Abstract
Background: We studied the clinicopathologic features of superficial CD34-positive fibroblastic tumor (SCPFT), which is a newly described neoplasm, to enhance the recognition and diagnostic level of the disease. Case presentation: We herein report two cases of superficial CD34-positive fibroblastic tumors in a 33-year-old man and a 30-year-old man. The 33-year-old man presented with a slow-growing subcutaneous nodule 5.0 cm in diameter on the right thigh, and the 30-year-old man developed a painful lump 4.0 cm in diameter on his right thigh. Histologically, the tumor was located in superficial soft tissue with relative circumscription. Tumors had abundant spindled polygonal cells, which were arranged in fascicular or sheet-like patterns. Neoplastic cells were characterized by polymorphic nuclei, granular cytoplasm, intranuclear cytoplasmic pseudoinclusions, and an extremely low mitotic rate. Immunohistochemically, neoplastic cells showed diffuse and strong CD34 expression and focal cytokeratin staining. The expression of S-100 protein, SMA, CD99, H-caldesmon, ALK-1, and bcl-2 were all negative. The Ki-67 index was low. Conclusions: SCPFT is a rare low-grade fibroblastic tumor that has typical morphologic features and unique biologic behavior. Familiarity with clinicopathologic characteristics of the tumor contributes to the differential diagnosis of similar tumors.
Keywords: CD34, fibroblastic tumor, immunohistochemistry, diagnosis, differential diagnosis
Introduction
Superficial CD34-positive fibroblastic tumor (SCPFT) was recently reported as an intermediate malignancy of mesenchymal origin, which was first presented by Carter et al. in 2014 [1]. These tumors occurred mostly in superficial soft tissues of the lower limbs of adults, with morphologic features of spindle cells arranged in sheets and striking nuclear pleomorphism, but without a high mitotic rate. Additionally, tumor cells presented diffuse and strong positivity for CD34 and focal cytokeratin (CK) positivity [2-5]. Due to the risk of local recurrence of this tumor, the most appropriate treatment for SCPFT was extended resection of the mass [6]. In this study, we retrospectively analyzed two cases of SCPFT with clinicopathologic features, diagnosis, and differential diagnosis, treatment and prognosis, in addition to a review of the literature.
Case presentation
First case
A 33-year-old man presented with a gradually increasing subcutaneous mass of the right thigh in the past three months, which was accidentally noticed two years ago. The nodule was located in the subcutaneous soft tissue, which was firm and tender. Skin on the surface of the nodule was not ruptured, red or swollen. Then, the patient underwent simple resection of the nodule, which was sent to the pathology department for histopathologic examination.
Second case
A 30-year-old male patient presented to our outpatient clinic with a 2-month history of a painless nodule in the right thigh. The nodule was 4 cm in diameter and had the same features as in the first case. Pathological examination was performed after surgical resection.
Materials and methods
The tissues were fixed in 4% buffered formalin solution and embedded in paraffin. Then, the paraffin-embedded blocks were cut into 4-μm-thick sections for hematoxylin and eosin and immunohistochemical staining. In this study, a set of antibodies was used, including CD34 (catalog no. ab157304, Abcam, USA), vimentin (catalog no. ab227081, Abcam, USA), Cytokeratin Pan (catalog no. 10R-2096, Fitzgerald, USA), S100 (catalog no. ab166649, Abcam, USA), SMA (Abcam, USA), CD99 (catalog no. ab108297, Abcam, USA), H-caldesmon (Abcam, USA), ALK-1 (Abcam, USA), INI1 (catalog no. ab12167, Abcam, USA), bcl-2 (catalog no. ab117115, Abcam, USA) and Ki-67 (Abcam, USA). The staining steps were strictly in accordance with the guidelines of the Elivision™ Plus Detection Kit instructions (Lab Vision, USA). Vimentin, CK, S-100, SMA, and H-caldesmon positive staining was mainly confined to the cytoplasm of tumor cells; CD34, CD99, ALK-1, bcl-2, and CD68 positive staining was mainly confined to the membrane of tumor cells. INI1 and Ki-67 positive staining was in the chromosomes of tumor cells.
