggesting 5-LO translocation occurs independently of FLAP activities [52]. Once FLAP has been inhibited, 5-LO activation for translocation was demonstrated to be by phosphorylation, leading to AA metabolism into LTA4 [13], [52]. LTA4 actions have been associated with smooth muscle contractions and leukocyte recruitment, however LTA4’s exact roles in these mechanisms are unclear and knowledge of LTA4 metabolites only casts more doubt. LTA4 can undergo one of two different pathways, all of which occur within the perinuclear membrane, allowing leukotriene and lipoxin close proximity to their ligand activated transcription factors. This evidence supports prior knowledge of upregulated levels of transcription and translation within activated leukocytes during an acute phase response.
Plant models of LTA4 production reference a cytosolic and ferrous 5-LO which derives LTA4 from 5-HpETE intermediates that had been obtained from ALA or LA, which plants have the ability to produce from unsaturated fatty acids. This enzyme’s independent self-maintenance is unique from mammalian 5-LO pathways, as the plant Fe2+ within 5-LO is responsible for facilitating the redox reactions required to return 5-LO to its active form after each reaction. Alternatively, mammalian 5-LO requires an independent lipid hydroperoxide reaction to occur in order to restore 5-LO to its active form after reacting with AA [52].
Leukotriene A4-hydrolase (LTA4-H) is responsible for metabolizing LTA4 into leukotriene B4 (LTB4), which is a marker of atherosclerosis with known mechanisms that induce pro-inflammatory behaviors. Further oxidation of LTB4 by 5-LO produces 5(S)-HpETE, a precursor for identified anti-inflammatory lipoxins. If LTA4 is not metabolized into LTB4 then leukotriene C4-synthase (LTC4-S) can metabolize LTA4 into LTC4 , which is a major precursor of glutamic acid (Glu) and two more isoforms involved in resolving inflammation. Next, γ – glutamyl transpeptidase (GGT) is responsible for the conversion of LTC4 to Glu and LTD4, which is followed by membrane-bound zinc metalloprotein dipeptidase (MBD) facilitating LTD4’s metabolism into LTE4. LTE4 is a cysteinyl of notable interest for identifying risk for atherosclerosis because it can be analyzed in urine samples and, therefore, has been associated with chronic inflammation deriving from lupus erythematosus [53], [54], [17], [55], [56], [57]. LTE4, as well as TXB2 and PGE2, have been shown to have a negative correlation with vitamin B6 through clinical studies supplementing different combinations of B vitamins and assessing homocysteine levels.
GGT is activated by glycosylation and inhibited when acetylated by aspirin, which allows LOX enzymes to compensate by oxidizing other essential PUFAs or eicosanoids into characterized anti-inflammatory lipoxins, protectins, resolvins and HODEs. Identified anti-atherogenic products resulting from GGT acetylation, have been shown to be products of hydroperoxyl-eicosatetraenoic acids (HpETEs) derived by cyP450’s metabolism of AA, although both LOX enzymes and cyP450 have been shown to produce various HETE isomers. 5-LO, 12-LO and 15-LO derive 5(S)-HETE, 12(S)-HETE and 15(S)-HETE accordingly from 5/12/15(S)-HpETEs. 15(S)-HETE is the precursor Lipoxins (i.e. LXA4 and LXB4) and can also be produced by 15-LO independently of cyP450 and aspirin.
Other products associated with aspirin treatment include 13-HODE, 17(S)-HpDHA, 15-epi-LXA4 and PD1 and RvE, which possess potent anti-atherogenic affects, supporting aspirin’s role as an anti-inflammatory drug lipid [52]. 15-epi-LXA2 is also referred to as aspirin-triggered LXA4 (ATLa9), inspiring the molecular structure of synthetic anti-inflammatory drugs [58]. PD1 and RvE have specifically been characterized for their capacity to protect tissues from injuries associated with extensive inflammation and resolving the inflammation of an acute phase response, respectively (see Fig. 1).
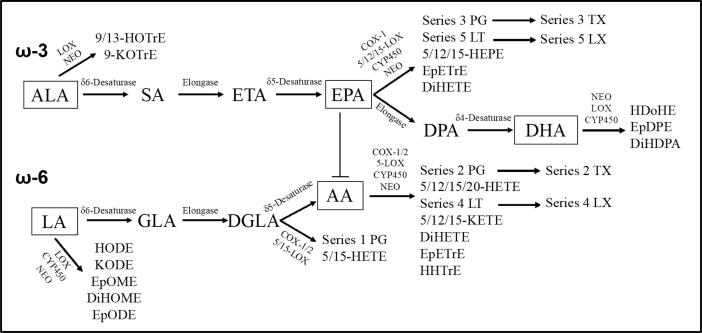
Fig. 1.
ω-3 and ω-6 PUFAs and their subsequent products Omega 3& 6 PUFAs metabolic pathways in mammals leading to eicosanoid production. ALA and LA are the major precursors of eicosanoids, derived mainly from plants. Desaturation and elongation reactions mediated by specific enzymes generates AA, EPA and DHA, which are the direct precursors of prostaglandins (PGs), thromboxanes (TXAs) and leukotrienes (LTs). EPA production inhibits AA metabolism as EPA derivatives are widely known for anti-inflammatory and anti-atherogenic effects.
