Abstract
Reprogramming of adult somatic cells into induced pluripotent stem cells (iPSCs) has revolutionized the complex scientific field of disease modelling and personalized therapy. Cardiac differentiation of human iPSCs into cardiomyocytes (hiPSC-CMs) has been used in a wide range of healthy and disease models by deriving CMs from different somatic cells. Unfortunately, hiPSC-CMs have to be improved because existing protocols are not completely able to obtain mature CMs recapitulating physiological properties of human adult cardiac cells. Therefore, improvements and advances able to standardize differentiation conditions are needed. Lately, evidences of an epigenetic memory retained by the somatic cells used for deriving hiPSC-CMs has led to evaluation of different somatic sources in order to obtain more mature hiPSC-derived CMs.
Keywords: hiPSC-CMs, epigenetic memory, maturation, cell modelling, drug testing
1. Introduction
Human induced pluripotent stem cells (hiPSCs) have assumed a pivotal role in research since their discovery in 2007 [1]. The possibility to differentiate them into functional cardiomyocytes (hiPSC-CMs) awakened excitement for the potential use of those cells in repairing and regenerating damaged cardiac tissue [2,3]; however, even though hiPSC-CMs represent an autologous source that overcomes the immunological limitations and ethical concerns belonging to embryonic stem cells (ESCs), the risk of tumor formation and uncontrolled differentiation have restricted this kind of approach. The possibility to characterize specific phenotypes associated with patient-specific genotypes allows the use of hiPSC-derived cells for disease modelling and drug development with very promising results [4,5,6].
Several works in the past have reported that hiPSCs are similar to ESCs, but it was recently demonstrated that, because of their somatic origin, epigenetic memory can influence their differentiation and maturation processes [7]. Furthermore, quite a few studies have demonstrated that hiPSC-CMs are molecularly and functionally immature and resemble embryonic and neonatal CMs [8,9,10,11]. Differences in structural morphology, gene and protein expression, as well as calcium handling and ionic patterns, have been described using a time-course of hiPSC-CMs maturation in-vitro; electrical properties and physiology of derived CMs can dramatically change in a time-dependent way, thus leading to the crucial need to optimize time and culture conditions during differentiation [12].
The focus of this review is to raise the issue of the different limitations and strengths affecting hiPSC-CMs derived from different somatic sources by the same patient, with particular attention to the role of cell origin and the advantages of CMs derived from a cardiac source.
2. Reprogramming
The discovery by Takahashi and Yamanaka in 2006 demonstrated that a defined set of factors is able to directly reprogram a somatic cell to an ESC-like state [13]. Out of 24 candidate ESC-associated genes, just four (i.e., Oct4, Sox2, Klf4, and c-Myc) have been determined sufficient to convert fibroblasts to a pluripotent cell type, iPSCs. These four “Yamanaka factors” were first constitutively expressed using retroviral vectors in both mouse [13] and human [1] fibroblasts, inducing these terminally differentiated cells to express genes that are typical of ESCs.
The original iPSCs reprogramming strategy is still being used and remains mostly unaltered, but some advances have been made in the delivery of the four “Yamanaka factors” to improve efficiency. iPSCs have been successfully generated using both integrating and non-integrating methods, but the latter seems to have advantages regarding safety due to a reduced risk of genotoxicity and insertional mutagenesis [14]. Integrating methods include retroviral [13] and lentiviral delivery [15], while non-integrating methods include Sendai viruses [16,17], episomal plasmid transfer [18,19], co-MIP [20], piggyBac transposons [21], small molecules [22], miRNAs [23], and protein-mediated delivery [24].
Many cell types have been successfully reprogrammed to pluripotency, including mononuclear cells from blood [25], umbilical cord and placenta [26], urine-derived cells [27], hair keratinocytes [28] and cardiac progenitor cells [29].
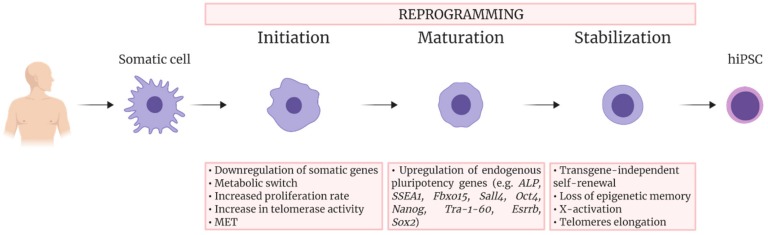
The process to attain pluripotency has been described as consisting of three steps (Figure 1) [30,31]. The first one, called initiation, is characterized by the downregulation of signature somatic genes, a metabolic switch from oxidative phosphorylation to glycolysis, an increase in cell proliferation and reactivation of telomerase activity. This stage also requires changes in cell morphology, in particular a mesenchymal-to-epithelial transition (MET), which involves the acquisition of epithelial characteristics as cell polarity and expression of E-cadherin. These morphological changes are important since it is known that the cell shape itself is involved in epigenetic modifications regulating reprogramming [32].
Figure 1.
Schematic overview of the sequential events occurring during somatic cell reprogramming into human iPSCs. The process consists of three steps, Initiation, Maturation, and Stabilization. The main events occurring during each step are indicated.
The second phase of reprogramming, called maturation, involves the upregulation of endogenous pluripotency genes. These genes include the alkaline phosphatase, SSEA1, Fbxo15, Sall4, Oct4, Nanog, Tra-1-60, Esrrb, and finally Sox2 [33]. The maturation step of reprogramming is likely the cause of the low efficiency of the reprogramming process and, indeed, a great number of cells in this phase undergo apoptosis or reversion [34].
Only 1% of the cells that initiate reprogramming make it to the third and final step, called stabilization; these are the cells that manage to repress transgene expression and activate endogenous pluripotency genes, becoming “stabilization-competent” [35]. Other changes occurring during the stabilization phase involve, for example, rearrangements in DNA methylation [33].
The core pluripotency gene cocktail is constituted by Oct4, Sox2 and Nanog. These transcription factors form a circuitry for pluripotency which is autoregulatory, since all of them are able to regulate the expression of each other. Oct4, Sox2, and Nanog have the ability to activate genes necessary to maintain ESC-like pluripotency and to repress lineage-specific transcription factors, preventing the exit from the pluripotent state [36,37]. Other factors present in reprogramming cocktails, such as c-Myc or Glis1, are used to facilitate activation of this autoregulatory circuitry by stimulating gene expression and proliferation in general [38,39].
The original reprogramming strategy has been widely used, leading improvements in the cell reprogramming process. However, the translation of iPSC to a clinical setting is challenged by many obstacles, such as frequent incomplete reprogramming of the cells. Indeed, there are differences in the transcriptomes of iPSCs and ESCs and this may result from iPSCs either not activating pluripotency genes in the same way ESCs do, or not completely silencing somatic genes [40]. Moreover, de novo mutations may occur during the reprogramming process and the culture of generated iPSCs [41]. The lack of a rapid and precise test to evaluate the level of reprogramming in iPSCs aggravates this challenge.
To overcome these issues, an alternative approach that bypasses the pluripotent stage has been developed. This strategy, called transdifferentiation or direct reprogramming, allows for the reprogramming of one somatic cell type directly into another by delivery of single or multiple specific transcription factors of the desired lineage. Different studies have shown that, with this technique, fibroblasts can be directly converted to several other cell types including neurons [42], cardiomyocytes [43], endothelial cells [44], hepatocytes [45] and chondrocytes [46]. However, in these works transdifferentiation did not always translate to human cells as effectively as it does in murine cells [47]. Recently, it has been reported that fibroblasts from human donors can be efficiently converted to myoblasts by the overexpression of MYOD1 and MYCL [48,49]; the myotubes from this study seem a promising cell source for cell therapy when tested in-vitro, but have yet to be studied in-vivo.
3. Cardiac Differentiation
Cardiovascular diseases (CVDs) are the greatest cause of mortality among non-communicable and communicable diseases [50]. As such, modelling CVDs in vitro is of great importance to better understand these diseases and to develop new drugs and alternative therapies.
Human CMs can be isolated from patient-derived heart tissue specimens, but the possibility to have access to human cardiac biopsies is rare. Moreover, current protocols to obtain adult primary CMs are still technically challenging making it difficult to obtain large quantities of viable cells. Additionally, after 24–48 h of being kept in culture, in the absence of mechanical and electrical stimuli and of supporting cells (i.e., cardiac fibroblasts), CMs undergo de-differentiation, lose their sarcomeric structure and die [51,52].
The possibility to derive hiPSC-CMs, starting from minimally invasive bioptic samples such as skin tissue, enables the creation of an in-vitro disease- and patient-specific model suitable for preclinical drug screening [53,54], thus replacing non-human cellular and animal models. Indeed, there are several challenges with these models, including their poor predictive capacity owing to inter-species differences in cardiac electrophysiology and human biology [55]. In addition, cell lines such as CHO and HEK293 cells are not ideal models for cardiotoxicity because ectopic expression of a cardiac ion channels does not always recapitulate the physiology of human CMs [56,57].
The initial observation that stem cells could mature into beating CMs was reported when ESCs were first cultured in suspension. These cells spontaneously formed three-dimensional aggregates and inside these “embryoid bodies” (EBs) cells with functional and electrical properties of CMs could be found [58]. A similar process occurring with iPSCs was later reported [59]. Even if it is rather inefficient (~1% purity of CMs) and highly cell line-dependent, the EB method is currently being applied because of its simplicity.
