Abstract
Cooperative breeding animals frequently inhabit harsh environments. It is widely accepted that harsh environments hinder independent reproduction, and this constraint maintains individuals in family groups. Yet the assumption that harsh ecological conditions reduce the success of members of cooperative breeding groups when breeding independently has not been experimentally tested. We addressed this shortcoming using the socially polymorphic Alpine silver ant, Formica selysi. This species has single-queen (independent breeders) and multiple-queen (cooperative breeders) colonies coexisting within populations. We placed newly mated queens emerging from each type of colony to breed alone in either a harsh or mild winter condition and recorded their brood production and survival. Queens emerging from single-queen colonies were unaffected by the winter condition and were more successful at founding a nest independently than queens from multiple-queen colonies. By contrast, queens from multiple-queen colonies had higher mortality after a harsh than after a mild winter. These results support the long-held assumption that harsh environments constrain independent reproduction of members of cooperative breeding groups.
Keywords: cooperative breeding, ecological constraints, hibernation, ants, social polymorphism
1. Introduction
Many animals live in cooperative breeding groups, where offspring delay dispersal and help their parents produce siblings, or where multiple adults share reproduction [1–3]. Explaining the evolutionary emergence and maintenance of cooperative breeding have been longstanding quests in evolutionary biology, because individuals staying in parental nests often partially or completely forgo their own reproduction [1,4,5]. Theoretically, extreme environmental harshness constrains independent breeding, keeping offspring with their parents [2,6]. Correspondingly, cooperative breeders typically live in unpredictable and harsh environments [3,5,7–14]. It is generally accepted that individuals that live in family groups have become less successful at breeding solitarily than individuals adapted to reproducing alone [15–17], especially in harsh environments [1,2,6]. Although a reduced ability to breed alone in harsh environments would represent a selective pressure for the maintenance of cooperative breeding, it has not been experimentally demonstrated.
We addressed this shortcoming with the Alpine silver ant, Formica selysi. This species has two types of colonies coexisting within populations: some queens breed alone, forming single-queen colonies, whereas others breed cooperatively, forming multiple-queen colonies [18,19]. Social organization is controlled by a supergene [20]. Queens and workers from single-queen colonies are larger than those from multiple-queens colonies [18,21], while multiple-queen colonies have three times as many workers, and enjoy three times the lifespan of a single-queen colony [18]. F. selysi is an ideal system to test the costs of breeding alone in harsh environments for cooperative breeders, as queens from both social forms can found colonies independently [22], unlike queens from some cooperative breeding ant species, which have lost their ability to reproduce alone [15,17,23]. Furthermore, their small size allows the ecological conditions to be manipulated in the laboratory. Because there is a genetic basis to colony structure, we can compare the success of individuals adapted to living alone or to living in family groups, when reproducing independently. Although mature colonies of the cooperative breeding form always host multiple queens [20,24], young queens emerging from these colonies exhibit plasticity in whether they fly away from their maternal colony and breed independently or seek re-adoption [18,19,22,24]. This flexibility allows us to evaluate if harsh conditions constrain the independent reproduction of individuals that typically stay in family groups.
We experimentally mated young queens originating from each type of colony and placed them to breed alone in either a mild or a harsh winter condition. We tested two predictions. First, individuals adapted to stay in family groups have lower success when reproducing alone than individuals adapted to breeding independently (e.g. [16,17]). For this, we compared the success of queens emerging from single and from multiple-queen colonies. We predicted that the former would have higher survival than the later, particularly after a harsh winter. Second, ecological harshness reduces the success of members of cooperative breeding groups when reproducing alone. For this, we compared the success of queens emerging from multiple-queen colonies after a harsh or after a mild winter. We predicted that they would have lower survival after a harsh winter.
2. Material and methods
(a). Field collection
We collected sexual pupae and workers from a high-altitude (Derborence: 46.2806° N, 7.2157° E; 1450 m a.s.l.) and a low-altitude population (Finges, 46.3138° N, 7.6012° E; 400 m a.s.l.) in Switzerland, from marked colonies of known social form, determined via SNP genotyping [20]. Both forms inhabit both populations [25]. We kept them inside plastic boxes (15.5 × 13.5 × 5.5 cm) lined with Fluon, and a glass tube (length = 16 cm; ø = 5 mm), one-third filled with water. We fed each colony with sugar and egg-jelly.
