Abstract
Extreme heat events are becoming more common as a result of anthropogenic global change. Developmental plasticity in physiological thermal limits could help mitigate the consequences of thermal extremes, but data on the effects of early temperature exposure on thermal limits later in life are rare, especially for vertebrate ectotherms. We conducted an experiment that to our knowledge is the first to isolate the effect of egg (i.e. embryonic) thermal conditions on adult heat tolerance in a reptile. Eggs of the lizard Anolis sagrei were incubated under one of three fluctuating thermal regimes that mimicked natural nest environments and differed in mean and maximum temperatures. After emergence, all hatchlings were raised under common garden conditions until reproductive maturity, at which point heat tolerance was measured. Egg mortality was highest in the warmest treatment, and hatchlings from the warmest treatment tended to have greater mortality than those from the cooler treatments. Despite evidence that incubation temperatures were stressful, we found no evidence that incubation treatment influenced adult heat tolerance. Our results are consistent with a low capacity for organisms to increase their physiological heat tolerance via plasticity, and emphasize the importance of behavioural and evolutionary processes as mechanisms of resilience to extreme heat.
Keywords: development, reptile, climate change
1. Introduction
Phenotypic plasticity, defined as the ability of a genotype to express different phenotypes under different environmental conditions, is a potentially powerful mechanism by which organisms can mitigate the effects of anthropogenic global change [1–4]. In ectotherms, limits to physiological heat tolerance usually increase via plasticity when individuals are exposed to warmer temperatures [5]. Therefore, heat tolerance plasticity may be adaptive as extreme temperatures become more common in the future [6]. For example, heat tolerance plasticity decreases the frequency of overheating events under current climatic conditions [7] and may delay extinction for many populations under projected future thermal regimes [8]. Nonetheless, thermal tolerance plasticity is not fully compensatory, meaning that heat tolerance only increases a fraction of a degree for every degree of increase in body temperature [9–11]. This means that thermal tolerance plasticity can slow, but not prevent, increases in overheating risk as temperatures rise [7].
Most heat tolerance plasticity data come from short-term acclimation studies that expose animals (usually adults) to temperature treatments for brief time intervals, after which heat tolerance is immediately measured [9]. However, temperature exposure during early-life stages can also affect heat tolerance later in life (here referred to as ‘developmental plasticity’ [12]). For example, several studies have shown that higher rearing temperatures can lead to higher heat tolerance in adult Drosophila [11,13,14]. Developmental plasticity is generally not considered in broad-scale assessments of plasticity effects under warming [3,8,9] because these data are simply lacking for most species. The lack of data on developmental plasticity is particularly acute for reptiles [15]. We are aware of only three studies that have tested for effects of egg incubation temperature on reptile heat tolerance. In one study, eggs exposed to warmer temperature produced hatchlings with lower heat tolerance than those exposed to cooler temperatures, a potentially maladaptive response [16]. Conversely, the other studies found that egg incubation temperature had either no effect on hatchling heat tolerance [17], or a positive effect on hatchling heat tolerance that was not maintained later in life [18].
To reliably integrate physiological plasticity into models of the effect of extreme future conditions, we need more data on how early thermal experiences shape the thermal tolerances of reproductive adults. To help fill the gap in our understanding of thermal plasticity, we tested for developmental plasticity in heat tolerance of the Cuban brown anole Anolis sagrei. To isolate the influence of egg incubation temperature on adult thermal physiology, we incubated eggs under thermal regimes that matched three different empirical patterns of natural nest temperatures, then raised hatchlings to adulthood under common garden conditions before measuring thermal tolerance. To our knowledge, this is the first experiment to isolate egg incubation temperature as a potential driver of adult reptile heat tolerance. Therefore, our results provide insight to the little-known sensitivity of different reptile life stages to long-lasting organizational effects of thermal perturbation.