Another method for cardiac differentiation was inspired by embryological cardiovascular development, where the anterior endoderm has a central role in the induction of cardiac mesoderm [60,61,62]. This method is based on the coculture of iPSCs with END-2 cells, an endoderm-like cell line from mouse carcinoma cells, which may result in the formation of beating clusters [61,63,64,65,66,67,68,69,70]. The preparations resulting from this protocol have a 20–25% purity of CMs.
Different signaling pathways and growth factors have been found involved in successfully inducing cardiac mesoderm in culture [71,72,73]. Combinations of BMP4, Wnt3a, and Activin A induce gastrulation-like events in iPSCs cultured in a high-density monolayer with a serum- and feeder cell-free system [74]. Spontaneously contracting areas are generally observed after 10 days from induction with BMP/Activin A and, after three weeks, these cell preparations typically consist of ~30% CMs [75]. A similar protocol uses factors that activate the canonical Wnt/β-catenin signaling pathway instead of BMP/Activin A to induce cardiac mesoderm [76,77,78]; this methodology has been described to produce up to 50% CMs [79]. Since all these growth factors don’t elicit optimal transcript levels to induce cardiogenesis if used outside the right time frames [80], time-dependent media supplementation is crucial to obtain an efficient lineage-specific differentiation. Commercial kits provide standardized and simplified protocols to increase the reproducibility of the differentiation process [54,81].
4. Functional Properties of hiPSC-CMs: Overview and Limitations
The spontaneous beating that appears at the beginning of the differentiation process is generally accepted as sign for the expression, within newly developing hiPSC-CMs, of functional cardiac ion channels and transporters related to generation of action potential (AP) and contractility. Unfortunately, hiPSC-CMs generated with current protocols are still quite immature and existing differentiation techniques appear to work efficiently only with specific cell lines [82,83,84].
The characterization of electrophysiological properties of differentiating, beating CMs is key to define the level of electrical and mechanical cell maturation. Several ionic currents have been characterized in single hiPSC-CMs by using the patch-clamp technique, such as the sodium (INa), the calcium (ICa,L and ICa,T) and the potassium ones (Ito, IKr and IKs) [85,86,87,88,89,90]. In particular, sodium and calcium inward components contribute to the depolarizing phases of the electrical activity; while the former is responsible of the fast depolarizing process, the latter has a functional role during the slower depolarization of spontaneous automatic cells together with If pacemaker current, or during the plateau in stimulated AP, critical phase for the cell contraction. Otherwise, repolarizing process is due to the outward potassium current contribution of the AP. The balance between inward and outward currents determine the AP duration (APD) and then the refractoriness period, that are crucial in developing arrhythmic events.
The biophysical properties that characterize voltage dependence and activation/inactivation kinetics of each of these ion channels have been studied in relation to time of culture. Furthermore, their current density was found to increase from day 30 to 80 of the differentiation process. Consequently, temporal changes of these properties determine different ionic contribution to the cardiac AP (INa, ICaL, IK1), leading to heterogeneous AP profiles and parameters (diastolic membrane potential, Ediast; AP amplitude, APA; AP duration, APD) [91,92,93].
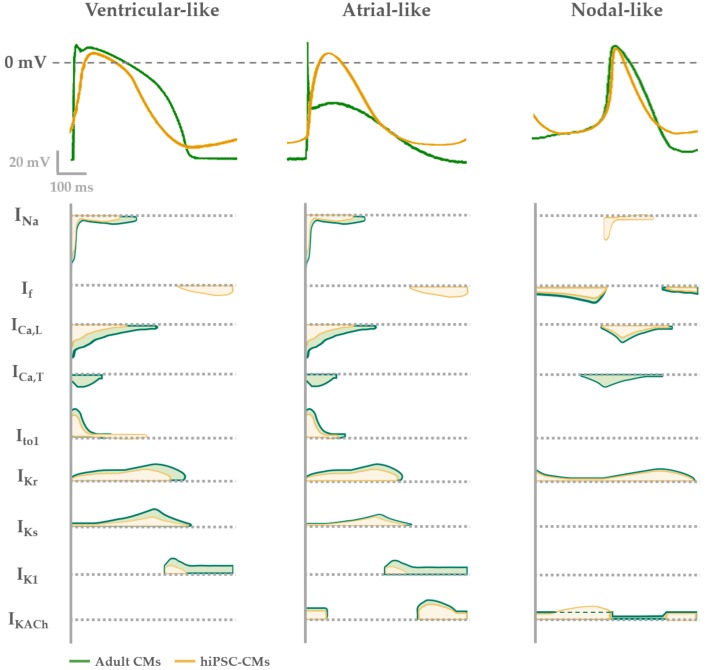
Based on the AP properties, CMs deriving from a single clone of differentiating iPSCs, frequently results in a mix of cells that can be classified as atrial-, ventricular- and nodal-like CMs [53,59,86]. However, this kind of classification is biased by being operator-dependent and may result in misleading interpretation when comparing CMs with prolonged APD (e.g., hiPSC-CMs from Long QT Syndrome patients) to healthy ones. In this context, tools can be used to identify and/or isolate atrial- or ventricular-like hiPSC-CMs. Recently, Schwach et al. have described a specific marker which is highly enriched in human atrial CMs, but not in ventricular ones, the so called chick ovalbumin upstream promoter transcription factors I and II (COUP-TFI and II) that regulates atrial-specific ion channels gene expression such as KCNA5 encoding Kv 1.5 (IKur current) and KCNJ3 encoding Kir 3.1 (IKACh current) [94,95,96]. By fusing this promoter with fluorescent reporter genes (mCherry) and combining it with the well-established human cardiac NKX2.5EGFP/+ reporter, they were able to sort a pure atrial cell population [97].
In Figure 2 the typical features of adult human CM APs are compared to the ones of hiPSC-CMs. In general, nodal-like hiPSC-CMs and sinoatrial CMs APs are comparable, showing spontaneous electrical activity thanks to the contribution of the funny (If) and calcium (ICaL) currents and the absence of the inward-rectifier potassium channels (IK1) that usually maintains negative Ediast. Major differences between adult and hiPSC-CM AP shapes are present when atrial and ventricular APs are analyzed. Indeed, hiPSC-CMs show more depolarized Ediast and they often still have a spontaneous electrical activity, because If is still functional and the IK1 expression is not enough to maintain an hyperpolarized Ediast [98]. As a consequence of the depolarized Ediast, AP upstroke velocity and APD in hiPSC-CMs are not superimposable to those of adult CMs.
Figure 2.
Electrophysiological phenotypes of hiPSC-derived (yellow) compared with adult CMs (green). AP shape (upper panel) described in each phenotype (ventricular-, atrial- or nodal-like) is determined by different contribution of cardiac ion currents, represented over time in the lower panel.
To overcome the lack of IK1 expression, the overexpression of Kir 2.1, IK1 encoding gene, [99] or an “electronic” maturation by injection of computational IK1 in a real time mode (dynamic clamp technique) have been designed [100,101]. In both cases, Ediast of derived-CMs hyperpolarizes and the activation of all expressed ion channels allows to develop an AP profile more similar to the one of atrial or ventricular adult CMs. This optimized physiological condition has been used to investigate mechanisms of cardiac cellular disease [4] and predict pharmacological approaches [5,6]. Overall, by adding IK1 (through dynamic clamp or channel overexpression), hiPSC-CMs AP becomes more similar to the adult one, suggesting that from the electrophysiological point of view the lack of this channel may be the main reason for the hiPSC-CM immaturity.
Additionally, hiPSC-CMs repolarization reserve is lower in comparison to adult CMs because of the low expression of the slow delayed rectifier channel IKs. Indeed, the functional contribution of this current to the hiPSC-CM AP has been usually seen under β-adrenergic stimulation and reduced repolarization reserve by blocking the rapid component IKr [102,103,104]. Only in few papers IKs has been recorded in basal conditions in hiPSC-CMs [4], a sign of a good cell maturation level. For this reason, the expression of IKs together with the one of IK1 in hiPSC-CMs are usually seen as functional maturation markers of these cells.
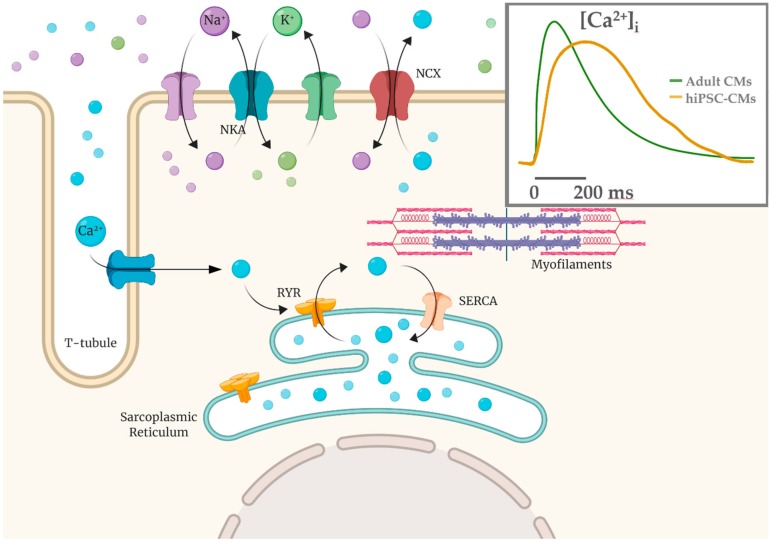
Several works have studied Ca2+ handling proteins (L-type Ca2+ channels, RyR2 in sarcoplasmic reticulum, SERCA2a pump-based Ca2+ uptake) and Ca2+ transient parameters, as well SR Ca2+ release events (Ca2+ sparks) [19,105,106]. In hiPSC-CMs there is an immature condition due to a poorly developed sub-cellular T-tubules system and sarcomeric structure; these are crucial elements for Ca2+-handling, contractile force and relaxation processes [10,107,108,109,110]. U-shaped Ca2+ transients in hiPSC-CMs suggest the presence of an immature functional excitation-contraction (EC) coupling compared to native CMs (Figure 3), thus implying that kinetic properties of calcium handling process are slower compared to the adult CMs [106,111].