In addition endothelial nitric oxide (NO) and foam cell ROS, nearly all leukotrienes and their products are capable of being involved in extracellular redox reactions, which can also oxidize membrane lipids or lipoproteins. ROS are produced within macrophages for the lysis and oxidation of phagocytosed pathogens or debris.
Novel drug development
Aspirin is still the most successful NSAID used in treating inflammatory acute episodes and even its products have inspired the structure of novel synthetic drugs (e.g. ATLa) [58]. Given the restorative and protective effects of these anti-inflammatory lipoxins resulting from treatment with aspirin, ATLa’s (an aspirin-triggered lipoxin A4 synthetic analog) have inspired the design of novel drugs that mimic their active sites. In one successful study, the design of numerous isomers resulted in two extremely successful molecules that mimic the triene-nature of ATLa’s [58].
Similar to MK886 mechanisms, inhibiting FLAP translocation and the subsequent activation of 5-LO, derivatives of ibuprofen (BRP-7, also referred to as 6 FLAP inhibitor) have been designed to imitate this action by inhibiting cPLA2 from releasing AA [59]. Currently, the most successful agonist of cPLA2 is a derivative of BRP-7, which as a nitrile group added to the C(5) position of the BI-ring that allows this compound the highest affinity for cPLA2 and most significantly lowered levels of AA’s immediate precursors [60]. The implications of a drug that successfully limits AA metabolism would be applicable to diets high in AA, such as the Western diet, and for those afflicted with a chronic inflammatory disease, such as obesity, diabetes, lupus, irritable bowel syndrome (IBS) or rheumatoid arthritis (Table 4).
Table 4.
Eicosanoids and other lipids precursor’s modulators of cell signaling.
| Lipoxin | Molecular Formula | Action |
|---|---|---|
| LXA4 | 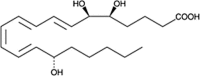 |
Vasodilation, competes with LTD4 receptors to regulate neutrophil recruitment |
| LXB4 | 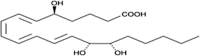 |
Vasodilation |
| 13-HODE | 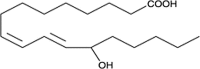 |
Activate/deactivate tissue-specific transcription factors involved in cell growth |
| 17(S)-HpDHA | 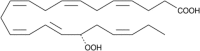 |
Precursor of PD1 and RvD1 |
| PD1 |  |
Prevent (Mφ) apoptosis by enhancing PMN recruitment |
| RvE1 | 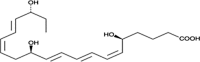 |
Tissue regeneration, lesion restoration |
| 15-epi-LXA4(ATLa9) | 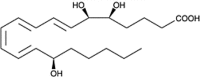 |
Reduces leukocyte infiltration to site of inflammation |
Nutrition
NSAID treatments have been correlated with increased risk of developing CAD and current evidence suggests COX inhibition also hinders the conversion of ALA or LA into AA, EPA or DHA [60], [61], [62], [63]. Overall, this knowledge only drives the need for more studies to identify alternative therapies via nutrition. Currently, essential n-3 and n-6 PUFAs are the focal point of these nutritional studies identifying their regulation of eicosanoid production and atherosclerosis due to their involvement in redox reactions as antioxidants that delay the occurrence of oxLDLs and subsequent formation of eicosanoids. Ideally, n-3 and n-6 consumption would be accomplished in a balanced fashion; however, most diets tend to favor certain essential PUFAs over others, especially AA in diets consisting mainly of meat and dairy or DHA in diets that include an abundance of fish [64], [65], [66], [67], [68].
Dietary consumption of AA, EPA and DHA increases the availability of these essential PUFAs for our tissues, as our bodies are not extremely efficient in metabolizing ALA into AA or LA into EPA then DHA. Additionally, competition for enzymes involved in producing EPA actually reduces production of AA [13]. In fact, one clinical study supports this as female participants on a vegan diet had the lowest levels of AA and LDL [69], [70] the biomarkers of atherosclerosis compared to males/females on regular Western diets or vegetarian diets with consistent dairy consumption [71], [72], [73].
AA, EPA and DHA are derived from ALA and LA through a series of desaturation and elongation events, mediated by δ6-desaturase, elongase and lastly, δ5-desaturase. δ6-desaturase and δ5-desaturase are also referred to as fatty acid desaturase 1 (FADS1) and fatty acid desaturase 2 (FADS2), respectively, which are also the COX-1/2 enzymes. The elongation process involved is also unique to these essential fatty acids, as elongase has selective specificity for long fatty acid chains (10–24 carbon chains) [74], [75], [76].
The enzymes involved in the production of AA, EPA, DHA and eicosanoids also rely upon the involvement of specific vitamins and minerals as the active donor for reactivating their active sites or as the active site in catalytic metalloproteases. FADS1 relies on vitamin B6 [77], Mg2+ and Zn2+, while FADS2 activity is dependent upon niacin, Zn2+ and vitamin C, and the involvement of these vitamins and minerals can be supported by studies that demonstrate an association between their supplementation or deficiency and relative levels of inflammation or rates of atherosclerosis in vivo [78], [79], [80]. In the LOX pathway, LTA4-H, GGT and MBD are all Zn2+ dependent metalloproteases. Studies that specifically supplemented niacin, folic acid and/or folate have distinguished niacin’s role in promoting inflammation resolution and the combined effect of folic acid and folate in lowering the aforementioned homocysteine levels (LTE4, LXB4 and PGE4) [81], [82]. A few common foods that are rich in niacin/vitamin B3 include poultry, peanuts, mushrooms, liver, tuna and peas.
