Abstract
Broadcast-spawning scleractinian corals annually release their gametes with high levels of synchrony, both within and among species. However, the timing of spawning can vary inter-annually. In particular, the night of spawning relative to the full moon phase can vary considerably among years at some locations. Although multiple environmental factors can affect the night of spawning, their effects have not been quantitatively assessed at the multi-regional level. In this study, we analysed environmental factors that are potentially correlated with spawning day deviation, in relation to the full moon phase, in Acropora corals inhabiting seven reefs in Australia and Japan. We accordingly found that sea surface temperature and wind speed within one to two months prior to the full moon of the spawning month were strongly correlated with spawning day deviations. In addition, solar flux had a weak effect on the night of spawning. These findings indicate that Acropora have the capacity to adjust their development and physiology in response to environmental factors for fine-tuning the timing of synchronous spawning, thereby maximizing reproductive success and post-fertilization survival.
Keywords: coral reef, spawning synchrony, reproduction, Acropora, sea surface temperature, full moon
1. Introduction
Inter-annual variations in environmental conditions, e.g. temperature and photoperiod, can lead to shifts in the phase and amplitude of the seasonal oscillations of biological events. Scleractinian corals exhibit conspicuous spawning synchrony [1–3], which was first documented in several populations on the Great Barrier Reef, Australia, where the majority of corals spawn synchronously over a few evenings following the full moon phase in late spring or early summer [4]. However, although the spawning pattern of corals has become one of the most well-studied annual reproduction events, it remains unclear how these corals adjust to inter-annual variations in environmental factors.
The synchronous spawning of corals is plausibly regulated by successive environmental cues at multiple timescales—spawning month (or season), day and hour [5]. Although the month of spawning has been linked to a number of environmental factors, including temperature, rainfall, solar insolation and wind speed [6–9], an investigation assessing all these variables at the scale of the Indo-Pacific basin indicated that the peak month of spawning is most closely associated with seasonal rises in sea surface temperature (SST) [10]. The day of spawning is generally in tune with lunar periodicity, and assumed to be determined by variations associated with the lunar cycle, e.g. in moonlight, pressure or water motion [2]. For determining the spawning hour, the duration of darkness after sunset is reportedly a potential proximate cue [11,12].
Despite the high synchrony of spawning that occurs each year, there remains substantial inter-annual variation. In particular, the night of spawning in relation to the full moon phase, which is critical for spawning synchrony, has been shown to exhibit inter-annual variation at some sites. Several local-scale studies have indicated that high SSTs in the months prior to spawning are correlated with the lunar spawning day [13–16]. However, how and to what extent environmental factors influence inter-annual variations in the night of spawning have not been explored. To address this issue, we generated statistical models to analyse the multi-year-long and multi-regional data of the spawning days of the coral genus Acropora, the species of which are dominant in most shallow-water coral assemblages throughout the Indo-Pacific Ocean [10].
2. Material and methods
(a). Multi-year-long dataset of spawning days in Acropora species
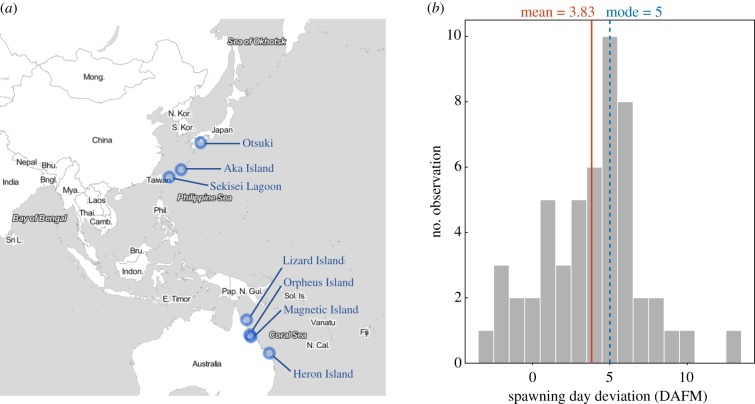
We obtained information on the spawning days of Acropora species from seven reefs in Australia and Japan, where field observations of coral spawning have been conducted for more than 5 years (electronic supplementary material, tables S1 and S2). Four of these reefs are located in the Great Barrier Reef (Heron Island, Lizard Island, Magnetic Island and Orpheus Island) and three reefs are located in the southwest of Japan (Sekisei Lagoon, Aka Island and Otsuki; figure 1a). To select a single variable to represent the timing of spawning for a focal year, we focused on the date at which spawning was observed for the first time in each of the years (hereafter referred to as ‘first spawning’) and calculated the deviation between first spawning and the full moon of that month for each year and each reef (electronic supplementary material, table S1 and figure S1).