Pathologic findings
Grossly, the tumor was a soft tissue mass with an ellipse of skin attached. On the cut surface, a grayish-white and firm node was identified; the first case measured 5.0 cm×3.5 cm×2.5 cm, and the second was 5.0 cm×3.5 cm×2.5 cm. The boundary between the nodular and surrounding tissues was clear. There was no obvious hemorrhage or necrosis in the tumor.
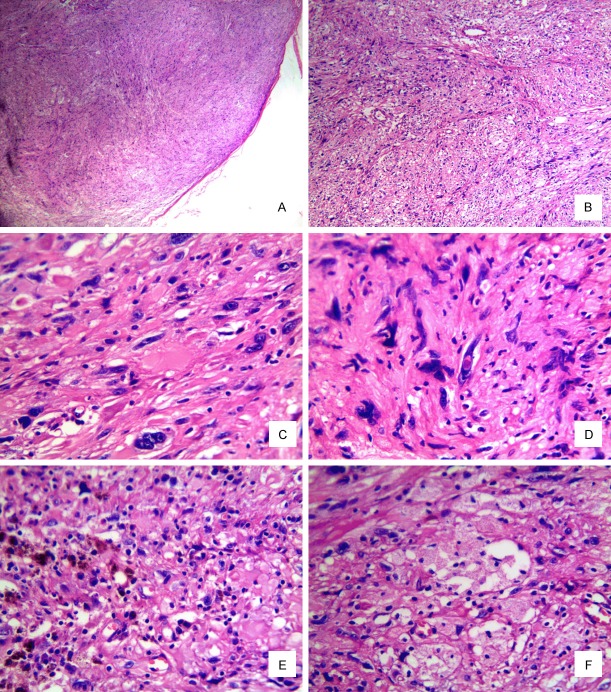
Microscopically, the lesion was a well-circumscribed nodule and had not invaded into the subcutaneous tissue. The tumor was composed of spindle-shaped or oval-shaped cells arranged in a fascicular or sheet-like pattern. There were also a few multinucleate giant cells scattered in the tumor tissue. The pleomorphism of the tumor nucleus was prominent, with macronuclei and irregular nuclei, some of which possess intranuclear cytoplasmic pseudoinclusions. The nucleoli of some tumor cells were single and obvious, and the chromatin was rough, but the mitotic activity was extremely low (<1/50 HPF), and no atypical mitosis was found. The tumor stroma was sparse, showing fissure-like thin-walled vessels and focally clustered xanthomatic-like foam cells in the background, and a small amount of scattered mast cells and lymphoplasmacytic infiltration. There was no neoplastic necrosis or hemorrhage in the tumor (Figure 1A-F).
Figure 1.
Histology of the tumor. (A) Well-circumscribed nodule. (B) Spindle-shaped or oval-shaped cells arranged in a fascicular or sheet-like pattern. (C) Epithelioid to polygonal cells with glassy cytoplasm. (D) Intranuclear cytoplasmic pseudoinclusion (A. H&E×40. B. H&E×100, C-F. H&E×400).
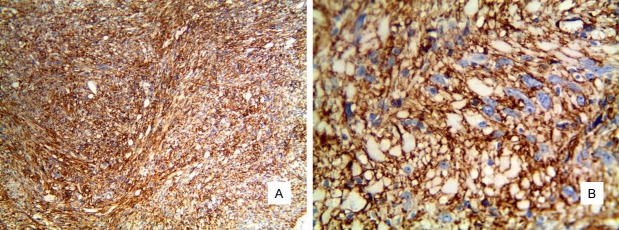
Immunohistochemistry showed that the tumor cells were strongly and diffusely positive for CD34 (Figure 2A, 2B) and vimentin and focal positive for cytokeratin. However, S-100 protein, SMA, CD99, H-caldesmon, INI1, ALK-1 and bcl-2 expression were negative. The Ki-67 labeling index was low, approximately 5%.
