Abstract
Background: Lung cancer is one of the most common human cancers. Long noncoding RNA-activated by DNA damage (NORAD) is often upregulated and promotes cell progression in various human cancers; however, its function and possible mechanism in lung cancer remain largely unknown. Methods: The expression levels of NORAD, miR-30a-5p and a disintegrin and metalloproteinase 19 (ADAM19) were evaluated by quantitative real-time polymerase chain reaction (qRT-PCR). 3-(4, 5)-dimethylthiazole-2-y1)-2, 5-biphenyl tetrazolium bromide (MTT) assay, flow cytometry, and transwell assay were employed to detect cell proliferation, apoptosis, migration, and invasion abilities, respectively. Western blot was used to detect the protein expression of ADAM19. The interaction between miR-30a-5p and NORAD or ADAM19 was predicted by online software and confirmed by the dual-luciferase reporter assay. Results: The expression levels of NORAD and ADAM19 were increased and the expression level of miR-30a-5p was decreased in lung cancer tissues and cells. Knockdown of NORAD could inhibit cell proliferation, migration and invasion but promote apoptosis in lung cancer cells. In addition, NORAD directly interacted with miR-30a-5p and its overexpression reversed the anti-cancer role of miR-30a-5p in lung cancer. Moreover, miR-30a-5p directly targeted ADAM19 and its inhibition attenuated the inhibitory effect of ADAM19 knockdown on progression of lung cancer cells. Furthermore, NORAD functioned as a competing endogenous RNA (ceRNA) through sponging miR-30a-5p to regulate ADAM19 expression. Conclusion: NORAD knockdown suppressed cell proliferation, migration and invasion but promoted cell apoptosis in lung cancer cells by regulating miR-30a-5p/ADAM19, providing a possible therapeutic strategy for lung cancer patients.
Keywords: Lung cancer, NORAD, miR-30a-5p, ADAM19, cell progression
Introduction
Lung cancer is the leading cause of cancer death around the world, with an estimated 1.4 million deaths in 2008 [1,2]. At present, despite tremendous advances in diagnosis and treatment over the past few decades, including surgery, chemotherapy, and radiotherapy, the overall survival rate remains unfavorable, with an overall 5-year survival rate for the lung cancer patients of all stages of approximately 15% [3,4]. Additionally, little is known about the pathogenesis of lung cancer, and early diagnosis and effective strategies for the patients are deficient. Hence, obtaining a clear understanding of the underlying mechanisms of lung cancer is crucial for developing novel therapeutic targets and improving the efficacy of clinical treatment.
Long noncoding RNAs (lncRNAs) are kinds of endogenous cellular RNAs (>200 nucleotides) that lack the ability protein coding. Recently, numerous studies demonstrated that lncRNAs were frequently dysregulated in various diseases and played essential roles in biologic processes, such cell growth, differentiation, cell cycle, migration, invasion, apoptosis, and autophagy [5-7]. Many lncRNAs have been shown to be correlated with the progression and development of lung cancer. For instance, Liu et al. showed that HOTAIR overexpression was closely associated with lung cancer advanced pathologic stage and poor prognosis [8]. Huang et al. proved that a high level of PVT1 had an evidently poorer overall survival time and its knockdown suppressed cell metastasis in lung cancer cells [9]. In terms of long noncoding RNA-activated by DNA damage (NORAD), it serves as an oncogene and is linked to overall survival in a variety of cancers, such as gastric cancer [10], colorectal cancer [11], and cervical cancer [12]. NORAD level was also markedly enhanced in non-small cell lung cancer (NSCLC) tissues and cells [13]. However, the impact of NORAD on progression of lung cancer and its possible mechanisms are still largely unknown.
Emerging evidence revealed that lncRNAs functioned as competing endogenous RNAs (ceRNAs), namely miRNA sponges or antagomirs, to bind to miRNAs and regulate their functions [14,15]. MiR-30a-5p was considered to be a tumor suppressor in some cancers, such as colon carcinoma [16], colorectal cancer [17], and hepatocellular carcinoma [18]. In addition, Zhu et al. pointed out that the miR-30a-5p abundance was notably reduced in lung cancer tissues [19]. A Disintegrin and Metalloproteinase 19 (ADAM19), a member of an ADAM family, has been suggested to be overexpressed in NSCLC cells [20]. Nevertheless, the relationships among NORAD, miR-30a-5p and ADAM19 in lung cancer has not been reported.
Here, we measured the abundance of NORAD, miR-30a-5p, and ADAM19 in lung cancer tissues and cells. Moreover, we explored the biological effects of them on cell proliferation, apoptosis, migration and invasion. Additionally, we also explored the ceRNA regulatory network of NORAD/miR-30a-5p/ADAM19 in lung cancer cells to better understand the molecular mechanism of lung cancer.
Materials and methods
Clinical specimens
Human lung cancer tissues and their matched normal tissues from 31 patients were provided by Department of Thoracic Surgery, Bayannaoer City Hospital. The lung cancer patients had not received chemotherapy, radiotherapy, or other therapy before surgery, and each tissue specimen was then instantly frozen in liquid nitrogen and kept in a refrigerator at -80°C until further processing. All patients signed the informed consent and this research was approved by the ethics committee of Department of Thoracic Surgery, Bayannaoer City Hospital.