Some studies suggest increasing n-3 consumption while others favor n-6 consumption [83], [84], [85], [86], [51]. However, numerous studies shows that simply increasing one or the other only increases expression levels of COX enzymes and subsequently their primary products, which tend to be pro-atherogenic [87], [88]. In this specific study, Wister rats were fed either a strict flaxseed oil diet or palm oil diet. The flaxseed oil diets enhanced COX enzyme expression resulted in heightened aortic sensitivity to phenylephrine, a vasodepressor often utilized clinically to lower blood pressure or as a nasal decongestant [89]. In light of this study and given the antioxidative nature of n-3/n-6’s, preventing the oxidization of LDLs during an acute phase response, it can be questioned as to whether or not these heightened levels of n-3/n-6 PUFAs also potentiate the conversion of primary eicosanoids to their anti-atherogenic counterparts. Additionally, a new source of n-3 PUFAs from botanical oils (e.g. primrose essential oil) has been identified to have equally anti-atherogenic capacities as fish oil or echium oil [25]. Primrose essential oil is known to have higher levels of GLA, which is rare in nature, and could be of more clinical importance given the potentially potent anti-inflammatory effects of its series 1 prostaglandins.
Foods identified to have high levels of essential omega-fatty acids can be found in studies involving PUFA’s that are abundant in walnuts, flaxseeds, botanical oils and conjugated LA (CLA) are most geared towards nutritional therapies than other novel therapies. In one study investigating nutritional therapy for metabolic syndrome, cinnamaldehyde demonstrated antioxidative and anti-inflammatory effects that preserved the vascular endothelium from fructose-induced cardiovascular damage [90], [91], [92], [93].
Other dietary methods that have been identified to reduce inflammation related to atherosclerosis cite anthocyanins and other polyphenolic compounds, found mostly in highly pigmented fruits and vegetables that are dark red, purple or blue. These polyphenols compounds demonstrated that they can prevent atherogenesis by lowering levels of TXB2 and 12-HETE and showing reduced platelet aggregation [94], [95], [96], [97], [98]. The proposed involvement of anthocyanins and polyphenols are not specific to COX or LOX, but their affinity for redox reactions could theoretically attenuate overall lipid oxidation levels, which can been associated with overall lower levels of eicosanoids and subsequently less robust acute phase responses altogether [98], [99], [100], [101], [102] (see Table 5).
Table 5.
Selected drug molecules with potential action on atherosclerosis.
| Active molecule | Experimental Design/ Model | Target Molecule | Results | Future Implications |
|---|---|---|---|---|
| BRP-7 | Human PMNs – ex vivo | FLAP | Novel inhibitors of leukotriene biosynthesis by targeting 5-lipoxygenase-activating protein | Improved MK886-like drugs |
| BRP-7 with nitrile at C(5)-BI position | HEK293s – in vitro | FLAP | Addition of nitrile group to BRP-7 significantly enhances FLAP binding | Improved FLAP inhibitors – in vitro |
| PGF2α | Micromini pigs | Estrogen Progesterone |
Corpus luteum regression results from treatment lowered estrogen and progesterone | PGF2α-like eicosanoid derived contraceptive trials |
| Varespladib: Inhibitors of PLA2 | Patients with Cardiovascular Disease | PLA2 | Reduces inflammation, atherogenic lipids | FDA considered varespladib an orphan drug for its potential to treat patients with sickle cell disease |
| VIA‐2291 (atreleuton) | Patients with recent acute coronary syndrome (ACS) | 5‐lipoxygenase inhibitor | Slowed plaque progression | Atreleuton has been used in trials studying the treatment of Atherosclerosis and Coronary Artery Disease. |
| Darapladib: Inhibitors of PLA2 | Double-blind trial, assigned patients with stable coronary heart disease to receive once-daily darapladib | Lp-PLA2 | Reduces inflammation, atherogenic lipids | The study failed to reduce the risk of coronary heart disease death |
Conclusion
The role of chronic inflammatory diseases linked to atherosclerosis often involves cardiometabolic dysfunctions; the relatively reactive nature of eicosanoids is responsible for their specifically local actions in an acute phase response; only supporting the need for more studies identifying nutritional therapies. Additionally, AA levels in the Western diet raises questions involving its activation-dependent COX-2 metabolism in leukocytes in relation to constitutive COX-1 activities, which produces mainly GLA from LA. The effect of excess AA on these enzymes would be useful in identifying potential improvement targets related to a Western diet. The association of high dietary AA with atherogenesis further supports evidence for critical nutrition’s intervention in atherosclerosis and highlights the need for nutritional therapy development over pharmaceutical drugs intervention for the treatment of eicosanoids driven inflammation and oxidative stress responsible for CVD. Ideally, diets that balance n-3 and n-6 in combination with the vitamins or minerals associated with their enhanced metabolism and bioavailability would be beneficial to these aforementioned diseases or biomarkers for atherosclerosis. Additionally, current initiatives to increase consumption of fruits and vegetables could potentially lower future rates of heart disease especially in Westernize societies.