2. Methods
(a). Egg collection
Adults (93 females and 28 males) were collected on 21 and 22 June 2018 in Palm Coast, FL, USA (29′60″ N – 81′20″ W), and transferred to an animal room at Auburn University. Females were housed individually in plastic cages (length: 450 mm, width: 280 mm, height: 300 mm), and each male was randomly assigned to two to four females and moved from one cage to another every 10 days to enhance female egg production. Each cage contained reptile cage carpet (Zoo Med Laboratories Inc., San Luis Obispo, CA, USA), bamboo perches, artificial leaves and a plastic nesting pot filled with soil. Cages were covered with a mesh screen. Artificial light (Reptisun 5.0 UVB lamp, Zoo Med) was set to allow 12 h of light per day with the room temperature varying from 24 to 28°C. Lizards were fed two crickets (size approx. 13 mm) dusted with vitamins (1 : 1 ratio of ‘Herptivite’ and ‘Calcium with Vit.D3’, Rep-Cal Research Labs, Los Gatos, CA, USA) twice a week and were misted with water every day. Nesting pots were checked for eggs every 2 days. When an egg was found, it was placed in a Petri dish (top radius: 28 mm, bottom radius: 26 mm, height: 15 mm) half-filled with vermiculite (−150 kPa [19]). The Petri dish was then sealed with Parafilm to minimize evaporation. Each egg was randomly assigned to one incubation treatment and placed into the corresponding incubator (Peltier-cooled Incubator IPP55 Plus, Memmert GmbH, Schwabach, Germany). One to seven eggs were incubated per female.
(b). Egg incubation and animal husbandry
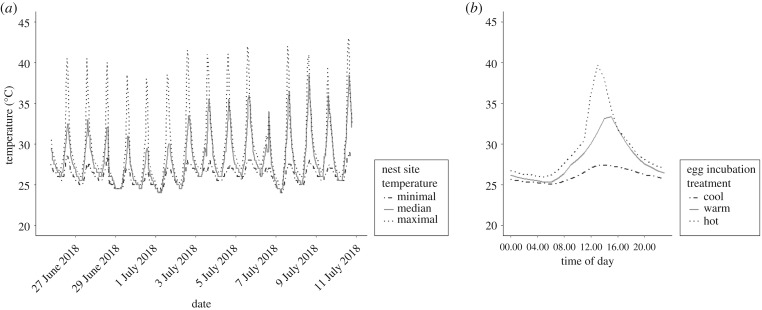
Three incubation treatments differed in mean and maximum temperature (‘cool’ treatment: mean = 26.2°C ± 0.79 s.d., min = 25.1°C, max = 27.4°C; ‘warm’ treatment: mean = 28.0°C ± 2.52 s.d., min = 25.3°C, max = 33.4°C; ‘hot’ treatment: mean = 29.4°C ± 3.92 s.d., min = 25.9°C, max = 39.7°C; figure 1). These incubation treatments mimicked natural nest temperatures of A. sagrei in Palm Coast, FL, USA (figure 1a). Nests were found by searching through leaf litter and ground cover, and nest temperatures were monitored hourly with iButton data loggers for 16 days between 25 June and 10 July 2018. We selected three nests corresponding to approximate minimum, median and maximum thermal conditions as templates for our treatments. For each hour of the day for each treatment, template nest temperature was averaged at that specific hour across all days (figure 1b).
Figure 1.
(a) Temperatures measured in natural A. sagrei nests used as templates for our experimental treatments. (b) Temperature treatments under which eggs were incubated in our experiments.
During the experiment, we noted higher mortality in the warm and hot treatments, so we allocated more eggs to those treatments to balance sample sizes of hatchlings across treatments (table 1). Variation in egg mortality was analysed using a generalized linear model with a binomial distribution; mortality was the dependent variable and incubation treatment was the independent variable.
Table 1.
Number of eggs incubated and egg mortality associated with each incubation treatment.
| treatment | total | dead | mortality rate |
|---|---|---|---|
| cool | 51 | 5 | 0.098 |
| warm | 70 | 30 | 0.429 |
| hot | 78 | 47 | 0.603 |
From the eggs that survived (n = 117), hatchlings were individually housed in the same way as adults, but in smaller cages (length: 205 mm, width: 160 mm, height: 142.5 mm) without nesting pots. They were housed in the same animal room as the adults with the same light and room temperature regimes. Within the first month after hatching, individuals were fed approximately 20 fruit flies three times a week. They were then fed twice a week with approximately four small crickets (size approx. 3 mm) and once a week with approximately 20 fruit flies until they were large enough (approx. six months old) to be fed twice a week with two larger crickets (size approx. 13 mm). All food items used were dusted with the same vitamins used for adults. Animals were misted with water daily. During the experiment, 37 animals died (n = 33) or escaped (n = 4). We tested for an effect of egg incubation treatment on hatchling mortality using a generalized linear model with a binomial distribution; mortality was the dependent variable and incubation treatment was an independent variable.
