Abstract
Lymphadenopathy is an important characteristic of POEMS syndrome, and a Castleman disease (CD)-like pathologic change in the lymph nodes is one of the major diagnostic criteria. However, the characteristics of lymphadenopathy in POEMS still have not been completely elucidated. The lymph node biopsies are available only for a small proportion of patients. A simple and safe way is needed to rule CD in or out. This study aimed to analyse the features of lymphadenopathy and estimate the role of imaging methods, including computed tomography (CT) and positron emission tomography-CT (PET/CT), in the diagnosis of lymphadenopathy in patients with POEMS syndrome. We conducted a retrospective analysis of 23 patients with confirmed POEMS syndrome. All of the patients received chest and abdominal CT scan and/or superficial ultrasound examinations. Four patients underwent PET/CT examinations, and 6 patients received lymph node biopsies. Enlarged lymph nodes (short diameter ≥ 1 cm) were found in 48% (11/23) of patients, but only 1 patient had an enlarged lymph node with a diameter ≥ 2 cm. Lymph nodes with CD-like pathologic changes from 2 patients showed increased maximum standard uptake values (SUVmax) of 18F-deoxyglucose (18FDG) on PET/CT, while lymph nodes with reactive pathologic changes from 2 other patients showed a normal metabolic PET/CT profile. The extent of lymph node enlargement in patients with POEMS was less than that in patients with CD per se. We draw the conclusion that most of the enlarged lymph nodes had diameters ≤ 2 cm, which is less than that in cases of CD per se and PET/CT may be helpful in determining whether enlarged lymph nodes are characterized by CD-like changes or not.
Keywords: PET/CT, POEMS syndrome, Castleman disease, lymphadenopathy
Introduction
Polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes (POEMS) syndrome is an underlying plasma cell dyscrasia [1]. One of the major criteria [1,2] for the diagnosis of POEMS syndrome is Castleman disease (CD, also called angiofollicular hyperplasia or giant lymph node hyperplasia) which has two forms: unicentric and multicentric CD. Because the POEMS syndrome involves multiple organs, multiple systems, and multiple disciplines, diagnosis is difficult and misdiagnosis is common [3,4]. Confirmation of CD is critical for the diagnosis, treatment, and prognosis of POEMS [3,5]. Nearly half of patients with POEMS exhibit lymph node enlargement, while only a small portion undergo lymph node biopsy for various reasons [3,5]. Even with lymph node biopsy, only a half of biopsied lymph nodes showed CD [3], while the rest were reactive or had other changes. Are there any ways to predict the properties of the lymph nodes beforehand to reduce the need for unnecessary open biopsy surgeries? Positron emission tomography-computed tomography (PET/CT) is a noninvasive imaging technique that combines functional imaging with anatomic location. It is widely used in the diagnosis of malignant diseases including CD per se [6-8]. PET/CT has been rarely used for the evaluation of lymphadenopathy in POEMS syndrome [9], although frequently used for examination of plasmacytomas and osteolystic lesions [10,11]. It is unclear whether PET/CT can be used to predict a lymphadenopathy to be a CD or not. In this study, we investigated 23 patients with POEMS syndrome and examined the role of PET/CT in the evaluation of CD.
Materials and methods
Twenty-three patients were admitted to our hospitals between 2004 and 2017 who met the established diagnosis criteria for POEMS syndrome according to Dispenzieri et al. [12] in 2003. In brief, the diagnosis consisted of 2 major criteria (i.e., polyneuropathy and monoclonal plasma proliferative disorder) and 7 minor criteria (i.e., bone lesions, CD, organomegaly, oedema, endocrinopathy, skin changes, and papilledema). Two major criteria and at least one minor criterion were required for such a diagnosis. The group consisted of 18 males and 5 females with an average age of 49.2 (range 31-76). All 23 patients underwent thoracoabdominopelvic computerized tomography (CT) scans and/or an ultrasound of the involved regions or superficial lymph nodes. The size and location of the lymph nodes were analyzed and an enlarged lymph node was defined as one with the short diameter of ≥ 1 cm.