Figure 2.
Diffuse and strong expression of CD34 (A. ElivisionTM ×100. B. ElivisionTM ×400).
Based on the above morphologic and immunohistochemical findings, a diagnosis of SCPFT was established. Ten months after the resection, no local recurrence or distal metastasis was observed.
Discussion
As a type of soft tissue tumor, spindle cell fibroblastic (myofibroblastic) tumors have always been a diagnostic difficulty in surgical pathology. According to the different biological behaviors, these tumors can be divided into three categories: benign, intermediate and malignant [7].
As an uncommon intermediate mesenchymal neoplasm, SCPFT has characteristic clinical, histopathologic, and immunohistochemical features, with a superficial site and strong CD34 positivity on immunohistochemistry. To date, approximately 42 cases of SCPFT have been reported. The actual incidence rate is most likely much higher due to the lack of sufficient recognition of this tumor.
These 42 cases of SCPFT reported in the previous literature were reviewed, and their clinical features and prognosis are summarized in Table 1. Among all the cases, there were 26 males and 18 females, with a sex ratio of approximately 1.5:1. The age of the patients ranged from 16 to 76 years (median, years). The most common sites were lower extremities (28/44), especially in thigh and buttock. The tumors were also located in the upper limb, neck, and vulva. The patient most commonly presented with a painless, slow-growing nodule in the superficial layer of the skin. The duration time before the operation was usually several years.
Table 1.
Clinicopathologic features
| Case | Age/Sex | Site | Size (mm) | Duration | follow-up (month) |
|---|---|---|---|---|---|
| 1 [4] | 23/M | Buttock | 20 | 4 years | ANED, 15 |
| 2 [4] | 38/M | Left thigh | 30 | 10 years | ANED, 12 |
| 3 [4] | 33/F | Right buttock | 16 | unknown | ANED, 12 |
| 5 [4] | 28/M | Right upper arm | 20 | 1 year | ANED, 6 |
| 6 [4] | 18/M | Left thigh | 35 | unknown | ANED, 4 |
| 7 [4] | 30/M | Right thigh | 20 | 16 months | ANED, 3 |
| 8 [4] | 19/M | Right thigh | 40 | 1 week | ANED, 2 |
| 9 [4] | 42/M | Right waist | 25 | 3 years | ANED, 2 |
| 10 [4] | 42/F | Right shoulder | 19 | 1 year | ANED, 2 |
| 11 [4] | 39/F | Right buttock | 50 | 6 years | ANED, 1 |
| 12 [1] | 20/M | Thigh | 31 | unknown | ANED/104 RLNM |
| 13 [1] | 21/F | Leg | 20 | unknown | ANED/30 |
| 14 [1] | 25/M | Groin | 75 | unknown | ANED/1 |
| 15 [1] | 25/M | Foot | 38 | 4 years | ANED/17 |
| 16 [1] | 26/M | Thigh | 65 | unknown | ANED/7 |
| 17 [1] | 26/M | Thigh | 22 | 7 years | NA |
| 18 [1] | 28/M | Thigh | 15 | unknown | ANED/24 |
| 19 [1] | 32/M | Shoulder | unknown | 14 years | ANED/2 |
| 20 [1] | 37/F | Vulva | 10 | unknown | NA |
| 21 [1] | 38/F | Neck | 15 | 6 years | ANED/38 |
| 22 [1] | 44/F | Popliteal fossa | 65 | 1 year | ANED/20 |
| 23 [1] | 45/M | Knee | unknown | unknown | NA |
| 24 [1] | 46/M | Hip | 20 | unknown | NA |
| 25 [1] | 48/M | Arm | 27 | unknown | ANED/4 |
| 26 [1] | 51/F | Arm | unknown | unknown | NA |
| 27 [1] | 53/F | Groin | 34 | 20 years | ANED/53 |
| 28 [1] | 57/F | Thigh | 74 | unknown | ANED/3 |
| 29 [1] | 76/F | Buttock | 20 | Many years | ANED/3 |
| 30 [5] | 48/F | Left thigh | 15 | unknown | NA |
| 31 [2] | 16/F | Right arm | 30 | 2 years | ANED/6 |
| 32 [3] | 29/F | Achilles tendon | 52 | 3 years | ANED/3 |
| 33 [3] | 25/M | Neck | 26 | unknown | ANED/3 |
| 34 [8] | 18/M | Thigh | 90 | 18 months | ANED/5 |
| 35 [9] | 32/M | Hip | 40 | 10 months | ANED/6 |
