Abstract
Intraoperative pathologic diagnosis for central nervous system (CNS) tumors is important to determine the neurosurgery procedure. But sometimes the differential diagnosis between glioma and lymphoma, or glioma and metastatic tumors is difficult for a pathologist during a short time, especially when the specimen is small or the frozen section has ice crystals. Immunohistochemistry (IHC) is a very useful method for diagnosis, but the traditional immunohistochemical method is time-consuming and not suitable intraoperatively. In this study, we chose Cytokeratin-pan, GFAP, and LCA as three immunohistochemical indicators. Intraoperative IHC was done by Novodiax ihcDirect technology combined with Leica Bond auto-staining. Compared with the manual method recommended for the reagents (Novodiax ihcDirect), the results show that auto-staining has better stability and high reproducibility in coloration, which has broad prospects for future application.
Keywords: Immunohistochemistry, intraoperative, pathologic diagnosis, CNS
Introduction
Intraoperative pathologic diagnosis of central nervous system (CNS) tumors is an essential tool for deciding the surgical procedure including details such as the resection boundary [1]. Today, the standard of practice is to utilize hematoxyline-eosin (H&E) and toluidine blue (smear preparation) staining during intraoperative surgery. A pathologist can make a diagnosis based solely upon the morphologies observed on the H&E stain. Difficulties of intraoperative diagnosis have been reported in distinguishing low-grade glioma (LGG) from gliosis, high-grade glioma (HGG) from LGG [2] and primary CNS lymphoma (PCNSL) from HGG, and CNS metastatic tumor from HGG. Cellularity, nuclear atypia, mitosis, and necrosis in tumors are evaluated, but pathologists sometimes face diagnostic difficulties because of the similarity of their morphology in frozen sections. Therefore, for a long time pathologists and oncologists have been searching for rapid immunohistochemistry or other molecular testing to help clarify the diagnosis. The rapid intraoperative immunocytochemistry (R-IHC) method was based on Novodiax ihcDirect technology with Leica Bond III fully automated IHC stainer. During the staining process, the Novodiax ihcDirect technology uses a polymerized HRP conjugated directly onto primary antibodies, which results in a simpler and faster IHC procedure that minimizes the time and workflow steps. As R-IHC takes only 20 min, it is able to provide immunohistochemical information for intraoperative pathologic diagnosis.
In this study, glial fibrillary acidic protein (GFAP), cytokeratin (CK)-pan, and leukocyte common antigen (LCA) immunostaining in frozen sections was undertaken by this new R-IHC method, followed by a reliable protocol we made; and we evaluated its usefulness for intraoperative diagnosis of CNS tumors.
Materials and methods
30 CNS tumor cases were chosen into the study and underwent intraoperative diagnosis at Tianjin Huanhu hospital from December 2016 to March 2017. Frozen sections were sliced at thicknesses of 5 μm and stained with H&E stain and R-IHC (manually and automatically). Additionally, resected tumors (same case) were fixed with 10% neutralized buffered formalin and embedded with paraffin. The formalin-fixed paraffin embedded (FFPE) tissues, referred to as permanent sections, were subjected to H&E stain and conventional IHC.
H&E staining protocols
For H&E staining performed by hand, slides were fixed in ethanol for 1 min and washed in running tap water for 2 min. Slides were then transferred to Harris’ haematoxylin for 1 min. Slides were then washed in running tap water for 1 min, cleared in 0.5% hydrochloric acid in 99% industrial methylated spirit for 30 sec, and transferred to running tap water for 2 min. The slides were then ‘blued’ in 0.4% ammonia alcohol. The slides were then washed in running tap water for 1 min and placed in 0.5% eosin for 1 min, before being washed in tap water and dehydrated in alcohol and cleared through xylene.
R-IHC protocols manually
For frozen tissue, freshly sectioned tissue must be immediately fixed in fixative solution (25 ml glacial acetic acid, 100 ml of 37% formaldehyde, 375 ml of methyl alcohol) for at least 30 seconds. Other procedures are shown (Table 1).