Figure 3.
Calcium-induced calcium release mechanism (CICR) schematized with T-tubule and sarcomere structures. Ca2+ influx via the L-type calcium channels is able to cause a release of the SR Ca2+ store via the Ca2+-sensitive ryanodine receptors (RYR2). In hiPSC-CMs the Ca2+ entry is mainly the extracellular one and calcium handling kinetics are slower (yellow in the inset) compared to adult CMs (green).
Single cells recordings with the patch clamp technique are still the most informative and accurate technique to disclose mechanisms underlying abnormal electrical activity in hiPSC-CMs. However, global electrophysiological information can also be acquired by the multielectrode array (MEA) system by plating spontaneous beating clusters of hiPSC-CM. This technique is useful to evaluate changes in AP rate, duration and conduction velocity.
Platforms of hiPSC-CM to test drug safety by analyzing their proarrhythmic effects have been recently developed. Cardiac electrophysiology models have been applied more and more in the emerging discipline of quantitative system pharmacology (QSP) for cardiac safety prediction [112]. hiPSC-CMs have been applied in screening the proarrhythmic potential of drugs; Sotalol, Dofetilide, and E4031 for hERG channel blockade, Quinidine and Flecainide as sodium channel blockers, and Verapamil and Diltiazem as calcium channel inhibitors represent the main examples [113,114,115]. These drugs, in conjunction with in silico modelling, have been indeed the major focus of the FDA’s Comprehensive In Vitro Proarrythmia Assay (CiPA) initiative [116]. In the last years, the CiPA has been a remarkable initiative that uses in silico models for the assessment of potential proarrhythmic effects of drugs that are then classified into high, intermediate and low risk for Torsade de Pointes (TdP) tachycardia. In particular, the potential torsadogenic effect of drugs is based on hERG (IKr) or IKs (slow-delayed rectifier) outward currents inhibition with or without Nav1.5 (transient/late sodium currents, INa/INaL) and Cav1.2 (L-Type Ca2+) inward currents enhancement. The result is a delayed AP repolarization with increased incidence of early afterdepolarization (EADs), leading to ventricular arrhythmias such as TdP and ventricular fibrillation [112,117,118,119]. Furthermore, a computational approach has been recently developed to recapitulate the human AP profile and drug-induced TdP [120,121,122].
The CiPA in silico system represents a predictive strategy applied on hiPSC-CMs for development of therapeutic drugs potentially safety in term of cardiac function. Anyway, care must be taken with conclusions about healthy and pathological phenotypes of CMs, that may result misleading because of their immature functional state [89,92,123].
5. Pluripotency and Cardiac Differentiation of hiPSCs Derived from Cardiac vs. Non-Cardiac Sources
The reprogramming process can be applied to all type of somatic cells, such as placenta [26] mononuclear cells from blood [25], and keratinocytes [28], from which it is possible to address the differentiation process toward cardiac phenotype. The somatic source may influence the phenotype of iPSCs by affecting both reprogramming and differentiation efficiency. For example, it has been shown how blood-derived iPSCs differentiate into hematopoietic cells more easily in comparison to fibroblast-derived ones [7]; in addition, beta cell derived-iPSCs were more prone to differentiate into insulin-producing cells if compared to ESCs [124].
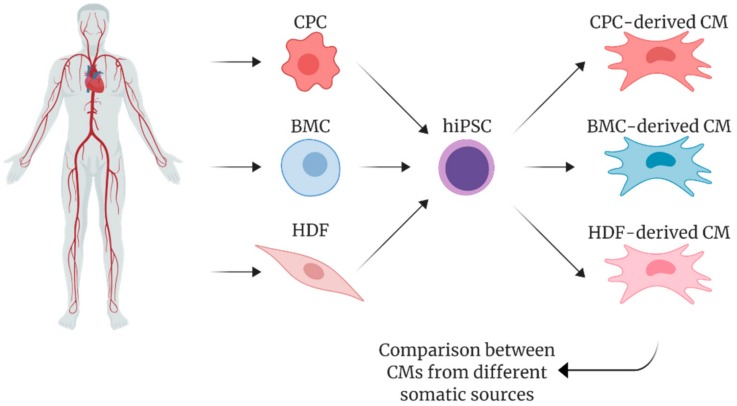
In agreement with these observations, it has been recently reported the possibility to reprogram explant-derived cells, elsewhere referred as cardiac progenitor cells (CPCs) [125], from human cardiac biopsies obtain functional and terminal differentiated CMs [29]. As schematized in Figure 4, CPC-derived hiPSCs account for improvements in differentiation to CMs in comparison to patient-matched hiPSCs from other somatic sources, such as bone marrow-derived mesenchymal stem cells (BMC) and dermal fibroblasts (HDF) both if cultured in monolayers [126] or EBs [127,128].
Figure 4.
Schematic overview of human induced pluripotent stem cells (hiPSCs-CMs) generated from different somatic sources: cardiac progenitor cells (CPCs) from cardiac tissue, bone marrow cells (BMs) from sternal region, and dermal fibroblasts (HDFs) from skin.
These works emphasized the existence of an epigenetic memory retained by iPSCs from their tissue of origin. Reprogramming of somatic cells to pluripotency undergo a reversal in DNA modifications that characterize the cell development, but in some cases these modifications remain unaltered, representing a residual tissue-specific DNA methylation that influences the differentiation potential of iPSCs [129].
Although hiPSC derived from HDFs have been described to produce a higher number of colonies that appear earlier in time, the expression level of pluripotency markers (e.g., Nanog, Oct4) resulted significantly enhanced in hiPSC from CPCs as compared to both hiPSC from HDFs and from BMCs [126,128]. Inversely, Sanchez-Freire et al. [127] showed that the expression of pluripotency markers was not different between the two hiPSC lineages from different tissues. In both cases, the ability of reprogrammed cells to form three germ layers (i.e., mesoderm, ectoderm, and endoderm), which is considered a hallmark for pluripotency in iPSCs, is not affected by the cell source.
As for the specification potential toward cardiac phenotype, hiPSC derived from cardiac somatic sources showed higher efficiency during the re-differentiation process compared to non-cardiac ones in terms of genes expression for early (NKX 2.5, ISL1) and late cardiogenic transcription factors (HAND2, TBX5, GATA4 and MEF2C) [127,128]. Genes encoding for late cardiac specific markers, such as MYLC2.a, MYH6, TNNI3 and TNNT2, were also overexpressed in cardiac hiPSC-CMs, as well as those encoding for cardiac specific ion channels (HCN1-4, CACNA1C and 1G, RyR2, Cx43) [126,128].
Accordingly, a higher percentage of Troponin T (cTnT)-positive CMs in beating cardiac Sca1-iPSC-CMs (cardiac) compared to HDF-iPSC-CMs (non-cardiac) has been reported both by Sanchez-Freire et al. (15 days) [127] and Meraviglia et al. (18–20 days) [128] as a late differentiation marker. Taken together these data support the hypothesis that the cardiac origin of somatic cells to be reprogrammed influences the transcription of cardiac genes during the differentiation of iPSCs.
While Meraviglia et al. and Pianezzi et al. observed that hiPSC-CMs started beating at 10 days of differentiation, Sanchez-Freire et al. needed five more days to detect the first spontaneous events in their Sca1- and HDF-derived CMs. Furthermore, in the studies by Meraviglia et al. and Pianezzi et al. this correlated with an upregulation of cTnI expressed in a sarcomeric pattern. In addition, CPC-derived CMs from Pianezzi et al. are the first population to exhibit early spontaneous beating (at 10 days of differentiation) compared to patient-matched HDF- and BMC-derived ones (15 days), thus suggesting precocity in cell differentiation from cardiac source.
Functionally, Meraviglia et al. and Sanchez-Freire et al. did not observe any differences in term of beating rates between cardiac and non-cardiac sources derived-CMs at 30 days of differentiation. Interestingly, in urine-derived hiPSC-CMs [111] the adaptation of AP to stimulation rates was not observed until 90 days of maturation, while in our hand CPC-derived cells showed APD90 shortening when stimulated from 2 to 4 Hz already at 35 days of differentiation (unpublished). Meraviglia et al. noticed that the maturation process affected especially the maximum diastolic potential (MDP) values, that resulted more hyperpolarized in CPC-CMs at 60 day of differentiation. However, Sanchez-Freire et al. did not observe any electrical difference between cardiac- and fibroblast-derived CMs at day 30.
In recent work, it has been observed variability in electrical properties and sensitivity to ion channel blockers in CMs derived from different sources [130]. Accordingly, the MEA measurements by Pianezzi et al. pointed out a higher maturation degree of CPC-CMs by highlighting the presence of IKs, a current more expressed and more functional in CPC-derived CMs in comparison to HDF- and BMC-derived cells. A higher repolarization reserve in CPC-CMs has been demonstrated by highlighting the contribution of IKs with the specific blocker JNJ303 under IKr blockade with E4031. In support of this, a JNJ303-dependent QT prolongation resulted strongly enhanced in CPC-CMs in comparison to HDF- and BMC-CMs [126].
