Highlights
-
•
This study examined neural alterations related to reward processing in IGD.
-
•
IGD participants showed blunted caudate activity associated with received loss magnitude relative to controls.
-
•
IGD did not show significant neural alterations related to gain processing.
-
•
Decreased loss sensitivity might be a general feature across addictions.
Keywords: Behavioral addiction, Reward processing, Loss aversion, Monetary incentive delay task, Internet gaming disorder
Abstract
Current models of addiction biology highlight altered neural responses to non-drug rewards as a central feature of addiction. However, given that drugs of abuse can directly impact reward-related dopamine circuitry, it is difficult to determine the extent to which reward processing alterations are a trait feature of individuals with addictions, or primarily a consequence of exogenous drug exposure. Examining individuals with behavioral addictions is one promising approach for disentangling neural features of addiction from the direct effects of substance exposure. The current fMRI study compared neural responses during monetary reward processing between drug naïve young adults with a behavioral addiction, internet gaming disorder (IGD; n = 22), and healthy controls (n = 27) using a monetary incentive delay task. Relative to controls, individuals with IGD exhibited blunted caudate activity associated with loss magnitude at the outcome stage, but did not differ from controls in neural activity at other stages. These findings suggest that decreased loss sensitivity might be a critical feature of IGD, whereas alterations in gain processing may be less characteristic of individuals with IGD, relative to those with substance use disorders. Therefore, classic theories of altered reward processing in substance use disorders should be translated to behavioral addictions with caution.
1. Introduction
Reward processing is a critical component of adaptive functioning and plays a key role in hedonic feelings and learning from past experience. Accordingly, alterations in reward processing have been implicated in several different classes of psychiatric disorders, particularly addictions. Accumulating evidence suggests that the corticostriatal neural network, including the striatum, ventromedial prefrontal cortex (vmPFC) and adjacent orbitofrontal cortex, plays a key role in neural reward processing (Clithero and Rangel, 2014) and that aberrant corticostriatal reward processing may partially underlie maladaptive behaviors such as continued substance-use (Volkow and Morales, 2015).
Congruently, several classic theories of substance use disorders (SUDs) identify altered reward processing as a key element underlying the development and maintenance of addictions. For example, reward deficiency syndrome (Blum et al., 2000) proposes that blunted neural response to reward might serve as a risk factor for initiating addictive behaviors due to seeking of pleasant sensations. Alternatively, reward hypersensitivity theory (Hommer et al., 2011) suggests that hypersensitivity of neural reward circuitry may put certain individuals at higher risk for addiction. Further still, incentive sensitization theory (Robinson and Berridge, 1993) proposes that extended substance use may impact reward function by enhancing the salience of cues predicting drug rewards while non-drug rewards become relatively less reinforcing.
A key challenge for evaluating these existing theories lies in the fact that most drugs of abuse strongly impact dopamine release (Nutt et al., 2015) and neural reward circuit functioning (Clark et al., 2019). Therefore, in the context of SUDs, it is difficult to determine whether reward processing dysfunction is mainly caused by exposure to an exogenous drug or else is a general feature of addiction. Behavioral addictions therefore offer a unique window into the underlying neurobiology of addiction without the confound of substance exposure (Yip et al., 2018).
Within this context, previous studies have examined monetary reward processing in gambling disorder, a canonical behavioral addiction (Balodis and Potenza, 2015; Clark et al., 2019; Luijten et al., 2017). However, it should be noted that money is an addiction-relevant stimulus for gambling disorder (Luijten et al., 2017). Thus, assessment of monetary reward processing in other types of behavioral addictions is needed to determine the extent to which alterations in reward processing may be a general feature of addiction independent of prolonged drug exposure or in response to addiction-relevant cues. Here, we assessed reward processing using a monetary incentive delay (MID) task among young adults with internet gaming disorder (IGD).
IGD is included in the current version of the DSM-5 as a candidate behavioral addiction warranting further study, in large part because it shares several clinical symptoms with SUDs, including craving, tolerance, and continued use/engagement despite severe negative consequences ( American Psychiatric Association, 2013). Existing evidence shows alterations in value-based decision-making (Ko et al., 2017; Yao et al., 2015) and related neural activity in frontal and striatal regions in IGD (Dong et al., 2011; Liu et al., 2017b; Seok et al., 2015; Wei et al., 2017; Weinstein et al., 2017). However, reward-processing independent of decision-making in IGD still remains to be examined. In addition, studies parsing distinct components of reward processing, such as valence (e.g., gains and losses) and stage (e.g., anticipation and outcome)(Clark et al., 2019), are needed to gain a more precise understanding of monetary reward processing in IGD.
