Abstract
In this work, the recent advances for rapid prototyping in the orthoprosthetic industry are presented. Specifically, the manufacturing process of orthoprosthetic aids are analysed, as thier use is widely extended in orthopedic surgery. These devices are devoted to either correct posture or movement (orthosis) or to substitute a body segment (prosthesis) while maintaining functionality. The manufacturing process is traditionally mainly hand-crafted: The subject’s morphology is taken by means of plaster molds, and the manufacture is performed individually, by adjusting the prototype over the subject. This industry has incorporated computer aided design (CAD), computed aided engineering (CAE) and computed aided manufacturing (CAM) tools; however, the true revolution is the result of the application of rapid prototyping technologies (RPT). Techniques such as fused deposition modelling (FDM), selective laser sintering (SLS), laminated object manufacturing (LOM), and 3D printing (3DP) are some examples of the available methodologies in the manufacturing industry that, step by step, are being included in the rehabilitation engineering market—an engineering field with growth and prospects in the coming years. In this work we analyse different methodologies for additive manufacturing along with the principal methods for collecting 3D body shapes and their application in the manufacturing of functional devices for rehabilitation purposes such as splints, ankle-foot orthoses, or arm prostheses.
Keywords: rapid prototyping, additive manufacturing, orthoses, prostheses, fused deposition modeling, laminated object manufacturing, selective laser sintering
1. Introduction
Assistive technologies, such as orthotic or prosthetic devices, have existed for many centuries. Orthotic devices have been widely used not only to provide immobilization, support, correction, or protection, but also to treat musculoskeletal injuries or dysfunctions [1]. In the 1970s, new techniques like plastic coating were developed, due to the demand of orthotic devices with a more attractive appearance, by applying a tinted rubber-based plastic film [2], allowing the improvement of orthoses appearance and comfort. In the early 1980s the rise of additive manufacturing technologies (AMT), popularly known as 3D printing technologies in a manufacturing environment, with the introduction of the stereolithography technique, based on the cure of photopolymer resin in thin layers with a UV laser allowed construction of 3D models. In the following years, other AMT were introduced, such as: fused deposition modeling (FDM), laminated object manufacturing (LOM), selective laser sintering (SLS), 3D printing, and variable rapid prototyping (Polyjet Technology), among others.
The AMTs are included in the field of rapid prototyping techniques (RPT), producing fully functional parts directly from a three-dimensional model without a machining process. A radical change in manufacturing of orthotic devices is already happening due to the exponential growth of RPT over recent decades [3,4]. A quick search in Scopus of 3D printing provides only 122 results before year 2000, 303 results between 2001 and 2005, 756 results from 2006 and 2010, 4521 results for the period 2011–2015, and 22,513 results in the last five years. In the biomedical engineering context, the developments have evolved quickly due to the need for individualized devices able to adapt properly to the patient’s anatomical shapes [5,6,7,8,9]. For this reason, RPT may be helpful in the orthoprosthetic industry, as these devices must adapt perfectly to the body, not only to accomplish their rehabilitative function, but to avoid disuse, as many of these devices produce blistering, ulcers, or discomfort [10,11]. These techniques have been already applied to the manufacturing of spinal braces [11,12], exoskeleton parts [13,14], and passive orthoses [15,16,17], and the application in the medical and dental industry represents one of largest serving industries in the world [18]. Moreover, RPT offer advantages in the design of custom orthotic devices (Figure 1): The orthotic and prosthetic devices are highly customizable, as in Zuniga et al. [19], it is possible to fit the devices to complex geometrical features, with high accuracy, and these devices are manufactured efficiently in terms of cost, lead-time, and product quality [20].
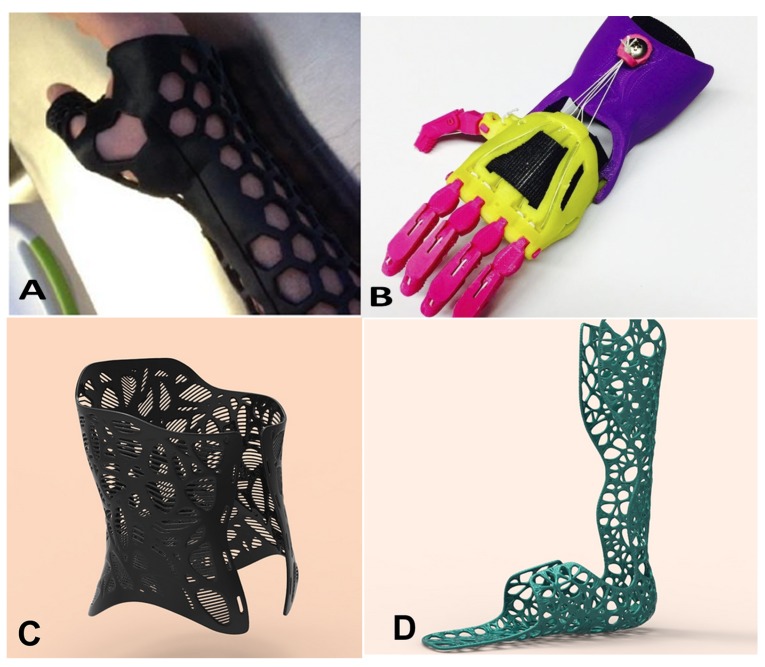
Figure 1.
Examples of 3D printed orthotics (a) Forearm static fixation (courtesy of Fitzpatrick et al. [21]). (b) Cyborg beast hand prosthesis—a low-cost 3D-printed prosthetic hand for children licensed under the CC-BY-NC license (courtesy of Zuniga et al. [19]). (c) Spinal brace (courtesy of Andiamo company [22]). (d) Ankle-foot orthosis (courtesy of Andiamo company [22]).
Currently, most rehabilitation devices are designed and hand-crafted by orthopaedists. Therefore, the quality of the product depends on the specialists’ skills and experience [23]. The manufacturing process requires time and depends on the expertise of the specialist to obtain products with functional features that match the unique gait dynamics of each subject. Thus, the need for custom-made products such as orthoses and assistive devices is an explicit need considering the evolution of the technology during the beginning of this century [6,24,25].
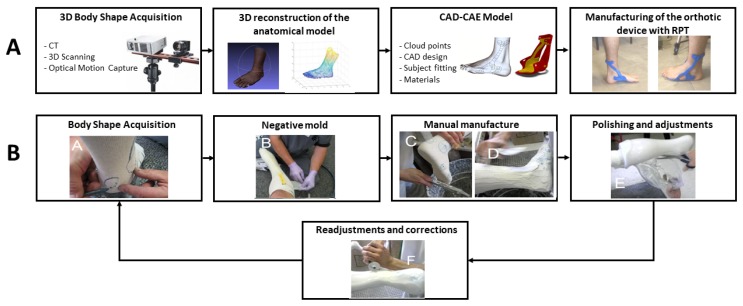
Regarding orthoprosthetic manufacturing, the first step is to acquire the morphology of the body segment. In the traditional manufacturing process, the subject’s morphology is usually acquired by means of foam or plaster moulds. A prototype is obtained by using a computerized numerical control (CNC) or a milling machine in the thermosetting polyurethane model obtained by the mould. Lastly, the specialist performs several modifications in the device to adjust it to the subject (Figure 2a). However, CNC and milling present some limitations as they are not able to reproduce complex surface designs or to deal with different thickness and materials.
Figure 2.
Phases of the manufacturing process of custom-fit orthotic devices. (A) Rapid prototyping techniques (RPT) methodology (courtesy of J. Barrios-Muriel). (B) Traditional methodology (courtesy of Mavroidis et al. [26] under CC-BY License.) Computer aided design (CAD)-computed aided engineering (CAE), computed tomography (CT).