Figure 1.
Location of the coral reefs and spawning day deviations from full moon in the genus Acropora. (a) Location of the coral reefs from which coral spawning data were collected in this study. The map was created using the leaflet function of R v. 3.5.1. (b) Histogram of spawning day deviation from full moon. Solid and dashed lines with numbers show the mean and mode values, respectively. Data for all seven reefs were combined. (Online version in colour.)
(b). Environmental variables
We selected four environmental parameters that are reportedly related to the timing of spawning: SST [10], solar insolation [7,8], wind speed [9,10] and rate of precipitation [6] (electronic supplementary material, table S3). The change in SST between days (ΔSST) was also used as a variable, as a previous study has reported that the rate of ΔSST is highly correlated with the spawning month [10]. In total, we used five variables in our model. Daily SST (°C) values were obtained from NOAA High-resolution Blended Analysis at a 0.25° spatial resolution [17]. The global daily 1° × 1° gridded Adjusted All-Sky Surface Spectral Shortwave Down Flux (hereafter, simply referred to as ‘solar flux’) data were obtained from NASA's Clouds and the Earth's Radiant Energy System Synoptic (CERES-SYN1 deg) Edition 4A, used as a measure of solar insolation. Solar fluxes (W m−2) are surface solar irradiances in the shortwave region (wavelength between 0.1754 and 4 µm) [18]. The daily means of 10 m above surface wind speed (m s−1) and rate of precipitation (mm h−1) at a 0.25° spatial resolution were derived from the Tropical Rainfall Measuring Mission's (TRMM) Microwave Imager (TMI) by the NASA Earth Sciences Program and were recorded using Remote Sensing Systems. These daily environmental parameters were obtained for the years 2003–2014 and used in subsequent analyses (electronic supplementary material, table S3). The mean values of each variable were calculated for four different time ranges: (1) −1 to −30, (2) −31 to −60, (3) −61 to −90 and (4) −91 to −120 days after the full moon of the month of the first spawning (DAFM) for each year (see electronic supplementary material, figure S1 for details). These time ranges were selected so as to cover the late stage of gametogenesis in Acropora corals [2,14], allowing us to examine in which range the environmental factors well explained the spawning day deviation. Monthly means of parameters (SST, ΔSST, solar flux, wind speed and rate of precipitation) were standardized to Z-score values (a mean of zero and a standard deviation of one) for each time range.
(c). Statistical analysis
To identify the factors affecting the night of Acropora coral spawning, we constructed linear mixed models (LMMs) with a Gaussian error structure, including Z-scores of the five assessed environmental parameters as explanatory variables and deviation from the full moon (by the DAFM) for each year as the response variable. No multi-collinearity between the environmental variables was found using variance inflation factors [19] (i.e. all five variables had variance inflation factors less than 3), and thus all variables were included in full models. The difference in reefs was included in the models as a random intercept. LMMs that included all experimental parameters (full models) were generated for each of the four different time ranges. To select the best-fit models (best LMMs) for each time range, all possible pairs of models were compared based on the Akaike information criterion (AIC) and the models with lowest AICs were selected. The data from the selected best-fit models were visualized for analytical use to examine the normality and homoscedasticity of the residuals (electronic supplementary material, figures S2–S5). Subsequently, we calculated two types of R2-values for the selected best-fit models: marginal R2 , which indicated the variance explained in the response variable by fixed effects, and conditional R2 , which indicated the variance explained by both fixed and random effects [20]. We also calculated the proportional change in variance at the reef level (PCV[Reef]) for the selected models to evaluate how the addition of fixed effects to the intercept model affected the variance component at the reef level [20]. Positive and negative PCV[Reef] values indicate that the variance at the reef level has decreased and increased, respectively. All analyses were performed using R v. 3.5.1 [21].