In Pianezzi et al. CMs derived from cardiac somatic cells showed differences from an early stage of maturation in calcium handling. Here, not only CPC-, but also HDF- and BMC-CMs at 35 days of differentiation were able to elicit RyR-mediated Ca2+ release when exposed to caffeine. However, the quantification of the number of responsive CMs clearly showed that the percentage of CPC-derived ones was significantly greater than the percentages of CMs derived from the other two cell types. On the other hand, the molecular expression of RyR2 and SERCA2a proteins were not different among the three groups. Thus, the “caffeine responsiveness” may represent a functional index, over the expression of cardiac specific genes, for the identification of differentiating CMs. Despite of this, in Sanchez-Freire et al., 30 days of differentiation were not sufficient to evince any differences between CPC- and HDF-CMs, equally immature in Ca2+ transient properties.
In general, we can say that, although different somatic cells show a cardiogenic potential when exposed to appropriate cardiac stimuli, cardiac precursor cells seem to be temporally and/or qualitatively more prone to differentiate into functional cardiac cells. Moreover, it must be clarified whether maturation of reprogrammed cells from cardiac sources represents also at late time points a better cellular substrate for cardiac disease modelling, drug testing and tissue regeneration.
6. Conclusions
To date, it has been widely described how hiPSC-CMs are able to recapitulate molecular and functional aspects of human heart pathophysiology, thus providing a good tool for disease modelling and development of personalized therapy that involves a pharmacological treatment. A wide range of genetic cardiomyopathies has been modelled using hiPSC-CMs [131], for example familiar long QT (LQT) syndromes [4,85,86,87,132,133], Brugada syndrome [134,135], Catecholaminergic polymorphic tachycardia (CPVT) [136,137] and atrial fibrillation [138,139].
Unfortunately, the physiological phenotype of iPSC-CMs is heterogeneous both in term of sub-populations of CMs and in term of maturation degree during differentiation protocol, potentially leading to an incorrect interpretation of data. To avoid this, the comparison of their functional parameters with the native and adult counterpart is crucial. The cellular size and morphology, together with the expression of structural proteins and a T-tubular system that ensure the electrical conduction, must be evaluated in order to perform accurate functional analysis and develop 3D platforms; electrophysiological parameters and Ca2+ handling features, contractile force, responses to beta-adrenergic stimulation, metabolic profile and conduction velocity must be verified to assess the ability of hiPSC-CM-based models to recapitulate diseases and pathological phenotypes. Furthermore, populations of cells differentiated from iPSCs contain non-cardiomyocyte cells that may interfere with maturation levels, electrophysiological properties and conduction velocity of differentiating CMs, therefore affecting the sensitivity to tested drugs. Standardization of methods and techniques from one laboratory to another is needed for a reliable comparison between healthy and pathological cell models.
Current differentiation protocols that are being tested to optimize the structural and functional maturation degree of hiPSC-CMs use addition of physiological substrates, prolongation of culture time, coculture with endothelial cells or fibroblasts, 3D cell platforms (“organoids”) and mechanical and electrical stimulation (dynamic clamp); these techniques, combined with purification methods such as pre-plating or substitution of glucose with lactate in the early maturation phase of CMs, can produce up to 90% cTnT-positive hiPSC-CMs [140,141,142,143,144,145,146,147].
Despite their limitations, thanks to molecular, structural and functional correlations with primary adult CMs, hiPSC-CMs can be considered reliable tool for disease modelling and it represents a valid platform for pharmacological screening [53,54]. Moreover, it is crucial to consider the somatic origin of hiPSC-CMs since it has been clearly demonstrated to impact on time of development and maturation degree of derived CMs in a patient-matched comparison.
The selection of a somatic donor tissue has to be adjusted according to the goal of the study. Since CPCs are derived from cardiac biopsies of patients who undergo heart surgery, the accessibility to human material can be limited. For these reasons, the use of cardiac derived cells as source to generate hiPSCs represents a compromise between the possibility to obtain a more mature CM and the invasiveness and risks of cardiac procedures.
Acknowledgments
Figure 1, Figure 3 and Figure 4 are created with Biorender.com.
Abbreviations
| hiPSC | Human Induced Pluripotent Stem Cell |
| CM | Cardiomyocyte |
| ESC | Embryonic Stem Cell |
| MET | Mesenchymal to Epithelial Transition |
| CVD | Cardiovascular Disease |
| CPC | Cardiac Progenitor Cell |
| BMC | Bone Marrow-derived stem Cell |
| HDF | Human Dermal Fibroblast |
| Na+ | Sodium |
| K+ | Potassium |
| Ca2+ | Calcium |
| Ediast | Diastolic Membrane Potential |
| AP | Action Potential |
| APA | Action Potential Amplitude |
| APD | Action Potential Duration |
| MDP | Maximum Diastolic Potential |
| MEA | Multielectrode Array |
| ECG | Electrocardiogram |
| TdP | Torsade de Pointes |
| LQTS | Long QT Syndrome |
| CPVT | Catecholaminergic Polymorphic Ventricular Tachycardia |
| DCM | Dilated Cardiomyopathies |
| HCM | Hypertrophic Cardiomyopathies |
| QSP | Quantitative System Pharmacology |
| CiPA | Comprehensive In Vitro Proarrythmia Assay |
Author Contributions
Writing and original draft preparation, A.M.L. and C.A.; figure preparation, A.M.L.; review and editing, C.A., M.R., and L.B. All authors have read and agreed to the published version of the manuscript.
Funding
L.B. was supported by research grant of Velux Stiftung (1127), Zurich and by research grant of Swiss National Science Foundation (IZCOZ0_182948) (Switzerland).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Li J., Minami I., Shiozaki M., Yu L., Yajima S., Miyagawa S., Shiba Y., Morone N., Fukushima S., Yoshioka M., et al. Human Pluripotent Stem Cell-Derived Cardiac Tissue-like Constructs for Repairing the Infarcted Myocardium. Stem Cell Rep. 2017;9:1546–1559. doi: 10.1016/j.stemcr.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Funakoshi S., Miki K., Takaki T., Okubo C., Hatani T., Chonabayashi K., Nishikawa M., Takei I., Oishi A., Narita M., et al. Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep. 2016;6:19111. doi: 10.1038/srep19111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocchetti M., Sala L., Dreizehnter L., Crotti L., Sinnecker D., Mura M., Pane L.S., Altomare C., Torre E., Mostacciuolo G., et al. Elucidating arrhythmogenic mechanisms of long-QT syndrome CALM1-F142L mutation in patient-specific induced pluripotent stem cell-derived cardiomyocytes. Cardiovasc. Res. 2017;113:531–541. doi: 10.1093/cvr/cvx006. [DOI] [PubMed] [Google Scholar]
- 5.Laksman Z., Wauchop M., Lin E., Protze S., Lee J., Yang W., Izaddoustdar F., Shafaattalab S., Gepstein L., Tibbits G.F., et al. Modeling Atrial Fibrillation using Human Embryonic Stem Cell-Derived Atrial Tissue. Sci. Rep. 2017;7:5268. doi: 10.1038/s41598-017-05652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schweizer P.A., Darche F.F., Ullrich N.D., Geschwill P., Greber B., Rivinius R., Seyler C., Müller-Decker K., Draguhn A., Utikal J., et al. Subtype-specific differentiation of cardiac pacemaker cell clusters from human induced pluripotent stem cells. Stem Cell Res. Ther. 2017;8:229. doi: 10.1186/s13287-017-0681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noguchi H., Miyagi-Shiohira C., Nakashima Y. Induced Tissue-Specific Stem Cells and Epigenetic Memory in Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2018;19:930. doi: 10.3390/ijms19040930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robertson C., Tran D.D., George S.C. Concise review: Maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells. 2013;31:829–837. doi: 10.1002/stem.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aigha I., Raynaud C. Maturation of pluripotent stem cell derived cardiomyocytes: The new challenge. Glob. Cardiol. Sci. Pract. 2016;2016:e201606. doi: 10.21542/gcsp.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedada F.B., Wheelwright M., Metzger J.M. Maturation status of sarcomere structure and function in human iPSC-derived cardiac myocytes. Biochim. Biophys. Acta. 2016;1863:1829–1838. doi: 10.1016/j.bbamcr.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu D., Linders A., Yamak A., Correia C., Kijlstra J.D., Garakani A., Xiao L., Milan D.J., van der Meer P., Serra M., et al. Metabolic Maturation of Human Pluripotent Stem Cell-Derived Cardiomyocytes by Inhibition of HIF1α and LDHA. Circ. Res. 2018;123:1066–1079. doi: 10.1161/CIRCRESAHA.118.313249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar N., Dougherty J.A., Manring H.R., Elmadbouh I., Mergaye M., Czirok A., Greta Isai D., Belevych A.E., Yu L., Janssen P.M.L., et al. Assessment of temporal functional changes and miRNA profiling of human iPSC-derived cardiomyocytes. Sci. Rep. 2019;9:13188. doi: 10.1038/s41598-019-49653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Athanasopoulos T., Munye M.M., Yáñez-Muñoz R.J. Nonintegrating Gene Therapy Vectors. Hematol. Oncol. Clin. North. Am. 2017;31:753–770. doi: 10.1016/j.hoc.