(c). Heat tolerance measurements
We measured heat tolerance as the critical thermal maximum (CTmax), the body temperature at which an animal loses neuromuscular coordination and can no longer right itself during a thermal ramp [20]. Measurements were conducted following the protocol of Leal & Gunderson [21]. Lizards were warmed under a heat lamp while monitoring their body temperatures with a wire thermocouple probe placed inside the cloaca. Lizards were flipped onto their backs at 1°C body temperature intervals starting at 36°C, and CTmax was recorded as the body temperature at which they lost righting ability. We had a low number of surviving males in the hot treatment (N = 6, see Results), so we first confirmed that males and females did not differ in thermal tolerance (t-test, p = 0.996) and then combined sexes to test for treatment effects with a mixed-effects linear model with dam as a random term using the lme function in the nlme package in R [22].
3. Results
(a). Egg and hatchling mortality
Of the 199 eggs that were incubated, 82 died. Egg mortality differed among incubation treatments, with higher mortality in the warm and hot treatments than the cool treatment ( p < 0.001; table 1). Hatchling mortality did not differ among egg incubation treatments ( p = 0.1425; table 2).
Table 2.
Mortality of males and females that hatched from the different incubation treatments.
| treatment | sex | total | dead | mortality rate |
|---|---|---|---|---|
| cool | male | 19 | 4 | 0.211 |
| female | 27 | 9 | 0.333 | |
| warm | male | 19 | 3 | 0.158 |
| female | 20 | 5 | 0.250 | |
| hot | male | 12 | 6 | 0.500 |
| female | 16 | 6 | 0.375 |
(b). Heat tolerance
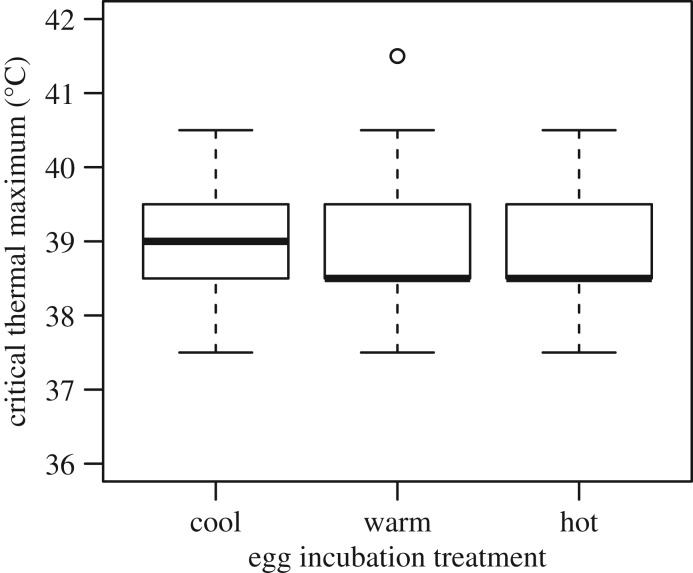
The mean CTmax values of adult lizards from eggs incubated in the cool, warm and hot treatment were 38.9 ± 0.9°C, 38.9 ± 1.0°C, and 38.9 ± 1.0°C, respectively (figure 2). There were no significant differences in thermal tolerance among treatments (F2,16 = 0.020, p = 0.982).
Figure 2.
Heat tolerance of adult A. sagrei hatched from eggs incubated under different thermal treatments.