(b). Mating
We placed young queens and non-nest-mate males from the same population, from either the same or the alternative social form, inside a plastic box (26.5 × 42 × 20 cm), with a meshed lid. We housed each queen inside a glass tube (length = 16 cm; ø = 5 mm), one-third filled with water. This mimicked claustral colony-founding conditions, when queens rely on their fat and protein reserves, and histolysed flight muscles to produce their first brood [23]. After a month we transferred them alongside their brood into a plastic box (10.5 × 13.5 × 5.5 cm) filled with approximately 300 g of sand (Spielsand, Collibri®), and provided them with water and food ad libitum. At this point, workers would procure food for their queens in nature. We followed the same procedure with some virgin queens to increase the survival sample size (table 1).
Table 1.
Sample sizes in the two stages of the experiment and two hibernation treatments (mild or harsh winter conditions). The number of queens is indicated with respect to the queen social origin, mate social origin (single-queen: s-q, multiple-queen: m-q), for low-elevation population (Finges, left) and high-elevation population (Derborence, right), at the start of the experiment (top), and in the hibernation phase of the experiment (bottom). The number of colonies from which queens originated is indicated in parentheses.
| start of the experiment (June, 2017) | ||||||||
|---|---|---|---|---|---|---|---|---|
| low elevation (Finges) |
high elevation (Derborence) |
|||||||
| queen social origin: | single-queen | multiple-queen | single-queen | multiple-queen | ||||
| mated to: | ||||||||
| s-q male | 46 (12) | 3 (1) | 119 (24) | 36 (14) | ||||
| m-q male | 14 (7) | 6 (3) | 279 (33) | 139 (26) | ||||
| unknown | 6 (6) | 0 | 17 (5) | 6 (6) | ||||
| virgin queens | 41 (18) | 3 (3) | 82 (33) | 71 (25) | ||||
| total number of queens | 107 | 12 | 497 | 252 | ||||
| in hibernation (November, 2017) | ||||||||
|---|---|---|---|---|---|---|---|---|
| queen social origin: | single-queen |
multiple-queen |
single-queen |
multiple-queen |
||||
| winter condition: | mild | harsh | mild | harsh | mild | harsh | mild | harsh |
| mated to: | ||||||||
| s-q male | 15 (10) | 16 (8) | 0 | 0 | 28 (14) | 27 (13) | 5 (4) | 6 (5) |
| m-q male | 4 (4) | 5 (4) | 1 | 1 | 72 (27) | 75 (28) | 35 (18) | 33 (16) |
| unknown | 2 (2) | 3 (3) | 0 | 0 | 1 | 1 | 0 | 2 (2) |
| virgin queens | 13 (9) | 15 (12) | 1 | 0 | 18 (13) | 21 (18) | 18 (13) | 14 (12) |
| total number of queens | 34 | 39 | 2 | 1 | 119 | 124 | 58 | 55 |
(c). Hibernation in mild or harsh winter conditions
Approximately five months after the beginning of the experiment (when snow falls in nature), we placed all queens and their brood in controlled-climate chambers. We removed their food, and pseudo-randomly divided all queens into two equal-sized treatment groups (considering their mating status, social origin, mate social origin and population; table 1). The temperature dropped 1–2° weekly (humidity: 70%), until 8°C (mild winter) or 3°C (harsh winter). After two weeks the temperature increased weekly until 22°C. At the end of the experiment (after 1 year), we counted the number of queens alive and their workers and pupae.