| 36 [10] | 31/F | Forearm | 50 | unknown | R four times/24 |
| 37 [10] | 53/F | Ankle | 40 | unknown | ANED/22 |
| 38 [10] | 33/M | Thigh | 55 | unknown | ANED/13 |
| 39 [11] | 51/F | Thigh | 15 | 24 months | NA |
These tumors had well-defined borders and solid textures, with grayish-white or grayish-yellow cut surfaces. The tumor sizes ranged from 15 to 100 mm (mean, 35 mm; median, 26 mm). Histopathologically, almost all the tumors were well-circumscribed and located in the superficial fascia of subcutis. Nevertheless, a few cases showed focal infiltration to adjacent fibrous and adipose tissues. The tumors were composed of epithelioid to spindle cells arranged in fascicles or sheets. The tumor cells were abundant in granular or eosinophilic glassy cytoplasm and had bizarre, hyperchromatic nuclei. Intranuclear pseudoinclusions were frequently observed. The atypia of the nucleus is obvious, but nuclear mitotic activity is very low (1/50 high-powered fields). No necrosis was observed. Otherwise, a small number of thin-walled vessels, focal xanthomatous changes, and inflammatory cells were identified in the lesions [1,8].
Immunohistochemistry showed that the tumor cells were all strongly and diffusely positive for CD34, and approximately half expressed cytokeratins. Other markers, including vascular endothelial markers (CD34, CD31, FLI-1), myogenic markers (SMA, desmin), melanin markers (S-100, HMB45, Melan-A), dendritic cells and histiocytic markers, as well as EMA and p53, were all negative. The Ki-67 labeling index was approximately below 5%. Cytogenetic studies revealed that chromosomal translocation of t(2;5)(q31;q31) was found in tumor cells [8].
There was no significant gender difference. Only one case had distant lymph node metastasis, and there was no recurrence after complete resection. The remaining cases were at follow-up time, and there was no recurrence of the tumor. Superficial CD34-positive fibroblastic tumors have histopathological features similar to those of other fibroblast tumors. Differential diagnosis includes the following diseases: 1. The most significant differential diagnosis is probably fibrosarcoma arising in dermatofibrosarcoma protuberans (DFSP). DFSP has obvious invasiveness and distant metastasis. Both of them can have pleomorphic and CD34-positive tumor cells, while DFSP transformation usually has a typical striate tissue structure background and spindle cells resulting in a distinctive storiform or cartwheel pattern, and the sarcoma-like transformation region has obvious mitotic figures and decreased immunohistochemical CD34 expression and significantly increased p53 protein and Ki-67 expression [12,13]. 2. Juvenile xanthogranuloma (JXG) is a rare disease that may occur at birth or in infancy. It can also occur in adults of all ages. The lesions may be single or multiple nodules a few millimeters in diameter. The most common site is the head and neck, but it can also occur in the limbs and torso. It may also involve internal organs, such as the lungs, kidneys, and gastrointestinal tract. The most common external location of the skin is the eye. Histologically, JXG consists of tissue cells, Touton giant cells and foam cells. Immunohistochemistry results are positive for CD68, S-100 protein is weakly focally positive, and langerin is negative. The diagnosis of JXG is primarily clinical but sometimes requires biopsy analysis [6,14]. 