In the current study, we compared neural responses to monetary gains and losses between individuals with IGD and healthy controls (HC) using a MID task (Andrews et al., 2011), which separates valence and phase of reward processing. As this task only requires a simple motor response, it enables examination of different components of monetary reward processing without the interference of other cognitive functions such as learning and decision-making (Balodis and Potenza, 2015). Based on the above mentioned findings using value-based decision-making tasks in IGD (Dong et al., 2011; Liu et al., 2017a; Seok et al., 2015), and consistent with neural reward alterations reported in SUDs (Luijten et al., 2017), we hypothesized that individuals with IGD would show altered neural responses during the anticipation and receipt of rewards and losses in relevant brain regions, such as the striatum and vmPFC.
2. Methods
2.1. Participants
Participants were recruited via advertisements in Beijing, China. Potential participants first completed an online questionnaire including demographic information, as well as questions regarding their internet and substance use. Individuals who appeared eligible based on the online questionnaire were then invited to the behavioral laboratory to participate in a clinical interview. Presence or absence of IGD was determined by DSM-5 diagnostic criteria, and other psychiatric disorders were assessed using the MINI International Neuropsychiatric Interview (Sheehan et al., 1998). The inclusion criteria for the IGD group were: (1) meeting at least 5 DSM-5 IGD criteria (Ko et al., 2014); (2) playing one online game for at least 20 hours per week; and (3) scoring at least 50 on Young's Internet addiction test (IAT) (Young, 2009). The inclusion criteria for the HC group were: (1) no DSM-5 diagnosis of IGD; (2) never or occasional engaging (< 2 h per week) in internet gaming (Yao et al., 2017); and (3) scoring less than 50 on the IAT. Participants with a history of brain injury, SUDs, gambling disorder, and other psychiatric disorders were excluded from both groups.
A total of 28 male young adults with IGD and 30 age- and sex-matched healthy control (HC) participants underwent fMRI scanning. fMRI data from 5 IGD and 3 HC participants was excluded due to excess head motion (i.e., > 3mm of displacement of 3 degrees of rotation in any of the six head motion parameters from realignment in both runs of the task), and behavioral data from 1 IGD participant was not recorded due to a computer crash. Therefore, a final sample of 22 IGD and 27 HC participants was included in the current analyses. Participants’ demographics are shown in Table 1. This study complied with the Declaration of Helsinki and was approved by the Institutional Review Board of the State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University. All participants provided written informed consent and received financial compensation for their participation.
Table 1.
Demographics and Internet gaming characteristics of IGD and HC participants.
| IGD (n = 22) mean ± SD | HC (n = 27) mean ± SD | t/χ2 | p | Effect sizea | |
|---|---|---|---|---|---|
| Age | 22.27 ± 1.55 | 22.00 ± 1.92 | 0.54 | 0.593 | 0.16 |
| YIAT | 67.23 ± 11.61 | 25.30 ± 6.11 | 16.23 | <0.001 | 4.66 |
| Barratt impulsiveness scale | 77.00 ± 9.56 | 67.35 ± 7.01 | 4.03 | <0.001 | 1.17 |
| Beck depression inventory | 7.64 ± 7.97 | 2.89 ± 4.16 | 2.46 | <0.05 | 0.77 |
| Beck anxiety inventory | 5.91 ± 5.46 | 2.00 ± 2.63 | 2.88 | <0.01 | 0.94 |
| Tobacco smoker (n, %) | 2 (9.09) | 0 (0.00) | 2.56 | 0.11 | 0.23 |
| Any alcohol use (n, %)b | 13 (48.15) | 7 (33.33) | 1.07 | 0.30 | 0.15 |
| FTND | 0.18 ± 0.66 | 0.00 ± 0.00 | – | – | – |
| AUDITb | 1.10 ± 1.81 | 1.11 ± 1.72 | −0.03 | 0.975 | 0.01 |
YIAS: Young Internet addiction scale; AUDIT: alcohol use disorder identification test; FTND: Fagerstöm test for nicotine dependence.
Cohen's d for t-tests and Cramer's V for χ2 test.
Data missing for 1 IGD participant.
2.2. MID task
The version of the MID task used in this study was modified from the paradigm developed by Knutson et al. (2001) and has been widely used in previous studies measuring reward function in substance and gambling disorders (Andrews et al., 2011; Balodis and Potenza, 2015; Yip et al., 2016). As shown in Fig. 1, at the start of each trial, participants were presented with a cue (anticipation stage) indicating an amount of money to be won or lost (e.g., ‘WIN 5’ or ‘LOSE 1’, range: 0, 1, or 5), followed by a fixation cross. Participants were then presented with a target stimulus. In order to win (or avoid losing) money on each trial, participants need to respond with a single button press before the disappearance of the target. The duration of the target is individually calibrated based on practice data to maintain accuracy around 67%. Following the target and another fixation cross, participants then received feedback about their response (outcome stage). The task includes 2 runs, each with 11 trials for each combination of valence (win or loss) and magnitude (1 or 5), as well as 11 neutral trials (win or loss 0), resulting in a total of 110 trials. The task was presented using E-prime 2.0 and each run was approximately 12 min (Andrews et al., 2011). To motivate participants, they were explicitly told that they could get the total amount of money earned as a bonus in Chinese Yuan (CNY) after finishing the task.