On the contrary, in the new RPT approach, the manufacturing process (Figure 2b) begins with the acquisition of subject’s morphology by means of 3D scanning technologies. Then, computer aided design (CAD)-computed aided engineering (CAE) tools are applied to obtain subject specific designs; whereas, functionality is studied by testing different materials and structures. Lastly, the design is easily exported to an additive manufacturing machine where the prototype is obtained. Manufacturing time may vary between several weeks in the traditional process, to a couple of days in the RPT approach. Therefore, the use of RPT, together with the new 3D acquisition methodologies, represents an alternative in the orthoprosthetic industry.
Many other applications of additive manufacturing (AM) and RPT are in the field of manufacturing of medical instruments [27], drug delivery systems [28], engineered tissues [29,30], scaffolds for bone regeneration [31,32], dental implants [33,34], prosthetic sockets, [35] or surgery [36,37,38]. In this work, we present a review of the developments in the manufacturing of orthotic devices, especially those related to the use of RPT to improve the quality and manufacture times in the rehabilitation field, as in splints, ankle-foot orthoses, or arm prostheses. This review provides comprehensive coverage on the different methodologies ready to be used in the orthotic and prosthetic industry. New 3D data acquisition techniques and the use of different materials are also referred to. This work is intended to be a reference guide on the techniques in this field for practitioners, but also for experienced readers who are interested in pursuing further research.
2. 3D Anatomical Data Acquisition Technologies
Applications of RPT combined with different techniques for measuring and modelling the human body are useful to generate new criteria for the orthotic device design. Depending on the data acquisition method used, the data can be expressed as a point cloud, voxels (3D volumetric pixels), or three dimensional coordinates of different anatomical points. Up to date, there is no standardized morphology acquisition procedure, however, there are several acquisition methods to support fabrication using RPT within the field of orthotic devices modelling, including computer tomography, 3D scanning, and different optical motion capture systems.
2.1. Computed Tomography
Computed tomography (CT) is a powerful technique to facilitate diagnostics and for surgical planning. Traditionally, the recorded images were in the axial or transverse plane. Currently, modern scanners record images along different planes, enabling volumetric reconstructions for 3D representations. Several studies have applied CT for the manufacture of orthotic devices. For example, Tang et al. [39] recently proposed the use of CT combined with AM techniques to manufacture insoles for diabetes. In their work, they studied pressure and tissue strain along the plantar foot to correlate these variables with the therapeutic effect of footwear and custom-made orthotic inserts, being able to reduce peak plantar pressure by 33.67%. Artioli et al. [40] studied the use of different acquisition techniques to manufacture 3D printed silicone ear prostheses, concluding that the use of CT and AM (using polylactic acid or polylactide, PLA, resolution 100 m) produce differences of 0.1% between the manufactured prosthesis and the objective model. Liacouras et al. [41,42] used CT to acquire the morphology of the patients stump and to develop the strategies for designing transtibial prosthetic sockets. Moreover, the data of CT allowed a finite element analysis of the prosthetic model socket to calculate the structural stresses and strain at the sockets, as well as the contact pressure at the fibula head. The high image resolution between tissues is one of the greatest advantages of CT, along with the capacity to improve contrast and reduce noise. However, several drawbacks are worthy of mentioning. Radiation is the main concern and the exposition is directly proportional to the duration of the scanning. Other drawbacks are the partial pixel effect, leading to a blurred boundary, as the different densities share common pixels [43].
2.2. 3D Scanning
To capture human topography or the external shape, 3D scanning arise as the most practical and comfortable solution. 3D scanning systems use light based techniques to determine the three-dimensional position in space of the different points that integrate the surface of an object. Computer software is then used to reconstruct surfaces from the point cloud and then, the CAD model is obtained.
Currently, 3D scanners for human measurement are available, including the use of single image for reconstruction, structured light technologies, lasers, and different algorithms for stereo reconstruction [44,45,46]. The most common technologies used to reconstruct human body shape are laser and structured light technologies [44]. The laser technique uses a projected laser dot or line from a hand- held device. A sensor measures the distance to the surface, typically a charge-coupled device or a position sensitive device. For static objects, data is collected in relation to an internal coordinate system and, for dynamic conditions, the position of the scanner must be determined to correctly define the point cloud [47]. Structured light methods use a projector-camera system with pre-defined light patterns projected on the moving object. However, a drawback of this technology is the inability to capture certain topography sections of human anatomy with intricate creases and folds, such as between fingers when the hand is in a neutral position, the back of the knee when flexed, or the armpits. The gathered information is more precise, however, and noise is reduced. Recent techniques explore the feasibility of 4D acquisition [48], but to the best knowledge of the authors, there is no report on its use for the design of orthotic and prosthetic devices yet.
Processing time is significantly reduced compared to magnetic resonance imaging (MRI) and CT, as well as the size of the data files [44]. The use of MRI and CT is mostly used to reconstruct of internal organs or tumours with high accuracy for surgical guidance [49]. Recording time and resolution may vary between different 3D scanners, ranging from 3–5 min and a tenth of millimetres for high accuracy systems to a couple of minutes [50] and millimetres for low cost systems [51]. Other advantages of 3D scanning methods are affordable hardware and software, minimal training requirement, availability, accessibility, and efficiency [44].
Several authors suggest the use of reverse engineering software to obtain a refined model by repairing the data. In the pioneering work of Chee Kai et al. [52], a 3D scanning method was selected over the traditional methods for prosthesis modeling, such as plaster-of-Paris impressions, MRI, and CT. Mavroidis et al. [26] used 3D laser scanning to create patient-specific foot orthoses. Surface data of the patient anatomy was manipulated to an optimal form using computer aided design (CAD) software and was fabricated using a rapid prototyping machine. The prototype properly fit the subject’s anatomy compared to a commercial ankle foot orthosis. Paterson [53] investigated the 3D anatomical data acquisition methods to establish a clinically valid, standardized method. He concluded that laser scanning appears to be the most suitable method to reduce the acquisition of ambiguous data and with a high performance in terms of cost, resolution, speed, accuracy, patient safety, cost, and overall efficiency. More recent works, such as those of Mali and Vasistha [54] or Agudelo-Ardila et al. [55], present efficient solutions for the manufacturing of lower and upper limb orthoses, respectively, using reverse engineering.
2.3. Optical Motion Capture System
Recently, techniques to measure the topography of the human body in dynamic movements are receiving attention, as the design of orthotic devices should not be designed only for static conditions, as most of them will be used in dynamic conditions to increase rehabilitation. As previously mentioned, there are many commercial 3D systems that are able to measure 3D shapes with high accuracy; however, most of them cannot acquire human motion. An optical motion capture system is a popular technology to capture human movement. These optical systems use several cameras recording in 2D to reconstruct the 3D position of a set of reflective markers placed in anatomical landmarks. These markers should be seen by two or more cameras calibrated to provide overlapped projections. However, the optical motion capture system has a drawback due to the strong limitations related to the number and density of markers [56]. Although only three markers are needed to reconstruct each body segment as a rigid body (e.g., to perform a kinematic analysis), this number is increased if body shape must be also retrieved. The number usually depends on the camera resolution but it does not exceed 60–80 markers per body segment. The main application of the marker-based acquisition technology is in the assessment of the manufactured devices due to the standardized protocols to acquire kinematics [57].