4. Discussion
We investigated whether natural egg incubation temperatures generate variation in offspring mortality and induce lasting plastic shifts in heat tolerance that carry into adulthood in the lizard A. sagrei. Egg mortality was highest in the hot treatment (table 1) indicating that our warmest incubation regime reached stressful levels. Nonetheless, CTmax did not vary among adults that hatched from eggs incubated under different treatments (figure 2). This does not mean that the CTmax of A. sagrei is not plastic, as adults acclimated to warm temperatures attain higher CTmax than those acclimated to cool temperatures [23]. Instead, our results show that there is no organizational effect of egg incubation temperature that patterns future heat tolerance limits. An alternative explanation is that our treatments imposed differential selection such that plastic individuals perished in the warmer treatments. We find this an unlikely explanation because, contrary to expectation [24–26], it would require that greater thermal fluctuation selects against individuals with thermal plasticity. Nonetheless, if selection did explain our result, the primary implication would be similar but for a more surprising reason: plasticity induced by incubation temperature does not lead to increased adult heat tolerance because plastic individuals are selected against. Interestingly, the CTmax that we measured is lower than that found in other A. sagrei populations, by 1.5–4°C depending on the study [23,27,28]. At this time, we cannot explain the differences among these studies.
Our results are consistent with recent studies on the effects of egg incubation temperature on reptile heat tolerance. While those studies focused on animals that had not yet reached sexual maturity and on taxa distantly related to A. sagrei (skinks and geckos), they nonetheless found that warmer incubation temperatures either had no lasting effect [17,18] or a negative effect [16], on heat tolerance. Although heat tolerance data are rare, it is not the only trait that yields insight into the link between egg temperature and adult responses to heat in reptiles. Much more is known about egg temperature and subsequent thermoregulatory behaviour, and the results are similar [15]. Most studies find either no effect of egg incubation temperature on preferred body temperatures (e.g. [29,30]) or a negative effect such that embryos exposed to high temperatures seek out cooler conditions after hatching (e.g. [31,32]). The overall pattern emerging is that embryonic exposure to high temperatures in reptiles does not increase resilience to high temperatures later in life [15].
Global warming is driving an increase in the frequency of extreme heat events that induce physiological stress [33], cause mass die-offs [34] and increase extinction risk [8]. Developmental plasticity has the potential to reduce the negative effects of extreme temperatures by increasing heat tolerance, especially given that nest temperatures are projected to rise along with body temperatures of free-ranging animals [35]. However, our results and those in other ectotherm systems indicate that developmental plasticity has a limited effect on adult heat tolerance [11,13,14,36]. This is consistent with the finding that short-term, reversible acclimation also has a small effect on heat tolerance in most instances [9]. Though small plastic changes in heat tolerance can decrease overheating risk [7,8], the capacity for plasticity to fully compensate for exposure to extreme heat seems slim. Nonetheless, the relative lack of data on developmental, as well as transgenerational, plasticity in thermal tolerance leave broad generalizations difficult to come by. We urgently need more data on plasticity in temperature-dependent thermal tolerance to assess the full scope of organismal capacity to adjust to changing thermal conditions [37].
Supplementary Material
Ethics
All research was conducted with the approval of the Auburn University Institutional Animal Care and Use Committee (protocol no. 2017-3027).
Data accessibility
Data are accessible as electronic supplementary material.
Authors' contributions
A.R.G., A.F. and D.A.W. conceived, designed and executed the project. A.R.G. and A.F. conducted the statistical analyses. A.R.G., A.F. and D.A.W. wrote the paper. All authors agree to be held accountable for the content herein and approve the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This project was funded by the National Science Foundation (DEB-1564563 to DAW).