Six patients with enlarged lymph nodes underwent biopsies. Of these patients, 4 received a whole-body PET/CT examination, which was performed after the patients had fasted for 4 h and 60-90 min after intravenous administration of 18F-FDG. 18F-FDG PET/CT images were obtained using a combined PET/CT biograph. All scans were acquired in 3-dimensional mode in the craniocaudal direction from the top of the skull to the middle thigh. The maximum standard uptake values (SUVmax) of 18F deoxyglucose (18FDG) were calculated.
All images were reviewed in consensus by two radiologists with 15 and 12 years of experience in the field of radiologic diagnosis. Biopsies from sections of involved tissues and organs were reviewed by two senior pathologists from our pathology department.
Results
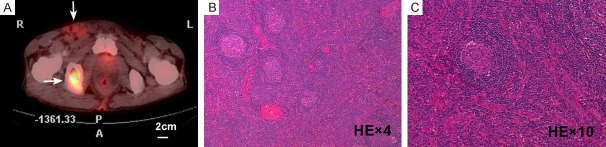
In our group of patients, the median time from the appearance of symptoms to a confirmed POEMS diagnosis was 12 months (range from 2 to 24 months). In 19 patients (19/23), lymph nodes were visible as assessed by CT and/or ultrasound; of these patients, 11 had lymph nodes ≥ 1 cm in short-axis diameter (11/23, 48%). The remaining 8 had multiple small lymph nodes (Table 1). Only one patient possessed a lymph node ≥ 2 cm (Figure 1). Of the 11 patients with enlarged lymph nodes, 8 patients were classified as multicentric CD, and the remaining 3 patients were diagnosed as unicentric CD (Table 1).
Table 1.
Detailed data of 23 patients with POEMS syndrome
| Patients | No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | No. 7 | No. 8 | No. 9 | No. 10 | No. 11 | No. 12 |
|
| ||||||||||||
| Gender/age | M/64 | M/32 | M/61 | F/47 | M/58 | M/31 | M/54 | M/49 | M/42 | M/57 | M/40 | M/45 |
| Deep LN | + | + | + | + | + | + | + | + | + | + | + | |
| Mediastinal | + | + | + | + | + | + | + | + | ||||
| Retroperitoneal | + | + | + | + | + | |||||||
| Superficial LN | + | + | + | + | + | + | + | + | ||||
| Axillary | + | + | + | + | ||||||||
| Cervical and Subclavicular | + | + | + | + | + | + | ||||||
| Inguinal | + | + | + | |||||||||
| Multi-/Uni- | Multi- | Multi- | Multi- | Multi- | Multi- | - | Uni- | |||||
| Size | < 1 cm | 1~2 cm | < 1 cm | < 1 cm | < 1 cm | 1~2 cm | > 2 cm | < 1 cm | 1~2 cm | 1~2 cm | - | 1~2 cm |
|
| ||||||||||||
| Patients | No. 13 | No. 14 | No. 15 | No. 16 | No. 17 | No. 18 | No. 19 | No. 20 | No. 21 | No. 22 | No. 23 | |
|
| ||||||||||||
| Gender/age | M/33 | M/38 | M/41 | F/47 | M/54 | M47 | M/62 | F/62 | M/76 | F/46 | F/46 | |
| Deep LN | + | + | + | + | ||||||||
| Mediastinal | ||||||||||||
| Retroperitoneal | + | + | + | + | ||||||||
| Superficial LN | + | + | + | + | + | + | + | |||||
| Axillary | + | + | + | + | + | + | + | |||||
| Cervical and Subclavicular | + | + | + | |||||||||
| Inguinal | + | + | + | + | ||||||||
| Multi-/Uni- | Multi- | Multi- | - | - | Uni- | Uni- | Multi- | - | ||||
| < 1 cm | 1~2 cm | 1~2 cm | < 1 cm | - | - | 1 cm | 1 cm | 1~2 cm | - | < 1 cm | ||
M, male; F, female; LN, lymph nodes; Multi-, multicentric; Uni-: unicentric.