Research has also been focused into structured light using this method [58]. Unkovskiy et al. [59] used a portable structured light scanner to retrieve the topology of the nose cavity and the face to design and manufacture a prosthetic nose. A portable projector was used to project in the region of interest an arbitrary light pattern with a colour code. The system performs the triangulation between the projection pattern and the camera image and to retrieve the correspondence between images. The advantage of this system is the noise reduction compared to the video capture image. The matching of the stereo projection pattern and the camera recorded image is less affected by noise than multiple stereo matching of camera images. Regretfully, synchronous measurement of the entire body shape using multiple projector-camera systems has not been reported yet using this technology. This is essential for capturing the 3D shape motion and to analyse, in the case of gait for example, foot width, length, circumferences, and arch changes during movement [60]. However, there is no commercial system including this technology and thus, more studies should be performed with different methods to compare accuracy and precision between these technologies. The cutting edge technologies in this field are the markerless systems. Chatzitofis et al. [61] proposed recently a low-cost robust and fast system to acquire body kinetics. Although this work still uses reflective straps it could be combined with 4D scanning systems, such as those proposed by Joo et al. [62], to obtain accurate topology and motion of the body segment to rehabilitate.
3. Rapid Prototyping Technologies for Orthotic Devices
The combination of CAD and computed aided manufacturing (CAM) is a well-known approach that is receiving increasing attention in the field of orthotic devices to replace the traditional craft practices. As stated by Ciobanu et al. [63], customized manufacturing through RPT requires: 3D scanning of the anatomic surface, 3D surface reconstruction, CAD modeling, conversion to stereolithography format (STL) and, lastly, machining using a special rapid prototyping machine (i.e., a 3D printer) controlled through computer. RPT offer advantages in manufacturing processes of custom-fit orthotic devices, in terms of greater design freedom, ability to create functional elements, superior accuracy and cost efficiency, shorter delivery time, and better user experience of the final product.
In an RPT manufacturing process, a representative virtual 3D CAD model is formed layer upon layer to form a physical object [9]. In RPT, a virtual model of the part is designed through CAD and is converted to a STL file format, which is the default standard file format for RP systems [64,65]. AMT can be categorized in different ways depending on the nature of the fabrication process, such as laser, printer technology, and extrusion technology [7]. There are many different AM processes. Kruth [66] proposed in the early 90’s the use of different methodologies for additive manufacturing, classified according to the material used for the prototype (see Table 1). Nevertheless, Paterson et al. [67] demonstrated that only a few of them could be used to manufacture orthotic and prosthetic devices.
Table 1.
Rapid prototyping techniques available for orthoprosthetics.
| Material | Process |
|---|---|
| Liquid base | Stereolithography (SLA) |
| Solid ground curing (SGC) | |
| UV light-curing (ULC) | |
| Ballistic particle manufacturing (BPM) | |
| Solid base | Laminated object manufacturing (LOM) |
| Fused deposition modeling (FDM) | |
| Powder base | Selective laser sintering (SLS) |
| 3D printing (Polymer injection) |
Liquid based process, such as stereolitography (SLA), solid ground curing (SGC), UV light curing (ULC), and ballistic particle manufacturing (BPM); or solid based process, such as laminated object manufacturing (LOM) are methodologies used in the manufacturing of orthoses and prostheses. Nevertheless, the most used methodologies to manufacture orthotic and prosthetic devices are fused deposition modelling (FDM), selective laser sintering (SLS), and powder bed and inkjet head 3D printing (3DP). These methodologies represent an optimized trade-off between cost, delivery time, accuracy, and comfort.
3.1. Fused Deposition Modeling (FDM)
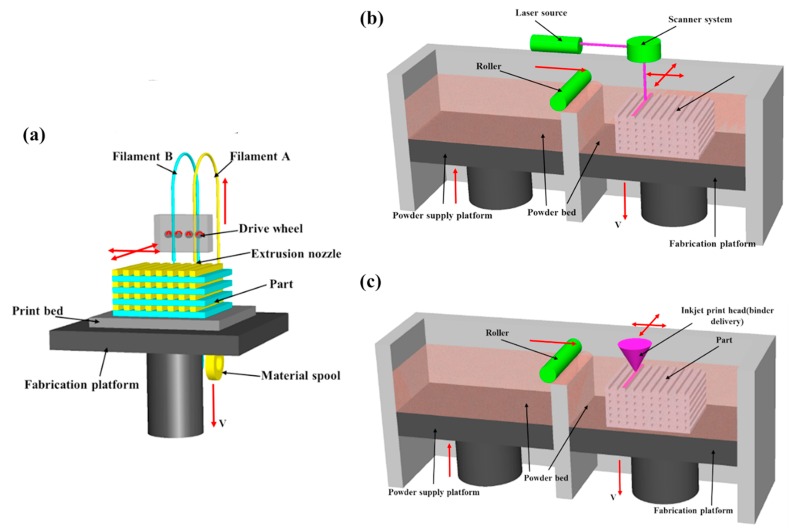
In the FDM process (see Figure 3a), a semi-molten material is extruded through an extrusion head that traverses in the X and Y axes to create each two dimensional layer of the piece to be manufactured. Two extrusion nozzles compose the movable extrusion head: one to deposit the build material and the other one that contains the support material [68].
Figure 3.
Comparison of the proposed schemes dor rapid prototyping. (a) Fused deposition modeling (FDM). (b) Selective laser sintering (SLS). (c) 3DP. Image adapted from Wang et al. [74] with permission of Elsevier Ltd.
In general, the perimeter of each layer is extruded first and then the delimited zone by the previous extrusion is filled by the extruder head by following a pre-defined pattern [68]. Once the layer is completed, the support platform lowers and another layer is extruded. The process continues layer by layer until the piece is complete.
The most common materials for FDM are polycarbonate (PC) and Acrylonitrile butadiene styrene (ABS) or a mixture of them. These materials have similar properties to thermoplastic material for injection moulding [69]. Other materials such as polymers or nylon-based materials may be used. The main advantage of FDM technology is in the use of low-cost materials. Tan et al. [70] were pioneers in the use of used FDM for tibial prosthesis manufacturing and concluded that the functional characteristics of prosthesis were valid for clinical purposes. On the contrary, manufacturing times are high. Since then, an increasing number of applications have arisen for FDM in the biomedical field for upper [17] and lower limb orthoses [50,71], hand prostheses [19], facial prosthesis [40,72], and drug delivery systems [73].
3.2. Selective Laser Sintering (SLS)
The DTM Corporation (now a part of 3D Systems) introduced the first SLS system in the 90s [75]. The SLS technique creates three-dimensional solid objects or parts by selectively fusing powdered polymer-based materials such as nylon/polyamide with a CO laser, turning powder material into solid objects (Figure 3b). A CO laser selectively sinters defined regions by traversing across the powder bed in the X and Y-axes to form a 2D profile [76]. Once the 2D profile has been completed, the platform lowers, a new layer of powder is distributed and the sintering process is repeated. Subsequently, the process is termed a powder-based fusion process. In general, all materials used are thermoplastics, the most common being polyamide 12 (PA), acrylonitrile butadiene styrene (ABS), and polycarbonate (PC) [77]. These materials lead to a considerable weight reduction improving the usability of the rehabilitation devices.
As an example of the use of this technology in the manufacturing of custom orthoses, Schrank and Stanhope [10] evaluated the accuracy of SLS manufacturing process of ankle foot orthoses (AFO). In this work the discrepancy between the CAD model and final product manufactured with SLS was measured through the Faroarm 3D scanner (accuracy ±25 m). The results showed values below 1.5 mm (SD = 0.39 mm). Deckers et al. [78] developed and tested an SLS-based AFO, highlighting the need to properly characterize the mechanical characteristics of the AFO such as strength, fatigue, and resistance to impacts. Vasiliauskaite et al. [79] tested a polyamide-based orthoses manufactured with SLS and concluded that the features were similar to a thermoformed polypropylene orthosis, the first one being stiffer than the second but enough for the purpose of rehabilitation.