(d). Statistical analyses: general procedures
Analyses were carried out in R [26] v. 3.5.1, with generalized linear mixed models (glmm) (‘glmer’ function [27]), unless otherwise stated. We always included as covariables the population, the date of mating as a continuous variable, and their colony of origin as a random effect. Non-significant interactions were removed one by one, based on their significance level. All other variables were kept in the models. We verified that there was no over-dispersion [28], and evaluated the residuals (‘DHARMa’ [29]). We obtained estimates with type III SS for models with interactions (‘Anova’ function [30]), and estimates, s.e. and p values with the ‘summary’ function for models without interactions [26]. We did post hoc analyses with FDR p-value adjustment (‘lsmeans’ function [31]). Analyses and results regarding queens' survival and reproductive success before winter can be found in the electronic supplementary material.
(e). Queens’ success after mild or harsh winters
We compared queen survival from the start of hibernation (after five months) until the end of the experiment (after 1 year) with a glmm with binomial error distribution. We included the social origin of the queen, the winter condition and the interaction. We included potentially confounding covariables, including the population or origin, the interaction between the winter condition and the population, and the mating status of the queen. We repeated this analysis with mated queens only and included the social origin of her mate, and the number of workers she had before winter as covariables instead of her mating status.
We compared the overall success of queens at founding a nest using a glmm with binomial error distribution, considering as successful only queens that survived and had a brood at the end of the experiment (n = 222). Finally, we used the size of the brood at the end of the experiment as a response variable in a MCMC generalized mixed effects model to account for over-dispersion [28,32,33] with zero-truncated Poisson error distribution. We used (uninformative) inverse-Wishart priors for the random effects and residual variance (V = 1; nu = 0.002), set the number of iterations to 4 100 000, preceded by a burn-in of 100 000, and saved every 2000th iterations to avoid autocorrelation between draws. We verified that the chain converged and that all autocorrelations were below 0.1 [32,33].
3. Results
(a). Queens’ success after mild or harsh winters
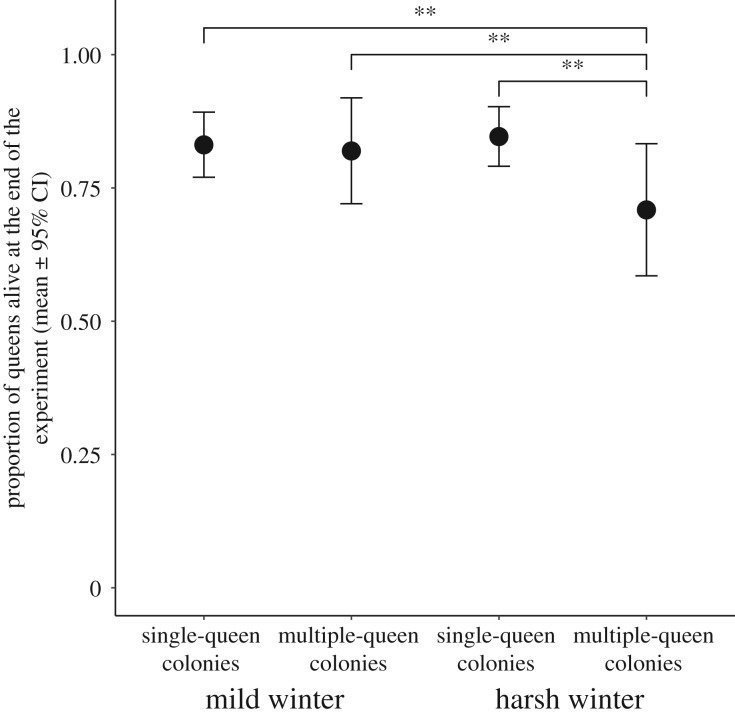
The harsh winter condition decreased the survival of queens from multiple-queen colonies, whereas it had no impact on queens from single-queen colonies (interaction between social origin and winter condition: χ2 = 3.92; p = 0.04; figure 1). A higher proportion of queens from multiple-queen colonies died after a harsh winter, compared to queens from single-queen colonies after a harsh winter (post hoc comparison: estimate = −1.38; s.e. = 0.42; z = −3.27; p = 0.003). Similarly, a higher proportion of queens