3. Results
Observational records of the spawning day on the seven examined reefs are summarized in electronic supplementary material, table S1 and histograms for these variables are presented in figure 1b (for total observations) and electronic supplementary material, figure S6 (for each reef region). Deviation from the full moon ranged from −3 (for Sekisei Lagoon in 2005) to +13 DAFM (for Otsuki in 2003), the mean and mode values were +3.83 and +5, respectively (solid and dashed vertical lines, respectively, in figure 1b). The spawning day deviation varied across reefs, with the mean deviation in Otsuki being highest among all seven reefs (+7.167 DAFM) and that in Sekisei Lagoon being lowest (+0.8571).
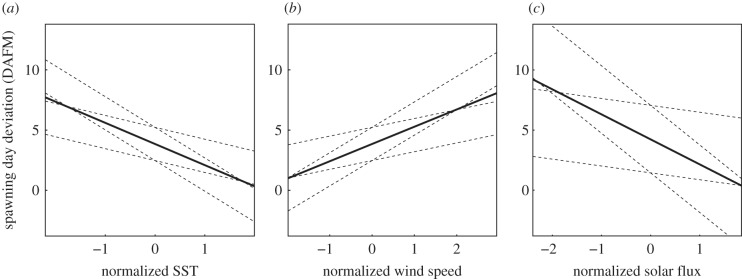
Table 1 summarizes the best LMMs selected based on the AIC values for different time ranges. The best LMMs for three of the four time ranges included the monthly mean SSTs, which had negative coefficients in all selected models. This indicated that when the monthly SST was low, the night of spawning was significantly delayed (i.e. it occurred later relative to the full moon; figure 2a and electronic supplementary material, figure S7). Wind speed was selected in the models for time ranges (1) and (2) and was found to positively contribute to the models (table 1; figure 2b and electronic supplementary material, figure S7), although the contribution was less clear in the models for time range (2) (table 1). Moreover, solar flux was selected in the best model for time range (4) and had a positive coefficient (table 1 and figure 2c; electronic supplementary material, figure S7). When we assessed the difference in the AIC values between the selected best model and the null model (ΔAIC), the best model of time range (1) showed the highest ΔAIC value among the four time ranges. The fixed effects of the best model of time range (1) explained 43.86% of the variance in the response variable , and when considering a random effect (reef) in addition to the fixed effects, 67.26% of the variance was explained . Moreover, the residual of the model fitted well with a normal distribution (electronic supplementary material, figure S2). The PCV[reef] values of the best-fit models for all time ranges were positive, which indicated that the inclusion of selected environmental parameters reduced the variance component at the reef level. A summary of the statistical results of the full models for the four different time ranges is shown in electronic supplementary material, table S4.
Table 1.
Summary of the best-fit linear mixed models (LMMs) having lowest AIC value for each of four time ranges. The results of full models were summarized in electronic supplementary material, table S4 and the diagnostic plots of these models were showed in electronic supplementary material, figure S2–S5. CI: confidence interval; VC: variance components; AIC: Akaike Information Criterion; ΔAIC: the difference of AIC between the best-fit model and the intercept model; : marginal R2; : conditional R2. AIC values were calculated using maximum likelihood (ML) and other parameters were generated from restricted maximum likelihood (REML).