Table 1.
R-IHC protocols manually
| Procedure | Time in minute |
|---|---|
| Wash twice with PBS-T | 0.25 |
| Blocking with Common IHC Blocker | 1 |
| PolyHRP-Anti GFAP/LCA/CK-pan conjugate | 3 |
| Wash twice with PBS-T | 0.25 |
| DAB working solution | 3 |
| Wash twice with PBS-T | 0.25 |
| Hematoxylin counterstaining | 0.25 |
| Wash twice with PBS-T | 0.25 |
| Mounting with aqueous medium & coverslipping | 0.75 |
| Total | About 10 minutes |
R-IHC protocols by Leica Bond III fully automated IHC stainer
The solutions, such as fixative solution, common IHC blocker and PolyHRP-Anti GFAP/LCA/CK-pan, were filled into the proprietary bottle-Bond Open Container (7 ml). All the bottles were registered on computer with Leica Biosystem, then we ranthe protocols we designed (see Table 2).
Table 2.
R-IHC protocols by Leica Bond III stainer
| Procedure | Time in minute |
|---|---|
| Fixative solution | 1 |
| Bond wash solution | 0 (3 times) |
| Blocking with Common IHC Blocker | 1 |
| Bond wash solution | 0 (3 times) |
| PolyHRP-Anti GFAP/LCA/CK-pan conjugate | 5 |
| Bond wash solution | 0 (3 times) |
| MIXDAB Refine | 5 |
| Deionized Water | 0 (3 times) |
| Hematoxylin | 1 |
| Deionized Water | 0 (1 time) |
| Mounting with aqueous medium & coverslipping | 0.75 |
| Total (include washing time) | About 20 minutes |
Conventional IHC for FFPE tissues
FFPE tissues were sectioned at 4 μm thicknesses, placed on slide glasses, and baked at 68°C for 15 min. We transferred the slides into the Bond III stainer and ran the standard IHC protocol.
Results
Of all the 30 cases, 10 cases were glioma, 10 cases were metastatic tumor, and 10 cases were PCNSL at final diagnosis. Immunohistochemical staining showed that the positive signal location was accurate, and there was no false negative or false positive. As shown in Figure 1, we found that GFAP, CK-pan, and LCA immunostaining had fine staining results using the R-IHC method. The protocol by Leica Bond III was better than staining by hand, that is to say, with conventional IHC. Although staining with the automated IHC stainer took a little longer time than manually (manually 10 min vs. Bond III 20 min), and had a antibody cost (manually 50-70 μl vs. Bond III 150 μl), the result was better. Compared with R-IHC staining by hand, auto-staining showed no folds, no edge effect, breakage or inhomogeneous coloration, and also automatic staining had good repeatability and quality control. From these results we chose R-IHC auto-staining method for the further study.
Figure 1.