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Somers A., Jean J.C., Sommer C.A., Omari A., Ford C.C., Mills J.A., Ying L., Sommer A.G., Jean J.M., Smith B.W., et al. Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette. Stem Cells. 2010;28:1728–1740. doi: 10.1002/stem.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fusaki N., Ban H., Nishiyama A., Saeki K., Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn. Acad. Ser. B. 2009;85:348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Churko J.M., Burridge P.W., Wu J.C. Generation of human iPSCs from human peripheral blood mononuclear cells using non-integrative Sendai virus in chemically defined conditions. Methods Mol. Biol. 2013;1036:81–88. doi: 10.1007/978-1-62703-511-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu J., Hu K., Smuga-Otto K., Tian S., Stewart R., Slukvin I.I., Thomson J.A. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burridge P.W., Thompson S., Millrod M.A., Weinberg S., Yuan X., Peters A., Mahairaki V., Koliatsos V.E., Tung L., Zambidis E.T. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS ONE. 2011;6:e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diecke S., Lu J., Lee J., Termglinchan V., Kooreman N.G., Burridge P.W., Ebert A.D., Churko J.M., Sharma A., Kay M.A., et al. Novel codon-optimized mini-intronic plasmid for efficient, inexpensive, and xeno-free induction of pluripotency. Sci. Rep. 2015;5:8081. doi: 10.1038/srep08081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woltjen K., Michael I.P., Mohseni P., Desai R., Mileikovsky M., Hämäläinen R., Cowling R., Wang W., Liu P., Gertsenstein M., et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou P., Li Y., Zhang X., Liu C., Guan J., Li H., Zhao T., Ye J., Yang W., Liu K., et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 23.Anokye-Danso F., Trivedi C.M., Juhr D., Gupta M., Cui Z., Tian Y., Zhang Y., Yang W., Gruber P.J., Epstein J.A., et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou H., Wu S., Joo J.Y., Zhu S., Han D.W., Lin T., Trauger S., Bien G., Yao S., Zhu Y., et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loh Y.H., Agarwal S., Park I.H., Urbach A., Huo H., Heffner G.C., Kim K., Miller J.D., Ng K., Daley G.Q. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–5479. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai J., Li W., Su H., Qin D., Yang J., Zhu F., Xu J., He W., Guo X., Labuda K., et al. Generation of human induced pluripotent stem cells from umbilical cord matrix and amniotic membrane mesenchymal cells. J. Biol. Chem. 2010;285:11227–11234. doi: 10.1074/jbc.M109.086389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue Y., Cai X., Wang L., Liao B., Zhang H., Shan Y., Chen Q., Zhou T., Li X., Hou J., et al. Generating a Non-Integrating Human Induced Pluripotent Stem Cell Bank from Urine-Derived Cells. PLoS ONE. 2013;8:e70573. doi: 10.1371/journal.pone.0070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novak A., Lorber A., Itskovitz-Eldor J., Binah O. Modeling Catecholaminergic Polymorphic Ventricular Tachycardia using Induced Pluripotent Stem Cell-derived Cardiomyocytes. Rambam Maimonides Med. J. 2012;3:e0015. doi: 10.5041/RMMJ.10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altomare C., Pianezzi E., Cervio E., Bolis S., Biemmi V., Benzoni P., Camici G.G., Moccetti T., Barile L., Vassalli G. Human-induced pluripotent stem cell-derived cardiomyocytes from cardiac progenitor cells: Effects of selective ion channel blockade. Europace. 2016;18:iv67–iv76. doi: 10.1093/europace/euw352. [DOI] [PubMed] [Google Scholar]
- 30.Samavarchi-Tehrani P., Golipour A., David L., Sung H., Beyer T.A., Datti A., Woltjen K., Nagy A., Wrana J.L. Functional Genomics Reveals a BMP-Driven Mesenchymal-to-Epithelial Transition in the Initiation of Somatic Cell Reprogramming. Cell Stem Cell. 2010;7:64–77. doi: 10.1016/j.stem.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 31.David L., Polo J.M. Phases of reprogramming. Stem Cell Res. 2014;12:754–761. doi: 10.1016/j.scr.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Downing T.L., Soto J., Morez C., Houssin T., Fritz A., Yuan F., Chu J., Patel S., Schaffer D.V., Li S. Biophysical regulation of epigenetic state and cell reprogramming. Nat. Mater. 2013;12:1154. doi: 10.1038/nmat3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polo J.M., Anderssen E., Walsh R.M., Schwarz B.A., Nefzger C.M., Lim S.M., Borkent M., Apostolou E., Alaei S., Cloutier J., et al. A Molecular Roadmap of Reprogramming Somatic Cells into iPS Cells. Cell. 2012;151:1617–1632. doi: 10.1016/j.cell.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanabe K., Nakamura M., Narita M., Takahashi K., Yamanaka S. Maturation, not initiation, is the major roadblock during reprogramming toward pluripotency from human fibroblasts. Proc. Natl. Acad. Sci. USA. 2013;110:12172–12179. doi: 10.1073/pnas.1310291110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golipour A., David L., Liu Y., Jayakumaran G., Hirsch C.L., Trcka D., Wrana J.L. A Late Transition in Somatic Cell Reprogramming Requires Regulators Distinct from the Pluripotency Network. Cell Stem Cell. 2012;11:769–782. doi: 10.1016/j.stem.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Pan G., Li J., Zhou Y., Zheng H., Pei D. A negative feedback loop of transcription factors that controls stem cell pluripotency and self-renewal. FASEB J. 2006;20:1730–1732. doi: 10.1096/fj.05-5543fje. [DOI] [PubMed] [Google Scholar]
- 37.Jaenisch R., Young R. Stem Cells, the Molecular Circuitry of Pluripotency and Nuclear Reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahl P.B., Lin C.Y., Seila A.C., Flynn R.A., McCuine S., Burge C.B., Sharp P.A., Young R.A. c-Myc Regulates Transcriptional Pause Release. Cell. 2010;141:432–445. doi: 10.1016/j.cell.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maekawa M., Yamaguchi K., Nakamura T., Shibukawa R., Kodanaka I., Ichisaka T., Kawamura Y., Mochizuki H., Goshima N., Yamanaka S. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature. 2011;474:225–229. doi: 10.1038/nature10106. [DOI] [PubMed] [Google Scholar]
- 40.Bilic J., Izpisua Belmonte J.C. Concise review: Induced pluripotent stem cells versus embryonic stem cells: Close enough or yet too far apart? Stem Cells. 2012;30:33–41. doi: 10.1002/stem.700. [DOI] [PubMed] [Google Scholar]
- 41.Saric T., Hescheler J. Stem cells and nuclear reprogramming. Minim. Invasive Ther. Allied Technol. 2008;17:64–78. doi: 10.1080/13645700801969303. [DOI] [PubMed] [Google Scholar]
- 42.Vierbuchen T., Ostermeier A., Pang Z.P., Kokubu Y., Südhof T.C., Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ieda M., Fu J.D., Delgado-Olguin P., Vedantham V., Hayashi Y., Bruneau B.G., Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morita R., Suzuki M., Kasahara H., Shimizu N., Shichita T., Sekiya T., Kimura A., Sasaki K., Yasukawa H., Yoshimura A. ETS transcription factor ETV2 directly converts human fibroblasts into functional endothelial cells. Proc. Natl. Acad. Sci. USA. 2015;112:160–165. doi: 10.1073/pnas.1413234112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sekiya S., Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 46.Yin S., Cen L., Wang C., Zhao G., Sun J., Liu W., Cao Y., Cui L. Chondrogenic transdifferentiation of human dermal fibroblasts stimulated with cartilage-derived morphogenetic protein 1. Tissue Eng. Part A. 2010;16:1633–1643. doi: 10.1089/ten.tea.2009.0570. [DOI] [PubMed] [Google Scholar]
- 47.Pang Z.P., Yang N., Vierbuchen T., Ostermeier A., Fuentes D.R., Yang T.Q., Citri A., Sebastiano V., Marro S., Südhof T.C., et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boularaoui S.M., Abdel-Raouf K.M.A., Alwahab N.S.A., Kondash M.E., Truskey G.A., Teo J.C.M., Christoforou N. Efficient transdifferentiation of human dermal fibroblasts into skeletal muscle: Efficient human skeletal muscle transdifferentiation. J. Tissue Eng. Regen. Med. 2018;12:e918–e936. doi: 10.1002/term.2415. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu K., Ohsumi S., Kishida T., Mazda O., Honda H. Fabrication of contractile skeletal muscle tissues using directly converted myoblasts from human fibroblasts. J. Biosci. Bioeng. 2019 doi: 10.1016/j.jbiosc.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization . Noncommunicable Diseases Country Profiles 2018. World Health Organization; Geneva, Switzerland: 2018. [Google Scholar]
- 51.Hoppe U.C., Beuckelmann D.J. Characterization of the hyperpolarization-activated inward current in isolated human atrial myocytes. Cardiovasc. Res. 1998;38:788–801. doi: 10.1016/S0008-6363(98)00047-9. [DOI] [PubMed] [Google Scholar]