| time range | selected best models based on AIC |
|||
|---|---|---|---|---|
| (1) | fixed effects | βcoefficent | lower 95% CI | upper 95% CI |
| (intercept) | 3.84 | 2.47 | 5.23 | |
| SST | −1.76 | −2.55 | −0.99 | |
| wind speed | 1.43 | 0.73 | 2.11 | |
| random effect | VC | |||
| Reef | 2.69 | |||
| AIC | 236.01 | |||
| ΔAIC | 32.11 | |||
| (%) | 43.86 | |||
| (%) | 67.26 | |||
| PCV[Reef] (%) | 29.06 | |||
| (2) | fixed effects | βcoefficent | lower 95% CI | upper 95% CI |
| (intercept) | 3.94 | 2.29 | 5.59 | |
| SST | −1.88 | −2.89 | −0.85 | |
| wind speed | 0.77 | −0.16 | 1.63 | |
| random effect | VC | |||
| Reef | 3.87 | |||
| AIC | 258.36 | |||
| ΔAIC | 9.75 | |||
| (%) | 27.1 | |||
| (%) | 56.15 | |||
| PCV[Reef] (%) | 11.66 | |||
| (3) | fixed effect | βcoefficent | lower 95% CI | upper 95% CI |
| (intercept) | 3.99 | 2.54 | 5.47 | |
| SST | −1.52 | −2.64 | −0.41 | |
| random effect | VC | |||
| Reef | 2.77 | |||
| AIC | 263.15 | |||
| ΔAIC | 4.96 | |||
| (%) | 19.4 | |||
| (%) | 42.51 | |||
| PCV[Reef] (%) | 4.12 | |||
| (4) | fixed effect | βcoefficent | lower 95%CI | upper 95% CI |
| (intercept) | 4.23 | 1.44 | 7.05 | |
| solar flux | −2.08 | −3.29 | −0.57 | |
| random effect | VC | |||
| Reef | 11.8 | |||
| AIC | 262.89 | |||
| ΔAIC | 5.22 | |||
| (%) | 19.84 | |||
| (%) | 74.03 | |||
| PCV[Reef] (%) | 13.07 | |||
Figure 2.
The relationships between environmental parameters and spawning day deviations from full moon estimated using best linear mixed models. Solid and dashed lines represent the regressions based on the intercepts and slopes calculated from estimated partial coefficients (solid lines) and its upper and lower 95% confidence intervals (dashed lines) under the best-fit model (see table 1 for details). Plots of spawning day deviation against standardized environmental parameters, i.e. (a) SSTs of time range (1), (b) wind speed of time range (1) and (c) solar flux of time range (4), are shown.
4. Discussion
Although Acropora corals spawn with high levels of synchrony, the night of spawning relative to the full moon phase varies inter-annually, suggesting some interplay between environmental and endogenous (developmental and physiological) factors. Although this study was not designed to establish a causal relationship between environmental parameters and spawning events, we did use statistical measures to explain inter-annual variation in the spawning days. Our results showed that high SST and low wind speed were clearly correlated with earlier spawning events, where the mean SST and wind speed explained approximately 50% of the deviation from a full moon. A weak correlation was detected between the solar flux four months prior to a full moon and the spawning days.
Correlations between SST and spawning day have been shown in several field observations [13,14,16] and an empirical study [22], all of which reported that high seawater temperature accelerated the spawning day in Acropora corals, which is consistent with our results. SST potentially affects the day of spawning in two ways, namely, the progression of gamete maturation and the proximate trigger for spawning events. In corals, temperature influences gametogenesis and the reproductive cycles, and thus functions as a determinant for the spawning season [2]. The eggs in Acropora species become pigmented (matured) approximately three weeks prior to spawning, and spermatids appear in testes four to six weeks prior to spawning [2]. These periods correspond to time ranges (1) and (2) in the present study, in which SST had a significant effect, suggesting that high SSTs accelerate the development of gametes and subsequently the day of spawning. Several studies have proposed that hormone pathways and G protein-coupled receptors are involved in gamete release [11,23–25], which awaits further studies in light of the role of SST.
Keith et al. [10] found that wind speeds were weakly correlated with the month of spawning in Acropora spp. in the Indo-Pacific, such that spawning is more probable when wind speeds are at intermediate levels [10]. Our results also indicated that high winds in the period immediately prior to spawning might delay the night of spawning. One plausible advantage of synchronous spawning is to maximize the probability of fertilization [26–28], which high wind speed and subsequent high wave action can negate, by increasing the dispersal of gametes before fertilization, in addition to the formation of white caps that can damage naked coral embryos floating at the sea surface [29]. Corals may sense water movements and regulate their spawning behaviours to avoid periods of high wave energy. In addition, strong winds leading high waves and surface currents may change the mixed layer depth as well as sunlight reflectivity, and thus affect seawater temperature. Interaction of these factors will be a subject of future empirical studies.