On the other hand, [80] manufactured a splint for upper extremities using the SLS method. They also concluded that the results of this manufacturing technique were good but did not make any clinical validation of the device. Another similar application of splints using SLS is the design proposed by Evill [81]. In this work, several aspects such as ventilation, hygiene, and aesthetics were improved through CAD. Although there are not conclusive results that confirm these purposes, the new design parameters considerably improve comfort.
3.3. Powder Bed and Inkjet Head 3D Printing: 3DP
3DP refers to three dimensional printing, which is understood as the process in which the manufactured product is made by means of powder layers stuck with adhesive. In this process, first, a powder layer is spread on the build platform. Second, a liquid binder is deposited selectively through an inkjet printhead by following a patterned layer in the XY plane. Once the 2D pattern is formed, the platform lowers, the next powder layer is spread and so on. This process is sometimes referred as powder bed and inkjet head 3D printing (or 3DP). It should not be confused with the widespread definition of 3D printing, that involves all additive manufacturing process that result in the manufacturing of tree-dimensional objects. The 3DP process is somewhat similar to SLS (see Figure 3b,c): In 3DP, a printing head places liquid adhesive in the material; whereas, in SLS a CO laser is used to fuse the layers. The accuracy of this process is lower than in SLS; nonetheless, this method is preferred due to its low cost and quickness. These qualities have lead 3DP to have a predominant role in the prototyping industry.
The employed materials (mainly thermoplastics as ABS) have the required properties to be used in orthotic and prosthetic applications. Herbert et al. [82] investigated whether this technology was suitable to produce functional prosthesis, and they suggested that, although the manufacturing levels were limited, patients felt more comfortable with prostheses made with 3DP machine (Corporation Z402) than the traditional handmade ones. Regretfully, the resistance was not studied in that work and therefore the durability of the product is unknown. Saijo et al. [83] used this technology to develop patient-specific maxilofacial implants reporting a reduction in operation times. As stated by Ventola [84], 3DP is particularly interesting in tissue engineering and regenerative medicine because of its digital precision, control, and versatility.
To summarize, Table 2 shows a comparison of the different RPT by using the commercial models used in the works described in the bibliography.
Table 2.
Characteristics of the most used machines for AMT.
| FDM | SLS | 3DP | |
|---|---|---|---|
| Model | Dimension STT 768 | spro 60 SD SLS | uPrint System |
| Production Time (h) | 7 | 3 | 7 |
| Active volume (mm) | 203 × 203 × 305 | 381 × 330 × 457 | 203 × 152 × 152 |
| Material | ABS P400 | Duraform PA (Nylon 12) | ABS P430 |
| Material consumption (g) | 40 | 20.15 | 55 |
| Cost ($/kg) | 190 | 90 | 30 |
4. Variable Property Rapid Prototyping
Variable rapid prototyping differs from other AMT as it aims at producing objects of varied properties. In this sense, each material used to manufacture the object provides specific values of strength, strain, heat deflection temperature, etc. [85]. Object geometries recently introduced 3D printers that use polyjet MatrixTM technology to allow the generation of composite material prototypes of varying stiffness and dual material prototypes. The Connex500TM 3D printer operates by using inkjet heads with two or more photopolymer materials. The material is extruded in 16 m thick layers. Each photopolymer layer is cured by UV light immediately after the extrusion [86].
A carpal skin was produced by Oxman [80], exploring the multiple material building capabilities available with this technology. Campbell et al. [87] explored the benefits of multiple materials integrated into a wrist splint compared to traditional custom-fitted wrist splints of qualified and experienced clinicians. The work focused on the attempt to place multiple materials to behave as hinges or cushioned features as opposed to traditional fabrication processes where a similar approach would be very difficult to replicate. A drawback for this technology is that the actual commercial CAD software is not efficient to apply the design potential and few computation tools manage the physical interaction between material properties. Nevertheless, recent advances in the use of additive manufacturing of hybrid composites, as recently presented for dental implants [88], may lead to a substantial revolution in the field of orthotics, were hybrid exoskeletons are currently changing from rigid structures to wearable garments.
5. Material Selection for Orthotic Devices
The choice of material when designing an orthotic device is vital to its success. Physical properties of the orthotic materials include their elasticity, hardness, density, response to temperature, durability, flexibility, compressibility, and resilience [89]. It should be mentioned that each physical property cannot be used alone as a single factor for assessing materials for orthotic devices. A hard material as well as an incorrect aspect in the design may result in an uncomfortable device or a biomechanically detrimental orthotic device. Thermoplastics, composites, and foams are the main materials used to manufacture orthotic devices through AMT [89].
The most known materials used in rapid prototyping manufacturing are ABS (Acrylonitrile butadiene styrene) and PLA (polylactic acid) [90]. ABS is a polymer commonly used to produce car bumpers due to its toughness and strength. PLA is a biodegradable thermoplastic that has been derived from renewable resources such as starch prepared from the grains of corn. These materials are used for the majority 3D printing machines. Rigid and semi-rigid structures can be manufactured with these materials. Depending on the designed thickness, these materials may have different properties.
Soft parts and some semi-rigid parts of orthotic devices are made with foamed materials, usually with open or closed cell structure. The first type allows the movement of gas between the cells whereas the second encloses the gas within the cell walls allowing for a water-tight material. This is desirable for orthotic manufacture as sweat will not be able to penetrate into the material to cause premature degradation. Paton et al. [91] investigated the physical properties of soft materials used to fabricate orthoses designed for the prevention of neuropathic diabetic foot ulcers. They concluded that the most clinically desirable dampening materials tested were Poron®96 and Poron®4000 (thickness of 6 mm) and the material with the best properties for motion control was ethylene vinyl acetate (EVA). With the evolution of AMT, insoles with variable porous structure and adjustable elastic modulus are being manufactured to adapt to the different needs of patients with diabetes [92].
In the SLS manufacture process, polymer powders and ceramics are mixed to form composites. RilsanTM D80 DuraFormTM PA and DuraFormTM GF are examples of materials used in SLS techniques. Faustini et al. [93] explored the feasibility of using RPT for AFO manufacturing process. The study determined that the optimal SLS material for AFO to store and release elastic energy was RilsanTM D80, considering minimizing energy dissipation through internal friction is a desired material characteristic. A relatively recent work from Walbran et al. [94] compares yield stress of custom made orthoses made by SLS of nylon with carbon fibres and FDM with PLA. Although PLA was chosen in terms of mechanical properties and cost, they concluded that the AMT have a strong potential not only to obtain consistent and repeatable models of the subjects affected limb independently of the operator’s skill and restraint of the subject, but to further automate the AFO design process.
Materials used in FDM are those with similar properties to thermoplastic materials for injection moulding. In general polycarbonate (PC) and ABS or their combinations, as mentioned above (PC-ABS, PC-ISO or ABSi), nylon-based materials and other polymers can be used. The main advantage of these materials is the low cost. Finally, in the case of variable properties rapid prototyping base materials, digital materials or composite digital materials can be used. The material properties can be modified by combinations and distribution of different types.
6. Discussion
The traditional manufacturing processes for orthotic and prosthetic devices is still mostly hand-crafted and requires special abilities from the othopaedist to obtain a quality product. Nevertheless, this manufacturing process, in general, produces discomfort to the patient. The acquisition of the morphology of the subject is not a clean process as the use of plaster is required to obtain the mould. Additionally, the final product may produce blistering on the subject’s skin, as the morphology is acquired in static conditions.