Figure 1.
18F-FDG PET/CT and histologic findings in lymph nodes > 2 cm in diameter from a 54-year-old male patient. (A) 18F-FDG PET/CT showing increased FDG metabolic activity in the inguinal lymph nodes (SUVmax = 4) (up arrow). An osteolytic lesion in the right ischium (SUVmax = 22) (down arrow). (B and C) Histologic results of the right inguinal lymph nodule biopsy (H&E, 4× objective for B and 10× objective for C). The biopsy is characterized by the presence of atrophic germinal centers that are surrounded by expanded mantle zones of small lymphocytes forming concentric rings (“onion-skinning”), and hyalinized blood vessels penetrating into follicles.
Six patients underwent open biopsy operations; 3 of them were diagnosed with CD, and 3 were diagnosed with reactive changes. With regards to the pathologic subtypes of CD, the hyaline-vascular type was found in 2 patients, while the plasma-cell type was found in 1 patient. Lymph node biopsies were unavailable for the remaining 5 patients with enlarged lymph nodes due to the following reasons: difficulties in obtaining tissues (n = 1), patients’ fear of the potential adverse effects of the surgery (n = 2), and unfavorable performance status (n = 2). In 1 patient, only a deep mediastinal lymph node was found (Patient No. 12 in Table 1). Of the 6 patients receiving biopsies, 4 received PET/CT examination. A right inguinal lymph node, which was determined to be hyaline-vascular CD on biopsy, showed hypermetabolism on PET/CT with a SUVmax of 4 (Figure 1). In the same patient, a significantly high metabolism with a SUVmax of 22 was also shown in the osteolytic lesion (Figure 1). Another enlarged lymph node diagnosed histologically as plasma-cell type CD by a CT-guided percutaneous puncture displayed a high FDG uptake (SUVmax = 4.5) on PET/CT (Figure 2). However, the enlarged lymph nodes from the remaining 2 patients with reactive changes on biopsy demonstrated a normal metabolic profile (Figure 3) on PET/CT.
Figure 2.
18F-FDG PET/CT and histologic findings of lymph node from a 42-year-old male patient. (A) 18F-FDG PET/CT shows increased FDG metabolism of the internal iliac lymph nodes (SUVmax = 4.5) (arrow). (B-L). Histology of the lymph node biopsies (4× objective for B, E, G, I and K; 10× objective for C, F, H, J and L; 40× objective for D). (B-D) Retained lymph node architecture and variable germinal center hyperplasia with expanded mantle zones (H&E staining). (E-L) Immunostaining results. The center of the follicles expressed B cell markers (CD20+ and CD79) and dendritic cell markers (CD23+). The mantle zone expressed plasmacytic markers (CD138).
Figure 3.

PET/CT reveals normal 18F-FDG metabolism of enlarged axillary lymph nodes indicating a reactive change on biopsy from a 47-year-old female. (A) Axial section PET image. (B) Axial section CT image. (C) Fused PET/CT image. (D) Coronal section whole-body PET image. Osteosclerosis is also shown in the thoracic vertebrae.
Discussion
Lymphadenopathy is one of the characteristics of POEMS syndrome, and CD is one of the major diagnostic criteria for POEMS. According to the literature, 11-30% [1] or 11-25% [13] of POEMS patients have documented CD. However, this rate may be underestimated because only a small portion of patients with lymphadenopathy undergo lymph node biopsy due to the small size and deep sites of the lymph nodes. In addition, not all enlarged lymph nodes demonstrate CD-like changes. Thus, the probability of negative results and unnecessary iatrogenic surgical damage causes further dilemma for many candidates and leads them away from undergoing surgery. Increasing the detection rate of biopsies and reducing unnecessary suffering are important. PET/CT is widely used in malignant or moderately malignant diseases, including CD per se; however, there is limited information on the use of PET/CT in CD in POEMS patients [9,10]. In this study, we hypothesized predictive role of PET/CT for CD-like changes in the diagnosis of POEMS syndrome.