Cell culture and transfection
The lung cancer cell lines (H460, H1299, A549, and SCLC-21H) and epithelial cell line (HBE) were bought from BeNa Culture Collection (Beijing, China). All cells were maintained in RPMI 1640 medium (Hyclone, Logan, Utah, USA) containing 10% fetal bovine serum (FBS; Hyclone). These cells were incubated in a moist atmosphere of 95% air and 5% CO2 at 37°C.
Small interfering RNAs (siRNAs) against NORAD (si-NORAD) or ADAM19 (si-ADAM19) and their negative control (si-NC), NORAD overexpression vector (NORAD) and vector, mimics or inhibitor of miR-30a-5p (miR-30a-5p or anti miR-30a-5p) and their negative control (miR-NC or anti-miR-NC) were bought from GenePharma (Shanghai, China). The transfection was subsequently performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Trizol (Invitrogen) was used to isolate total RNA from tissues or cells in accordance with the instructions. For NORAD and ADAM19 mRNA detection, RNA was reverse transcribed to cDNA with a PrimeScript RT-PCR Kit (Takara, Dalian, China). For miR-30a-5p detection, reverse transcription was performed with a TaqMan microRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). The reaction process for PCR were done with SYBR Green Supermix (Bio-Rad, CA, USA) on ABI 7500 real-time PCR system (Applied Biosystems). The 2-ΔΔCt method was employed to evaluate gene expression. The expression levels of NORAD and ADAM19 were normalized to GAPDH, and miR-30a-5p was normalized to U6. Primer sequences were: NORAD (Forward, 5’-TGATAGGATACATCTTGGACATGGA-3’; Reverse, 5’-AACCTAATGAACAAGTCCTGACATACA-3’), miR-30a-5p (Forward, 5’-ACACTCCAGCTGGGTGTAAACATCCTCGAC-3’; Reverse, 5’-CAGTGCGTGTCGTGGAGT-3’), ADAM19 (Forward, 5’-CTGAAGGCTGTGGGAAGAAG-3’; Reverse, 5’-AGCTACCACAGGACCCACAC-3’), GAPDH (Forward, 5’-GAAGAGAGAGACCCTCACGCTG-3’; Reverse, 5’-ACTGTGAGGAGGGGAGATTCAGT-3’), U6 (Forward, 5’-GCTTCGGCAGCACATATACTAAAAT-3’; Reverse, 5’-CGCTTCACGAATTTGCGTGTCAT).
Cell proliferation assay
A549 and SCLC-21H cells were sowed in 96-well plates in triplicate and cultured overnight. After that, si-NORAD, miR-30a-5p, miR-30a-5p + NORAD, si-ADAM19, si-ADAM19 + anti-miR-30a-5p, or their matched negative controls were transfected into A549 and SCLC-21H cells for 24 h, 48 h or 72 h. Methyl thiazolyl tetrazolium (MTT; 5 mg/mL, 20 μL, Beyotime, Shanghai, China) was added to the wells and the plates were placed in the incubator (4 h, 37°C). The medium was discarded followed by adding 150 μl of dimethyl sulfoxide. A microtiter plate reader (Bio-Rad, Hercules, CA, USA) was used to measure the optical density at 450 nm.
Cell apoptosis assay
Annexin V-FITC/PI Apoptosis Assay Kit (Sangon Biotech, Shanghai, China) was used to examine apoptosis. Briefly, after transfection for 48 h, A549 and SCLC-21H cells were stained with Annexin V-FITC (10 µL) and PI (5 µL) for 15 min in the dark. Finally, cell apoptosis was analyzed by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Transwell assay
For the migration assay, transfected A549 and SCLC-21H cells (2×104 cells/well) were placed into the upper chamber and cultured in RPMI 1640 medium alone, while RPMI 1640 with 10% FBS was placed into the lower chamber, followed by incubation in an incubator for 24 h. After that, non-migrated cells were carefully removed with a cotton swab, and migrated cells were fixed using 95% ethanol and then stained using 0.1% crystal violet, and counted by microscope (Olympus, Tokyo, Japan). For invasion assay, the upper chamber was pre-coated using Matrigel (BD, San Jose, CA, USA) and the experiment was performed following a similar approach.
Dual-luciferase reporter assay
The putative binding sites of miR-30a-5p and NORAD or ADAM19 were predicted using starBase or TargetScan software online. The NORAD fragment and 3’-UTR of ADAM19 that contained predicted wild-type or mutant binding sites of miR-30a-5p were amplified and inserted into pGL3 vector (Promega, Madison, WI, USA). The vector was named wild-type (WT-NORAD, WT-ADAM19) or mutant-type (MUT-NORAD, MUT-ADAM19) plasmids, respectively. A549 and SCLC-21H cells were co-transfected with WT (WT-NORAD, WT-ADAM19) or MUT (MUT-NORAD, MUT-ADAM19) with miR-30a-5p or miR-NC for 48 h. After that, luciferase activity was examined with the dual-luciferase reporter assay system (Promega, Madison, WI, USA), followed by normalizing to Renilla luciferase activity.