- 52.Cerbai E., Sartiani L., DePaoli P., Pino R., Maccherini M., Bizzarri F., DiCiolla F., Davoli G., Sani G., Mugelli A. The properties of the pacemaker current I(F)in human ventricular myocytes are modulated by cardiac disease. J. Mol. Cell. Cardiol. 2001;33:441–448. doi: 10.1006/jmcc.2000.1316. [DOI] [PubMed] [Google Scholar]
- 53.Burridge P.W., Matsa E., Shukla P., Lin Z.C., Churko J.M., Ebert A.D., Lan F., Diecke S., Huber B., Mordwinkin N.M., et al. Chemically defined generation of human cardiomyocytes. Nat. Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dell’Era P., Benzoni P., Crescini E., Valle M., Xia E., Consiglio A., Memo M. Cardiac disease modeling using induced pluripotent stem cell-derived human cardiomyocytes. World J. Stem Cells. 2015;7:329–342. doi: 10.4252/wjsc.v7.i2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dixon J.A., Spinale F.G. Large animal models of heart failure: A critical link in the translation of basic science to clinical practice. Circ. Heart Fail. 2009;2:262–271. doi: 10.1161/CIRCHEARTFAILURE.108.814459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu H.R., Vlaminckx E., Hermans A.N., Rohrbacher J., Van Ammel K., Towart R., Pugsley M., Gallacher D.J. Predicting drug-induced changes in QT interval and arrhythmias: QT-shortening drugs point to gaps in the ICHS7B Guidelines. Br. J. Pharmacol. 2008;154:1427–1438. doi: 10.1038/bjp.2008.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathur A., Loskill P., Shao K., Huebsch N., Hong S., Marcus S.G., Marks N., Mandegar M., Conklin B.R., Lee L.P., et al. Human iPSC-based Cardiac Microphysiological System for Drug Screening Applications. Sci. Rep. 2015;5:1–7. doi: 10.1038/srep08883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Itskovitz-Eldor J., Schuldiner M., Karsenti D., Eden A., Yanuka O., Amit M., Soreq H., Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 2000;6:88–95. doi: 10.1007/BF03401776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang J., Wilson G.F., Soerens A.G., Koonce C.H., Yu J., Palecek S.P., Thomson J.A., Kamp T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Synnergren J., Åkesson K., Dahlenborg K., Vidarsson H., Améen C., Steel D., Lindahl A., Olsson B., Sartipy P. Molecular Signature of Cardiomyocyte Clusters Derived from Human Embryonic Stem Cells. Stem Cells. 2008;26:1831–1840. doi: 10.1634/stemcells.2007-1033. [DOI] [PubMed] [Google Scholar]
- 61.Cao F., Wagner R.A., Wilson K.D., Xie X., Fu J.-D., Drukker M., Lee A., Li R.A., Gambhir S.S., Weissman I.L., et al. Transcriptional and Functional Profiling of Human Embryonic Stem Cell-Derived Cardiomyocytes. PLoS ONE. 2008;3:e3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu X.Q., Soo S.Y., Sun W., Zweigerdt R. Global Expression Profile of Highly Enriched Cardiomyocytes Derived from Human Embryonic Stem Cells. Stem Cells. 2009;27:2163–2174. doi: 10.1002/stem.166. [DOI] [PubMed] [Google Scholar]
- 63.Mummery C., Ward-van Oostwaard D., Doevendans P., Spijker R., van den Brink S., Hassink R., van der Heyden M., Opthof T., Pera M., de la Riviere A.B., et al. Differentiation of human embryonic stem cells to cardiomyocytes: Role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 64.Passier R., Oostwaard D.W., Snapper J., Kloots J., Hassink R.J., Kuijk E., Roelen B., de la Riviere A.B., Mummery C. Increased Cardiomyocyte Differentiation from Human Embryonic Stem Cells in Serum-Free Cultures. Stem Cells. 2005;23:772–780. doi: 10.1634/stemcells.2004-0184. [DOI] [PubMed] [Google Scholar]
- 65.Willems E., Bushway P.J., Mercola M. Natural and Synthetic Regulators of Embryonic Stem Cell Cardiogenesis. Pediatr. Cardiol. 2009;30:635–642. doi: 10.1007/s00246-009-9409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freund C., Davis R.P., Gkatzis K., Ward-van Oostwaard D., Mummery C.L. The first reported generation of human induced pluripotent stem cells (iPS cells) and iPS cell-derived cardiomyocytes in the Netherlands. Neth. Heart J. 2010;18:51–54. [PMC free article] [PubMed] [Google Scholar]
- 67.Kang Y., Nagy J.M., Polak J.M., Mantalaris A. Proteomic Characterization of the Conditioned Media Produced by the Visceral Endoderm-Like Cell Lines HepG2 and END2: Toward a Defined Medium for the Osteogenic/Chondrogenic Differentiation of Embryonic Stem Cells. Stem Cells Dev. 2008;18:77–92. doi: 10.1089/scd.2008.0026. [DOI] [PubMed] [Google Scholar]
- 68.Arrell D.K., Niederländer N.J., Faustino R.S., Behfar A., Terzic A. Cardioinductive Network Guiding Stem Cell Differentiation Revealed by Proteomic Cartography of Tumor Necrosis Factor α-Primed Endodermal Secretome. Stem Cells. 2008;26:387–400. doi: 10.1634/stemcells.2007-0599. [DOI] [PubMed] [Google Scholar]
- 69.Freund C., Oostwaard D.W., Monshouwer-Kloots J., van den Brink S., van Rooijen M., Xu X., Zweigerdt R., Mummery C., Passier R. Insulin Redirects Differentiation from Cardiogenic Mesoderm and Endoderm to Neuroectoderm in Differentiating Human Embryonic Stem Cells. Stem Cells. 2008;26:724–733. doi: 10.1634/stemcells.2007-0617. [DOI] [PubMed] [Google Scholar]
- 70.Xu X.Q., Graichen R., Soo S.Y., Balakrishnan T., Bte Rahmat S.N., Sieh S., Tham S.C., Freund C., Moore J., Mummery C., et al. Chemically defined medium supporting cardiomyocyte differentiation of human embryonic stem cells. Differentiation. 2008;76:958–970. doi: 10.1111/j.1432-0436.2008.00284.x. [DOI] [PubMed] [Google Scholar]
- 71.Winnier G., Blessing M., Labosky P.A., Hogan B.L. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 72.Marvin M.J., Di Rocco G., Gardiner A., Bush S.M., Lassar A.B. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naujok O., Diekmann U., Lenzen S. The Generation of Definitive Endoderm from Human Embryonic Stem Cells is Initially Independent from Activin A but Requires Canonical Wnt-Signaling. Stem Cell Rev. Rep. 2014;10:480–493. doi: 10.1007/s12015-014-9509-0. [DOI] [PubMed] [Google Scholar]
- 74.Xu C., Police S., Hassanipour M., Gold J.D. Cardiac Bodies: A Novel Culture Method for Enrichment of Cardiomyocytes Derived from Human Embryonic Stem Cells. Stem Cells Dev. 2006;15:631–639. doi: 10.1089/scd.2006.15.631. [DOI] [PubMed] [Google Scholar]
- 75.Laflamme M.A., Chen K.Y., Naumova A.V., Muskheli V., Fugate J.A., Dupras S.K., Reinecke H., Xu C., Hassanipour M., Police S., et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 76.Naito A.T., Shiojima I., Akazawa H., Hidaka K., Morisaki T., Kikuchi A., Komuro I. Developmental stage-specific biphasic roles of Wnt/β-catenin signaling in cardiomyogenesis and hematopoiesis. Proc. Natl. Acad. Sci. USA. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ueno S., Weidinger G., Osugi T., Kohn A.D., Golob J.L., Pabon L., Reinecke H., Moon R.T., Murry C.E. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tran T.H., Wang X., Browne C., Zhang Y., Schinke M., Izumo S., Burcin M. Wnt3a-Induced Mesoderm Formation and Cardiomyogenesis in Human Embryonic Stem Cells. Stem Cells. 2009;27:1869–1878. doi: 10.1002/stem.95. [DOI] [PubMed] [Google Scholar]
- 79.Yang L., Soonpaa M.H., Adler E.D., Roepke T.K., Kattman S.J., Kennedy M., Henckaerts E., Bonham K., Abbott G.W., Linden R.M., et al. Human cardiovascular progenitor cells develop from a KDR + embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 80.Schlange T., Andrée B., Arnold H.H., Brand T. BMP2 is required for early heart development during a distinct time period. Mech. Dev. 2000;91:259–270. doi: 10.1016/S0925-4773(99)00311-1. [DOI] [PubMed] [Google Scholar]
- 81.Ross S., Holliday M., Lim S., Semsarian C. Characterization of the first induced pluripotent stem cell line generated from a patient with autosomal dominant catecholaminergic polymorphic ventricular tachycardia due to a heterozygous mutation in cardiac calsequestrin-2. Stem Cell Res. 2019;37:101450. doi: 10.1016/j.scr.2019.101450. [DOI] [PubMed] [Google Scholar]
- 82.Matsa E., Burridge P.W., Wu J.C. Human stem cells for modeling heart disease and for drug discovery. Sci. Transl. Med. 2014;6:239ps6. doi: 10.1126/scitranslmed.3008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karakikes I., Ameen M., Termglinchan V., Wu J.C. Human induced pluripotent stem cell-derived cardiomyocytes: Insights into molecular, cellular, and functional phenotypes. Circ. Res. 2015;117:80–88. doi: 10.1161/CIRCRESAHA.117.305365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tu C., Chao B.S., Wu J.C. Strategies for Improving the Maturity of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ. Res. 2018;123:512–514. doi: 10.1161/CIRCRESAHA.118.313472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moretti A., Bellin M., Welling A., Jung C.B., Lam J.T., Bott-Flügel L., Dorn T., Goedel A., Höhnke C., Hofmann F., et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N. Engl. J. Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 86.Ma J., Guo L., Fiene S.J., Anson B.D., Thomson J.A., Kamp T.J., Kolaja K.L., Swanson B.J., January C.T. High purity human-induced pluripotent stem cell-derived cardiomyocytes: Electrophysiological properties of action potentials and ionic currents. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H2006–H2017. doi: 10.1152/ajpheart.00694.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yazawa M., Hsueh B., Jia X., Pasca A.M., Bernstein J.A., Hallmayer J., Dolmetsch R.E. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471:230–234. doi: 10.1038/nature09855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davis R.P., Casini S., van den Berg C.W., Hoekstra M., Remme C.A., Dambrot C., Salvatori D., Oostwaard D.W., Wilde A.A.M., Bezzina C.R., et al. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation. 2012;125:3079–3091. doi: 10.1161/CIRCULATIONAHA.111.066092. [DOI] [PubMed] [Google Scholar]