Solar flux is related to the energy acquisition of coral via the photosynthesis of symbiotic algae, which may accelerate the maturation of gametes and spawning days. Another possibility is that increasing solar flux at time range (4) affected the subsequent increase in SST at time ranges (1) and (2) and indirectly accelerated the spawning days. In our study, the SST was found to lag the solar flux cycle at all the seven reefs examined (electronic supplementary material, figure S8). The difference in two fluctuating factors (SST and solar flux) may account for the effects of these factors at different time ranges.
Overall, our data are consistent with the hypothesis that Acropora have the capacity to fine-tune the timing of spawning, by adjusting their development and physiology in response to the environmental factors, potentially leading to increases in reproductive success and post-fertilization survival rates. Recent climate change has been shown to have significant impacts on coral reef ecosystems [30–32], e.g. rising SST due to global warming may cause the mass bleaching of corals [33,34] and is likely to go along with unpredictable effects on environmental factors. The bleaching can lead to detrimental effects on coral reproduction such as decreasing number of gonads in polyp, reduction of gravid colonies and impaired sperm motility [35–37], and the negative effects on the reproduction may persist for several years [38]. Moreover, a recent study has reported that the spawning synchrony of Acropora eurystoma has been attenuated in the Red Sea, which might result in reducing the probability of fertilization success [39]. This study provides a basic framework for further data-driven studies to understand the responses of corals to environmental changes as well as the underlying mechanisms that regulate their reproductive cycles.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Prof. Andrew Baird for his assistance in accessing unpublished data used in the manuscript preparation as well as for reviewing the manuscript and for his helpful comments. The Coral Spawning Working Group is also acknowledged for providing the access to the data. We also thank Tomoko Hamabata, Yuu Ishii, Watal M. Iwasaki and Takuro Nakayama for their assistance in analysing the data.
Data accessibility
Data are provided as electronic supplementary material.
Authors' contributions
Y.S. carried out the data collection and statistical analyses, and drafted the manuscript; M.H. collected field data and participated in data analysis, and critically revised the manuscript; S.F. carried out the statistical analyses and critically revised the manuscript; M.K. and N.U. participated in the design of the study and data analysis, and critically revised the manuscript; S.M. conceived of the study, designed the study, coordinated the study and drafted the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by JSPS KAKENHI grant no. JP19K06786 (to S.M.).
References
- 1.Harrison PL, Babcock RC, Bull GD, Oliver JK, Wallace CC, Willis BL. 1984. Mass spawning in tropical reef corals. Science 223, 1186–1189. ( 10.1126/science.223.4641.1186) [DOI] [PubMed] [Google Scholar]
- 2.Babcock RC, Bull GD, Harrison PL, Heyward AJ, Oliver JK, Wallace CC, Willis BL. 1986. Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar. Biol. 90, 379–394. ( 10.1007/BF00428562) [DOI] [Google Scholar]
- 3.Hayashibara T, Shimoike K, Kimura T, Hosaka S, Heyward A, Harrison P, Kudo K, Omori M. 1993. Patterns of coral spawning at Akajima Island, Okinawa, Japan. Mar. Ecol. Prog. Ser. 101, 253–262. ( 10.3354/meps101253) [DOI] [Google Scholar]
