Abstract
AIM
To systematically review and Meta-analyze studies of managing open angle glaucoma (OAG) with gonioscopy-assisted transluminal trabeculotomy (GATT) and to evaluate its effectiveness and safety.
METHODS
Eligible studies were retrieved and screened from five main electronic databases. Mean difference (MD) was hired to show the pooled effectiveness of intraocular pressure (IOP) and medication decrease achieved by GATT. In addition, combined surgical success and reoperation rates were calculated, and complications were also summarized.
RESULTS
Ten studies were included for systematic review, but one study was not pooled for Meta-analysis due to the repeated data. The combined IOP decrease after GATT was 9.81 mm Hg (95%CI: 7.98-11.63 mm Hg) which showed significant reduction from the baselines (Z=10.52, P<0.0001). Similarly, the number of medications after GATT also decreased distinctly compared with that of medication before the surgery (Z=9.09, P<0.0001), and the pooled medication decrease was 1.68 (95%CI: 1.31-2.04). In addition, the combined surgical success rate was 85%, while the pooled reoperation rate was 20%. Sight-threatening complications occurred scarcely, whereas the pooled occurrence rate of hyphemia was as high as 36.0%.
CONCLUSION
GATT could effectively lower IOP and decrease medications for patients with OAG. Moreover, the procedure appears to be a safe and promising treatment for OAG due to its minimally-invasive and conjunctiva-sparing nature.
Keywords: gonioscopy-assisted transluminal trabeculotomy, open angle glaucoma, intraocular pressure, complication, Meta-analysis
INTRODUCTION
To date, filtration surgeries, both trabeculectomy and glaucoma drainage devices, are the mainstream means to lower intraocular pressure (IOP) for patients with open angle glaucoma[1]. These surgical procedures could decrease the IOP effectively through drainage the aqueous humor into the subconjunctival space (bleb) through thefiltration bypass, but scarring of the conjunctiva and scleral flap always causes the IOP out of control[2]. Additionally, severe intra- and post-operative complications, such as lens damage, vitreous hemorrhage, choroidal detachment, blebitis and corneal endothelium compensation etc., may lead to surgical failures and compromise the eye sights postoperatively[3].
Different to the filtration surgeries, angle-based procedures, like trabeculotomy, facilitate the aqueous outflow through the physical collector system, so these surgeries are more suitable for open angle glaucoma (OAG)[4]. However, the traditional metal trabeculotomy shows imperfect effectiveness of decreasing IOP due to part angle incision and limited Schlemm's canal opening[5]. To achieve 360-degree trabeculotomy, ab externo circumferential trabeculotomy (AECT) via either suture or illuminated microcatheter-assisted have been introduced to the clinical settings for several years[6]–[9]. Although these techniques have been proven to be more effective than metal trabeculotomy in decreasing IOP, conjunctiva incision and scleral flap making are still needed to expose the Schlemm's canal. Given that, scarring of conjunctiva may prevent the eye treated by AECT from another filtration surgery afterward.
To overcome the drawbacks of AECT, a novel ab interno trabeculotomy, named as gonioscopy-assisted transluminal trabeculotomy (GATT), was initiated by Grover et al[10] in 2014. The manipulations of this technique are performed in the anterior chamber under direct gonioscopic observation, which could provide aclear vision for canal identification and microcatheter/suture insertion. More importantly, the procedure approach is only two clear cornea wounds without violating conjunctiva and sclera[10]. Thus, GATT is a minimally-invasive procedure which seems to be promising for clinical applications. Till now, a few studies of treating OAG with GATT have been reported, and most of them are single-centered, retrospective case serials with small sample size[10]–[19]. Therefore, the effectiveness of GATT to decrease IOP and its safety assessed by the single study is not very reliable and conclusive. For this reason, it is necessary and meaningful to pool the current studies to evaluate the effectiveness and draw more definite conclusions by means of Meta-analysis.
The aim of this study is to systematically review the studies of managing OAG with GATT and Meta-analyzed the data regarding IOP and medication decrease, surgical success rate, reoperation rate and complications.