Western blot assay
Tissues and cells were lysed using RIPA lysis buffer containing protease inhibitors (Beyotime) to extract total protein. Protein content was determined by bicinchoninic acid (BCA) assay kit (Tanon, Shanghai, China). Equal amount of total protein (30-40 μg) was separated using 10% SDS-PAGE and transferred onto activated polyvinylidene fluoride membranes. After that, the membranes were incubated with specific the primary antibodies against ADAM19 (1:500, ab104800, Abcam, Cambridge, MA, USA) or GAPDH (1:1000, ab37168, Abcam) overnight at 4°C following blocking with skim milk. After incubation with secondary antibodies (Sangon Biotech) for 2 h, Enhanced Chemiluminescence (ECL; Tanon) chromogenic substrate was performed to visualize the bands, and the intensity of the bands was quantified by ImageJ software.
Statistical analysis
Statistical analyses in this study was performed using GraphPad Prism 4.0 (GraphPad Software, La Jolla, California, USA). All data were presented as the mean ± standard deviation from at least three independent experiments. The differences between groups were analyzed using Student’s t-test. Spearman’s correlation was employed to determine the correlation between miR-30a-5p expression and NORAD or ADAM19 mRNA level. Differences with P<0.05 were considered significant.
Results
NORAD expression was upregulated in lung cancer tissues and cells
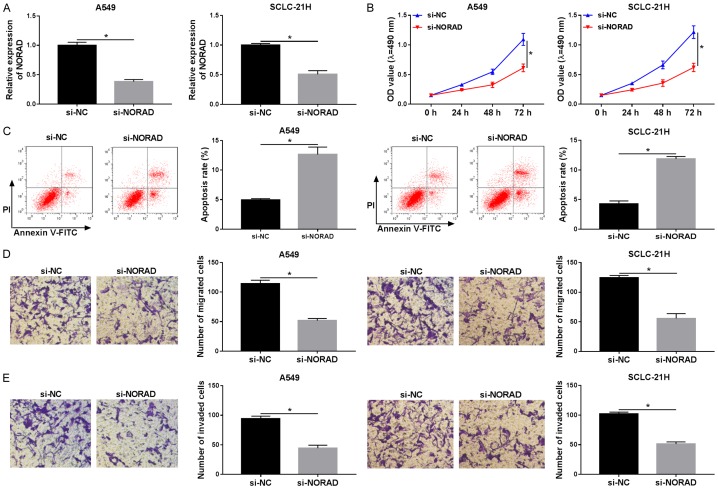
First, the expression level of NORAD in 31 paired samples (lung cancer specimens and corresponding adjacent non-tumor tissues) was measured by qRT-PCR. Results showed that the abundance of NORAD was dramatically increased in lung cancer tissues (Figure 1A). Similarly, NORAD expression was also markedly higher in lung cancer cells (H460, H1299, A549, SCLC-21H) than in HBE cells, especially in A549 and SCLC-21H cells (Figure 1B). Therefore, A549 and SCLC-21H cells were chosen for further analysis. These data indicated that NORAD might play an oncogenic role in lung cancer.
Figure 1.
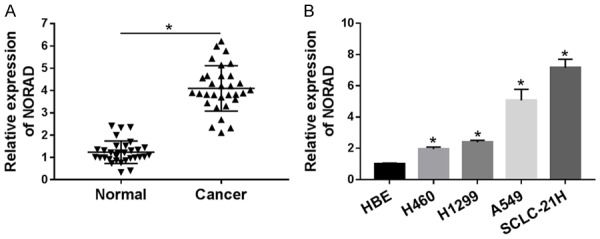
Expression level of NORAD in lung cancer tissues and cells. A. The expression level of NORAD in lung cancer tissues and adjacent normal samples (n=31) was tested by qRT-PCR. B. The abundance of NORAD was detected in lung cancer tissue, cells and HBE cells by qRT-PCR. *P<0.05.
Knockdown of NORAD inhibited cell proliferation, migration, and invasion but induced apoptosis in lung cancer cells
To explore the possible biologic effects of NORAD on the proliferation, apoptosis, migration, and invasion of lung cancer cells, si-NC or si-NORAD was transfected into A549 and SCLC-21H cells. Then, knockdown efficiency of si-NORAD was evaluated by RT-qPCR. As displayed in Figure 2A, compared with si-NC group, introduction of si-NORAD induced an obvious reduction of NORAD level in A549 and SCLC-21H cells, suggesting that si-NORAD could be used for the following experiments. MTT assay revealed that NORAD inhibition markedly limited cell proliferation when compared with the NC group in A549 and SCLC-21H cells (Figure 2B). Subsequent flow cytometry analysis proved that absence of NORAD led to an evident elevation of apoptotic rate in A549 and SCLC-21H cells (Figure 2C). Besides, transwell assay manifested that NORAD downregulation notably inhibited the migration and invasion of A549 and SCLC-21H cells (Figure 2D and 2E). These results suggested that inhibition of NORAD suppressed lung cancer cell progression.
Figure 2.
Knockdown of NORAD suppressed progression in lung cancer. A549 and SCLC-21H cells were transfected with si-NC or si-NORAD. A. The level of NORAD was determined by qRT-PCR. B. MTT assay was used to estimate cell proliferation. C. Flow cytometry was performed to assess cell apoptosis. D and E. The number of migrated and invaded cells was determined by transwell assay. *P<0.05.