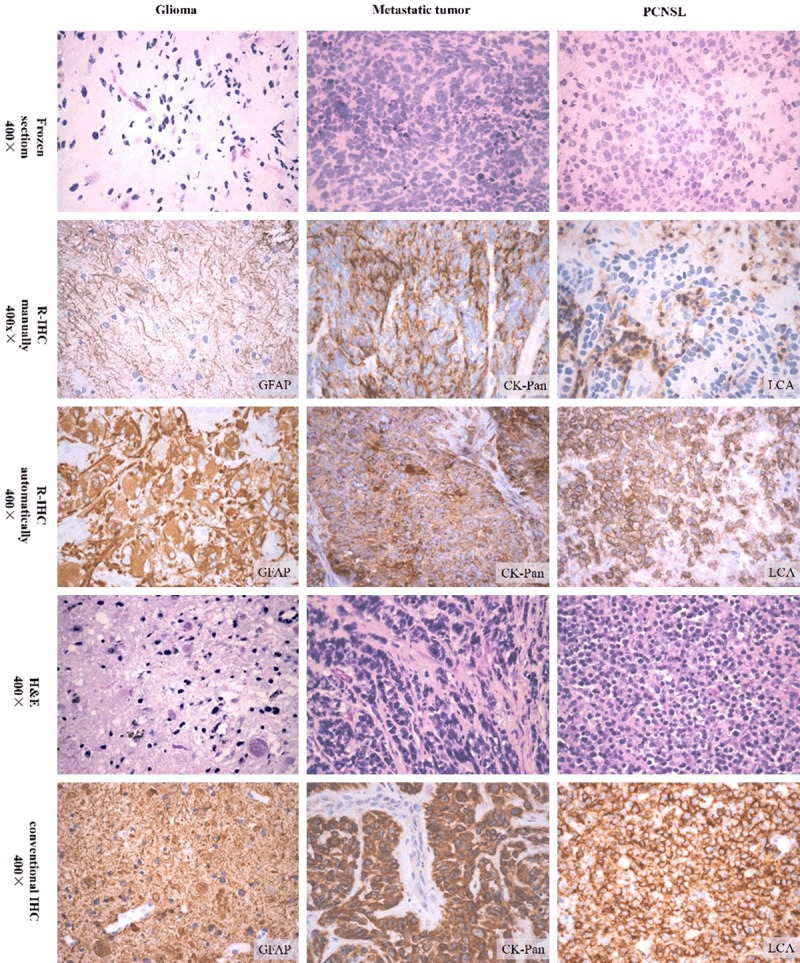
R-IHC staining.
Discussion
H&E stain of frozen tumor tissue sections constitutes the main diagnostic technique, but the diagnostic accuracy in the intraoperative diagnosis for CNS tumors using frozen sections is not always high, at rates of 50-96% [3-5]. The reason for the discrepant diagnosis is partly due to the high content of water as well as fat in fresh brain tumor, and the innately soft nature of brain tumor. Poor quality in frozen sections causes diagnostic difficulties. But sometimes it is difficult for the pathologist to make a differential diagnosis on standard H&E staining in a short time during the operation.
IHC is a very useful technique in tumor diagnosis; however, conventional IHC processes require more than 120 minutes to complete and are thereby impractical for intraoperative applications. In the past few years people had also explored numbers of rapid IHC methods such as R-ICC (rapid immunocytochemistry) based on AC electric field-facilitated antigen-antibody reaction [2], Roche Ventana BenchMark Ultra automated platform employing a rapid frozen section protocol. R-ICC by a microwave-based method for intraoperative peritoneal washing cytology of pancreatic or biliary carcinoma was previously reported [6]. Recently, the usefulness of R-ICC for cytokeratin in touch prints of sentinel lymph nodes for evaluating metastasis of breast cancer was reported [7,8].
In this study, we used Novodiax ihcDirect technology combined with Leica Bond III autostaining, so the whole IHC procedure could be controlled in under 20 min, and that made rapid intraoperative IHC (R-IHC) possible. The Novodiax ihcDirect technology uses a polymerized HRP conjugated directly onto primary antibodies that results in a simpler and faster IHC procedure that minimizes the time and workflow steps. This innovation as direct IHC with corresponding branded product line is labeled as ihcDirectTM. The ihcDirect method yields a revolutionary technology that opens a spectrum of new clinical applications including intraoperative surgery. Using the Novodiax PolyHRP technology, Intraoperative IHC tests can now be completed in a short time using fresh frozen tissue.
To evaluate the usefulness of intraoperative R-IHC in CNS tumors, the results were compared to those in both R-IHC in frozen sections and conventional IHC in permanent sections. Compared to R-IHC staining by hand, the auto-stainer used universal cover tiles which made the reaction liquid well-distributed, and the reaction plane was more flat. Flushing of the large flow wash buffer ensured that there was no residue after each reaction step which made the final staining background clear. Thus auto-staining showed a more fine staining result which was similar to conventional IHC. In conclusion, we confirmed that Novodiax ihcDirect technology combined with Leica Bond IIIauto-staining was feasible and practical, and the auto-staining protocol we established was exercisable. On the other hand, auto-staining also has shortcomings, such as a little longer reaction time and more antibody cost than staining by hand, but these were acceptable. Automatic staining had good repeatability, quality control, and also saved labor. It was easy to realize the standardization process.