References
- 1.Fox RJ, Donelson JM, Schunter C, Ravasi T, Gaitán-Espitia JD. 2019. Beyond buying time: the role of plasticity in phenotypic adaptation to rapid environmental change. Phil. Trans. R. Soc. B 374, 20180174 ( 10.1098/rstb.2018.0174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huey RB, Kearney MR, Krockenberger A, Holtum JA, Jess M, Williams SE. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil. Trans. R. Soc. B 367, 1665–1679. ( 10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seebacher F, White CR, Franklin CE. 2015. Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Change 5, 61–66. ( 10.1038/nclimate2457) [DOI] [Google Scholar]
- 4.Somero G. 2010. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912–920. ( 10.1242/jeb.037473) [DOI] [PubMed] [Google Scholar]
- 5.Somero GN, Lockwood BL, Tomanek L. 2017. Biochemical adaptation: response to environmental challenges, from life's origins to the Anthropocene. Sunderland, MA: Sinauer Associates, Incorporated Publishers. [Google Scholar]
- 6.Stillman JH. 2003. Acclimation capacity underlies susceptibility to climate change. Science 301, 65 ( 10.1126/science.1083073) [DOI] [PubMed] [Google Scholar]
- 7.Gunderson AR, Dillon ME, Stillman JH. 2017. Estimating the benefits of plasticity in ectotherm heat tolerance under natural thermal variability. Funct. Ecol. 31, 1529–1539. ( 10.1111/1365-2435.12874) [DOI] [Google Scholar]
- 8.Morley S, Peck L, Sunday J, Heiser S, Bates A. 2019. Physiological acclimation and persistence of ectothermic species under extreme heat events. Glob. Ecol. Biogeogr. 28, 1018–1037. ( 10.1111/geb.12911) [DOI] [Google Scholar]
- 9.Gunderson AR, Stillman JH. 2015. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B 282, 20150401 ( 10.1098/rspb.2015.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schou MF, Mouridsen MB, Sørensen JG, Loeschcke V. 2016. Linear reaction norms of thermal limits in Drosophila: predictable plasticity in cold but not in heat tolerance. Funct. Ecol. 31, 934–945. ( 10.1111/1365-2435.12782) [DOI] [Google Scholar]
- 11.van Heerwaarden B, Kellermann V, Sgrò C.M. 2016. Limited scope for plasticity to increase upper thermal limits. Funct. Ecol. 30, 1947–1956. ( 10.1111/1365-2435.12687) [DOI] [Google Scholar]
- 12.Beaman JE, White CR, Seebacher F. 2016. Evolution of plasticity: mechanistic link between development and reversible acclimation. Trends Ecol. Evol. 31, 237–249. ( 10.1016/j.tree.2016.01.004) [DOI] [PubMed] [Google Scholar]
- 13.Kellermann V, Sgrò CM. 2018. Evidence for lower plasticity in CTmax at warmer developmental temperatures. J. Evol. Biol. 31, 1300–1312. ( 10.1111/jeb.13303) [DOI] [PubMed] [Google Scholar]
- 14.MacLean HJ, Sørensen JG, Kristensen TN, Loeschcke V, Beedholm K, Kellermann V, Overgaard J. 2019. Evolution and plasticity of thermal performance: an analysis of variation in thermal tolerance and fitness in 22 Drosophila species. Phil. Trans. R. Soc. B 374, 20180548 ( 10.1098/rstb.2018.0548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Refsnider JM, Clifton IT, Vazquez TK. 2019. Developmental plasticity of thermal ecology traits in reptiles: trends, potential benefits, and research needs. J. Therm. Biol 84, 74–82. ( 10.1016/j.jtherbio.2019.06.005) [DOI] [PubMed] [Google Scholar]
- 16.Dayananda B, Murray BR, Webb JK. 2017. Hotter nests produce hatchling lizards with lower thermal tolerance. J. Exp. Biol. 220, 2159–2165. ( 10.1242/jeb.152272) [DOI] [PubMed] [Google Scholar]
- 17.Llewelyn J, Macdonald SL, Moritz C, Martins F, Hatcher A, Phillips BL. 2018. Adjusting to climate: acclimation, adaptation and developmental plasticity in physiological traits of a tropical rainforest lizard. Integr. Zool. 13, 411–427. ( 10.1111/1749-4877.12309) [DOI] [PubMed] [Google Scholar]
- 18.Abayarathna T, Murray BR, Webb JK. 2019. Higher incubation temperatures produce long-lasting upward shifts in cold tolerance, but not heat tolerance, of hatchling geckos. Biol. Open 8, bio042564 ( 10.1242/bio.042564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Packard GC, Packard MJ, Miller K, Boardman TJ. 1987. Influence of moisture, temperature, and substrate on snapping turtle eggs and embryos. Ecology 68, 983–993. ( 10.2307/1938369) [DOI] [Google Scholar]