Lymphadenopathy has been associated with 26-74% of patients with POEMS syndrome [13]. In our study, 11/23 (48%) patients had enlarged lymph nodes, and the average diameter of the enlarged lymph nodes was 1-2 cm, which is smaller than that in cases of CD without POEMS [14]. In unicentric CD per se, the mean and median nodal sizes were 6.1 and 4.7 cm, respectively and in multicentric CD per se these sizes were 4.5 and 4.1 cm, respectively [7]. Pan et al. reported the maximum short diameter of lymph nodes in patients with POEMS syndrome was less than 2.7 cm [9]. Dispenzieri [12] believed that patients with POEMS syndrome lack any massive lymphadenopathies. Of the 11 patients evaluated in this study, 10 possessed enlarged lymph nodes infiltrating the superficial tissues, which was convenient from a surgical standpoint. Only one patient had a unicentric lymph node involving a deep site, thus requiring a CT-guided percutaneous puncture.
Only 6 of the 23 patients underwent lymph node biopsies. In these 6 patients, half were diagnosed with CD histologically, while half had reactive changes. These findings were similar to a report wherein 58% of the biopsies exhibited CD and 42% showed reactive changes [3]. The enlargement of some lymph nodes may result from extravascular volume overload due to increased vascular permeability.
Small lymph nodes less than 1 cm are well visualized by PET/CT. SUVmax does not have a significant correlation with lesion size, according to the literature [8,15]. Therefore, this imaging trait may be more suitable for cases of POEMS syndrome with small lymph nodes. In this study we found lymph nodes with CD-like change histologically showed an increase of 18F-FDG accumulation on PET/CT with a SUVmax of 4-4.5. Alberti, et al. [10] reported that an inguinal lymph node which was diagnosed as hyaline-vascular CD in biopsy had a SUVmax of 4.5. Pan et al. [9] reported that two lymph nodes identified as hyaline-vascular type and mixed type in biopsy exhibited hypermetabolism on PET/CT with the SUVmax ranging from 1.0 to 10.0. Compared to the lymph nodes with CD-like changes, the lymph nodes with reactive changes histopathologically had a normal SUVmax in our study. The difference of SUVmax may be due to the different levels of proliferation between lymph nodes with CD-like changes and reactive changes.
Conclusion
Most of the enlarged lymph nodes had diameters ≤ 2 cm and not all of the enlarged lymph nodes showed CD-like histologic changes. PET/CT may be helpful in determining whether enlarged lymph nodes are characterized by CD-like changes or not. The limitation of our study is a small patient number because of the rarity of this disease. However, our results can be used as a baseline for further evaluations.
Acknowledgements
This study was funded by the National Natural Science Foundation of China [81700130]; Natural Science Foundation of Jiangsu Province of China [BK20150474]; the Science and Technology Commission of Zhenjiang Municipality [SH2017006] and Novo Nordisk Haemophilia Research Fund in China.
Disclosure of conflict of interest
None.