from multiple-queen colonies died after a harsh winter, compared to queens from single-queen colonies after a mild winter (post hoc comparison: estimate = −1.75; s.e. = 0.50; z = −3.48; p = 0.002). A higher proportion of queens from multiple-queen colonies died after a harsh than after a mild winter (post hoc comparison: estimate = −1.52; s.e. = 0.58; z = −2.59; p = 0.01). Queens emerging from multiple-queen colonies had similar survival after a mild winter as queens from single-queen colonies after a harsh or mild winter (post hoc comparisons: p = 0.77 and p = 0.70, respectively). Queens from single-queen colonies were unaffected by the winter condition (post hoc comparison: p = 0.52). Similarly, when considering mated queens only, the harsh winter decreased the survival of queens from multiple-queen colonies but had no impact on the survival of queens from single-queen colonies (interaction: χ2 = 4.38; p = 0.03; post hoc comparison: estimate = −1.60; s.e. = 0.51; z = −3.09; p = 0.01). Differences in survival were not due to potential confounding factors, like queen mating status (interaction: χ2 = 2.30; p = 0.12; effect of her mating status: estimate(virgin) = 0.63; s.e. = 0.34; z = 1.82; p = 0.06), population (see electronic supplementary material), or, for mated queens, her mate social origin (p = 0.36), or her number of workers (p = 0.42).
Figure 1.
Survival of queens from the start of hibernation to the end of the experiment, according to their social origin (single or multiple-queen colonies) and experimental winter condition (mild or harsh). Queens emerging from multiple-queen colonies had higher mortality after a harsh than after a mild winter condition and had higher mortality than queens from single-queen colonies in both winter conditions. Post hoc FDR corrected-p** = <0.01. Other groups did not differ.
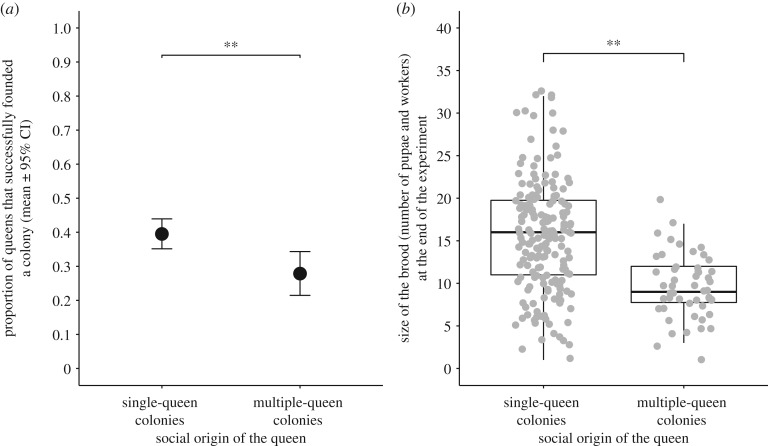
Queens from single-queen colonies were more successful at founding a nest than queens from multiple-queen colonies (estimate = 0.62; s.e. = 0.25; z = 2.48; p = 0.01; figure 2a) and had larger broods at the end of the experiment (posterior mean = 0.47; 95% credible intervals = 0.29–0.64; pMCMC < 0.0005; figure 2b). Differences in nest-founding success and brood size were not due to potential confounding factors like the social origin of their mate (nest founding success: interaction: χ2 = 2.15; p = 0.14; effect of the male: p = 0.64; effect of the male on brood size: pMCMC = 0.63), their population, whether they mated earlier or later in the season, and for brood size, the winter condition (all p or pMCMC > 0.17).
Figure 2.
(a) Proportion of queens that successfully founded a colony throughout the experiment. **p = 0.02. These data include queens that died before winter. (b) Size of the brood at the end of the experiment. Each point represents a queen. Only live queens with at least one worker are included. The data inside each box include the median and the 1st and 3rd quartiles. **MCMCglmm-p < 0.001. The winter condition did not predict brood size.