- 4.Willis BL, Babcock RC, Harrison PL, Oliver TK. 1985. Patterns in the mass spawning of corals on the Great Barrier Reef from 1981 to 1984. Proc. 5th. Int. Coral Reef Congr. 4, 343–348. [Google Scholar]
- 5.Oliver JK, Babcock RC, Harrison PL, Willis BL. 1988. Geographic extent of mass coral spawning: clues to ultimate causal factors. Proc. 6th. Int. Coral Reef Symp. 2, 803–810. [Google Scholar]
- 6.Mendes JM, Woodley JD. 2002. Timing of reproduction in Montastraea annularis: relationship to environmental variables. Mar. Ecol. Prog. Ser. 227, 241–251. ( 10.3354/meps227241) [DOI] [Google Scholar]
- 7.Penland L, Kloulechad J, Idip D, Van Woesik R. 2004. Coral spawning in the western Pacific Ocean is related to solar insolation: evidence of multiple spawning events in Palau. Coral Reefs 23, 133–140. ( 10.1007/s00338-003-0362-x) [DOI] [Google Scholar]
- 8.Van Woesik R, Lacharmoise F, Köksal S. 2006. Annual cycles of solar insolation predict spawning times of Caribbean corals. Ecol. Lett. 9, 390–398. ( 10.1111/j.1461-0248.2006.00886.x) [DOI] [PubMed] [Google Scholar]
- 9.Van Woesik R. 2010. Calm before the spawn: global coral spawning patterns are explained by regional wind fields. Proc. R. Soc. B 277, 715–722. ( 10.1098/rspb.2009.1524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keith SA, et al. 2016. Coral mass spawning predicted by rapid seasonal rise in ocean temperature. Proc. R. Soc. B 283, 20160011 ( 10.1098/rspb.2016.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashibara T, Iwao K, Omori M. 2004. Induction and control of spawning in Okinawan staghorn corals. Coral Reefs 23, 406–409. ( 10.1007/s00338-004-0406-x) [DOI] [Google Scholar]
- 12.Brady AK, Hilton JD, Vize PD. 2009. Coral spawn timing is a direct response to solar light cycles and is not an entrained circadian response. Coral Reefs 28, 677–680. ( 10.1007/s00338-009-0498-4) [DOI] [Google Scholar]
- 13.Shimoike K. 1999. [Observation of coral spawning in Akajima Island with newly found spawning patterns]. Midoriishi 10, 29–31. [In Japanese.] [Google Scholar]
- 14.Nozawa Y. 2012. Annual variation in the timing of coral spawning in a high-latitude environment: influence of temperature. Biol. Bull. 222, 192–202. ( 10.1086/BBLv222n3p192) [DOI] [PubMed] [Google Scholar]
- 15.Lin CH, Nozawa Y. 2017. Variability of spawning time (lunar day) in Acropora versus merulinid corals: a 7-yr record of in situ coral spawning in Taiwan. Coral Reefs 36, 1269–1278. ( 10.1007/s00338-017-1622-5) [DOI] [Google Scholar]
- 16.Fujiwara S, Kezuka D, Ishimizu H, Tabata S, Nojima S. 2015. [Condition for mass spawning of scleractinian coral Acropora in the Sekisei Lagoon, Ryukyu Islands]. Bull. Jpn. Soc. Fish. Ocean. 79, 130–140. [In Japanese.] [Google Scholar]
- 17.Reynolds RW, Smith TM, Liu C, Chelton DB, Casey KS, Schlax MG. 2007. Daily high-resolution-blended analyses for sea surface temperature. J. Clim. 20, 5473–5496. ( 10.1175/2007JCLI1824.1) [DOI] [Google Scholar]
- 18.Rutan DA, Kato S, Doelling DR, Rose FG, Nguyen LT, Caldwell TE. 2015. CERES synoptic product: methodology and validation of surface radiant flux. J. Atmos. Ocean. Technol. 32, 1121–1143. ( 10.1175/jtech-d-14-00165.1) [DOI] [Google Scholar]
- 19.Zuur AF, Ieno EN, Elphick CS. 2010. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 1, 3–14. ( 10.1111/j.2041-210X.2009.00001.x) [DOI] [Google Scholar]
- 20.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 21.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 22.Paxton CW, Baria MVB, Weis VM, Harii S. 2016. Effect of elevated temperature on fecundity and reproductive timing in the coral Acropora digitifera. Zygote 24, 511–516. ( 10.1017/S0967199415000477) [DOI] [PubMed] [Google Scholar]