Authors’ contributions
Both authors Kimberly Piper and Mahdi Garelnabi participated in the manuscript preparation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcte.2020.100216.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Greenlund K, Giles W, Keenan N. Heart disease and stroke mortality in the 20th century. Ward J, Warren C, eds. Silent victories: the history and practice of public health in twentieth century America. (2006).
- 2.Capewell Simon. Cardiovascular risk factor trends and potential for reducing coronary heart disease mortality in the United States of America. Bull World Health Organ. 2010;88:120–130. doi: 10.2471/BLT.08.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parthasarathy Sampath, Litvinov Dmytri, Selvarajan Krithika, Garelnabi Mahdi. Lipid peroxidation and decomposition – conflicting roles in plaque vulnerability and stability. Biochim Biophys Acta. 2008;1781:221–231. doi: 10.1016/j.bbalip.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taleb S. Inflammation in atherosclerosis. Arch Cardiovasc Dis. 2016 Dec;109(12):708–715. doi: 10.1016/j.acvd.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Viola J., Soehnlein O. Atherosclerosis – a matter of unresolved inflammation. Semin Immunol. 2015;27(3):184–193. doi: 10.1016/j.smim.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P., Ridker P.M., Hansson G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 8.Cox RA, García-Palmieri MR. Cholesterol, Triglycerides, and Associated Lipoproteins. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 31.
- 9.Berg Jeremy M, Tymoczko John L, Gatto GJ Jr. Stryer: Biochemistry. WH Freeman and Company 5 (2002): 306–307.
- 10.Cooper, Geoffrey M, Hausman Robert E. The cell: molecular approach. 6th ed., 2013. Sinauer Associates: Sunderland, MA.
- 11.Funk Colin D. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nature Reviews Drug Discovery. 2005;4(8):664–672. doi: 10.1038/nrd1796. [DOI] [PubMed] [Google Scholar]
- 12.Seo Min-Ju, Oh Deok-Kun. Prostaglandin synthases: molecular characterization and involvement in prostaglandin biosynthesis. Prog Lipid Res. 2017;66:50–68. doi: 10.1016/j.plipres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Meade Elizabeth A., Smith William L., Dewitt David L. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993;268(9):6610–6614. [PubMed] [Google Scholar]
- 14.Dobrian Anca D. Functional and pathological roles of the 12-and 15-lipoxygenases. Prog Lipid Res. 2011;50(1):115–131. doi: 10.1016/j.plipres.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leikauf George D. Activation of eicosanoid metabolism in human airway epithelial cells by ozonolysis products of membrane fatty acids. Res Rep Health Eff Inst. 1995;71:1–15. [PubMed] [Google Scholar]
- 16.Schrör K. Prostaglandins, other eicosanoids and endothelial cells. Basic Res Cardiol. 1985;80(5):502–514. doi: 10.1007/BF01907914. [DOI] [PubMed] [Google Scholar]
- 17.Murphy Robert C., Gijón Miguel A. Biosynthesis and metabolism of leukotrienes. Biochem J. 2007;405(3):379–395. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- 18.Morita Ikuo. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002;68:165–175. doi: 10.1016/s0090-6980(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 19.Yu Y, Ricciotti E, Scalia R, Tang SY, Grant G, Yu Z, Landesberg G, Crichton I, Wu W, Puré E, Funk CD, FitzGerald GA. Vascular COX-2 modulates blood pressure and thrombosis in mice. Sci Transl Med. 2012 May 2;4(132):132ra54. doi: 10.1126/scitranslmed.3003787. PubMed PMID: 22553252; PubMed Central PMCID: PMC3882087. [DOI] [PMC free article] [PubMed]
- 20.Lara-Guzmán O.J., Gil-Izquierdo Á., Medina S., Osorio E., Álvarez-Quintero R., Zuluaga N., Oger C., Galano J.M., Durand T., Muñoz-Durango K. Oxidized LDL triggers changes in oxidative stress and inflammatory biomarkers in human macrophages. Redox Biol. 2018;15:1–11. doi: 10.1016/j.redox.2017.11.017. Epub 2017 Nov 22. PubMed PMID: 29195136; PubMed Central PMCID: PMC5723280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terao J., Asano I., Matsushita S. Preparation of hydroperoxy and hydroxyl derivatives of rat liver phosphatidylcholine and phosphatidylethanolamine. Lipids. 1985;20(5):312–317. doi: 10.1007/BF02534264. [DOI] [PubMed] [Google Scholar]
- 22.Gniwotta C., Morrow J.D., Roberts L.J., 2nd, Kühn H. Prostaglandin F2-like compounds, F2-isoprostanes, are present in increased amounts in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1997;17(11):3236–3241. doi: 10.1161/01.atv.17.11.3236. [DOI] [PubMed] [Google Scholar]
- 23.Berry K.A., Borgeat P., Gosselin J., Flamand L., Murphy R.C. Urinary metabolites of leukotriene B4 in the human subject. J Biol Chem. 2003 4;278(27):24449–24460. doi: 10.1074/jbc.M300856200. [DOI] [PubMed] [Google Scholar]