MiR-30a-5p was a target of NORAD
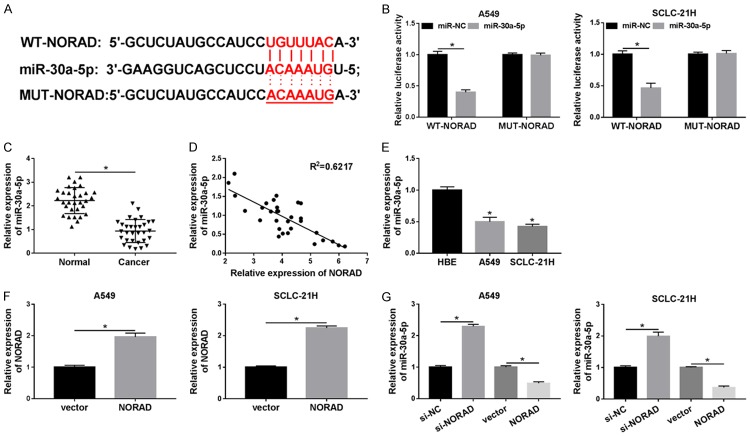
It is well known that lncRNAs act as ceRNAs of miRNAs to modulate target mRNAs expression. Thus, starBase online website was employed to search NORAD targets. miR-30a-5p was found to potentially bind to NORAD (Figure 3A). Subsequently, the prediction was validated by dual-luciferase activity assay. The effect of miR-30a-5p on the luciferase activity of the WT-NORAD or MUT-NORAD reporter plasmid was measured, and the results showed that introduction of miR-30a-5p mimics conspicuously reduced the luciferase activity of WT-NORAD reporter, compared with miR-NC, whereas miR-30a-5p had no effect on the luciferase activity of MUT-NORAD reporter in A549 and SCLC-21H cells (Figure 3B). We then investigated the level of miR-30a-5p in lung cancer tissues and cells. As presented in Figure 3C-E, the level of miR-30a-5p was drastically decreased in lung cancer tissues and cells, and an inverse correlation was noted between miR-30a-5p and NORAD expression in lung cancer tissues (R2=0.6217). Transfection of NORAD led to an obvious promotion of NORAD expression in A549 and SCLC-21H cells compared with transfection of vector, confirming the successful introduction of NORAD into the cells (Figure 3F). As expected, overexpression of NORAD resulted in a significant reduction of miR-30a-5p expression in A549 and SCLC-21H cells, whereas NORAD knockdown showed an opposite effect (Figure 3G). Taken together, our findings revealed that miR-30a-5p was a direct target of NORAD and negatively regulated by it.
Figure 3.
MiR-30a-5p is a target gene of NORAD. A. The binding sites between NORAD and miR-30a-5p were predicted by starBase. B. The luciferase activity was measured in A549 and SCLC-21H cells co-transfected with WT-NORAD or MUT-NORAD and miR-30a-5p or miR-NC. C. The level of miR-30a-5p was measured using qRT-PCR in lung cancer tissues and adjacent normal samples. D. The correlation between NORAD mRNA level and miR-30a-5p expression was analyzed in lung cancer tissues by Spearman’s correlation analysis. E. MiR-30a-5p level was detected by qRT-PCR in cells (HBE, A549 and SCLC-21H). F. The expression of NORAD was determined by qRT-PCR in A549 and SCLC-21H cells transfected with vector or NORAD. G. The level of miR-30a-5p was measured by qRT-PCR in A549 and SCLC-21H cells transfected with si-NC, si-NORAD, vector, or NORAD. *P<0.05.
NORAD reversed the inhibitory effect of miR-30a-5p on progression of lung cancer cells
Since the suppressive effect of NORAD on miR-30a-5p expression in lung cancer, we further investigated whether NORAD had the same effect on the biologic functions of miR-30a-5p. Then a torsion experiment was employed to confirm this speculation. The transfection efficiency of miR-30a-5p mimics was measured to ensure its effectiveness, and we found that transfection of miR-30a-5p mimics enhanced the expression level of miR-30a-5p and transfection of NORAD weakened the effect (Figure 4A). Then functional experiments indicated that restoration of miR-30a-5p prominently blocked the proliferation, migration, and invasion but contributed to apoptosis of A549 and SCLC-21H cells. These effects were abrogated by addition of NORAD (Figure 4B-E). Thus, our present results demonstrated that NORAD inhibited miR-30a-5p to play its role.
Figure 4.
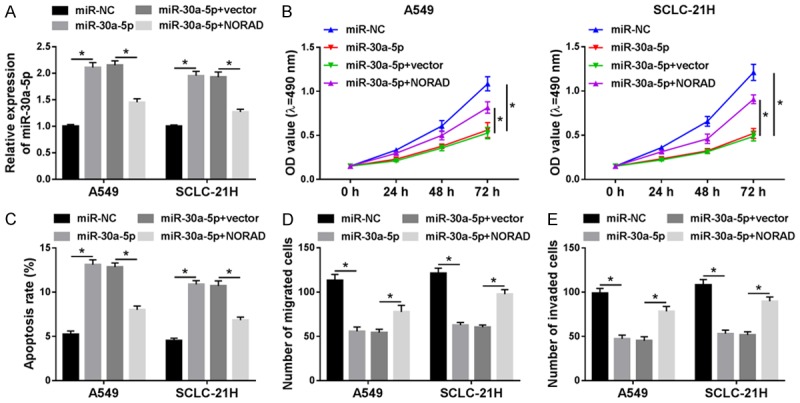
NORAD attenuated the inhibitory effect of miR-30a-5p on progression of lung cancer cells. A549 and SCLC-21H cells were transfected with miR-NC, miR-30a-5p, miR-30a-5p + vector, or miR-30a-5p + NORAD. A. MiR-30a-5p level was measured by qRT-PCR. B. MTT assay was used to estimate cell proliferation. C. Flow cytometry was performed to assess cell apoptosis. D and E. Transwell assay was employed to determine cell migration and invasion. *P<0.05.