References
- 1.Dispenzieri A. POEMS syndrome. Blood Rev. 2007;21:285–299. doi: 10.1016/j.blre.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Dispenzieri A, Armitage JO, Loe MJ, Geyer SM, Allred J, Camoriano JK, Menke DM, Weisenburger DD, Ristow K, Dogan A, Habermann TM. The clinical spectrum of Castleman’s disease. Am J Hematol. 2012;87:997–1002. doi: 10.1002/ajh.23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Zhou DB, Huang Z, Jiao L, Duan MH, Zhang W, Zhao YQ, Shen T. Clinical characteristics and long-term outcome of patients with POEMS syndrome in China. Ann Hematol. 2011;90:819–826. doi: 10.1007/s00277-010-1149-0. [DOI] [PubMed] [Google Scholar]
- 4.Shi X, Hu S, Yu X, Zhuang Q, Luo M, Jiang Q, Wang L, Lu Y, Fei X, Xi X, Zhu Y. Clinicopathologic analysis of POEMS syndrome and related diseases. Clin Lymphoma Myeloma Leuk. 2015;15:e15–21. doi: 10.1016/j.clml.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Zhou DB. New advances in the diagnosis and treatment of POEMS syndrome. Br J Haematol. 2013;161:303–315. doi: 10.1111/bjh.12236. [DOI] [PubMed] [Google Scholar]
- 6.Kligerman SJ, Auerbach A, Franks TJ, Galvin JR. Castleman disease of the thorax: clinical, radiologic, and pathologic correlation: from the radiologic pathology archives. Radiographics. 2016;36:1309–1332. doi: 10.1148/rg.2016160076. [DOI] [PubMed] [Google Scholar]
- 7.Hill AJ, Tirumani SH, Rosenthal MH, Shinagare AB, Carrasco RD, Munshi NC, Ramaiya NH, Howard SA. Multimodality imaging and clinical features in Castleman disease: single institute experience in 30 patients. Br J Radiol. 2015;88:20140670. doi: 10.1259/bjr.20140670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee ES, Paeng JC, Park CM, Chang W, Lee WW, Kang KW, Chung JK, Lee DS. Metabolic characteristics of Castleman disease on 18F-FDG PET in relation to clinical implication. Clin Nucl Med. 2013;38:339–342. doi: 10.1097/RLU.0b013e3182816730. [DOI] [PubMed] [Google Scholar]
- 9.Pan Q, Li J, Li F, Zhou D, Zhu Z. Characterizing POEMS syndrome with 18F-FDG PET/CT. J Nucl Med. 2015;56:1334–1337. doi: 10.2967/jnumed.115.160507. [DOI] [PubMed] [Google Scholar]
- 10.Alberti MA, Martinez-Yelamos S, Fernandez A, Vidaller A, Narvaez JA, Cano LM, Gamez C, Martinez-Matos JA. 18F-FDG PET/CT in the evaluation of POEMS syndrome. Eur J Radiol. 2010;76:180–182. doi: 10.1016/j.ejrad.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Montoriol PF, Cachin F, Michel JL, Soubrier M. Two more cases of evaluation of POEMS syndrome using 18-FDG PET/CT. Eur J Radiol. 2011;80:861–864. doi: 10.1016/j.ejrad.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 12.Dispenzieri A, Kyle RA, Lacy MQ, Rajkumar SV, Therneau TM, Larson DR, Greipp PR, Witzig TE, Basu R, Suarez GA, Fonseca R, Lust JA, Gertz MA. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101:2496–2506. doi: 10.1182/blood-2002-07-2299. [DOI] [PubMed] [Google Scholar]
- 13.Dispenzieri A. POEMS syndrome: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89:213–223. doi: 10.1002/ajh.23644. [DOI] [PubMed] [Google Scholar]
- 14.Luo JM, Li S, Huang H, Cao J, Xu K, Bi YL, Feng RE, Huang C, Qin YZ, Xu ZJ, Xiao Y. Clinical spectrum of intrathoracic Castleman disease: a retrospective analysis of 48 cases in a single Chinese hospital. BMC Pulm Med. 2015;15:34. doi: 10.1186/s12890-015-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enomoto K, Nakamichi I, Hamada K, Inoue A, Higuchi I, Sekimoto M, Mizuki M, Hoshida Y, Kubo T, Aozasa K, Hatazawa J. Unicentric and multicentric Castleman’s disease. Br J Radiol. 2007;80:e24–26. doi: 10.1259/bjr/93847196. [DOI] [PubMed] [Google Scholar]