- 89.Ivashchenko C.Y., Pipes G.C., Lozinskaya I.M., Lin Z., Xiaoping X., Needle S., Grygielko E.T., Hu E., Toomey J.R., Lepore J.J., et al. Human-induced pluripotent stem cell-derived cardiomyocytes exhibit temporal changes in phenotype. Am. J. Physiol. Heart Circ. Physiol. 2013;305:H913–H922. doi: 10.1152/ajpheart.00819.2012. [DOI] [PubMed] [Google Scholar]
- 90.Goversen B., van der Heyden M.A.G., van Veen T.A.B., de Boer T.P. The immature electrophysiological phenotype of iPSC-CMs still hampers in vitro drug screening: Special focus on IK1. Pharmacol. Ther. 2018;183:127–136. doi: 10.1016/j.pharmthera.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 91.Otsuji T.G., Minami I., Kurose Y., Yamauchi K., Tada M., Nakatsuji N. Progressive maturation in contracting cardiomyocytes derived from human embryonic stem cells: Qualitative effects on electrophysiological responses to drugs. Stem Cell Res. 2010;4:201–213. doi: 10.1016/j.scr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 92.Ben-Ari M., Naor S., Zeevi-Levin N., Schick R., Ben Jehuda R., Reiter I., Raveh A., Grijnevitch I., Barak O., Rosen M.R., et al. Developmental changes in electrophysiological characteristics of human-induced pluripotent stem cell-derived cardiomyocytes. Heart Rhythm. 2016;13:2379–2387. doi: 10.1016/j.hrthm.2016.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Veerman C.C., Mengarelli I., Lodder E.M., Kosmidis G., Bellin M., Zhang M., Dittmann S., Guan K., Wilde A.A.M., Schulze-Bahr E., et al. Switch from Fetal to Adult SCN5A Isoform in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Unmasks the Cellular Phenotype of a Conduction Disease-Causing Mutation. J. Am. Heart Assoc. 2017;6:e005135. doi: 10.1161/JAHA.116.005135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schwach V., Verkerk A.O., Mol M., Monshouwer-Kloots J.J., Devalla H.D., Orlova V.V., Anastassiadis K., Mummery C.L., Davis R.P., Passier R. A COUP-TFII Human Embryonic Stem Cell Reporter Line to Identify and Select Atrial Cardiomyocytes. Stem Cell Rep. 2017;9:1765–1779. doi: 10.1016/j.stemcr.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Devalla H.D., Schwach V., Ford J.W., Milnes J.T., El-Haou S., Jackson C., Gkatzis K., Elliott D.A., Chuva de Sousa Lopes S.M., Mummery C.L., et al. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol. Med. 2015;7:394–410. doi: 10.15252/emmm.201404757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Argenziano M., Lambers E., Hong L., Sridhar A., Zhang M., Chalazan B., Menon A., Savio-Galimberti E., Wu J.C., Rehman J., et al. Electrophysiologic Characterization of Calcium Handling in Human Induced Pluripotent Stem Cell-Derived Atrial Cardiomyocytes. Stem Cell Rep. 2018;10:1867–1878. doi: 10.1016/j.stemcr.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elliott D.A., Braam S.R., Koutsis K., Ng E.S., Jenny R., Lagerqvist E.L., Biben C., Hatzistavrou T., Hirst C.E., Yu Q.C., et al. NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat. Methods. 2011;8:1037–1040. doi: 10.1038/nmeth.1740. [DOI] [PubMed] [Google Scholar]
- 98.Doss M.X., Di Diego J.M., Goodrow R.J., Wu Y., Cordeiro J.M., Nesterenko V.V., Barajas-Martínez H., Hu D., Urrutia J., Desai M., et al. Maximum Diastolic Potential of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Depends Critically on IKr. PLoS ONE. 2012;7:e40288. doi: 10.1371/journal.pone.0040288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vaidyanathan R., Markandeya Y.S., Kamp T.J., Makielski J.C., January C.T., Eckhardt L.L. IK1-enhanced human-induced pluripotent stem cell-derived cardiomyocytes: An improved cardiomyocyte model to investigate inherited arrhythmia syndromes. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H1611–H1621. doi: 10.1152/ajpheart.00481.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Meijer van Putten R.M.E., Mengarelli I., Guan K., Zegers J.G., van Ginneken A.C.G., Verkerk A.O., Wilders R. Ion channelopathies in human induced pluripotent stem cell derived cardiomyocytes: A dynamic clamp study with virtual IK1. Front. Physiol. 2015;6:7. doi: 10.3389/fphys.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Verkerk A.O., Veerman C.C., Zegers J.G., Mengarelli I., Bezzina C.R., Wilders R. Patch-Clamp Recording from Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Improving Action Potential Characteristics through Dynamic Clamp. Int. J. Mol. Sci. 2017;18:1873. doi: 10.3390/ijms18091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeng H., Wang J., Clouse H., Lagrutta A., Sannajust F. Human-induced pluripotent stem cell-derived cardiomyocytes have limited IKs for repolarization reserve as revealed by specific KCNQ1/KCNE1 blocker. JRSM Cardiovasc. Dis. 2019;8 doi: 10.1177/2048004019854919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Braam S.R., Tertoolen L., Casini S., Matsa E., Lu H.R., Teisman A., Passier R., Denning C., Gallacher D.J., Towart R., et al. Repolarization reserve determines drug responses in human pluripotent stem cell derived cardiomyocytes. Stem Cell Res. 2013;10:48–56. doi: 10.1016/j.scr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 104.Jost N., Virág L., Comtois P., Ordög B., Szuts V., Seprényi G., Bitay M., Kohajda Z., Koncz I., Nagy N., et al. Ionic mechanisms limiting cardiac repolarization reserve in humans compared to dogs. J. Physiol. 2013;591:4189–4206. doi: 10.1113/jphysiol.2013.261198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mauritz C., Schwanke K., Reppel M., Neef S., Katsirntaki K., Maier L.S., Nguemo F., Menke S., Haustein M., Hescheler J., et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 106.Itzhaki I., Rapoport S., Huber I., Mizrahi I., Zwi-Dantsis L., Arbel G., Schiller J., Gepstein L. Calcium handling in human induced pluripotent stem cell derived cardiomyocytes. PLoS ONE. 2011;6:e18037. doi: 10.1371/journal.pone.0018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee Y.K., Ng K.M., Lai W.-H., Chan Y.C., Lau Y.-M., Lian Q., Tse H.F., Siu C.-W. Calcium homeostasis in human induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Rev. Rep. 2011;7:976–986. doi: 10.1007/s12015-011-9273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gherghiceanu M., Barad L., Novak A., Reiter I., Itskovitz-Eldor J., Binah O., Popescu L.M. Cardiomyocytes derived from human embryonic and induced pluripotent stem cells: Comparative ultrastructure. J. Cell. Mol. Med. 2011;15:2539–2551. doi: 10.1111/j.1582-4934.2011.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lundy S.D., Zhu W.Z., Regnier M., Laflamme M.A. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22:1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cadet J.S., Kamp T.J. A Recipe for T-Tubules in Human iPS Cell-Derived Cardiomyocytes. Circ. Res. 2017;121:1294–1295. doi: 10.1161/CIRCRESAHA.117.312177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pioner J.M., Guan X., Klaiman J.M., Racca A.W., Pabon L., Muskheli V., Macadangdang J., Ferrantini C., Hoopmann M.R., Moritz R.L., et al. Absence of full-length dystrophin impairs normal maturation and contraction of cardiomyocytes derived from human induced pluripotent stem cells. Cardiovasc. Res. 2019:cvz109. doi: 10.1093/cvr/cvz109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li Z., Garnett C., Strauss D.G. Quantitative Systems Pharmacology Models for a New International Cardiac Safety Regulatory Paradigm: An Overview of the Comprehensive In Vitro Proarrhythmia Assay In Silico Modeling Approach. Cpt Pharmacomet. Syst. Pharmacol. 2019;8:371–379. doi: 10.1002/psp4.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gibson J.K., Yue Y., Bronson J., Palmer C., Numann R. Human stem cell-derived cardiomyocytes detect drug-mediated changes in action potentials and ion currents. J. Pharmacol. Toxicol. Methods. 2014;70:255–267. doi: 10.1016/j.vascn.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 114.Lu H.R., Whittaker R., Price J.H., Vega R., Pfeiffer E.R., Cerignoli F., Towart R., Gallacher D.J. High Throughput Measurement of Ca ++ Dynamics in Human Stem Cell-Derived Cardiomyocytes by Kinetic Image Cytometery: A Cardiac Risk Assessment Characterization Using a Large Panel of Cardioactive and Inactive Compounds. Toxicol. Sci. 2015;148:503–516. doi: 10.1093/toxsci/kfv201. [DOI] [PubMed] [Google Scholar]
- 115.Patel D., Stohlman J., Dang Q., Strauss D.G., Blinova K. Assessment of Proarrhythmic Potential of Drugs in Optogenetically Paced Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Toxicol. Sci. 2019;170:167–179. doi: 10.1093/toxsci/kfz076. [DOI] [PubMed] [Google Scholar]