ADAM19 was a direct target of miR-30a-5p
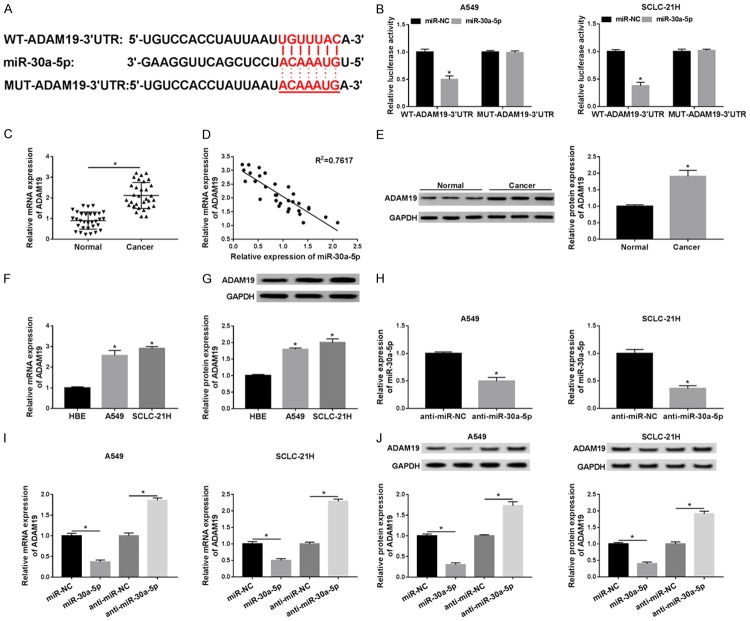
In order to explore the underlying mechanism of miR-30a-5p-mediated progression in lung cancer, we predicted the downstream targets of miR-30a-5p. As shown in Figure 5A, TargetScan online provided the putative binding sites of ADAM19 and miR-30a-5p, and dual-luciferase reporter assay further confirmed the prediction. The luciferase assay showed that transfection of miR-30a-5p mimics strikingly decreased the luciferase activity of WT-ADAM19 in both A549 and SCLC-21H cells, but did not affect the luciferase activity of MUT-ADAM19 (Figure 5B), indicating that ADAM19 was a target of miR-30a-5p. In addition, the mRNA and protein expression of ADAM19 were explored in lung cancer tissues and cells. As depicted in Figure 5C-G, the mRNA and protein levels of ADAM19 were notably reduced in lung cancer tissues and cells, representing a negative correlation between ADAM19 mRNA level and miR-30a-5p expression in lung cancer tissues (R2=7617). Moreover, the abundance of miR-30a-5p was apparently upregulated in A549 and SCLC-21H cells transfected with miR-30a-5p compared with miR-NC group (Figure 5H). Besides, miR-30a-5p overexpression led to a significant decrease of ADAM19 mRNA and protein expression and its knockdown presented an opposite effect (Figure 5I, 5J). The above results indicated that miR-30a-5p directly targeted ADAM19.
Figure 5.
ADAM19 was a direct target of miR-30a-5p. A. The binding sites between miR-30a-5p and ADAM19 were provided by TargetScan. B. The luciferase activity was measured in A549 and SCLC-21H cells co-transfected with WT-ADAM19 or MUT-ADAM19 and miR-30a-5p or miR-NC. C. The expression of ADAM19 was measured using qRT-PCR in lung cancer tissues and adjacent normal samples. D. The correlation between ADAM19 mRNA level and miR-30a-5p expression was analyzed in lung cancer tissues using Spearman’s correlation analysis. E. The protein expression of ADAM19 was examined by western blot in in lung cancer tissues and adjacent normal tissues. F and G. The mRNA and protein levels of ADAM19 were detected in cells (HBE, A549 and SCLC-21H) by qRT-PCR and western blot, respectively. H. The abundance of miR-30a-5p was measured by qRT-PCR in A549 and SCLC-21H cells transfected with anti-miR-NC or anti-miR-30a-5p. I and J. The mRNA and protein expression levels of ADAM19 were detected in A549 and SCLC-21H transfected with miR-NC, miR-30a-5p, anti-miR-NC, or anti-miR-30a-5p by qRT-PCR and western blot, respectively. *P<0.05.