- 24.Shewale S.V., Boudyguina E., Zhu X., Shen L., Hutchins P.M., Barkley R.M. Botanical oils enriched in n-6 and n-3 FADS2 products are equally effective in preventing atherosclerosis and fatty liver. J Lipid Res. 2015;56(6):1191–1205. doi: 10.1194/jlr.M059170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohn C.E., Fernandez-Solari J., De Laurentiis A., Prestifilippo J.P., de la Cal C., Funk R. The rapid release of corticosterone from the adrenal induced by ACTH is mediated by nitric oxide acting by prostaglandin E2. Proc Natl Acad Sci USA. 2005;102(17):6213–6218. doi: 10.1073/pnas.0502136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imig J.D., Walsh K.A., Hye Khan M.A., Nagasawa T., Cherian-Shaw M., Shaw S.M. Soluble epoxide hydrolase inhibition and peroxisome proliferator activated receptor γ agonist improve vascular function and decrease renal injury in hypertensive obese rats. Exp Biol Med (Maywood) 2012;237(12):1402–1412. doi: 10.1258/ebm.2012.012225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imig J.D. Epoxyeicosanoids in hypertension. Physiol Res. 2019;68(5):695–704. doi: 10.33549/physiolres.934291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hye Khan M.A., Stavniichuk A., Sattar M.A., Falck J.R., Imig J.D. Epoxyeicosatrienoic acid analog EET-A blunts development of lupus nephritis in mice. Front Pharmacol. 2019;10(10):512. doi: 10.3389/fphar.2019.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neckář J., Hye Khan M.A., Gross G.J., Cyprová M., Hrdlička J., Kvasilová A. Epoxyeicosatrienoic acid analog EET-B attenuates post-myocardial infarction remodeling in spontaneously hypertensive rats. Clin Sci (Lond) 2019;133(8):939–951. doi: 10.1042/CS20180728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hye Khan M.A., Schmidt J., Stavniichuk A., Imig J.D., Merk D. A dual farnesoid X receptor/soluble epoxide hydrolase modulator treats non-alcoholic steatohepatitis in mice. Biochem Pharmacol. 2019;166:212–221. doi: 10.1016/j.bcp.2019.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia V., Gilani A., Shkolnik B., Pandey V., Zhang F.F., Dakarapu R. 20-HETE signals through G-protein-coupled receptor GPR75 (G(q)) to affect vascular function and trigger hypertension. Circ Res. 2017;120(11):1776–1788. doi: 10.1161/CIRCRESAHA.116.310525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fava C., Bonafini S. Eicosanoids via CYP450 and cardiovascular disease: hints from genetic and nutrition studies. Prostaglandins Other Lipid Mediat. 2018;139:41–47. doi: 10.1016/j.prostaglandins.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Yu Y., Cheng Y., Fan J., Chen X.S., Klein-Szanto A., Fitzgerald G.A. Differential impact of prostaglandin H synthase 1 knockdown on platelets and parturition. J Clin Invest. 2005;115(4):986–995. doi: 10.1172/JCI23683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joo M, Sadikot RT. PGD synthase and PGD2 in immune response. Mediators Inflamm. 2012;2012:503128. doi: 10.1155/2012/503128. Epub 2012 Jun 25. PubMed PMID: 22791937; PubMed Central PMCID: PMC3389719. [DOI] [PMC free article] [PubMed]
- 35.Vallerie S.N., Kramer F., Barnhart S., Kanter J.E., Breyer R.M., Andreasson K.I. Myeloid cell prostaglandin E2 receptor EP4 modulates cytokine production but not atherogenesis in a mouse model of type 1 diabetes. PLoS ONE. 2016;11(6) doi: 10.1371/journal.pone.0158316. eCollection 2016. PubMed PMID: 27351842; PubMed Central PMCID: PMC4924840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noguchi M., Hirata M., Kawaguchi H., Tanimoto A. Corpus luteum regression induced by prostaglandin F(2α) in microminipigs during the normal estrous cycle. In Vivo. 2017;31(6):1097–1101. doi: 10.21873/invivo.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duffy Diane M. Novel contraceptive targets to inhibit ovulation: the prostaglandin E2 pathway. Human Reproduction Update. 2015;21(5):652–670. doi: 10.1093/humupd/dmv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori-Kawabe Mayumi. Role of Rho/Rho-kinase and NO/cGMP signaling pathways in vascular function prior to atherosclerosis. J Atherosclerosis Thrombosis. 2010;16(6):722–732. doi: 10.5551/jat.1875. [DOI] [PubMed] [Google Scholar]
- 39.Tang S.Y., Monslow J., Todd L., Lawson J., Puré E., FitzGerald G.A. Cyclooxygenase-2 in endothelial and vascular smooth muscle cells restrains atherogenesis in hyperlipidemic mice. Circulation. 2014;129(17):1761–1769. doi: 10.1161/CIRCULATIONAHA.113.007913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones David A., Fitzpatrick F.A. Thromboxane A2 synthase. Modification during “suicide” inactivation. J Biol Chem. 1991;266(34):23510–23514. [PubMed] [Google Scholar]