Alternatively, RPT and AMT have a strong potential to change not only the way in which orthotic and prosthetic products are designed, but also the manufacturing process and the specialist profile. The use of RPT in the orthoprosthetic industry may suppose a considerable change in the know-how; however, it also leads to important benefits. The goal is to accelerate the reconstruction process of 3D anatomical models and biomedical objects for the design and manufacturing of medical products and simulate 3D body shape to design the most suitable orthoses for the patient. Thus, the use of CAD and RPT facilitate the design and fabrication of custom-fit orthotic products with a number of advantages over traditional methods: the use of new materials, customized designs, virtual testing, etc.
The application of these technologies may lead to a significant improvement in the orthotic manufacturing process as production times are lower, morphology acquisition is faster and more pleasant for the patient, as plaster moulds are suppressed and manufacturing errors are minimized. Considerable effort has been applied in the application of AMT to the medical industry and specifically in the design and manufacturing of orthoses and prostheses for rehabilitation purposes [89], to mitigate the effects of aging [95], in the design of active wearable exoskeletons [96], and also to bring this technology closer to the general public [19,97].
The inclusion of these manufacturing methods requires a high investment in equipment, materials, and training that may cause hesitation from investors or orthotic and prosthetic technicians. Moreover, healthcare specialists show some reservations to change their own work routines [86]. Nevertheless, the eruption of 3D printers into the market and the continuous improvements made in this field will give an impulse to the implementation of this technology in the orthoprosthetic industry. In addition, the experience shown in other medical fields, such as in dental implant manufacturing will make possible the implementation of this technology to produce orthoses and prosthesis to reduce waiting lists.
The mass production of these aids must include a series of key points leading to high quality patterns in the manufacturing process. These key points are: acquisition of the subject’s anthropometric data, product design, material selection, manufacturing process planning, and product service, among others. The correct application of these steps reduces the total production time and, therefore, the delivery times. Thus, the inclusion of RPT to produce high quality and short delivery time orthotic and prosthetic devices, that also satisfies functionality and patients comfort is now a reality. Finally, the inclusion of manufacturing technology in a traditional environment such as the orthoprosthetic industry will lead to better products to satisfy the specifications required in rehabilitation.
7. Conclusions
In this work, a review of the different RPT applied to the orthoprosthetic industry has been presented. Specifically, the manufacturing process to manufacture orthoses and prostheses have been analysed and the main works in this field have also been presented. These techniques have been shown to have an exponential growth in the following years in the biomedical field. The new advances in the subject’s morphology acquisition as well as the use of RPT can improve the accuracy of the final device, leading to a better rehabilitation process. RPT will help us to optimize the manufacturing process and improve both the design and functionality of assistive devices. Thus, RPT combined with CAD-CAM tools provide a major control in the design and manufacture processes. Finally, the future lines of development in this field will be based on the design of new structures and materials to improve comfort, which will grant the success of the new orthoprosthetic aids.
Author Contributions
Conceptualization, J.B.-M. and F.R.-S.; Formal analysis, J.B.-M.; Funding acquisition, F.J.A.-S. and D.R.S.; Methodology, J.B.-M. and F.R.-S.; Project administration, F.J.A.-S. and D.R.S.; Supervision, F.R.-S., F.J.A.-S. and D.R.S.; Writing—original draft, J.B.-M.; Writing—review & editing, F.R.-S., F.J.A.-S. and D.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Consejería de Economía e Infraestructuras de la Junta de Extremadura and the European Regional Development Fund “Una manera de hacer Europa” under project IB18103.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Webster J. Atlas of Orthoses and Assistive Devices. Elsevier; Amsterdam, The Netherlands: 2019. [Google Scholar]
- 2.Meyer P.R., Jr. Lower limb orthotics. Clin. Orthop. Relat. Res. 1974;102:58–71. doi: 10.1097/00003086-197407000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Mikołajewska E., Macko M., Szczepański Z., Mikołajewski D. Encyclopedia of Information Science and Technology. 4th ed. IGI Global; Hershey PA, USA: 2018. Reverse Engineering in Rehabilitation; pp. 521–528. [Google Scholar]
- 4.Jiang R., Kleer R., Piller F.T. Predicting the future of additive manufacturing: A Delphi study on economic and societal implications of 3D printing for 2030. Technol. Forecast. Soc. Chang. 2017;117:84–97. doi: 10.1016/j.techfore.2017.01.006. [DOI] [Google Scholar]
- 5.Singh S., Ramakrishna S. Biomedical applications of additive manufacturing: present and future. Curr. Opin. Biomed. Eng. 2017;2:105–115. doi: 10.1016/j.cobme.2017.05.006. [DOI] [Google Scholar]
- 6.Gibson I., Srinath A. Simplifying medical additive manufacturing: Making the surgeon the designer. Proced. Technol. 2015;20:237–242. doi: 10.1016/j.protcy.2015.07.038. [DOI] [Google Scholar]
- 7.Thompson M.K., Moroni G., Vaneker T., Fadel G., Campbell R.I., Gibson I., Bernard A., Schulz J., Graf P., Ahuja B., et al. Design for Additive Manufacturing: Trends, opportunities, considerations, and constraints. CIRP Ann. 2016;65:737–760. doi: 10.1016/j.cirp.2016.05.004. [DOI] [Google Scholar]
- 8.Espalin D., Arcaute K., Rodriguez D., Medina F., Posner M., Wicker R. Fused deposition modeling of patient-specific polymethylmethacrylate implants. Rapid Prototyp. J. 2010;16:164–173. doi: 10.1108/13552541011034825. [DOI] [Google Scholar]
- 9.Lantada A.D., Morgado P.L. Rapid Prototyping for Biomedical Engineering: Current Capabilities and Challenges. Ann. Rev. Biomed. Eng. 2012;14:73–96. doi: 10.1146/annurev-bioeng-071811-150112. [DOI] [PubMed] [Google Scholar]
- 10.Schrank E.S., Stanhope S.J. Dimensional accuracy of ankle-foot orthoses constructed by rapid customization and manufacturing framework. J. Rehabil. Res. Dev. 2011;48:31–42. doi: 10.1682/JRRD.2009.12.0195. [DOI] [PubMed] [Google Scholar]
- 11.Cobetto N., Aubin C.E., Clin J., Le May S., Desbiens-Blais F., Labelle H., Parent S. Braces Optimized With Computer-Assisted Design and Simulations Are Lighter, More Comfortable, and More Efficient than Plaster-Cast Braces for the Treatment of Adolescent Idiopathic Scoliosis. Spine Deform. 2014;2:276–284. doi: 10.1016/j.jspd.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Redaelli D.F., Storm F.A., Biffi E., Reni G., Colombo G. International Conference on Design, Simulation, Manufacturing: The Innovation Exchange. Springer; Berlin, Germany: 2019. A Virtual Design Process to Produce Scoliosis Braces by Additive Manufacturing; pp. 860–870. [Google Scholar]