Fig. 1.
A. Graphical illustration of the MIDT gain and loss trials. B. Individual and group-averaged response times to target for different reward valences and magnitudes. Error bars indicate standard error of mean, and each black point represents the response of a participant.
2.3. Behavioral data analyses
Between-group differences in demographics, internet gaming characteristics, clinical measures, and total amount of money earned during the MID task were assessed using independent t-tests. An analysis of variance (ANOVA) with repeated measures on response time (RT) on the MID task was conducted with group (IGD and HC) as a between-subject variable and valence (win/loss) and magnitude (0, 1, 5) as within-subject variables. Analyses were conducted using R (https://www.r-project.org/) and JASP (https://jasp-stats.org).
2.4. Imaging data acquisition
Imaging data were acquired using a 3.0 T SIEMENS Trio scanner in the Imaging Center for Brain Research of Beijing Normal University using the following parameters: repetition time (TR) = 1500 ms, echo time (TE) = 27 ms, flip angle (FA) = 60°, field of view (FOV) = 22 cm, acquisition matrix = 64 × 64, voxel size = 3.44 × 3.44 mm, slice thickness = 4 mm, number of slices = 26. A T1-weighted sagittal scan was acquired as a structural reference with following parameters: TR = 2530 ms, TE = 3.39 ms, TI = 1100 ms, FA = 7°, FOV = 256 × 256 mm2, voxel size = 1 × 1 mm, slice = 144).
2.5. Image data preprocessing and analyses
Preprocessing was conducted using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and DPABI (Yan et al., 2016). Functional data were realigned, coregistered with the structural images, segmented and normalized to Montreal Neurological Institute (MNI) standard space, and smoothed with a 6mm full-width-half-maximum (FWHM) Gaussian kernel.
A general linear model (GLM) was used to examine the neural activity related to reward processing for different valences and stages. Four event regressors were modeled using a canonical hemodynamic response function for: gain anticipation, loss anticipation, gain receipt, and loss receipt. In addition, a parametric modulator was included for each event. These parametric modulators modelled the neural activity associated with the absolute value of potential gains or losses (0, 1, or 5) during anticipation stages, and with the absolute value of received gains or losses (0, 1, or 5) during outcome stages, respectively. Ineffective trials (i.e., those without any response) and response stage (i.e., target responses) of effective trials were modelled separately as regressors of no interest. Six parameters of head motion from realignment were also included in the GLM as covariates of no interest. Primary analyses were performed on results from the modulated regressors. Specifically, coefficients of parametric modulators were estimated at the individual level and then taken to group-level random-effect analyses. One-sample t-tests were used to examine the neural activity associated with parametric modulators in IGD and HC groups respectively. Two-sample t-tests were used to examine between-group differences. The whole-brain unthresholded statistical maps are available at NeuroVault (https://identifiers.org/neurovault.collection:6215).
Given their critical roles in reward processing, the striatum and vmPFC were chosen as our two a priori regions of interest (ROI) (Clark et al., 2019; Clithero and Rangel, 2014; Sescousse et al., 2013). The striatum mask was created by combining the bilateral nucleus accumbens, caudate, and putamen, based on the Harvard-Oxford structural atlas (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). The vmPFC mask was provided by Luke Chang on NeuroVault (https://identifiers.org/neurovault.image:18650). To control for multiple comparisons, the small volume correction (SVC; threshold: voxel-level family-wise error (FWE) corrected p < 0.05) was conducted within a combined mask including both vmPFC and striatum. Moreover, mean parameter estimates of the parametric modulators within the vmPFC and striatum for each participant were calculated and entered into Bayesian two-sample t-test analyses using JASP with default settings. The Bayes factor (BF10) quantifies the relative support for the H1, compared to the H0, given the data. By convention, BF10 scores between 1 and 3 and those above 3 denote anecdotal or noteworthy evidence for H1 over H0, respectively, and BF10 scores below 1 suggest that H0 is more supported by the data than the H1 (Wagenmakers et al., 2017).