We believe that with the application of this technique, intraoperative diagnosis process may be changed. Pathologists could select appropriate indicators after gross examination, and the R-IHC auto-staining procedure could be carried out directly using frozen sections, which reduces time consumption. Furthermore, when the conventional frozen H&E staining sections and R-IHC can be performed at the same time, it is very helpful and useful for pathologists to diagnose. Currently, Novodiax is also developing more ihcDirectTM antibodies, such as isocitrate dehydrogenase (IDH) antibody, and reproductive system tumor related antibodies, which give R-IHC auto-staining technology broader application in the future.
Acknowledgements
The present study was approved by the Medical Ethics Committee of Tianjin Huanhu Hospital, and all patients provided written informed consent. The study participants provided their consent for the publication of any data/associated images.
Disclosure of conflict of interest
None.
References
- 1.Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW, Berger MS. Role of extent of resection in the long-term outcome of lowgrade hemispheric gliomas. J. Clin. Oncol. 2008;26:1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 2.Moriya J, Tanino MA, Takenami T, Endoh T, Urushido M, Kato Y, Wang L, Kimura T, Tsuda M, Nishihara H, Tanaka S R-IHC Study Group. Rapid immunocytochemistry based on alternating current electric field using squash smear preparation of central nervous system tumors. Brain Tumor Pathol. 2016;33:13–18. doi: 10.1007/s10014-015-0238-0. [DOI] [PubMed] [Google Scholar]
- 3.Plesec TP, Prayson RA. Frozen section discrepancy in the evaluation of central nervous system tumors. Arch Pathol Lab Med. 2007;131:1532–1540. doi: 10.5858/2007-131-1532-FSDITE. [DOI] [PubMed] [Google Scholar]
- 4.Uematsu Y, Owai Y, Okita R, Tanaka Y, Itakura T. The usefulness and problem of intraoperative rapid diagnosis in surgical neuropathology. Brain Tumor Pathol. 2007;24:47–52. doi: 10.1007/s10014-007-0219-z. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa E, Yamamoto T, Satomi K, Matsuda M, Akutsu H, Shibuya M, Nakai K, Sakamoto N, Takano S, Matsumura A. Intraoperative pathological diagnosis in 205 glioma patients in the pre-BCNU wafer era: retrospective analysis with intraoperative implantation of BCNU wafers in mind. Brain Tumor Pathol. 2014;31:156–161. doi: 10.1007/s10014-014-0177-1. [DOI] [PubMed] [Google Scholar]
- 6.Nomoto S, Nakao A, Takeuchi Y, Nonami T, Harada A, Ichihara T, Takagi H. Intraoperative peritoneal washing cytology with the rapid immunoperoxidase method using microwave irradiation. J Surg Oncol. 1995;60:30–34. doi: 10.1002/jso.2930600107. [DOI] [PubMed] [Google Scholar]
- 7.Johnston EI, Beach RA, Waldrop SM, Lawson D, Cohen C. Rapid intraoperative immunohistochemical evaluation of sentinel lymph nodes for metastatic breast carcinoma. Appl Immunohistochem Mol Morphol. 2006;14:57–62. doi: 10.1097/01.pai.0000153722.21155.5f. [DOI] [PubMed] [Google Scholar]
- 8.Pugliese MS, Kohr JR, Allison KH, Wang NP, Tickman RJ, Beatty JD. Accuracy of intraoperative imprint cytology of sentinel lymph nodes in breast cancer. Am J Surg. 2006;192:516–519. doi: 10.1016/j.amjsurg.2006.05.014. [DOI] [PubMed] [Google Scholar]