Knockdown of miR-30a-5p alleviated the si-ADAM19-mediated suppressive effect on progression of lung cancer cells
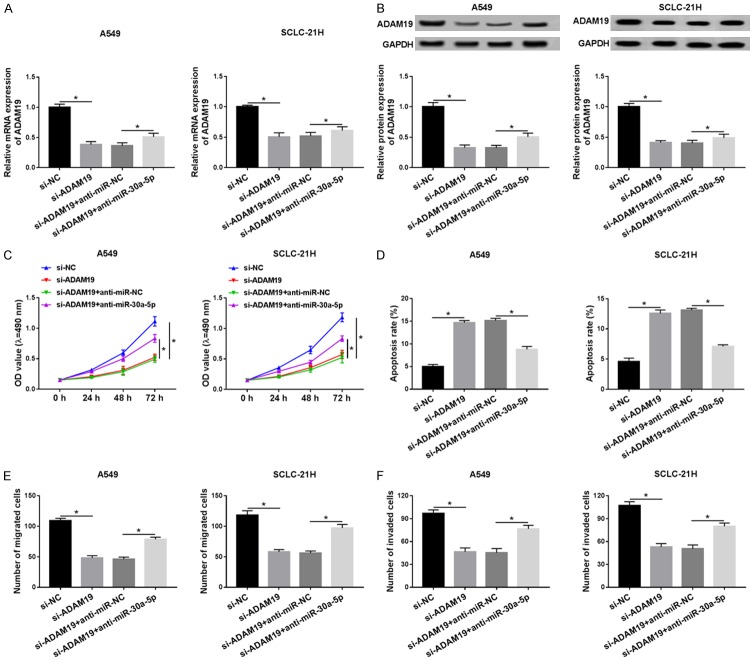
In order to investigate whether miR-30a-5p exerted its effects by inhibiting ADAM19, a torsion experiment was performed. qRT-PCR and western blot analysis indicated that the mRNA and protein expression of ADAM19 was dramatically decreased in A549 and SCLC-21H cells transfected with si-ADAM19, which was abolished by transfection of miR-30a-5p inhibitor (Figure 6A and 6B). The functional experimental results confirmed that proliferation, migration, and invasion abilities were greatly repressed but cell apoptosis was facilitated after the ADAM19 level was decreased, while these effects were weakened by inhibition of miR-30a-5p (Figure 6C-F). Collectively, these results demonstrated that miR-30a-5p exerted its biologic functions by decreasing ADAM19.
Figure 6.
MiR-30a-5p exerts its effects by decreasing ADAM19. A549 and SCLC-21H cells were transfected with si-NC, si-ADAM19, si-ADAM19 + anti-miR-NC, or si-ADAM19 + anti-miR-30a-5p. A and B. The mRNA and protein levels of ADAM19 were detected in A549 and SCLC-21H cells by qRT-PCR and western blot, respectively. C. MTT assay was performed to measure the proliferation of A549 and SCLC-21H cells. D. Flow cytometry was performed to evaluate apoptosis of A549 and SCLC-21H cells. E and F. The migration and invasion capabilities of H1299 and A549 cells were determined using transwell assay. *P<0.05.
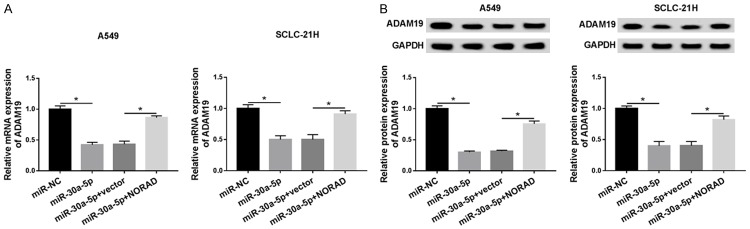
ADAM19 was regulated by NORAD and miR-30a-5p
As NORAD was negatively regulated miR-30a-5p, the effect of NORAD on the ADAM19 expression was expected to be confirmed. Results showed that the addition of miR-30a-5p inhibited ADAM19 mRNA and protein expression, whereas it was abrogated by upregulating NORAD (Figure 7A and 7B), suggesting that NORAD functioned as a ceRNA of miR-30a-5p to regulate ADAM19 expression.
Figure 7.
ADAM19 is regulated by NORAD and miR-30a-5p. A549 and SCLC-21H cells were transfected with miR-NC, miR-30a-5p, miR-30a-5p + vector, or miR-30a-5p + NORAD. A and B. The mRNA and protein expression levels of ADAM19 were detected using qRT-PCR and western blot, respectively. *P<0.05.
Discussion
Lung cancer, with high morbidity and mortality, is one of the most frequent malignancies around the world [21]. More recent evidence has proven that lncRNAs play essential roles in development and malignant progression of cancers, and aberrant expression of lncRNAs is tightly related to malignant tumors [22,23]. In our study, we focused on the involvement of NORAD in lung cancer and explored its underlying molecular mechanism.
NORAD, a critical cancer-associated lncRNA, has been found to be expressed at a high level and play an oncogenic role in various cancers. For instance, Tong et al. proved that NORAD expression was remarkably elevated in epithelial ovarian cancer cell lines and tumors [24]. Zhang et al. showed that downregulation of NORAD evidently suppressed colorectal cancer cell growth, migration, and invasion, and facilitated apoptosis through inhibiting miR-202-5p [11]. Also, Chen et al. disclosed that the abundance of NORAD was enhanced in NSCLC tissues and cells, and NORAD could act as a growth-promoting lncRNA by inhibiting the biologic functions of miR-136-5p in NSCLC [25]. Cao et al. proved that NORAD expression level was obviously enhanced in patients with NSCLC compared with that in the control group [13]. As the first step of our research, we evaluated the abundance of NORAD in lung cancer tissues and cells. The results suggested that NORAD level was obviously elevated in both lung cancer tissue samples and cells, which was in line with previous research. Additionally, we revealed that downregulation of NORAD markedly repressed cell growth, migration, and invasion capabilities but accelerated apoptosis in lung cancer cells. Thus, these results indicated that NORAD played positive roles in the development of lung cancer.