4. Discussion
The evolution and maintenance of cooperative breeding have long been evolutionary conundrums, as individuals staying in parental groups usually forgo at least partially their own reproduction [1,4,23]. Cooperative breeding species typically live in harsh and unpredictable environments [5,7–9,13,14]. It is assumed that harsh environments hinder independent reproduction, helping retain cooperative breeders in family groups [2,6]. While a lower ability to breed alone in harsh environments would represent a selective pressure for the maintenance of cooperative breeding, it had not been experimentally investigated. Here, we show that colder winters reduce the survival of queens that typically breed cooperatively, when they attempt to breed alone. Hence, our study provides empirical evidence that harsh environments hinder independent reproduction of cooperative breeding animals. Our experiment also shows that queens that typically breed alone are more successful at breeding independently than queens that typically reproduce cooperatively, as expected for genetically determined social forms.
Queens from multiple-queen colonies belong to a genetically determined social form and had a long evolutionary history of cooperative breeding and are likely adapted to reproducing in families (although they can reproduce independently [19,22]). While we think our results generalize to other cooperative breeding species, including vertebrates, we acknowledge that this remains an open empirical question, and the results may depend on each species' life history. Although we did not test this, queens from multiple-queen colonies would probably have higher survival in a harsh environment when staying with their families than when reproducing alone. Previous studies have shown that large ant colonies survive winters better than small colonies [10,11,34], that workers provide food and protection to queens [23], and that they thermoregulate the nest [35,36].
In many species multiple-queen colonies are more common in high-altitude/latitude populations, which has been attributed to a greater mortality risk when breeding independently in these sites [10–14]. Surprisingly, multiple-queen colonies in our species are rarer and have fewer queens at higher altitude [25]. This pattern may be caused by source–sink or extinction–recolonization dynamics [37], with high-elevation populations being ephemeral and at the margin of the distribution [25]. Under this view, the larger queens emerging from single-queen colonies [21] may regularly disperse from low-elevation and colonize high-elevation populations, while queens from multiple-queen colonies may fail to disperse from low-elevation and establish colonies alone in high-elevation populations. Accordingly, our experiment revealed that colder winters reduced the success of queens originating from low-elevation populations compared to queens from high elevation (see electronic supplementary material, figure S1), and of queens from multiple-queen colonies compared to queens from single-queen colonies. Additionally, high-elevation populations may be too fragmented or too ephemeral to sustain large multiple-queen colonies.
To summarize, our results provide empirical support to the long-held assumption that harsh environments limit the success of independent reproduction of individuals that typically stay at home [1–4]. This need not mean that cooperative breeding evolved as a response to harsh environments. In fact, ancestral state reconstructions in the bird clade show that cooperative breeding evolves before colonizing harsh environments [5]. What drove the evolution of cooperative breeding in ants remains unknown. But independently of the evolutionary route leading to cooperative breeding, our results suggest that once individuals are adapted to staying with their families, they lose at least partially their ability to reproduce independently. This is particularly true in harsh environments, where the challenges of life alone are intensified.
Supplementary Material
Acknowledgements
We thank Sagane Dind, Jason Buser and Julie Guenat for their help with laboratory work, and Shana Caro, Santiago Herce Castañón and three anonymous reviewers for their constructive feedback on the manuscript.
Ethics
This work complied with the legal requirements of the University of Lausanne and Switzerland. F. selysi is not an endangered species.
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.0vt4b8gv0 [38].
Authors' contribution
O.D.G. designed the experiment. O.D.G., P.B. and G.G. performed the experiment. O.D.G. analysed the data. All authors discussed the interpretation of the results and wrote the paper and agree to be held accountable for all aspects of the work and for the content therein and approve the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Swiss National Science Foundation (grant no. 31003A-173189/1).