MATERIALS AND METHODS
Strategy for Searching Studies
Studies of treating OAG with GATT were retrieved from five main electronic databases, including PubMed, EMBASE, OVID Medline, Cochrane Library and Clinical Trial gov. Two independent reviewers employed PI principle (participant+intervention) to search relevant literatures. Patients with OAG were the participants without considering the OAG was primary or secondary. To expand the search results, alternative text words and acronyms for GATT were used for searching as it is a new technique that has not been indexed by a Mesh word. After database searching, the reviewers compared their searching results and solved discrepancies by discussion.
Study Selection
The retrieved literatures were selected by the reviewers based on the PI principle. Briefly, the literatures were first screened by indexing titles, and studies that obviously irrelevant to OAG or GATT were excluded. Then details of the remaining literatures were checked out carefully through browsing abstracts and full-texts after which eligible studies were picked out for further quality assessment. The process was carried out independently by the two reviewers and discussion was conducted until consensus achieved.
Studies, Participants and Intervention
Considering GATT is a relatively new surgical procedure that initiated by Grover et al[10] in 2014 and few studies have been reported till now, studies on managing OAG through the procedure were all included no matter what the study design was. Patients with OAG combined with cataract or not were all the participants. The demographics of the patients (e.g. age and gender ratio), the severity before GATT (IOP and medication usage) and prior anti-glaucoma surgeries were not considered. GATT was defined as gonioscopy-assisted ab interno 360° trabeculotomy via threading suture or illuminated microcatheter into the Schlemm's canal.
Clinical Outcomes
IOP decrease at the end of follow-up was chosen as the primary outcome. The unit of IOP was expressed as mm Hg. In addition, medication decrease after GATT, surgical success rate, reoperation rate and complications were the secondary outcomes. The surgical success was defined as achieving 360° trabeculotomy during the surgery regardless of postoperative IOP decrease. And the complications included both intraoperative and postoperative complications.
Data Extraction and Management
Characteristics of each eligible study, including study design, region, sample size, demographics of the participants, details of the procedure (initial goniotomy, gonioscopy and cannulation), the definition of successful IOP control and follow-up, were extracted and managed by the two reviewers independently. The results were compared, and discussion was carried out if discrepancies existed between the reviewers.
Study Quality Evaluation
Given the included studies were either prospective or retrospective studies, a valid 14-item checklist (http://links.lww.143com/ICO/A265) that developed by the Review Body for Interventional Procedures was employed to evaluate the quality of these studies, which covers the main elements of an interventional study. The items on the checklist are questions regarding study design, randomization, mask, clinical outcomes, and follow-up etc. Each item would be answered by three options: “Yes”, “No” or “Unclear”. One study could be deemed as “High quality” in case of more than 8 “Yes” obtained. According to the checklist, important prognostic factors (Item 5) defined in this study were the classification of OAG (primary OAG, secondary OAG or congenital/juvenile OAG), prior anti-glaucoma surgeries, IOP and medications before GATT. Important outcomes (Item 11) included IOP decrease, medication reduction, surgical success rate and complications. In addition, the long follow-up period was defined as ≥1y.
Size Effects and Statistical Analyses
In the present study, all statistical analyses were conducted by using Stata 14.0 (Stata Corp LLC, College Station, TX, USA). Mean difference (MD) was chosen as the size effects to assess the effectiveness to decrease IOP and medication after GATT when considering these data were continuous. On the other hand, pooled rates were calculated for the proportional data (surgical success rate, reoperation rate and complication occurrence). All size effects were expressed as mean and 95% confidence interval (CI). Either fixed-effects model or random-effects model was adopted based on how the heterogeneity was. In addition, when distinct heterogeneity existed (P value for Chi-square less than 0.1 or I2>50%), the overall size effects were sub-grouped by related covariates (e.g. follow-ups and cannulations). P<0.05 was considered as the significant threshold.