Recently, a ceRNA hypothesis has been proposed that lncRNAs may exert their biologic functions by acting as miRNA sponges, eventually resulting in repression of miRNA targets [26]. To explore whether NORAD acted as a miRNA sponge, bioinformatics analysis was performed, and results suggested that NORAD contained binding sites for miR-30a-5p, and this interaction was confirmed by the dual luciferase reporter assay. MiR-30a-5p has been shown to be a tumor suppressor in many cancers by modulating target expression. For example, Park et al. presented that miR-30a-5p suppressed metastasis in colorectal cancer cell lines by targeting transmembrane-4-L-six-family protein (TM4SF1) [27]. In addition, Ye et al. found that high level of miR-30a-5p limited cell growth, migration, and invasion in gallbladder cancer cells by targeting E2F7 [28]. In this research, we proved that miR-30a-5p abundance was reduced in lung cancer tissues and cells, which is also consistent with previous research [19]. Mechanistically, we found that restoration of miR-30a-5p prominently blocked the cell growth, migration, and invasion, andcontributed to apoptosis in lung cancer cells. Our data suggested that miR-30a-5p acted as a tumor inhibitor in lung cancer.
To analyze how miR-30a-5p affected progression of lung cancer, potential targets were predicted by TargetScan, revealing that ADAM19 and miR-30a-5p had a potential binding site. Additionally, dual-luciferase reporter assay further verified the binding relationship between them. The emerging evidence suggested that ADAM19 was overexpressed in various cancers, such as colon cancer [29], retinoblastoma [30], primary brain tumors [31]. Moreover, Shan et al. showed that downregulation of ADAM19 repressed migration and invasion of NSCLC cells [32]. Here, we found that ADAM19 knockdown suppressed proliferation, migration, and invasion abilities but accelerated apoptosis, while the effect was abated by inhibiting miR-30a-5p. Moreover, we displayed that ADAM19 expression was positively regulated by NORAD and negatively regulated by miR-30a-5p. Therefore, our findings revealed that NORAD functioned as a ceRNA to positively regulate ADAM19 expression by sponging miR-30a-5p.
In summary, our research presented first evidence that NORAD regulated lung cancer cell growth, apoptosis, migration and invasion through the miR-30a-5p/ADAM19 axis, clarifying the underlying mechanism of NORAD as an oncogene in lung cancer. Thus, NORAD/miR-30a-5p/ADAM19 axis played a key role in the pathogenesis and development of lung cancer, with possible therapy implications for lung cancer patients.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Crino L, Weder W, Van Meerbeeck J, Felip E. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v103–15. doi: 10.1093/annonc/mdq207. [DOI] [PubMed] [Google Scholar]
- 4.Rosell R, Bivona TG, Karachaliou N. Genetics and biomarkers in personalisation of lung cancer treatment. Lancet. 2013;382:720–731. doi: 10.1016/S0140-6736(13)61715-8. [DOI] [PubMed] [Google Scholar]
- 5.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–9. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 6.Jiang C, Li X, Zhao H, Liu H. Long non-coding RNAs: potential new biomarkers for predicting tumor invasion and metastasis. Mol Cancer. 2016;15:62. doi: 10.1186/s12943-016-0545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi X, Sun M, Liu H, Yao Y, Kong R, Chen F, Song Y. A critical role for the long non-coding RNA GAS5 in proliferation and apoptosis in non-small-cell lung cancer. Mol Carcinog. 2015;54(Suppl 1):E1–E12. doi: 10.1002/mc.22120. [DOI] [PubMed] [Google Scholar]
- 8.Liu XH, Liu ZL, Sun M, Liu J, Wang ZX, De W. The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer. 2013;13:464. doi: 10.1186/1471-2407-13-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Liu S, Wang H, Zhang Z, Yang Q, Gao F. LncRNA PVT1 overexpression is a poor prognostic biomarker and regulates migration and invasion in small cell lung cancer. Am J Transl Res. 2016;8:5025–5034. [PMC free article] [PubMed] [Google Scholar]
- 10.Miao Z, Guo X, Tian L. The long noncoding RNA NORAD promotes the growth of gastric cancer cells by sponging miR-608. Gene. 2019;687:116–124. doi: 10.1016/j.gene.2018.11.052. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Li XY, Hu P, Ding YS. LncRNA NORAD contributes to colorectal cancer progression by inhibition of miR-202-5p. Oncol Res. 2018 doi: 10.3727/096504018X15190844870055. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huo H, Tian J, Wang R, Li Y, Qu C, Wang N. Long non-coding RNA NORAD upregulate SIP1 expression to promote cell proliferation and invasion in cervical cancer. Biomed Pharmacother. 2018;106:1454–1460. doi: 10.1016/j.biopha.2018.07.101. [DOI] [PubMed] [Google Scholar]