References
- 1.Koenig WD, Dickinson JL. 2016. Cooperative breeding in vertebrates: studies of ecology, evolution, and behavior. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Emlen ST. 1982. The evolution of helping. I. An ecological constraints model. Am. Nat. 119, 29–39. ( 10.1086/283888) [DOI] [Google Scholar]
- 3.Keller L. 1995. Social life: the paradox of multiple-queen colonies. Trends Ecol. Evol. 10, 355–360. ( 10.1016/S0169-5347(00)89133-8) [DOI] [PubMed] [Google Scholar]
- 4.Reeve HK, Keller L. 2001. Tests of reproductive-skew models in social insects. Annu. Rev. Entomol. 46, 347–385. ( 10.1146/annurev.ento.46.1.347) [DOI] [PubMed] [Google Scholar]
- 5.Cornwallis CK, Botero CA, Rubenstein DR, Downing PA, West SA, Griffin AS. 2017. Cooperation facilitates the colonization of harsh environments. Nat. Ecol. Evol. 1, 0057 ( 10.1038/s41559-016-0057) [DOI] [PubMed] [Google Scholar]
- 6.McLeod DV, Wild G. 2014. The relationship between ecology and the optimal helping strategy in cooperative breeders. J. Theor. Biol. 354, 25–34. ( 10.1016/j.jtbi.2014.03.003) [DOI] [PubMed] [Google Scholar]
- 7.Rubenstein DR, Lovette IJ. 2007. Temporal environmental variability drives the evolution of cooperative breeding in birds. Curr. Biol. 17, 1414–1419. ( 10.1016/j.cub.2007.07.032) [DOI] [PubMed] [Google Scholar]
- 8.Jetz W, Rubenstein DR. 2011. Environmental uncertainty and the global biogeography of cooperative breeding in birds. Curr. Biol. 21, 72–78. ( 10.1016/j.cub.2010.11.075) [DOI] [PubMed] [Google Scholar]
- 9.Lukas D, Clutton-Brock T. 2017. Climate and the distribution of cooperative breeding in mammals. R. Soc. open sci. 4, 160897 ( 10.1098/rsos.160897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiroto A, Satoh T, Hirota T. 2011. The importance of workers for queen hibernation survival in Camponotus ants. Zool. Sci. 28, 327–331. ( 10.2108/zsj.28.327) [DOI] [PubMed] [Google Scholar]
- 11.Shiroto A, Satoh T, Hirota T. 2014. Polygyny increases survival of minor workers and mortality of major workers in overwintering Camponotus yamaokai (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 107, 702–707. ( 10.1603/AN13063) [DOI] [Google Scholar]
- 12.Bourke AF, Heinze J. 1994. The ecology of communal breeding: the case of multiple-queen leptothoracine ants. Phil. Trans. R. Soc. Lond. B 345, 359–372. ( 10.1098/rstb.1994.0115) [DOI] [Google Scholar]
- 13.Heinze J, Rueppell O. 2014. The frequency of multi-queen colonies increases with altitude in a Nearctic ant. Ecol. Entomol. 39, 527–529. ( 10.1111/een.12119) [DOI] [Google Scholar]
- 14.Heinze J. 1993. Life histories of subarctic ants. Arctic 46, 354–358. ( 10.14430/arctic1363) [DOI] [Google Scholar]
- 15.Schrempf A, Heinze J. 2007. Back to one: consequences of derived monogyny in an ant with polygynous ancestors. J. Evol. Biol. 20, 792–799. ( 10.1111/j.1420-9101.2006.01235.x) [DOI] [PubMed] [Google Scholar]
- 16.Keller L, Passera L. 1989. Size and fat content of gynes in relation to the mode of colony founding in ants (Hymenoptera; Formicidae). Oecologia 80, 236–240. ( 10.1007/BF00380157) [DOI] [PubMed] [Google Scholar]
- 17.Stille MJO. 1996. Queen/worker thorax volume ratios and nest-founding strategies in ants. Oecologia 105, 87–93. [DOI] [PubMed] [Google Scholar]
- 18.Rosset H, Chapuisat M. 2006. Alternative life-histories in a socially polymorphic ant. Evol. Ecol. 21, 577–588. ( 10.1007/s10682-006-9139-3) [DOI] [Google Scholar]
- 19.Chapuisat M, Bocherens S, Rosset H. 2004. Variable queen number in ant colonies: no impact on queen turnover, inbreeding, and population genetic differentiation in the ant Formica selysi. Evolution 58, 1064–1072. ( 10.1111/j.0014-3820.2004.tb00440.x) [DOI] [PubMed] [Google Scholar]