- 41.Gitlin Jonathan M., Loftin Charles D. Cyclooxygenase-2 inhibition increases lipopolysaccharide-induced atherosclerosis in mice. Cardiovasc Res. 2008;81(2):400–407. doi: 10.1093/cvr/cvn286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diederichsen S.Z., Grønhøj M.H., Mickley H., Gerke O., Steffensen F.H., Lambrechtsen J. CT-detected growth of coronary artery calcification in asymptomatic middle-aged subjects and association with 15 biomarkers. JACC Cardiovasc Imaging. 2017;10(8):858–866. doi: 10.1016/j.jcmg.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Ray WA, Stein CM, Daugherty JR, Hall K, Arbogast PG, Griffin MR. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet. 2002;360(9339):1071-3. [DOI] [PubMed]
- 44.Frolov A., Yang L., Dong H., Hammock B.D., Crofford L.J. Anti-inflammatory properties of prostaglandin E2: deletion of microsomal prostaglandin E synthase-1 exacerbates non-immune inflammatory arthritis in mice. Prostaglandins Leukot Essent Fatty Acids. 2013;89(5):351–358. doi: 10.1016/j.plefa.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian X., Zhang J., Liu J. Tumor-secreted PGE2 inhibits CCL5 production in activated macrophages through cAMP/PKA signaling pathway. J Biol Chem. 2011;286(3):2111–2120. doi: 10.1074/jbc.M110.154971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loynes CA, Lee JA, Robertson AL, Steel MJ, Ellett F, Feng Y, Levy BD, Whyte MKB, Renshaw SA. PGE(2) production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo. Sci Adv. 2018;4(9):eaar8320. [DOI] [PMC free article] [PubMed]
- 47.Thompson D., Morrice N., Grant L., Le Sommer S., Ziegler K., Whitfield P. Myeloid protein tyrosine phosphatase 1B (PTP1B) deficiency protects against atherosclerotic plaque formation in the ApoE(-/-) mouse model of atherosclerosis with alterations in IL10/AMPKα pathway. Mol Metab. 2017;6(8):845–853. doi: 10.1016/j.molmet.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffin É.W., Skelly D.T., Murray C.L., Cunningham C. Cyclooxygenase-1-dependent prostaglandins mediate susceptibility to systemic inflammation-induced acute cognitive dysfunction. J Neurosci. 2013;33(38):15248–15258. doi: 10.1523/JNEUROSCI.6361-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirkby Nicholas S. et al. Systematic study of constitutive cyclooxygenase-2 expression: role of NF-κB and NFAT transcriptional pathways. Proc Natl Acad Sci 113(2)(2016): 434–39. [DOI] [PMC free article] [PubMed]
- 50.Griffin M.R. High-dose non-steroidal anti-inflammatories: painful choices. Lancet. 2013;382(9894):746–748. doi: 10.1016/S0140-6736(13)61128-9. [DOI] [PubMed] [Google Scholar]
- 51.Chistiakov D.A., Melnichenko A.A., Grechko A.V., Myasoedova V.A., Orekhov A.N. Potential of anti-inflammatory agents for treatment of atherosclerosis. Exp Mol Pathol. 2018;104(2):114–124. doi: 10.1016/j.yexmp.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Ahmetaj-Shala B., Kirkby N.S., Knowles R., Al'Yamani M., Mazi S., Wang Z. Evidence that links loss of cyclooxygenase-2 with increased asymmetric dimethylarginine: novel explanation of cardiovascular side effects associated with anti-inflammatory drugs. Circulation. 2015;131(7):633–642. doi: 10.1161/CIRCULATIONAHA.114.011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Call JT, Deliargyris EN, Newby LK. Focusing on inflammation in the treatment of atherosclerosis. Cardiol Rev. 2004;12(4):194–200. [DOI] [PubMed]
- 54.Werz O., Gerstmeier J., Libreros S., De la Rosa X., Werner M., Norris P.C. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat Commun. 2018;9(1):59. doi: 10.1038/s41467-017-02538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riccioni Graziano, Bäck Magnus. Leukotrienes as modifiers of preclinical atherosclerosis? Scientific World J. 2012;2012 doi: 10.1100/2012/490968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colazzo F, Gelosa P, Tremoli E, Sironi L, Castiglioni L. Role of the Cysteinyl Leukotrienes in the Pathogenesis and Progression of Cardiovascular Diseases. Mediators Inflamm 2017;2017:2432958. doi: 10.1155/2017/2432958. Epub 2017 Aug 28. Review. PubMed PMID: 28932020; PubMed Central PMCID: PMC5592403. [DOI] [PMC free article] [PubMed]
- 57.Devchand Pallavi R. The PPARα–leukotriene B4 pathway to inflammation control. Nature. 1996;384(6604):39. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 58.Kim Jeong A. A leukocyte type of 12-lipoxygenase is expressed in human vascular and mononuclear cells: evidence for upregulation by angiotensin II. Arterioscler Thromb Vasc Biol. 1995;15(7):942–948. doi: 10.1161/01.atv.15.7.942. [DOI] [PubMed] [Google Scholar]
- 59.Dessen Andréa. Crystal structure of human cytosolic phospholipase A2 reveals a novel topology and catalytic mechanism. Cell. 1999;97(3):349–360. doi: 10.1016/s0092-8674(00)80744-8. [DOI] [PubMed] [Google Scholar]