- 13.Bourell D., Stucker B., Cook D., Gervasi V., Rizza R., Kamara S., Liu X. Additive fabrication of custom pedorthoses for clubfoot correction. Rapid Prototyp. J. 2010;16:189–193. doi: 10.1108/13552541011034852. [DOI] [Google Scholar]
- 14.Langlois K., Moltedo M., Bacek T., Rodriguez-Guerrero C., Vanderborght B., Lefeber D. Design and development of customized physical interfaces to reduce relative motion between the user and a powered ankle foot exoskeleton; Proceedings of the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob); Enschede, The Netherlands. 26–29 August 2018; pp. 1083–1088. [Google Scholar]
- 15.Gibson K.S., Woodburn J., Porter D., Telfer S. Functionally Optimized Orthoses for Early Rheumatoid Arthritis Foot Disease: A Study of Mechanisms and Patient Experience. Arthritis Care Res. 2014;66:1456–1464. doi: 10.1002/acr.22060. [DOI] [PubMed] [Google Scholar]
- 16.Pallari J.H.P., Dalgarno K.W., Woodburn J. Mass customization of foot orthoses for rheumatoid arthritis using selective laser sintering. IEEE Trans. Biomed. Eng. 2010;57:1750–1756. doi: 10.1109/TBME.2010.2044178. [DOI] [PubMed] [Google Scholar]
- 17.Baronio G., Harran S., Signoroni A. A critical analysis of a hand orthosis reverse engineering and 3D printing process. Appl. Bionics Biomech. 2016;2016:8347478. doi: 10.1155/2016/8347478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell I., Diegel O., Kowen J., Wohlers T. Wohlers Report 2018: 3D Printing and Additive Manufacturing State of the Industry: Annual Worldwide Progress Report. Wohlers Associates; Fort Collins, CO, USA: 2018. [Google Scholar]
- 19.Zuniga J., Katsavelis D., Peck J., Stollberg J., Petrykowski M., Carson A., Fernandez C. Cyborg beast: A low-cost 3d-printed prosthetic hand for children with upper-limb differences. BMC Res. Notes. 2015;8:10. doi: 10.1186/s13104-015-0971-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Carvalho Filho I.F.P., Medola F.O., Sandnes F.E., Paschoarelli L.C. Proceedings of the International Conference on Applied Human Factors and Ergonomics; Springer; Berlin, Germany: 2019. Manufacturing Technology in Rehabilitation Practice: Implications for Its Implementation in Assistive Technology Production; pp. 328–336. [Google Scholar]
- 21.Fitzpatrick A.P., Mohanned M.I., Collins P.K., Gibson I. Design of a patient specific, 3D printed arm cast; Proceedings of the International Conference on Design and Technology; Geelong, Australia. 5–8 December 2016; pp. 135–142. [Google Scholar]
- 22.Parvez N., Parvez S. Andiamo Project. [(accessed on 30 October 2019)]; Available online: http://andiamo.io/
- 23.Chevalier T., Chockalingam N. Effects of foot orthoses: How important is the practitioner? Gait Posture. 2012;35:383–388. doi: 10.1016/j.gaitpost.2011.10.356. [DOI] [PubMed] [Google Scholar]
- 24.Dalgarno K., Pallari J., Woodburn J., Xiao K., Wood D., Goodridge R., Ohtsuki C. Mass customization of medical devices and implants: state of the art and future directions. Virtual Phys. Prototyp. 2006;1:137–145. doi: 10.1080/17452750601092031. [DOI] [Google Scholar]
- 25.Baumers M., Tuck C., Bourell D., Sreenivasan R., Hague R. Sustainability of additive manufacturing: Measuring the energy consumption of the laser sintering process. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2011;225:2228–2239. doi: 10.1177/0954405411406044. [DOI] [Google Scholar]
- 26.Mavroidis C., Ranky R.G., Sivak M.L., Patritti B.L., DiPisa J., Caddle A., Gilhooly K., Govoni L., Sivak S., Lancia M., et al. Patient specific ankle-foot orthoses using rapid prototyping. J. Neuroeng. Rehabil. 2011;8:1. doi: 10.1186/1743-0003-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Culmone C., Smit G., Breedveld P. Additive Manufacturing. Elsvier; Amsterdam, The Netherlands: 2019. Additive manufacturing of medical instruments: A state-of-the-art review. [Google Scholar]
- 28.Palo M., Holländer J., Suominen J., Yliruusi J., Sandler N. 3D printed drug delivery devices: perspectives and technical challenges. Expert Rev. Med. Devices. 2017;14:685–696. doi: 10.1080/17434440.2017.1363647. [DOI] [PubMed] [Google Scholar]
- 29.Jeon O., Lee Y.B., Jeong H., Lee S.J., Wells D., Alsberg E. Individual cell-only bioink and photocurable supporting medium for 3D printing and generation of engineered tissues with complex geometries. Mater. Horiz. 2019;9:1625–1631. doi: 10.1039/C9MH00375D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derakhshanfar S., Mbeleck R., Xu K., Zhang X., Zhong W., Xing M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018;3:144–156. doi: 10.1016/j.bioactmat.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koffler J., Zhu W., Qu X., Platoshyn O., Dulin J.N., Brock J., Graham L., Lu P., Sakamoto J., Marsala M., et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019;25:263. doi: 10.1038/s41591-018-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egan P.F. Integrated design approaches for 3D printed tissue scaffolds: Review and outlook. Materials. 2019;12:2355. doi: 10.3390/ma12152355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dawood A., Marti B.M., Sauret-Jackson V., Darwood A. 3D printing in dentistry. Brit. Dent. J. 2015;219:521. doi: 10.1038/sj.bdj.2015.914. [DOI] [PubMed] [Google Scholar]
- 34.Tahayeri A., Morgan M., Fugolin A.P., Bompolaki D., Athirasala A., Pfeifer C.S., Ferracane J.L., Bertassoni L.E. 3D printed versus conventionally cured provisional crown and bridge dental materials. Dent. Mater. 2018;34:192–200. doi: 10.1016/j.dental.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen K.T., Benabou L., Alfayad S. Systematic Review of Prosthetic Socket Fabrication using 3D printing; Proceedings of the 2018 ACM 4th International Conference on Mechatronics and Robotics Engineering; Valenciennes, France. 7–11 February 2018; pp. 137–141. [Google Scholar]
- 36.Chae M.P., Rozen W.M., McMenamin P.G., Findlay M.W., Spychal R.T., Hunter-Smith D.J. Emerging applications of bedside 3D printing in plastic surgery. Front. Surg. 2015;2:25. doi: 10.3389/fsurg.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malik H.H., Darwood A.R., Shaunak S., Kulatilake P., Abdulrahman A., Mulki O., Baskaradas A. Three-dimensional printing in surgery: A review of current surgical applications. J. Surg. Res. 2015;199:512–522. doi: 10.1016/j.jss.2015.06.051. [DOI] [PubMed] [Google Scholar]
- 38.Tack P., Victor J., Gemmel P., Annemans L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online. 2016;15:115. doi: 10.1186/s12938-016-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang L., Wang L., Bao W., Zhu S., Li D., Zhao N., Liu C. Functional gradient structural design of customized diabetic insoles. J. Mech. Behav. Biomed. Mater. 2019;94:279–287. doi: 10.1016/j.jmbbm.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Artioli B.O., Kunkel M.E., Mestanza S.N. World Congress on Medical Physics and Biomedical Engineering 2018. Springer; Berlin, Germany: 2019. Feasibility Study of a Methodology Using Additive Manufacture to Produce Silicone Ear Prostheses; pp. 211–215. [Google Scholar]