RESULTS
Study Selection
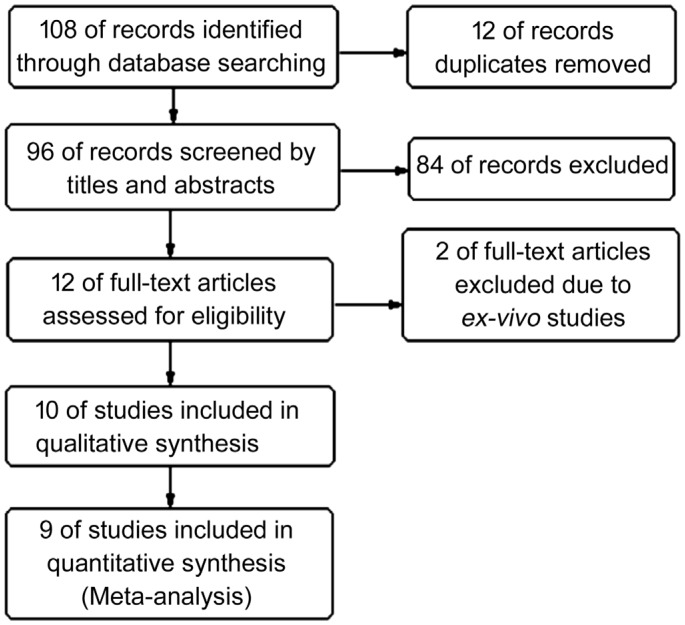
Figure 1 shows the diagram of selecting eligible studies. A total of 108 literatures were retrieved from the databases. After removing 12 duplicates, 96 records were screened by titles and abstracts, and 84 unrelated records were excluded. Then, the remaining 12 studies were assessed through browsing full-texts, and two ex-vivo studies were excluded. Finally, ten studies were included for systematic review, but one study[10] was not pooled for Meta-analysis due to the repeated data.
Figure 1. Flow diagram of selecting eligible studies.
Study Characteristics
Table 1 demonstrates the characteristics of the included studies. The publication year ranged from 2014 to 2018, and the regions only covered three countries: USA, Germany and Japan. Most study (n=8, 80%) were retrospective case serials, while the others (20%) were non-randomized prospective studies. A total of 523 patients with OAG (537 eyes) involved in these studies, and OAG classification included primary open angle glaucoma (POAG), secondary open angle glaucoma (SOAG), primary congenital glaucoma (PCG) and juvenile open angle glaucoma (JOAG). Of the ten studies, five combined with cataract extraction, while only GATT was carried out in the other five studies. Moreover, 25G microvitreoretinal blade (n=3, 30%), trabectome (n=2, 20%) and microsurgical blade (n=1, 10%) were commonly used to perform the initial goniotomy. Either suture (n=3, 30%) or illuminated microcatheter (n=3, 30%) or suture/microcatheter (n=3, 30%) were used in these studies. The criteria of successful IOP control were also slightly different among these studies, but most of them adopted the following routine standards: IOP<21 mm Hg, IOP reduction >20% or no further anti-glaucoma surgeries. However, the follow-up periods varied largely in the studies spanning from 2mo to 33mo.
Table 1. Characteristics of the included studies.