- 60.Dwyer James H. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350(1):29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 61.Deng Yangmei. Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB J. 2011;25(2):703–713. doi: 10.1096/fj.10-171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bannenberg Gerard L. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174(7):4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 63.Conti Pio. Mast cells emerge as mediators of atherosclerosis: Special emphasis on IL-37 inhibition. Tissue Cell. 2017;49(3):393–400. doi: 10.1016/j.tice.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Anand R., Kaithwas G. Anti-inflammatory potential of alpha-linolenic acid mediated through selective COX inhibition: computational and experimental data. Inflammation. 2014;37(4):1297–1306. doi: 10.1007/s10753-014-9857-6. [DOI] [PubMed] [Google Scholar]
- 65.Davis J.S., Lee H.Y., Kim J., Advani S.M., Peng H.L., Banfield E. Use of non-steroidal anti-inflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1) doi: 10.1136/openhrt-2016-000550. eCollection 2017. PubMed PMID: 28674622; PubMed Centra PMCID: PMC5471872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agca R., Heslinga S.C., Rollefstad S., Heslinga M., McInnes I.B., Peters M.J. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76(1):17–28. doi: 10.1136/annrheumdis-2016-209775. [DOI] [PubMed] [Google Scholar]
- 67.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barnard N.D., Willett W.C., Ding E.L. The misuse of meta-analysis in nutrition research. JAMA. 2017;318(15):1435–1436. doi: 10.1001/jama.2017.12083. [DOI] [PubMed] [Google Scholar]
- 69.O'Neill C.M., Minihane A.M. The impact of fatty acid desaturase genotype on fatty acid status and cardiovascular health in adults. Proc Nutr Soc. 2017;76(1):64–75. doi: 10.1017/S0029665116000732. [DOI] [PubMed] [Google Scholar]
- 70.Nettleton J.A., Lovegrove J.A., Mensink R.P., Schwab U. Dietary fatty acids: is it time to change the recommendations? Ann Nutr Metab. 2016;68(4):249–257. doi: 10.1159/000446865. [DOI] [PubMed] [Google Scholar]
- 71.Kris-Etherton P.M., Fleming J.A. Emerging nutrition science on fatty acids and cardiovascular disease: nutritionists’ perspectives. Adv Nutr. 2015;6(3):326S–337S. doi: 10.3945/an.114.006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vannice G., Rasmussen H. Position of the academy of nutrition and dietetics: dietary fatty acids for healthy adults. J Acad Nutr Diet. 2014;114(1):136–153. doi: 10.1016/j.jand.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 73.Huang Y.W., Jian Z.H., Chang H.C., Nfor O.N., Ko P.C., Lung C.C. Vegan diet and blood lipid profiles: a cross-sectional study of pre and postmenopausal women. BMC Womens Health. 2014;8(14):55. doi: 10.1186/1472-6874-14-55. PubMed PMID: 24712525; PubMed Central PMCID: PMC3996202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.James Michael J, Robert A Gibson, Leslie G Cleland. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr 71(1)(2000): 343s-348s. [DOI] [PubMed]
- 75.Kahleova H, Levin S, Barnard N. Cardio-metabolic benefits of plant-based diets. nutrients. 2017 Aug 9;9(8). pii: E848. doi: 10.3390/nu9080848. Review. PubMed PMID: 28792455; PubMed Central PMCID: PMC5579641. [DOI] [PMC free article] [PubMed]
- 76.Melina V., Craig W., Levin S. Position of the academy of nutrition and dietetics: vegetarian diets. J Acad Nutr Diet. 2016;116(12):1970–1980. doi: 10.1016/j.jand.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 77.Barr S.I. Vegetarian diets. World Rev Nutr Diet. 2015;111:53–57. doi: 10.1159/000362297. [DOI] [PubMed] [Google Scholar]
- 78.Khatun J., Loh T.C., Akit H., Foo H.L., Mohamad R. Fatty acid composition, fat deposition, lipogenic gene expression and performance of broiler fed diet supplemented with different sources of oil. Anim Sci J. 2017;88(9):1406–1413. doi: 10.1111/asj.12775. [DOI] [PubMed] [Google Scholar]
- 79.Igal RA. Stearoyl CoA desaturase-1: New insights into a central regulator of cancer metabolism. Biochim Biophys Acta 2016 Dec;1861(12 Pt A):1865–1880. [DOI] [PubMed]
- 80.Rezamand P., Watts J.S., Yavah K.M., Mosley E.E., Ma L., Corl B.A. Relationship between stearoyl-CoA desaturase 1 gene expression, relative protein abundance, and its fatty acid products in bovine tissues. J Dairy Res. 2014;81(3):333–339. doi: 10.1017/S0022029914000181. [DOI] [PubMed] [Google Scholar]
- 81.Zhao M., Ralat M.A., da Silva V., Garrett T.J., Melnyk S., James S.J. Vitamin B-6 restriction impairs fatty acid synthesis in cultured human hepatoma (HepG2) cells. Am J Physiol Endocrinol Metab. 2013;304(4):E342–E351. doi: 10.1152/ajpendo.00359.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J.Y., Kothapalli K.S., Brenna J.T. Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis. Curr Opin Clin Nutr Metab Care. 2016;19(2):103–110. doi: 10.1097/MCO.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee H., Park W.J. Unsaturated fatty acids, desaturases, and human health. J Med Food. 2014;17(2):189–197. doi: 10.1089/jmf.2013.2917. [DOI] [PubMed] [Google Scholar]