3. Atypical fibroma (AFX) is a rare mesenchymal tumor that occurs mainly in the head and neck of elderly patients. Clinically, tumors are characterized by rapid exogenous growth, and the epidermis often ulcerates. Histopathologically, AFX is a well-defined dermal tumor composed of large tissue-like cells, enlarged fusiform and epithelioid tumor cells, and multinucleated tumor giant cells. The nucleus is single and pleomorphic. Immunohistochemistry results show that traditional AFX tumor cells stain positive for vimentin, CD10, and p53 [15,16]. 4. In metastatic high-grade myxofibrosarcoma (MFS), the morphology of the tumor is characterized by extensive cell population, pleomorphic and mitotic activity, but with obvious histological features such as mucin-like stroma, nodular and curvilinear blood vessels. Immunohistochemistry results show that muscle-specific actin (MSA), alpha-smooth muscle actin (α-SMA), S-100 protein, desmin, CD34, and myogenin may prove useful in the diagnosis of MFS when they are negative [17-19]. 5. Adult-type fibrosarcoma (FS) was defined as a “malignant tumor, composed of fibroblasts with variable collagen and in classical cases, a herringbone architecture” in 2002 by the World Health Organization (WHO). It is composed of relatively uniform, hyperchromatic spindled cells arranged in a herringbone pattern [19].
Conclusion
In summary, superficial CD34-positive fibroblast tumors may be rare in our usual work. Similar tumors may be referred to as “low-grade malignant fibrous histiocytoma/undifferentiated pleomorphic sarcoma during routine practice diagnosis”, and these tumors are currently considered to be moderate (marginal) malignancies with rare metastatic potential. Therefore, accurately understanding and diagnosing this tumor has great practical significance for us to better understand its disease occurrence, tissue morphology, and biologic behavior. As the number of reports increases and research deepens, diagnosis based on pathologic features and immunohistochemical analysis will become clearer.
Acknowledgements
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the consent forms is available for review by the Editor of this journal.
Disclosure of conflict of interest
None.
Abbreviations
- SCPFT
superficial CD34-positive fibroblastic tumor
- CK
cytokeratin
- DFSP
dermatofibrosarcoma protuberans
- JXG
juvenile xanthogranuloma
- AFX
atypical fibroma
- MFS
metastatic high-grade myxofibrosarcoma
- MSA
muscle-specific actin
- FS
adult-type fibrosarcoma
- F
female
- RLNM
regional lymph node metastasis
- M
male
- NA
not available
- ANED
alive no evidence of disease
- R
recurred
References
- 1.Carter JM, Weiss SW, Linos K, DiCaudo DJ, Folpe AL. Superficial CD34-positive fibroblastic tumor: report of 18 cases of a distinctive lowgrade mesenchymal neoplasm of intermediate (borderline) malignancy. Mod Pathol. 2014;27:294–302. doi: 10.1038/modpathol.2013.139. [DOI] [PubMed] [Google Scholar]
- 2.Sood N, Khandelia BK. Superficial CD34-positive fibroblastic tumor: a new entity; case report and review of literature. Indian J Pathol Microbiol. 2017;60:377–380. doi: 10.4103/IJPM.IJPM_589_16. [DOI] [PubMed] [Google Scholar]
- 3.Hendry SA, Wong DD, Papadimitriou J, Robbins P, Wood BA. Superficial CD34-positive fibroblastic tumour: report of two new cases. Pathology. 2015;47:479–482. doi: 10.1097/PAT.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 4.Lao IW, Yu L, Wang J. Superficial CD34-positive fibroblastic tumour: a clinicopathological and immunohistochemical study of an additional series. Histopathology. 2017;70:394–401. doi: 10.1111/his.13088. [DOI] [PubMed] [Google Scholar]