- 23.Twan W-H, Hwang J-S, Lee Y-H, Wu H-F, Tung Y-H, Chang C-F. 2006. Hormones and reproduction in scleractinian corals. Comp. Biochem. Physiolosy ParA Mol. Integr. Physiol. 144, 247–253. ( 10.1016/j.cbpa.2006.01.011) [DOI] [PubMed] [Google Scholar]
- 24.Kaniewska P, Alon S, Karako-Lampert S, Hoegh-Guldberg O, Levy O. 2015. Signaling cascades and the importance of moonlight in coral broadcast mass spawning. Elife 4, e09991 ( 10.7554/eLife.09991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg Y, Doniger T, Harii S, Sinniger F, Levy O. 2017. Canonical and cellular pathways timing gamete release in Acropora digitifera, Okinawa, Japan. Mol. Ecol. 26, 2698–2710. ( 10.1111/mec.14062) [DOI] [PubMed] [Google Scholar]
- 26.Pierson WJ, Moskowitz L. 1964. A proposed spectral form for fully developed wind seas based on the similarity theory of S. A. Kitaigorodskii. J. Geophys. Res. 69, 5181–5190. ( 10.1029/jz069i024p05181) [DOI] [Google Scholar]
- 27.Levitan DR, Fukami H, Jara J, Kline D, McGovern TM, McGhee KE, Swanson CA, Knowlton N. 2004. Mechanisms of reproductive isolation among sympatric broadcast-spawning corals of the Montastraea annularis species complex. Evolution 58, 308–323. ( 10.1111/j.0014-3820.2004.tb01648.x) [DOI] [PubMed] [Google Scholar]
- 28.Baird AH, Guest JR, Willis BL. 2009. Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu. Rev. Ecol. Evol. Syst. 40, 551–571. ( 10.1146/annurev.ecolsys.110308.120220) [DOI] [Google Scholar]
- 29.Heyward AJ, Negri AP. 2012. Turbulence, cleavage, and the naked embryo: a case for coral clones. Science 335, 1064 ( 10.1126/science.1216055) [DOI] [PubMed] [Google Scholar]
- 30.Spalding MD, Brown BE. 2015. Warm-water coral reefs and climate change. Science 350, 769–771. ( 10.1126/science.aad0349) [DOI] [PubMed] [Google Scholar]
- 31.Heron SF, Maynard JA, Van Hooidonk R, Eakin CM. 2016. Warming trends and bleaching stress of the world's coral reefs 1985-2012. Sci. Rep. 6, 38402 ( 10.1038/srep38402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes TP, et al. 2003. Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933. ( 10.1126/science.1085046) [DOI] [PubMed] [Google Scholar]
- 33.Hughes TP, et al. 2017. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. ( 10.1038/nature21707) [DOI] [PubMed] [Google Scholar]
- 34.Hughes TP, et al. 2018. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83. ( 10.1126/science.aan8048) [DOI] [PubMed] [Google Scholar]
- 35.Baird AH, Marshall PA. 2002. Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 237, 133–141. ( 10.3354/meps237133) [DOI] [Google Scholar]
- 36.Mendes JM, Woodley JD. 2002. Effect of the 1995–1996 bleaching event on polyp tissue depth, growth, reproduction and skeltal band formation in Montastraea annularis. Mar. Ecol. Progr. Ser. 235, 93–102. ( 10.3354/meps235093) [DOI] [Google Scholar]
- 37.Hagedorn M, Carter VL, Lager C, Camperio Ciani JF, Dygert AN, Schleiger RD, Henley EM. 2016. Potential bleaching effects on coral reproduction. Reprod. Fertil. Dev. 28, 1061–1071. ( 10.1071/RD15526) [DOI] [Google Scholar]
- 38.Levitan DR, Boudreau W, Jara J, Knowlton N. 2014. Long-term reduced spawning in Orbicella coral species due to temperature stress. Mar. Ecol. Prog. Ser. 515, 1–10. ( 10.3354/meps11063) [DOI] [Google Scholar]
- 39.Shlesinger T, Loya Y. 2019. Breakdown in spawning synchrony: a silent threat to coral persistence. Science 365, 1002–1007. ( 10.1126/science.aax0110) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are provided as electronic supplementary material.