| Author, year | Region | Study design | Participants/eyes | Primary disease | Combinded surgeries | Age (SD) | F/M | Initial goniotomy | Gonioscopy | Cannulation | Tenonmeter | Success IOP-control | Follow-up |
| Smith et al[18], 2018 | USA | RCS | 8/8 | POAG | Cataract | 75.00 (10.80) | 5/3 | Trabectome (90°) | SJGL | MC | NA | NA | 2mo-2y |
| Grover et al[14], 2018 | USA | RCS | 119/119 | OAG | Cataract | 68.21 (11.17) | 66/53 | 25G MVRB | SJGL | 5-0 S/MC | GT | IOP reduction >20% and IOP<21 mm Hg/no further surgery at or after 6mo follow-up | 24mo |
| Rahmatnejad et al[11], 2017 | USA | RCS | 48/48 | POAG | Cataract | 59.90 (15.28) | 23/25 | 25G MVRB | NA | MC | GT | IOP reduction >20%/IOP=5-21 mm Hg/no need further surgery. | 11.9 (3-30)mo |
| Grover et al[17], 2017 | USA | RCS | 35/35 | OAG | Cataract | 67.7 (14.4) | 15/20 | MSB | SJGL | S/MC | NA | IOP reduction >20% and IOP<21 mm Hg/no further surgery at or after 6mo follow-up | 24mo |
| Moster et al[19], 2017 | USA | PCS | 76 | OAG | NA | 57.3 (17.8) | 34/42 | NA | NA | NA | NA | NA | 8.8 (6.6)mo |
| Seuthe et al[15], 2016 | Germany | RCS | 88/88 | OAG | NA | NA | NA | NA | OSGL | 10-0 S | NA | NA | 12mo |
| Voykov et al[16], 2015 | Germany | RCS | 31/31 | OAG | NA | 65 (15) | 13/18 | NA | OSGL | 10-0 S | NA | IOP≤21 mm Hg and IOP reduction >25%. | 24mo |
| Sato et al[12], 2015 | Japan | PCS | 12/12 | OAG | NA | 70.8 (8.1) | 9/3 | Trabectome (15°) | SJGL | 5-0 S | GT | NA | 5.5 (0.9)mo |
| Grover et al[13], 2015 | USA | RCS | 10/14 | PCG/JOAG | NA | 18.4 (10.44) | 5/5 | 25G MVRB | NA | MC | GT | NA | 20.4 (12-33)mo |
| Grover et al[10], 2014 | USA | RCS | 96/106 | OAG | Cataract | 64.48 (15.16) | 52/54 | MSB | SJGL | S/MC | NA | IOP reduction >20% and IOP<21 mm Hg/no further surgery at or after 6mo follow-up | 10.94 (3.29)mo |
RCS: Retrospective case serials; PCS: Prospective case serials; POAG: Primary open angle glaucoma; OAG: Open angle glaucoma; PCG: Primary congenital glaucoma; JOAG: Juvenile open angle glaucoma; MVRB: Microvitreoretinal blade; MSB: Microsurgical blade; SJGL: Swan Jacob goniolens; OSGL: Osher surgical goniolens; MC: Microcatheter; S: Suture; F: Female; M: Male; IOP: Intraocular pressure; GT: Goldmann tonometer.
Study Quality
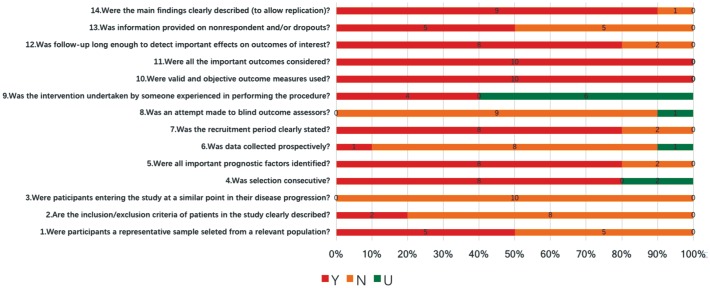
Figure 2 represents the results of assessing the studies by the 14-item checklist. Of the ten studies, eight obtained no less than 8 “Yes” by which could be deemed as high-quality; whereas the other two were poor-quality.
Figure 2. Study quality evaluation referring by the 14-item checklist.