Exploratory whole-brain analyses were also conducted using a voxel-level uncorrected threshold of p < 0.001 combined with a cluster-level FWE corrected threshold of p < 0.05 by the means of Gaussian Random Field Theory (GRFT). Finally, for any significant result revealed by the two-sample t-tests, parametric estimates in the identified region (with the peak point as the center and a radius of 6mm) were extracted, and Pearson correlation analysis was used to examine its association with IGD severity within the IGD group.
3. Results
3.1. Behavioral results
As shown in Fig. 1, there was a significant main effect of potential reward magnitude on reaction times (F(2, 94) = 24.14, p < 0.001, partial η2 = 0.34). Post-hoc tests indicate that participants responded to the target significantly faster in 5 CNY and 1 CNY relative to neutral (0 CNY) trials. Moreover, we found a significant interaction between valence and magnitude (F(2, 94) = 3.32, p = 0.04, partial η2 = 0.06), which is mainly driven by a quicker response in loss 0 CNY than gain 0 CNY trials. However, neither the main effect of group (F(1, 47) = 0.02, p = 0.89, partial η2 < 0.001), nor any interaction effect with group reached significance (ps > 0.2). Moreover, IGD and HC groups obtained similar total amount of money earned during the task (t(47) = 0.29, p = 0.77, Cohen's d = 0.08). These results suggest that individuals with IGD showed comparable behavioral performance on the MID task with healthy controls.
3.2. Imaging results
3.2.1. Within group results
We first examined neural activity modulated by the magnitude of gain or loss in the HC group. HC participants showed significant positive neural correlates of the magnitude of anticipated gains in the vmPFC, precentral gyrus, and supplementary motor area, and negative neural correlates of the magnitude of anticipated losses in the insula, prefrontal and parietal cortices. During outcome stages, HC participants showed positive neural correlates of the magnitude of received gains and losses in the striatum and several prefrontal regions (Table S1). The same analyses were also conducted within the IGD group. Neural correlates of gain magnitude at the outcome stage were similar to the HC group; however, the IGD group did not show significant neural activity associated with parametric modulators during gain anticipation, loss anticipation, or loss receipt. (Table S2).
3.2.2. Between-group results
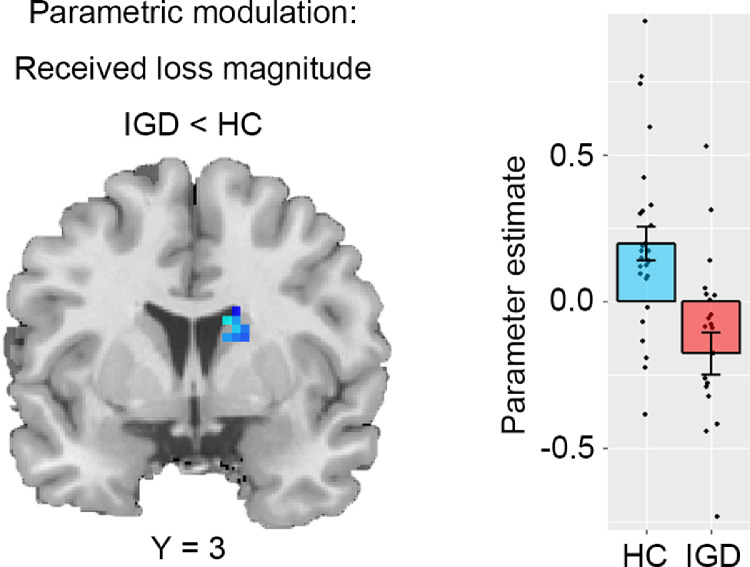
Next, we examined between-group differences in neural activity modulated by parametric regressors for different valences and stages. We found that, the IGD group showed lower neural activity associated with the magnitude of received losses in the right caudate (MNI coordinates: 15, 3, 18, t = -4.16, psvc = 0.035, BF10 = 127.904) compared to the HC group (Fig. 2). Follow-up correlations indicated no significant associations between parameter estimates from the right caudate and addiction severity (i.e., YIAS scores) in the IGD group.
Fig. 2.
The IGD group show blunted caudate activity (MNI coordinates: 15, 3, 18) associated with loss magnitude at the outcome stage compared to the HC group. Display threshold: uncorrected p < 0.005 at voxel level.
The IGD and HC groups did not show significantly different striatal or vmPFC activity modulated by the magnitude of anticipated gains, anticipated losses, or received gains. The BF10 scores for between-group differences in parameter estimates of modulators during these three stages were 0.286, 0.715, and 0.387 for the striatum, and 0.353, 0.290, and 0.288 for the vmPFC, respectively, suggesting moderate or small support for the null hypotheses that these two groups showed similar striatal and vmPFC activity associated with parametric modulators at gain anticipation, loss anticipation, and gain receipt. (Fig. 3).