- 20.Lutterschmidt WI, Hutchison VH. 1997. The critical thermal maximum: history and critique. Can. J. Zool. 75, 1561–1574. ( 10.1139/z97-783) [DOI] [Google Scholar]
- 21.Leal M, Gunderson AR. 2012. Rapid change in the thermal tolerance of a tropical lizard. Am. Nat. 180, 815–822. ( 10.1086/668077) [DOI] [PubMed] [Google Scholar]
- 22.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 23.Corn MJ. 1971. Upper thermal limits and thermal preferenda for three sympatric species of Anolis. J. Herpetol. 5, 17–21. ( 10.2307/1562838) [DOI] [Google Scholar]
- 24.Lande R. 2014. Evolution of phenotypic plasticity and environmental tolerance of a labile quantitative character in a fluctuating environment. J. Evol. Biol. 27, 866–875. ( 10.1111/jeb.12360) [DOI] [PubMed] [Google Scholar]
- 25.Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press. [Google Scholar]
- 26.Phillips BL, Munoz MM, Hatcher A, Macdonald SL, Llewelyn J, Lucy V, Moritz C. 2016. Heat hardening in a tropical lizard: geographic variation explained by the predictability and variance in environmental temperatures. Funct. Ecol. 30, 1161–1168. ( 10.1111/1365-2435.12609) [DOI] [Google Scholar]
- 27.Kolbe JJ, Ehrenberger JC, Moniz HA, Angilletta MJ Jr. 2013. Physiological variation among invasive populations of the brown anole (Anolis sagrei). Physiol. Biochem. Zool. 87, 92–104. ( 10.1086/672157) [DOI] [PubMed] [Google Scholar]
- 28.Gunderson AR, Mahler DL, Leal M. 2018. Thermal niche evolution across replicated Anolis lizard adaptive radiations. Proc. R. Soc. B 285, 20172241 ( 10.1098/rspb.2017.2241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alberts AC, Perry AM, Lemm JM, Phillips JA. 1997. Effects of incubation temperature and water potential on growth and thermoregulatory behavior of hatchling Cuban rock iguanas (Cyclura nubila). Copeia 1997, 766–776. ( 10.2307/1447294) [DOI] [Google Scholar]
- 30.Spotila JR, Zimmerman LC, Binckley CA, Grumbles JS, Rostal DC, List A Jr, Beyer EC, Phillips KM, Kemp SJ. 1994. Effects of incubation conditions on sex determination, hatching success, and growth of hatchling desert tortoises, Gopherus agassizii. Herpetol. Monogr. 8, 103–116. ( 10.2307/1467074) [DOI] [Google Scholar]
- 31.Goodman RM, Walguarnery JW. 2007. Incubation temperature modifies neonatal thermoregulation in the lizard Anolis carolinensis. J. Exp. Zool. A Ecol. Genet. Physiol. 307, 439–448. ( 10.1002/jez.397) [DOI] [PubMed] [Google Scholar]
- 32.Tamplin JW, Cyr AB. 2011. Effects of acclimation and egg-incubation temperature on selected temperature by hatchling western painted turtles (Chrysemys picta bellii). J. Therm. Biol 36, 507–514. ( 10.1016/j.jtherbio.2011.09.001) [DOI] [Google Scholar]
- 33.Stillman JH. 2019. Heat waves, the new normal: summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology 34, 86–100. ( 10.1152/physiol.00040.2018) [DOI] [PubMed] [Google Scholar]
- 34.Ratnayake H, Kearney MR, Govekar P, Karoly D, Welbergen JA. 2019. Forecasting wildlife die-offs from extreme heat events. Anim. Conserv. 22, 386–395. ( 10.1111/acv.12476) [DOI] [Google Scholar]
- 35.Levy O, Buckley LB, Keitt TH, Smith CD, Boateng KO, Kumar DS, Angilletta MJ. 2015. Resolving the life cycle alters expected impacts of climate change. Proc. R. Soc. B 282, 20150837 ( 10.1098/rspb.2015.0837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enriquez-Urzelai U, Sacco M, Palacio AS, Pintanel P, Tejedo M, Nicieza AG. 2019. Ontogenetic reduction in thermal tolerance is not alleviated by earlier developmental acclimation in Rana temporaria. Oecologia 189, 385–394. ( 10.1007/s00442-019-04342-y) [DOI] [PubMed] [Google Scholar]
- 37.Noble DW, Stenhouse V, Schwanz LE. 2018. Developmental temperatures and phenotypic plasticity in reptiles: a systematic review and meta-analysis. Biol. Rev. 93, 72–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are accessible as electronic supplementary material.