Effectiveness of Reducing IOP after GATT
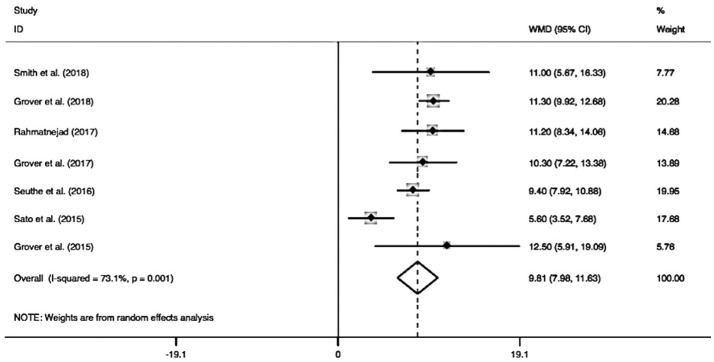
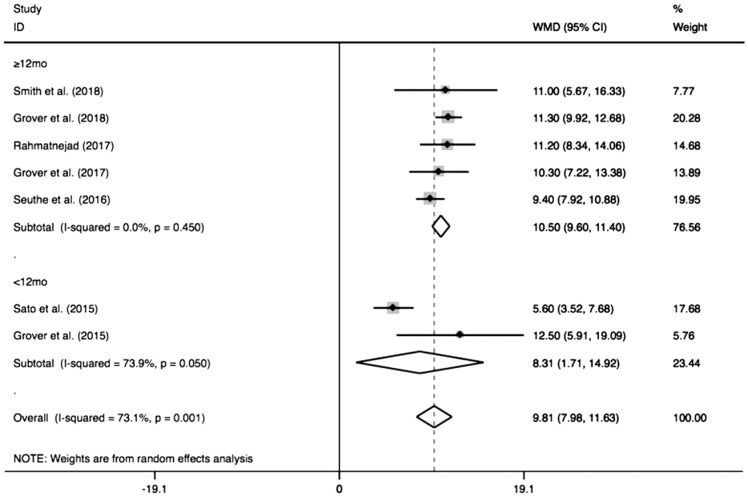
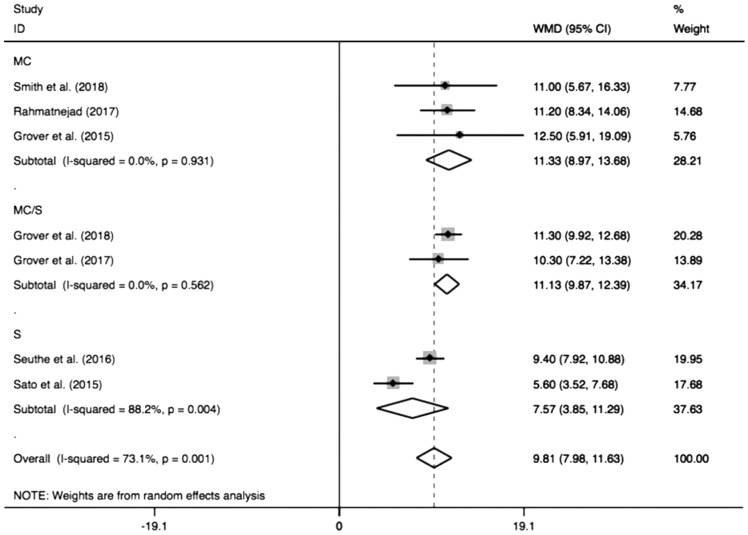
Figure 3 shows the pooled IOP decrease after GATT. The combined IOP decrease was 9.81 mm Hg (95%CI: 7.98-11.63), and the pooled IOP after GATT was significantly lower than the preoperative IOP (I2=73.1%, Z=10.52, P<0.0001). When the combined IOP decrease was sub-grouped by different follow-ups (Figure 4), the pooled results also showed distinct reduction in either longer follow-up (≥12mo, MD=10.50, 95%CI: 9.60-11.40, I2=0, Z=22.92, P<0.0001) or shorter follow-up (<12mo, MD=8.31, 95%CI: 1.71-14.92, I2=73.9%, Z=2.47, P=0.01), which suggesting shorter follow-up (<12mo) was a significant factor to generate the heterogeneity (I2=73.9%, P=0.05). Additionally, subgroup analysis of different cannulations (Figure 5) showed significant IOP decrease could be achieved by using either microcatheter (MD=11.33, 95%CI: 8.97-13.68, I2=0, Z=9.43, P<0.0001) or suture (MD=7.57, 95%CI: 3.85-11.29, I2=88.2%, Z=3.99, P<0.0001) or suture/microcatheter (MD=11.13, 95%CI: 9.87-12.39, I2=0, Z=17.28, P<0.0001). The results also indicated that suture was a significant confounding factor (I2=88.2%, P=0.004), because different sutures (5-0 or 10-0) were used in these studies.
Figure 3. Forest plot for IOP decrease after GATT.
Figure 4. Subgroup analysis for IOP decrease by different follow-ups.
Figure 5. Subgroup analysis for IOP decrease by different cannulations.