Fig. 3.
The IGD and HC groups show similar striatal and vmPFC activity associated with the magnitude of anticipated gains (A_gain), anticipated losses (A_loss), and received gains (R_gain), but differ in the striatal activity associated with the magnitude of received losses (R_loss).
Whole-brain analyses using our a voxel-level uncorrected threshold of p < 0.001 combined with a cluster-level family-wise error (FWE) corrected threshold of p < 0.05 indicated no other significant between-group differences.
4. Discussion
This study used a modified MID task to test whether reward processing is altered among individuals with IGD, relative to healthy controls. The IGD group showed blunted striatal activity associated with the magnitude of received losses relative to controls. However, contrary to our hypothesis, the IGD and HC groups exhibited similar neural activity modulated by the magnitude of anticipated or received gains. These findings suggest that reduced sensitivity to the experience of negative events might be a critical feature of IGD. Furthermore, this pattern of results also indicates that overall deficits in reward processing in IGD (and possibly other behavioral addictions) may be less evident relative to SUDs.
Processing of negative outcomes, including monetary losses, is crucial for changing maladaptive behaviors and preventing the development of bad habits (Cohen et al., 2009; Ersche et al., 2016), and healthy individuals are typically very sensitive to the experience of negative outcomes. In the current study, we found that, compared to healthy controls, IGD participants showed blunted neural activity associated with the magnitude of received losses in the right caudate, which is part of the dorsal striatum and critically involved in processing the salience of rewards (Bartra et al., 2013). Prior work indicates hypoactivation of the anterior cingulate cortex, another key region of the salience network (Alexander et al., 2015), in IGD participants when losing money during risky decision-making (Dong et al., 2011). Moreover, individuals with IGD also exhibited lower neural correlates of risk levels in the frontoparietal network during decision-making involving in losses (Liu et al., 2017a). These findings converge to suggest that reduced sensitivity to loss may be a core feature associated with IGD.
Insensitivity to losses at the outcome stage has also been shown in other addictions in studies using similar paradigms. For example, individuals with gambling disorder showed lower activation in the ventral striatum, insula, and frontal and temporal regions when receiving losses compared to healthy controls (Balodis et al., 2012). Similarly, reduced loss-related activity in the insula and prefrontal regions has been reported in individuals with binge eating disorder (Balodis et al., 2013). Moreover, reduced activation of the amygdala at the loss receipt stage was observed in individuals with a family history of alcoholism (Andrews et al., 2011). Taken together, reduced sensitivity to losses at the outcome stage appears to be a general feature across different addictions (Fauth-Bühler and Mann, 2017). However, affected brain regions do not converge to the caudate, which may be related to the state of addiction or contextual factors (Wilson et al., 2008), and more evidence is required to elucidate the role of the caudate in loss processing in individuals with IGD.
Alterations in positive reward processing (e.g., monetary gains) have been proposed to play a critical role in the psychopathology of addictive disorders by some classic theories (Blum et al., 2000; Hommer et al., 2011) and are consistently observed in SUDs (Luijten et al., 2017). Some studies suggested that IGD is also associated with such deficit (Dong et al., 2011; Dong et al., 2013; Liu et al., 2017a; Qi et al., 2016; Wang et al., 2017). For example, individuals with IGD relative to controls showed higher activation in the orbitofrontal cortex when receiving monetary gains (Dong et al., 2011) and higher activation in the superior frontal gyrus after continuous wins (Dong et al., 2013). By comparison, another study found hypoactivation of the insula, medial frontal gyrus, and precentral gyrus during monetary feedback processing in individuals with IGD (Kim et al., 2014). Contrary to previous studies and our hypotheses, we did not find any significant between-group difference in neural correlates of gain magnitude during anticipation or outcome stage. Bayesian analyses also indicate stronger evidence for the null hypotheses that the IGD and HC groups did not differ in striatal and vmPFC activity associated with the magnitude of anticipated or received gains. One possible reason for the inconsistency is that most of previous studies mentioned above used monetary decision-making paradigms, in which reward processing is difficult to be dissociated from other cognitive processes (e.g., risk evaluation). Thus, it is also understandable that the affected brain regions and directionality across these studies are relatively heterogeneous. Although direct comparisons are still needed to test the speculation, the lack of differences in gain processing between the IGD and HC groups might suggest that, in the absence of prolonged exposure to exogenous drugs with potential dopaminergic effects, gain-related reward processing is less impaired in IGD than SUDs.