- 41.Liacouras P., Garnes J., Roman N., Petrich A., Grant G.T. Designing and manufacturing an auricular prosthesis using computed tomography, 3-dimensional photographic imaging, and additive manufacturing: a clinical report. J. Prosthet. Dent. 2011;105:78–82. doi: 10.1016/S0022-3913(11)60002-4. [DOI] [PubMed] [Google Scholar]
- 42.Liacouras P.C., Sahajwalla D., Beachler M.D., Sleeman T., Ho V.B., Lichtenberger J.P. Using computed tomography and 3D printing to construct custom prosthetics attachments and devices. 3D Print. Med. 2017;3:8. doi: 10.1186/s41205-017-0016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diwakar M., Kumar M. A review on CT image noise and its denoising. Biomed. Signal Process. Control. 2018;42:73–88. doi: 10.1016/j.bspc.2018.01.010. [DOI] [Google Scholar]
- 44.Haleem A., Javaid M. 3D scanning applications in medical field: A literature-based review. Clin. Epidemiol. Global Health. 2019;7:199–210. doi: 10.1016/j.cegh.2018.05.006. [DOI] [Google Scholar]
- 45.Górski F., Zawadzki P., Wichniarek R., Kuczko W., Żukowska M., Wesołowska I., Wierzbicka N. International Conference on Computational & Experimental Engineering and Sciences. Springer; Berlin, Germany: 2019. Automated Design of Customized 3D-Printed Wrist Orthoses on the Basis of 3D Scanning; pp. 1133–1143. [Google Scholar]
- 46.Grazioso S., Caporaso T., Selvaggio M., Panariello D., Ruggiero R., Di Gironimo G. Using photogrammetric 3D body reconstruction for the design of patient–tailored assistive devices; Proceedings of the IEEE 2019 II Workshop on Metrology for Industry 4.0 and IoT (MetroInd4. 0&IoT); Naples, Italy. 4–6 June 2019; pp. 240–242. [Google Scholar]
- 47.Barrios-Muriel J., Romero Sánchez F., Alonso F., Salgado D. Design of Semirigid Wearable Devices Based on Skin Strain Analysis. J. Biomech. Eng. 2019;141:021008. doi: 10.1115/1.4040250. [DOI] [PubMed] [Google Scholar]
- 48.Bogo F., Romero J., Pons-Moll G., Black M.J. Dynamic FAUST: Registering human bodies in motion; Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition; Honolulu, HI, USA. 21–26 July 2017; pp. 6233–6242. [Google Scholar]
- 49.Thompson A., McNally D., Maskery I., Leach R.K. X-ray computed tomography and additive manufacturing in medicine: A review. Int. J. Metrol. Qual. Eng. 2017;8:17. doi: 10.1051/ijmqe/2017015. [DOI] [Google Scholar]
- 50.Dal Maso A., Cosmi F. 3D-printed ankle-foot orthosis: a design method. Mater. Today Proc. 2019;12:252–261. doi: 10.1016/j.matpr.2019.03.122. [DOI] [Google Scholar]
- 51.Parrilla E., Ballester A., Solves-Camallonga C., Nácher B., Antonio Puigcerver S., Uriel J., Piérola A., González J.C., Alemany S. Low-cost 3D foot scanner using a mobile app. Footwear Sci. 2015;7:S26–S28. doi: 10.1080/19424280.2015.1038308. [DOI] [Google Scholar]
- 52.Chee Kai C., Siaw Meng C., Sin Ching L., Seng Teik L., Chit Aung S. Facial prosthetic model fabrication using rapid prototyping tools. Integr. Manuf. Syst. 2000;11:42–53. doi: 10.1108/09576060010303668. [DOI] [Google Scholar]
- 53.Paterson A. Ph.D Thesis. Loughborough University; Loughborough, UK: 2013. Digitisation of the Splinting Process: Exploration and Evaluation of a Computer Aided Design Approach to Support Additive Manufacture. [Google Scholar]
- 54.Mali H.S., Vasistha S. Advances in Additive Manufacturing and Joining. Springer; Berlin, Germany: 2020. Fabrication of Customized Ankle Foot Orthosis (AFO) by Reverse Engineering Using Fused Deposition Modelling; pp. 3–15. [Google Scholar]
- 55.Agudelo-Ardila C., Prada-Botía G., PH R. Journal of Physics: Conference Series. Volume 1388. IOP Publishing; Bristol, UK: 2019. Orthotic prototype for upper limb printed in 3D: A efficient solution; p. 012016. [Google Scholar]
- 56.Kimura M., Mochimaru M., Kanade T. Measurement of 3D foot shape deformation in motion; Proceedings of the ACM 5th ACM/IEEE International Workshop on Projector Camera Systems; Bali Way, CA, USA. 20 August 2008; p. 10. [DOI] [Google Scholar]
- 57.Mo S., Leung S.H., Chan Z.Y., Sze L.K., Mok K.M., Yung P.S., Ferber R., Cheung R.T. The biomechanical difference between running with traditional and 3D printed orthoses. J. Sports Sci. 2019;37:2191–2197. doi: 10.1080/02640414.2019.1626069. [DOI] [PubMed] [Google Scholar]
- 58.Liberadzki P., Adamczyk M., Witkowski M., Sitnik R. Structured-Light-Based System for Shape Measurement of the Human Body in Motion. Sensors. 2018;18:2827. doi: 10.3390/s18092827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Unkovskiy A., Spintzyk S., Brom J., Huettig F., Keutel C. Direct 3D printing of silicone facial prostheses: A preliminary experience in digital workflow. Journal Prosthet. Dent. 2018;120:303–308. doi: 10.1016/j.prosdent.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Schmeltzpfenning T., Plank C., Krauss I., Aswendt P., Grau S. Dynamic foot scanning. Prospects and limitations of using synchronized 3D scanners to capture complete human foot shape while walking. In: Karwowski W., Salvendy G., editors. Advances in Applied Digital Human Modeling, Proceedings of the 3rd International Conference on Applied Human Factors and Ergonomics, Miami, FL, USA, 19–22 July, 2010. CRC Press; Boca Raton, FL, USA: 2010. [Google Scholar]
- 61.Chatzitofis A., Zarpalas D., Kollias S., Daras P. DeepMoCap: Deep Optical Motion Capture Using Multiple Depth Sensors and Retro-Reflectors. Sensors. 2019;19:282. doi: 10.3390/s19020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joo H., Simon T., Sheikh Y. Total capture: A 3d deformation model for tracking faces, hands, and bodies; Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition; Salt Lake City, UT, USA. 18–22 June 2018; pp. 8320–8329. [Google Scholar]
- 63.Ciobanu O., Ciobanu G., Rotariu M. Photogrammetric scanning technique and rapid prototyping used for prostheses and ortheses fabrication. Appl. Mech. Mater. 2013;371:230–234. doi: 10.4028/www.scientific.net/AMM.371.230. [DOI] [Google Scholar]
- 64.Kudelski R., Dudek P., Kulpa M., Rumin R. Using reverse engineering and rapid prototyping for patient specific orthoses; Proceedings of the IEEE 2017 XIIIth International Conference on Perspective Technologies and Methods in MEMS Design (MEMSTECH); Lviv, Ukraine. 20–23 April 2017; pp. 88–90. [Google Scholar]
- 65.Vitali A., Regazzoni D., Rizzi C., Colombo G. Design and additive manufacturing of lower limb prosthetic socket; Proceedings of the ASME 2017 International Mechanical Engineering Congress and Exposition; Tampa, FL, USA. 10 January 2017. [Google Scholar]
- 66.Kruth P.P. Material Incress Manufacturing by Rapid Prototyping Techniques. CIRP Ann. Manuf. Technol. 1991;40:603–614. doi: 10.1016/S0007-8506(07)61136-6. [DOI] [Google Scholar]