- 5.Wada N, Ito T, Uchi H, Nakahara T, Tsuji G, Yamada Y, Oda Y, Furue M. Superficial CD34-positive fibroblastic tumor: a new case from Japan. J Dermatol. 2016;43:934–936. doi: 10.1111/1346-8138.13327. [DOI] [PubMed] [Google Scholar]
- 6.Pajaziti L, Hapciu SR, Pajaziti A. Juvenile xanthogranuloma: a case report and review of the literature. BMC Res Notes. 2014;7:174. doi: 10.1186/1756-0500-7-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zambo I, Veselý K. WHO classification of tumours of soft tissue and bone 2013: the main changes compared to the 3rd edition. Cesk Patol. 2014;50:64–70. [PubMed] [Google Scholar]
- 8.Yamaga K, Fujita A, Osaki M, Osaki M, Kuwamoto S, Ishiguro N, Yamamoto T, Nagashima H. Detailed analysis of a superficial CD34-positive fibroblastic tumor: a case report and review of the literature. Oncol Lett. 2017;14:3395–3400. doi: 10.3892/ol.2017.6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamada T, Katsuki N, Hosokawa Y, Ayano Y, Ikeda M. Additional case of superficial CD34-positive fibroblastic tumor in a Japanese patient. J Dermatol. 2019;46:e134–e136. doi: 10.1111/1346-8138.14629. [DOI] [PubMed] [Google Scholar]
- 10.Batur S, Ozcan K, Ozcan G, Tosun I, Comunoglu N. Superficial CD34 positive fibroblastic tumor: report of three cases and review of the literature. Int J Dermatol. 2019;58:416–422. doi: 10.1111/ijd.14357. [DOI] [PubMed] [Google Scholar]
- 11.Lin TL, Yang CS, Juan CK, Weng YC, Chen YJ. Superficial CD34-positive fibroblastic tumor: a case report and review of the literature. Am J Dermatopathol. 2020;42:68–71. doi: 10.1097/DAD.0000000000001355. [DOI] [PubMed] [Google Scholar]
- 12.Malhotra B, Schuetze SM. Dermatofibrosarcoma protruberans treatment with platelet-derived growth factor receptor inhibitor: a review of clinical trial results. Curr Opin Oncol. 2012;24:419–424. doi: 10.1097/CCO.0b013e328353d78d. [DOI] [PubMed] [Google Scholar]
- 13.El Hachem M, Diociaiuti A, Latella E, Zama M, Lambiase C, Giraldi L, Surrenti T, Callea F. Congenital myxoid and pigmented dermatofibrosarcoma protuberans: a case report. Pediatr Dermatol. 2013;30:e74–77. doi: 10.1111/pde.12131. [DOI] [PubMed] [Google Scholar]
- 14.Israel MS, Carlos R, Pires FR. Oral juvenile xanthogranuloma: report of two cases. Pediatr Dent. 2017;39:238–240. [PubMed] [Google Scholar]
- 15.Mentzel T, Requena L, Brenn T. Atypical fibroxanthoma revisited. Surg Pathol Clin. 2017;10:319–335. doi: 10.1016/j.path.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Soleymani T, Aasi SZ, Novoa R, Hollmig ST. Atypical fibroxanthoma and pleomorphic dermal sarcoma: updates on classification and management. Dermatol Clin. 2019;37:253–259. doi: 10.1016/j.det.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Neagu TP, Sinescu RD, Enache V, Achim SC, Ţigliş M, Mirea LE. Metastatic high-grade myxofibrosarcoma: review of a clinical case. Rom J Morphol Embryol. 2017;58:603–609. [PubMed] [Google Scholar]
- 18.Haglund KE, Raut CP, Nascimento AF, Wang Q, George S, Baldini EH. Recurrence patterns and survival for patients with intermediateand high-grade myxofibrosarcoma. Int J Radiat Oncol Biol Phys. 2012;82:361–367. doi: 10.1016/j.ijrobp.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 19.Bahrami A, Folpe AL. Adult-type fibrosarcoma: a reevaluation of 163 putative cases diagnosed at a single institution over a 48-year period. Am J Surg Pathol. 2010;34:1504–1513. doi: 10.1097/PAS.0b013e3181ef70b6. [DOI] [PubMed] [Google Scholar]