The current study has several theoretical implications: first, as individuals with IGD did not exhibit significant neural alterations related to gain processing as proposed by some classic theories of SUDs (Blum et al., 2000; Hommer et al., 2011), it is important to exercise caution in applying these theories to the field of IGD. New theories of the neurobiological basis of IGD need to incorporate potential distinctions in gain processing between individuals with IGD and those with SUDs. Second, whereas existing theories of IGD focus predominantly on gain processing and largely neglect loss processing, reduced sensitivity to monetary losses and other negative experience seems to be a general feature that characterizes different types of addictive disorders, including SUDs, gambling disorder, and IGD (Balodis et al., 2012; Ersche et al., 2016; Fauth-Bühler and Mann, 2017; Yao et al., 2015; Yip et al., 2018). Therefore, a greater consideration of this aspect of reward processing might be valuable for both neurobiological and clinical research in the field of IGD. Finally, as reward processing is a multifaceted complex process, the use of multi-dimensional assessments (e.g., assessing reward and loss across different contexts) may be helpful in providing additional insight into its mechanisms in addictive disorders, such as IGD (Yao et al., 2017).
This study has some limitations. First, we only included male participants. While IGD is significantly more prevalent among men versus women, further work to extend these findings to female populations is needed. Second, in order to examine relatively pure reward processing in IGD, we minimized the interactions between reward processing and other cognitive functions by using the MID task. Thus, our findings may not generalize to other more complex contexts involving reward processing, such as reinforcement learning and value-based decision-making (Keiflin and Janak, 2015). Moreover, we did not observe any between-group differences in experiment performance, partly because the MID task has been designed to minimize individual differences in some behavioral measurements (e.g., accuracy). Thus, other paradigms designed for detection of behavioral differences may be needed to link reward-related behavioral alterations more explicitly to neural responses in IGD. Finally, this study did not examine reward processing for gaming-related stimuli. In previous studies, individuals with IGD consistently show enhanced reward responses to gaming-related cues or rewards (Brand et al., 2019; Ko et al., 2013; Liu et al., 2017b). Thus, a critical future direction will be to directly compare activity in response to non-gaming and gaming-specific stimuli within the same sample of individuals with IGD.
5. Conclusions
In summary, this study systematically examined neural responses associated with monetary gain and loss processing at anticipation and outcome stages. Participants with IGD mainly showed blunted neural sensitivity to loss magnitude at the outcome stage, but did not differ in gain processing compared to healthy controls. These findings suggest that reward processing deficits in IGD might be dominant in loss domain, which may be helpful for both theory update and treatment development in this field.
CRediT authorship contribution statement
Yuan-Wei Yao: Formal analysis, Investigation, Writing - original draft, Visualization. Lu Liu: Formal analysis, Investigation, Writing - review & editing. Patrick D. Worhunsky: Methodology, Writing - review & editing, Visualization. Sarah Lichenstein: Writing - review & editing. Shan-Shan Ma: Investigation. Lei Zhu: Investigation. Xin-Hui Shi: Investigation. Songshan Yang: Methodology. Jin-Tao Zhang: Conceptualization, Resources, Writing - review & editing, Supervision, Funding acquisition. Sarah W. Yip: Conceptualization, Resources, Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 31871122). YWY's involvement was supported by the PhD Fellowship from the Einstein Center for Neurosciences Berlin. SWY's involvement was supported by NIDA grants (K01DA039299 and K12DA000167), the National Center on Addiction and Substance Abuse (CASA) and an Open Project grant from the State Key Laboratory of Cognitive Neuroscience and Learning of Beijing Normal University. The funders had no role in the study design, collection and analysis of the data, or preparation of the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102202.