- 116.Colatsky T., Fermini B., Gintant G., Pierson J.B., Sager P., Sekino Y., Strauss D.G., Stockbridge N. The Comprehensive in Vitro Proarrhythmia Assay (CiPA) initiative—Update on progress. J. Pharmacol. Toxicol. Methods. 2016;81:15–20. doi: 10.1016/j.vascn.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 117.Weiss J.N., Garfinkel A., Karagueuzian H.S., Chen P.S., Qu Z. Early afterdepolarizations and cardiac arrhythmias. Heart Rhythm. 2010;7:1891–1899. doi: 10.1016/j.hrthm.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kussauer S., David R., Lemcke H. hiPSCs Derived Cardiac Cells for Drug and Toxicity Screening and Disease Modeling: What Micro- Electrode-Array Analyses Can Tell Us. Cells. 2019;8:1331. doi: 10.3390/cells8111331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kanda Y., Yamazaki D., Osada T., Yoshinaga T., Sawada K. Development of torsadogenic risk assessment using human induced pluripotent stem cell-derived cardiomyocytes: Japan iPS Cardiac Safety Assessment (JiCSA) update. J. Pharmacol. Sci. 2018;138:233–239. doi: 10.1016/j.jphs.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 120.O’Hara T., Virág L., Varró A., Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: Model formulation and experimental validation. PLoS Comput. Biol. 2011;7:e1002061. doi: 10.1371/journal.pcbi.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paci M., Hyttinen J., Aalto-Setälä K., Severi S. Computational models of ventricular- and atrial-like human induced pluripotent stem cell derived cardiomyocytes. Ann. Biomed. Eng. 2013;41:2334–2348. doi: 10.1007/s10439-013-0833-3. [DOI] [PubMed] [Google Scholar]
- 122.Passini E., Britton O.J., Lu H.R., Rohrbacher J., Hermans A.N., Gallacher D.J., Greig R.J.H., Bueno-Orovio A., Rodriguez B. Human in Silico Drug Trials Demonstrate Higher Accuracy than Animal Models in Predicting Clinical Pro-Arrhythmic Cardiotoxicity. Front. Physiol. 2017;8:668. doi: 10.3389/fphys.2017.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang X., Pabon L., Murry C.E. Engineering adolescence: Maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Res. 2014;114:511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bar-Nur O., Russ H.A., Efrat S., Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9:17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 125.Barile L., Lionetti V., Cervio E., Matteucci M., Gherghiceanu M., Popescu L.M., Torre T., Siclari F., Moccetti T., Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014;103:530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 126.Pianezzi E., Altomare C., Bolis S., Balbi C., Torre T., Rinaldi A., Camici G.G., Barile L., Vassalli G. Role of somatic cell sources in the maturation degree of human induced pluripotent stem cell-derived cardiomyocytes. Biochim. Biophys. Acta Mol. Cell Res. 2019;28:118538. doi: 10.1016/j.bbamcr.2019.118538. [DOI] [PubMed] [Google Scholar]
- 127.Sanchez-Freire V., Lee A.S., Hu S., Abilez O.J., Liang P., Lan F., Huber B.C., Ong S.G., Hong W.X., Huang M., et al. Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells. J. Am. Coll. Cardiol. 2014;64:436–448. doi: 10.1016/j.jacc.2014.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meraviglia V., Wen J., Piacentini L., Campostrini G., Wang C., Florio M.C., Azzimato V., Fassina L., Langes M., Wong J., et al. Higher cardiogenic potential of iPSCs derived from cardiac versus skin stromal cells. Front. Biosci. 2016;21:719–743. doi: 10.2741/4417. [DOI] [PubMed] [Google Scholar]
- 129.Kim K., Zhao R., Doi A., Ng K., Unternaehrer J., Cahan P., Huo H., Loh Y.-H., Aryee M.J., Lensch M.W., et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat. Biotechnol. 2011;29:1117–1119. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sheng X., Reppel M., Nguemo F., Mohammad F.I., Kuzmenkin A., Hescheler J., Pfannkuche K. Human pluripotent stem cell-derived cardiomyocytes: Response to TTX and lidocain reveals strong cell to cell variability. PLoS ONE. 2012;7:e45963. doi: 10.1371/journal.pone.0045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Giacomelli E., Mummery C.L., Bellin M. Human heart disease: Lessons from human pluripotent stem cell-derived cardiomyocytes. Cell. Mol. Life Sci. 2017;74:3711–3739. doi: 10.1007/s00018-017-2546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fatima A., Kaifeng S., Dittmann S., Xu G., Gupta M.K., Linke M., Zechner U., Nguemo F., Milting H., Farr M., et al. The disease-specific phenotype in cardiomyocytes derived from induced pluripotent stem cells of two long QT syndrome type 3 patients. PLoS ONE. 2013;8:e83005. doi: 10.1371/journal.pone.0083005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sala L., Gnecchi M., Schwartz P.J. Long QT Syndrome Modelling with Cardiomyocytes Derived from Human-induced Pluripotent Stem Cells. Arrhythm. Electrophysiol. Rev. 2019;8:105–110. doi: 10.15420/aer.2019.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Selga E., Sendfeld F., Martinez-Moreno R., Medine C.N., Tura-Ceide O., Wilmut S.I., Pérez G.J., Scornik F.S., Brugada R., Mills N.L. Sodium channel current loss of function in induced pluripotent stem cell-derived cardiomyocytes from a Brugada syndrome patient. J. Mol. Cell. Cardiol. 2018;114:10–19. doi: 10.1016/j.yjmcc.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ma D., Liu Z., Loh L.J., Zhao Y., Li G., Liew R., Islam O., Wu J., Chung Y.Y., Teo W.S., et al. Identification of an INa-dependent and Ito-mediated proarrhythmic mechanism in cardiomyocytes derived from pluripotent stem cells of a Brugada syndrome patient. Sci. Rep. 2018;8:11246. doi: 10.1038/s41598-018-29574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Itzhaki I., Maizels L., Huber I., Gepstein A., Arbel G., Caspi O., Miller L., Belhassen B., Nof E., Glikson M., et al. Modeling of catecholaminergic polymorphic ventricular tachycardia with patient-specific human-induced pluripotent stem cells. J. Am. Coll. Cardiol. 2012;60:990–1000. doi: 10.1016/j.jacc.2012.02.066. [DOI] [PubMed] [Google Scholar]
- 137.Zhang X.H., Haviland S., Wei H., Sarić T., Fatima A., Hescheler J., Cleemann L., Morad M. Ca2+ signaling in human induced pluripotent stem cell-derived cardiomyocytes (iPS-CM) from normal and catecholaminergic polymorphic ventricular tachycardia (CPVT)-afflicted subjects. Cell Calcium. 2013;54:57–70. doi: 10.1016/j.ceca.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Marczenke M., Fell J., Piccini I., Röpke A., Seebohm G., Greber B. Generation and cardiac subtype-specific differentiation of PITX2-deficient human iPS cell lines for exploring familial atrial fibrillation. Stem Cell Res. 2017;21:26–28. doi: 10.1016/j.scr.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 139.Marczenke M., Piccini I., Mengarelli I., Fell J., Röpke A., Seebohm G., Verkerk A.O., Greber B. Cardiac Subtype-Specific Modeling of Kv1.5 Ion Channel Deficiency Using Human Pluripotent Stem Cells. Front. Physiol. 2017;8:469. doi: 10.3389/fphys.2017.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shimko V.F., Claycomb W.C. Effect of mechanical loading on three-dimensional cultures of embryonic stem cell-derived cardiomyocytes. Tissue Eng. Part. A. 2008;14:49–58. doi: 10.1089/ten.a.2007.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chan Y.C., Ting S., Lee Y.K., Ng K.M., Zhang J., Chen Z., Siu C.W., Oh S.K.W., Tse H.F. Electrical stimulation promotes maturation of cardiomyocytes derived from human embryonic stem cells. J. Cardiovasc. Transl. Res. 2013;6:989–999. doi: 10.1007/s12265-013-9510-z. [DOI] [PubMed] [Google Scholar]
- 142.Kamakura T., Makiyama T., Sasaki K., Yoshida Y., Wuriyanghai Y., Chen J., Hattori T., Ohno S., Kita T., Horie M., et al. Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circ. J. 2013;77:1307–1314. doi: 10.1253/circj.CJ-12-0987. [DOI] [PubMed] [Google Scholar]
- 143.Tohyama S., Hattori F., Sano M., Hishiki T., Nagahata Y., Matsuura T., Hashimoto H., Suzuki T., Yamashita H., Satoh Y., et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013;12:127–137. doi: 10.1016/j.stem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 144.Correia C., Koshkin A., Duarte P., Hu D., Teixeira A., Domian I., Serra M., Alves P.M. Distinct carbon sources affect structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Sci. Rep. 2017;7:8590. doi: 10.1038/s41598-017-08713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lemoine M.D., Mannhardt I., Breckwoldt K., Prondzynski M., Flenner F., Ulmer B., Hirt M.N., Neuber C., Horváth A., Kloth B., et al. Human iPSC-derived cardiomyocytes cultured in 3D engineered heart tissue show physiological upstroke velocity and sodium current density. Sci. Rep. 2017;7:5464. doi: 10.1038/s41598-017-05600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ronaldson-Bouchard K., Ma S.P., Yeager K., Chen T., Song L., Sirabella D., Morikawa K., Teles D., Yazawa M., Vunjak-Novakovic G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556:239–243. doi: 10.1038/s41586-018-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ulmer B.M., Stoehr A., Schulze M.L., Patel S., Gucek M., Mannhardt I., Funcke S., Murphy E., Eschenhagen T., Hansen A. Contractile Work Contributes to Maturation of Energy Metabolism in hiPSC-Derived Cardiomyocytes. Stem Cell Rep. 2018;10:834–847. doi: 10.1016/j.stemcr.2018.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]