- 20.Purcell J, Brelsford A, Wurm Y, Perrin N, Chapuisat M. 2014. Convergent genetic architecture underlies social organization in ants. Curr. Biol. 24, 2728–2732. ( 10.1016/j.cub.2014.09.071) [DOI] [PubMed] [Google Scholar]
- 21.Meunier J, Chapuisat M. 2009. The determinants of queen size in a socially polymorphic ant. J. Evol. Biol. 22, 1906–1913. ( 10.1111/j.1420-9101.2009.01805.x) [DOI] [PubMed] [Google Scholar]
- 22.Reber A, Meunier J, Chapuisat M. 2010. Flexible colony-founding strategies in a socially polymorphic ant. Anim. Behav. 79, 467–472. ( 10.1016/j.anbehav.2009.11.030) [DOI] [Google Scholar]
- 23.Bourke AF, Franks NR. 1995. Social evolution in ants. Princeton, NJ: Princeton University Press. [Google Scholar]
- 24.Avril A, Purcell J, Brelsford A, Chapuisat M. 2019. Asymmetric assortative mating and queen polyandry are linked to a supergene controlling ant social organization. Mol. Ecol. 28, 1428–1438. ( 10.1111/mec.14793) [DOI] [PubMed] [Google Scholar]
- 25.Purcell J, Pellissier L, Chapuisat M. 2015. Social structure varies with elevation in an Alpine ant. Mol. Ecol. 24, 498–507. ( 10.1111/Mec.13042) [DOI] [PubMed] [Google Scholar]
- 26.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 27.Bates D, Maechler M, Bolker B. 2012. lme4: linear mixed-effects models using S4 classes. R package version 0.999375-42. 2011.
- 28.Zuur A, Ieno E, Walker N, Saveliev A, Smith G. 2009. Mixed effects models and extensions in ecology. New York, NY: Spring Science and Business Media. [Google Scholar]
- 29.Hartig F. 2018. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. See http://florianhartig.github.io/DHARMa/.
- 30.Fox J, et al. 2012. Package ‘car’. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 31.Lenth RV. 2016. Least-squares means: the R package lsmeans. J. Stat. Softw. 69, 1–33. ( 10.18637/jss.v069.i01) [DOI] [Google Scholar]
- 32.Hadfield J. 2017. MCMCglmm Course Notes https://cran.r-project.org/web/packages/MCMCglmm/vignettes/CourseNotes.pdf.
- 33.Hadfield J. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 34.Markin GP, Dillier JH, Collins H. 1973. Growth and development of colonies of the red imported fire ant, Solenopsis invicta. Ann. Entomol. Soc. Am. 66, 803–808. ( 10.1093/aesa/66.4.803) [DOI] [Google Scholar]
- 35.Jones JC, Oldroyd BP. 2006. Nest thermoregulation in social insects. Adv. Insect Physiol. 33, 153–191. ( 10.1016/S0065-2806(06)33003-2) [DOI] [Google Scholar]
- 36.Kadochová Š, Frouz J. 2013. Thermoregulation strategies in ants in comparison to other social insects, with a focus on red wood ants (Formica rufa group). F1000Res. 2, 280 ( 10.12688/f1000research.2-280.v2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pulliam HR. 1988. Sources, sinks, and population regulation. Am. Nat. 132, 652–661. ( 10.1086/284880) [DOI] [Google Scholar]
- 38.De Gasperin O, Blacher P, Grasso G, Chapuisat M. 2020. Data from: Winter is coming: harsh environments limit independent reproduction of cooperative-breeding queens in a socially polymorphic ant Dryad Digital Repository. ( 10.5061/dryad.0vt4b8gv0) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- De Gasperin O, Blacher P, Grasso G, Chapuisat M. 2020. Data from: Winter is coming: harsh environments limit independent reproduction of cooperative-breeding queens in a socially polymorphic ant Dryad Digital Repository. ( 10.5061/dryad.0vt4b8gv0) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.0vt4b8gv0 [38].