Appendix. Supplementary materials
References
- Alexander W.H., Fukunaga R., Finn P., Brown J.W. Reward salience and risk aversion underlie differential ACC activity in substance dependence. Neuroimage Clin. 2015;8:59–71. doi: 10.1016/j.nicl.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association. fifth ed. Arlington; VA: 2013. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Andrews M.M., Meda S.A., Thomas A.D., Potenza M.N., Krystal J.H., Worhunsky P., Stevens M.C., O’Malley S., Book G.A., Reynolds B. Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biol. Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis I.M., Kober H., Worhunsky P.D., Stevens M.C., Pearlson G.D., Potenza M.N. Diminished frontostriatal activity during processing of monetary rewards and losses in pathological gambling. Biol. Psychiatry. 2012;71:749–757. doi: 10.1016/j.biopsych.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis I.M., Kober H., Worhunsky P.D., White M.A., Stevens M.C., Pearlson G.D., Sinha R., Grilo C.M., Potenza M.N. Monetary reward processing in obese individuals with and without binge eating disorder. Biol. Psychiatry. 2013;73:877–886. doi: 10.1016/j.biopsych.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis I.M., Potenza M.N. Anticipatory reward processing in addicted populations: a focus on the monetary incentive delay task. Biol Psychiatry. 2015;77:434–444. doi: 10.1016/j.biopsych.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O., McGuire J.T., Kable J.W. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Braverman E.R., Holder J.M., Lubar J.F., Monastra V.J., Miller D., Lubar J.O., Chen T.J., Comings D.E. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J. Psychoactive Drugs. 2000;32:1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Brand, M., Wegmann, E., Stark, R., Müller, A., Wölfling, K., Robbins, T.W., Potenza, M.N., 2019. The Interaction of Person-Affect-Cognition-Execution (I-PACE) model for addictive behaviors: update, generalization to addictive behaviors beyond Internet-use disorders, and specification of the process character of addictive behaviors. Neurosc. Biobehav. Rev. [DOI] [PubMed]
- Clark L., Boileau I., Zack M. Neuroimaging of reward mechanisms in Gambling disorder: an integrative review. Mol. Psychiatry. 2019;24:674–693. doi: 10.1038/s41380-018-0230-2. [DOI] [PubMed] [Google Scholar]
- Clithero J.A., Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Soc. Cogn. Affect. Neurosci. 2014;9:1289–1302. doi: 10.1093/scan/nst106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.X., Nikolai A., Doris L., Elger C.E., Volker S., Schlaepfer T.E. Nuclei accumbens phase synchrony predicts decision-making reversals following negative feedback. J. Neurosci. Official J. Soc. Neurosci. 2009;29:7591–7598. doi: 10.1523/JNEUROSCI.5335-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G., Hu Y., Lin X. Reward/punishment sensitivities among internet addicts: implications for their addictive behaviors. Progr. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;46:139–145. doi: 10.1016/j.pnpbp.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Dong G., Huang J., Du X. Enhanced reward sensitivity and decreased loss sensitivity in Internet addicts: an fMRI study during a guessing task. J. Psychiatr. Res. 2011;45:1525–1529. doi: 10.1016/j.jpsychires.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Ersche K.D., Gillan C.M., Jones P.S., Williams G.B., Ward L.H., Luijten M., De W.S., Sahakian B.J., Bullmore E.T., Robbins T.W. Carrots and sticks fail to change behavior in cocaine addiction. Science. 2016;352:1468–1471. doi: 10.1126/science.aaf3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauth-Bühler M., Mann K. Neurobiological correlates of internet gaming disorder: Similarities to pathological gambling. Addict. Behav. 2017;64:349–356. doi: 10.1016/j.addbeh.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Sescousse G., Caldú X., Segura B., Dreher J.C. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci. Biobehav. Rev. 2013;37:681–696. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Hommer D.W., Bjork J.M., Gilman J.M. Imaging brain response to reward in addictive disorders. Ann. New York Acad. Sci. 2011;1216:50–61. doi: 10.1111/j.1749-6632.2010.05898.x. [DOI] [PubMed] [Google Scholar]
- Keiflin R., Janak P.H. Dopamine prediction errors in reward learning and addiction: from theory to neural circuitry. Neuron. 2015;88:247–263. doi: 10.1016/j.neuron.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Son J.W., Choi W.H., Kim Y.R., Oh J.H., Lee S., Kim J.K. Neural responses to various rewards and feedback in the brains of adolescent Internet addicts detected by functional magnetic resonance imaging. Psychiatry Clin. Neurosci. 2014;68:463–470. doi: 10.1111/pcn.12154. [DOI] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 2001;21:15. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C.-H., Yen J.-Y., Chen S.-H., Wang P.-W., Chen C.-S., Yen C.-F. Evaluation of the diagnostic criteria of Internet gaming disorder in the DSM-5 among young adults in Taiwan. J. Psychiatr. Res. 2014;53:103–110. doi: 10.1016/j.jpsychires.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Ko C.H., Liu G.C., Yen J.Y., Chen C.Y., Yen C.F., Chen C.S. Brain correlates of craving for online gaming under cue exposure in subjects with Internet gaming addiction and in remitted subjects. Addiction Biol. 2013;18:559–569. doi: 10.1111/j.1369-1600.2011.00405.x. [DOI] [PubMed] [Google Scholar]