- 13.Gao W, Weng T, Wang L, Shi B, Meng W, Wang X, Wu Y, Jin L, Fei L. Long non-coding RNA NORAD promotes cell proliferation and glycolysis in non-small cell lung cancer by acting as a sponge for miR-136-5p. Mol Med Rep. 2019;19:5397–5405. doi: 10.3892/mmr.2019.10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu XH, Sun M, Nie FQ, Ge YB, Zhang EB, Yin DD, Kong R, Xia R, Lu KH, Li JH, De W, Wang KM, Wang ZX. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92. doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the rosetta stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baraniskin A, Birkenkamp-Demtroder K, Maghnouj A, Zöllner H, Munding J, Klein-Scory S, Reinacher-Schick A, Schwarte-Waldhoff I, Schmiegel W, Hahn SA. MiR-30a-5p suppresses tumor growth in colon carcinoma by targeting DTL. Carcinogenesis. 2012;33:732–739. doi: 10.1093/carcin/bgs020. [DOI] [PubMed] [Google Scholar]
- 17.Wei W, Yang Y, Cai J, Cui K, Li RX, Wang H, Shang X, Wei D. MiR-30a-5p suppresses tumor metastasis of human colorectal cancer by targeting ITGB3. Cell Physiol Biochem. 2016;39:1165–76. doi: 10.1159/000447823. [DOI] [PubMed] [Google Scholar]
- 18.He R, Yang L, Lin X, Chen X, Lin X, Wei F, Liang X, Luo Y, Wu Y, Gan T. MiR-30a-5p suppresses cell growth and enhances apoptosis of hepatocellular carcinoma cells via targeting AEG-1. Int J Clin Exp Pathol. 2015;8:15632–41. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J, Zeng Y, Xu C, Qin H, Lei Z, Shen D, Liu Z, Huang JA. Expression profile analysis of microRNAs and downregulated miR-486-5p and miR-30a-5p in non-small cell lung cancer. Oncol Rep. 2015;34:1779–86. doi: 10.3892/or.2015.4141. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Lian Y, Ge C. MiR-145 changes sensitivity of non-small cell lung cancer to gefitinib through targeting ADAM19. Eur Rev Med Pharmacol Sci. 2019;23:5831–5839. doi: 10.26355/eurrev_201907_18323. [DOI] [PubMed] [Google Scholar]
- 21.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 22.Haemmerle M, Gutschner T. Long non-coding RNAs in cancer and development: where do we go from here? Int J Mol Sci. 2015;16:1395–405. doi: 10.3390/ijms16011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong L, Ao Y, Zhang H, Wang K, Wang Y, Ma Q. Long noncoding RNA NORAD is upregulated in epithelial ovarian cancer and its downregulation suppressed cancer cell functions by competing with miR-155-5p. Cancer Med. 2019;8:4782–4791. doi: 10.1002/cam4.2350. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Chen T, Qin S, Gu Y, Pan H, Bian D. Long non-coding RNA NORAD promotes the occurrence and development of non-small cell lung cancer by adsorbing MiR-656-3p. Mol Genet Genomic Med. 2019;7:e757. doi: 10.1002/mgg3.757. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Yoon JH, Abdelmohsen K, Gorospe M, editors. Functional interactions among microRNAs and long noncoding RNAs. Seminars in Cell & Developmental Biology. 2014;34:9–14. doi: 10.1016/j.semcdb.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park Y, Kim S, Lee M, Seo S, Lee J, Kim S, Kim I, Lee S, Lee S, Kim SW. MicroRNA-30a-5p (miR-30a) regulates cell motility and EMT by directly targeting oncogenic TM4SF1 in colorectal cancer. J Cancer Res Clin Oncol. 2017;143:1915–1927. doi: 10.1007/s00432-017-2440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye YY, Mei JW, Xiang SS, Li HF, Ma Q, Song XL, Wang Z, Zhang YC, Liu YC, Jin YP, Hu YP, Jiang L, Liu FT, Zhang YJ, Hao YJ, Liu YB. MicroRNA-30a-5p inhibits gallbladder cancer cell proliferation, migration and metastasis by targeting E2F7. Cell Death Dis. 2018;9:410. doi: 10.1038/s41419-018-0444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Q, Yu L, Qin D, Huang R, Jiang X, Zou C, Tang Q, Chen Y, Wang G, Wang X. Role of microRNA-30c targeting ADAM19 in colorectal cancer. PLoS One. 2015;10:e0120698. doi: 10.1371/journal.pone.0120698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Z, Zhang A, Jiang T, Du Z, Che C, Wang F. MiR-145 suppressed human retinoblastoma cell proliferation and invasion by targeting ADAM19. Int J Clin Exp Pathol. 2015;8:14521–7. [PMC free article] [PubMed] [Google Scholar]
- 31.Wildeboer D, Naus S, Amy Sang QX, Bartsch JW, Pagenstecher A. Metalloproteinase disintegrins ADAM8 and ADAM19 are highly regulated in human primary brain tumors and their expression levels and activities are associated with invasiveness. J Neuropathol Exp Neurol. 2006;65:516–27. doi: 10.1097/01.jnen.0000229240.51490.d3. [DOI] [PubMed] [Google Scholar]
- 32.Shan N, Shen L, Wang J, He D, Duan C. MiR-153 inhibits migration and invasion of human non-small-cell lung cancer by targeting ADAM19. Biochem Biophys Res Commun. 2015;456:385–91. doi: 10.1016/j.bbrc.2014.11.093. [DOI] [PubMed] [Google Scholar]