- 67.Paterson A.M., Bibb R., Campbell R.I., Bingham G. Comparing additive manufacturing technologies for customised wrist splints. Rapid Prototyp. J. 2015;21:230–243. doi: 10.1108/RPJ-10-2013-0099. [DOI] [Google Scholar]
- 68.Chua C.K., Leong K.F. 3D Printing and Additive Manufacturing: Principles and Applications (with Companion Media Pack) of Rapid Prototyping. 4th ed. World Scientific Publishing Company; Singapore: 2014. [Google Scholar]
- 69.Petrovic V., Vicente Haro Gonzalez J., Jorda Ferrando O., Delgado Gordillo J., Ramon Blasco Puchades J., Portoles Grinan L. Additive layered manufacturing: Sectors of industrial application shown through case studies. Int. J. Prod. Res. 2011;49:1061–1079. doi: 10.1080/00207540903479786. [DOI] [Google Scholar]
- 70.Tan K.C., Lee P.V.S., Tam K.F., Lye S.L. Automation of prosthetic socket design and fabrication using computer aided design/computer aided engineering and rapid prototyping techniques; Proceedings of the First National Symposium of Prosthetics and Orthotics; Singapore, Republic of Singapore. 28 October 1998; pp. 19–22. [Google Scholar]
- 71.Leite M., Soares B., Lopes V., Santos S., Silva M.T. Design for personalized medicine in orthotics and prosthetics. Proced. CIRP. 2019;84:457–461. doi: 10.1016/j.procir.2019.04.254. [DOI] [Google Scholar]
- 72.Abdullah A.M., Mohamad D., Din T.N.D.T., Yahya S., Akil H.M., Rajion Z.A. Fabrication of nasal prosthesis utilising an affordable 3D printer. Int. J. Adv. Manuf. Technol. 2019;100:1907–1912. doi: 10.1007/s00170-018-2831-y. [DOI] [Google Scholar]
- 73.Melocchi A., Parietti F., Loreti G., Maroni A., Gazzaniga A., Zema L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J. Drug Deliv. Sci. Technol. 2015;30:360–367. doi: 10.1016/j.jddst.2015.07.016. [DOI] [Google Scholar]
- 74.Wang X., Jiang M., Zhou Z., Gou J., Hui D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017;110:442–458. doi: 10.1016/j.compositesb.2016.11.034. [DOI] [Google Scholar]
- 75.Beaman J., Deckard C. Selective laser sintering with assisted powder handling. No. 4,938,816. U.S. Patent. 1990 Jul 3;
- 76.Gibson I., Rosen D.W., Stucker B. Additive Manufacturing Technologies. Springer; Berlin, Germany: 2015. [Google Scholar]
- 77.Kruth J.P., Mercelis P., Van Vaerenbergh J., Froyen L., Rombouts M. Binding mechanisms in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2005;11:26–36. doi: 10.1108/13552540510573365. [DOI] [Google Scholar]
- 78.Deckers J.P., Vermandel M., Geldhof J., Vasiliauskaite E., Forward M., Plasschaert F. Development and clinical evaluation of laser-sintered ankle foot orthoses. Plast. Rubber Compos. 2018;47:42–46. doi: 10.1080/14658011.2017.1413760. [DOI] [Google Scholar]
- 79.Vasiliauskaite E., Ielapi A., Deckers J., Vermandel M., De Beule M., Van Paepegem W., Forward M., Plasschaert F. 16th National Day on Biomedical Engineering. National Committee on Biomedical Engineering (NCBME); Brussels, Belgium: Dec 1, 2017. Selective laser sintered ankle foot orthosis can support drop foot gait; p. 23. [Google Scholar]
- 80.Oxman N. Ph.D Thesis. Massachusetts Institute of Technology; Cambridge, MA, USA: 2010. Material-Based Design Computation. [Google Scholar]
- 81.Evill J. Cortex. [(accessed on 29 October 2019)];2013 Available online: http://www.evilldesign.com/cortex.
- 82.Herbert N., Simpson D., Spence W.D., Ion W. A preliminary investigation into the development of 3-D printing of prosthetic sockets. J. Rehabil. Res. Dev. 2005;42:141–146. doi: 10.1682/JRRD.2004.08.0134. [DOI] [PubMed] [Google Scholar]
- 83.Saijo H., Igawa K., Kanno Y., Mori Y., Kondo K., Shimizu K., Suzuki S., Chikazu D., Iino M., Anzai M., et al. Maxillofacial reconstruction using custom-made artificial bones fabricated by inkjet printing technology. J. Artif. Org. 2009;12:200–205. doi: 10.1007/s10047-009-0462-7. [DOI] [PubMed] [Google Scholar]
- 84.Ventola C.L. Medical Applications for 3D Printing: Current and Projected Uses. Pharm. Ther. 2014;39:704–711. [PMC free article] [PubMed] [Google Scholar]
- 85.Oxman N. Variable property rapid prototyping. Virtual Phys. Prototyp. 2011;6:3–31. [Google Scholar]
- 86.Peng H., Mankoff J., Hudson S.E., McCann J. A Layered Fabric 3D Printer for Soft Interactive Objects; Proceedings of the ACM 33rd Annual ACM Conference on Human Factors in Computing Systems; Seoul, Korea. 18–23 April 2015; pp. 1789–1798. [DOI] [Google Scholar]
- 87.Campbell I., Bourell D., Gibson I. Additive manufacturing: Rapid prototyping comes of age. Rapid Prototyp. J. 2012;18:255–258. doi: 10.1108/13552541211231563. [DOI] [Google Scholar]
- 88.Silva M., Felismina R., Mateus A., Parreira P., Malça C. Application of a hybrid additive manufacturing methodology to produce a metal/polymer customized dental implant. Proced. Manuf. 2017;12:150–155. doi: 10.1016/j.promfg.2017.08.019. [DOI] [Google Scholar]
- 89.Vaish A., Vaish R. 3D printing and its applications in Orthopedics. J. Clin. Orthop. Trauma. 2018;9:S74–S75. doi: 10.1016/j.jcot.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ciobanu O., Soydan Y., Hizal S. Customized foot orthosis manufactured with 3D printers; Proceedings of the 8th International Symposium on Intelligent Manufacturing Systems; Antalya, Turkey. 27–28 September 2012; 7p. [Google Scholar]
- 91.Paton J., Jones R.B., Stenhouse E., Bruce G. The physical characteristics of materials used in the manufacture of orthoses for patients with diabetes. Foot Ankle Int. 2007;28:1057–1063. doi: 10.3113/FAI.2007.1057. [DOI] [PubMed] [Google Scholar]
- 92.Ma Z., Lin J., Xu X., Ma Z., Tang L., Sun C., Li D., Liu C., Zhong Y., Wang L. Design and 3D printing of adjustable modulus porous structures for customized diabetic foot insoles. Int. J. Lightweight Mater. Manuf. 2019;2:57–63. doi: 10.1016/j.ijlmm.2018.10.003. [DOI] [Google Scholar]
- 93.Faustini M.C., Neptune R.R., Crawford R.H., Stanhope S.J. Manufacture of passive dynamic ankle-foot orthoses using selective laser sintering. IEEE Trans. Biomed. Eng. 2008;55:784–790. doi: 10.1109/TBME.2007.912638. [DOI] [PubMed] [Google Scholar]
- 94.Walbran M., Turner K., McDaid A. Customized 3D printed ankle-foot orthosis with adaptable carbon fibre composite spring joint. Cogent Eng. 2016;3:1227022. doi: 10.1080/23311916.2016.1227022. [DOI] [Google Scholar]
- 95.Trauner K.B. The emerging role of 3D printing in arthroplasty and orthopedics. J. Arthroplast. 2018;33:2352–2354. doi: 10.1016/j.arth.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 96.Tsai W., Yang Y., Chen C.H. Smart Science, Design & Technology: Proceedings of the 5th International Conference on Applied System Innovation (ICASI 2019), Fukuoka, Japan, 12–18 April 2019. CRC Press; Boca, Raton, FL, USA: 2019. A wearable 3D printed elbow exoskeleton to improve upper limb rehabilitation in stroke patients; p. 231. [Google Scholar]
- 97.Ang B.W., Yeow C.H. Print-it-Yourself (PIY) glove: a fully 3D printed soft robotic hand rehabilitative and assistive exoskeleton for stroke patients; Proceedings of the 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS); Vancouver, BC, Canada. 24–28 September 2017; pp. 1219–1223. [Google Scholar]