- 84.Guillou H., Zadravec D., Martin P.G., Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog Lipid Res. 2010;49(2):186–199. doi: 10.1016/j.plipres.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Claar Dru, Hartert Tina V., Peebles Ray Stokes., Jr. The role of prostaglandins in allergic lung inflammation and asthma. Expert Rev Respiratory Med. 2015;9(1):55–72. doi: 10.1586/17476348.2015.992783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bønaa Kaare Harald. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 87.Wongwarawipat T., Papageorgiou N., Bertsias D., Siasos G., Tousoulis D. Olive oil-related anti-inflammatory effects on atherosclerosis: potential clinical implications. Endocr Metab Immune Disord Drug Targets. 2018;18(1):51–62. doi: 10.2174/1871530317666171116103618. [DOI] [PubMed] [Google Scholar]
- 88.Moss J.W., Ramji D.P. Nutraceutical therapies for atherosclerosis. Nat Rev Cardiol. 2016;13(9):513–532. doi: 10.1038/nrcardio.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khan R., Spagnoli V., Tardif J.C., L'Allier P.L. Novel anti-inflammatory therapies for the treatment of atherosclerosis. Atherosclerosis. 2015;240(2):497–509. doi: 10.1016/j.atherosclerosis.2015.04.783. [DOI] [PubMed] [Google Scholar]
- 90.Bäck M., Hansson G.K. Anti-inflammatory therapies for atherosclerosis. Nat Rev Cardiol. 2015;12(4):199–211. doi: 10.1038/nrcardio.2015.5. [DOI] [PubMed] [Google Scholar]
- 91.Silva Figueiredo P, Carla Inada A, Marcelino G, Maiara Lopes Cardozo C, de Cássia Freitas K, de Cássia Avellaneda Guimarães R, Pereira de Castro A, Aragão do Nascimento V, Aiko Hiane P. Fatty acids consumption: the role metabolic aspects involved in obesity and its associated disorders. Nutrients. 2017 Oct22;9(10). pii: E1158. doi: 10.3390/nu9101158. Review. PubMed PMID: 29065507;PubMed Central PMCID: PMC5691774. [DOI] [PMC free article] [PubMed]
- 92.Szostak-Wegierek D., Kłosiewicz-Latoszek L., Szostak W.B., Cybulska B. The role of dietary fats for preventing cardiovascular disease. A review. Rocz Panstw Zakl Hig. 2013;64(4):263–269. [PubMed] [Google Scholar]
- 93.Dieli Oliveira Nunes, Camila Cruz Pereira Almenara, Gilson Brás Broseghini-Filho, Marito Afonso Sousa Costa Silva, Ivanita Stefanon, Dalton V Vassallo, Alessandra S Padilha. Flaxseed oil increases aortic reactivity to phenylephrine through reactive oxygen species and the cyclooxygenase-2 pathway in rats. Lipids Health Dis. 2014; 13: 107. Published online 2014 Jul 3. doi: 10.1186/1476-511X-13-107 PMCID: PMC4226993. [DOI] [PMC free article] [PubMed]
- 94.Peterson L.D., Thies F., Calder P.C. Dose-dependent effects of dietary gamma-linolenic acid on rat spleen lymphocyte functions. Prostaglandins Leukot Essent Fatty Acids. 1999;61(1):19–24. doi: 10.1054/plef.1999.0067. [DOI] [PubMed] [Google Scholar]
- 95.Arisaka M., Arisaka O., Yamashiro Y. Fatty acid and prostaglandin metabolism in children with diabetes mellitus. II. The effect of evening primrose oil supplementation on serum fatty acid and plasma prostaglandin levels. Prostaglandins Leukot Essent Fatty Acids. 1991;43(3):197–201. doi: 10.1016/0952-3278(91)90169-6. [DOI] [PubMed] [Google Scholar]
- 96.Nassar B.A., Manku M.S., Huang Y.S., Jenkins D.K., Horrobin D.F. The influence of dietary marine oil (Polepa) and evening primrose oil (Efamol) on prostaglandin production by the rat mesenteric vasculature. Prostaglandins Leukot Med. 1987;26(3):253–263. doi: 10.1016/0262-1746(87)90035-7. [DOI] [PubMed] [Google Scholar]
- 97.Ramchurren N, Botha JH, Robinson KM, Leary WP. Effects of gamma-linolenic acid on murine cells in vitro and in vivo. S Afr Med J. 1985 Nov 23;68(11):795–8. [PubMed]
- 98.Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10(11). pii: E1618. doi: 10.3390/nu10111618. Review. PubMed PMID: 30400131; PubMed Central PMCID: PMC6266803. [DOI] [PMC free article] [PubMed]
- 99.Forino M., Pace S., Chianese G., Santagostini L., Werner M., Weinigel C. Humudifucol and bioactive prenylated polyphenols from hops (Humulus lupulus cv. “Cascade”) J Nat Prod. 2016;79(3):590–597. doi: 10.1021/acs.jnatprod.5b01052. [DOI] [PubMed] [Google Scholar]
- 100.Smit Ella N. Higher erythrocyte 22: 6n–3 and 22: 5n–6, and lower 22: 5n–3 suggest higher Δ-4-desaturation capacity in women of childbearing age. Br J Nutr. 2003;89(5):739–740. doi: 10.1079/BJN2003851. [DOI] [PubMed] [Google Scholar]
- 101.Pace-Asciak Cecil R. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta. 1995;235(2):207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- 102.Lila Mary Ann. Anthocyanins and human health: an in vitro investigative approach. Biomed Res Int. 2004;2004(5):306–313. doi: 10.1155/S111072430440401X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.