- Ko C.H., Wang P.W., Liu T.L., Chen C.S., Yen C.F., Yen J.Y. The adaptive decision-making, risky decision, and decision-making style of Internet gaming disorder. Eur. Psychiatry. 2017;44:189–197. doi: 10.1016/j.eurpsy.2017.05.020. [DOI] [PubMed] [Google Scholar]
- Liu L., Xue G., Potenza M.N., Zhang J.-T., Yao Y.-W., Xia C.-C., Lan J., Ma S.-S., Fang X.-Y. Dissociable neural processes during risky decision-making in individuals with Internet-gaming disorder. NeuroImage. 2017;14:741–749. doi: 10.1016/j.nicl.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Yip S.W., Zhang J.T., Wang L.J., Shen Z.J., Liu B., Ma S.S., Yao Y.W., Fang X.Y. Activation of the ventral and dorsal striatum during cue reactivity in Internet gaming disorder. Addiction Biol. 2017;22:791–801. doi: 10.1111/adb.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M., Schellekens A.F., Kühn S., Machielse M.W., Sescousse G. Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry. 2017;74:387. doi: 10.1001/jamapsychiatry.2016.3084. [DOI] [PubMed] [Google Scholar]
- Nutt D.J., Lingford-Hughes A., Erritzoe D., Stokes P.R.A. The dopamine theory of addiction: 40 years of highs and lows. Nat. Rev. Neurosci. 2015;16:305. doi: 10.1038/nrn3939. [DOI] [PubMed] [Google Scholar]
- Qi X., Yang Y., Dai S., Gao P., Du X., Zhang Y., Du G., Li X., Zhang Q. Effects of outcome on the covariance between risk level and brain activity in adolescents with internet gaming disorder. Neuroimage Clin. 2016;12:845–851. doi: 10.1016/j.nicl.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T.E., Berridge K.C. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Seok J.-W., Lee K.H., Sohn S., Sohn J.-H. Neural substrates of risky decision making in individuals with Internet addiction. Aust. N. Z. J. Psychiatry. 2015;49:923–932. doi: 10.1177/0004867415598009. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The MINI international neuropsychiatric interview (MINI): The development and validation of a structured interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Volkow N.D., Morales M. The brain on drugs: from reward to addiction. Cell. 2015;162:712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Wagenmakers E.J., Marsman M., Jamil T., Ly A., Verhagen J., Love J., Selker R., Gronau Q.F., Šmíra M., Epskamp S. Bayesian inference for psychology. Part I: theoretical advantages and practical ramifications. Psychon. Bull. Rev. 2017;25:1–23. doi: 10.3758/s13423-017-1343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wu L., Wang L., Zhang Y., Du X., Dong G. Impaired decision-making and impulse control in Internet gaming addicts: evidence from the comparison with recreational Internet game users. Addiction Biol. 2017;22:1610–1621. doi: 10.1111/adb.12458. [DOI] [PubMed] [Google Scholar]
- Wei L., Zhang S., Turel O., Bechara A., He Q. A tripartite neurocognitive model of internet gaming disorder. Front. Psychiatry. 2017;8:285. doi: 10.3389/fpsyt.2017.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein A., Livny A., Weizman A. New developments in brain research of internet and gaming disorder. Neurosci. Biobehav. Rev. 2017;75:314–330. doi: 10.1016/j.neubiorev.2017.01.040. [DOI] [PubMed] [Google Scholar]
- Wilson S.J., Sayette M.A., Delgado M.R., Fiez J.A. Effect of smoking opportunity on responses to monetary gain and loss in the caudate nucleus. J. Abnorm. Psychol. 2008;117:428. doi: 10.1037/0021-843X.117.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Wang X.D., Zuo X.N., Zang Y.F. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 2016;14:339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- Yao Y.W., Liu L., Ma S.S., Shi X.H., Zhou N., Zhang J.T., Potenza M.N. Functional and structural neural alterations in Internet gaming disorder: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2017;83:313. doi: 10.1016/j.neubiorev.2017.10.029. [DOI] [PubMed] [Google Scholar]
- Yao Y.-W., Wang L.-J., Yip S.W., Chen P.-R., Li S., Xu J., Zhang J.-T., Deng L.-Y., Liu Q.-X., Fang X.-Y. Impaired decision-making under risk is associated with gaming-specific inhibition deficits among college students with Internet gaming disorder. Psychiatry Res. 2015;229:302–309. doi: 10.1016/j.psychres.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Yip S.W., Devito E.E., Kober H., Worhunsky P.D., Carroll K.M., Potenza M.N. Anticipatory reward processing among cocaine-dependent individuals with and without concurrent methadone-maintenance treatment: relationship to treatment response. Drug Alcohol Dependence. 2016;166:134–142. doi: 10.1016/j.drugalcdep.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip S.W., Gross J.J., Chawla M., Ma S.S., Shi X.H., Liu L., Yao Y.W., Zhu L., Worhunsky P.D., Zhang J. Is neural processing of negative stimuli altered in addiction independent of drug effects? Findings from drug-naïve youth with internet gaming disorder. Neuropsychopharmacology. 2018;43:1364–1372. doi: 10.1038/npp.2017.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K. Internet addiction: diagnosis and treatment considerations. J. Contemporary Psychother. 2009;39:241–246. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.