Medication Decrease after GATT
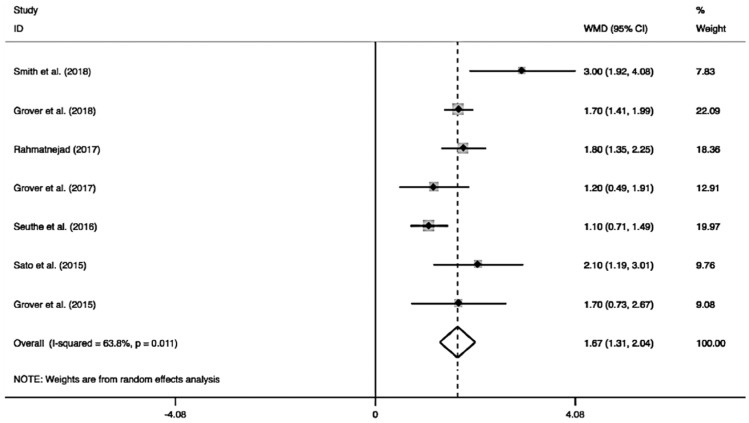
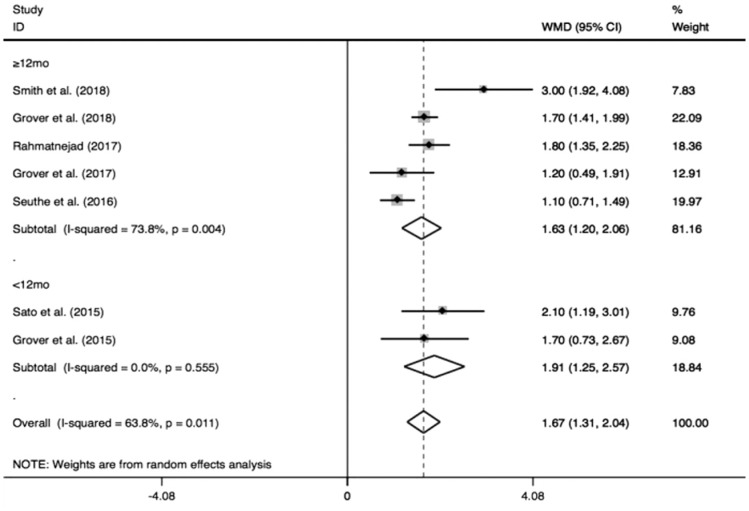
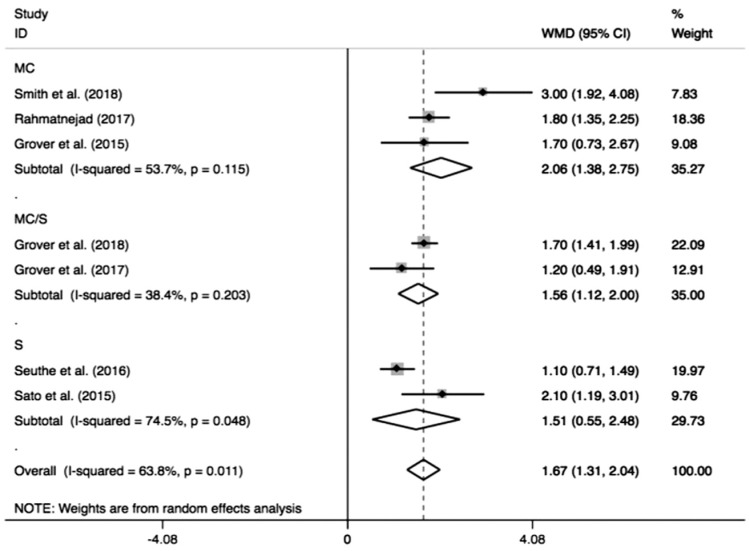
As shown in Figure 6, the pooled medication decreased by 1.67 (95%CI: 1.31-2.04) from the number before the surgery, and the medications after GATT was distinctly less than those before the operation (I2=63.8%, Z=9.09, P<0.0001). As the pooled data demonstrated in Figure 7, significant IOP reduction was found either in longer follow-up (≥12mo, MD=1.63, 95%CI: 1.20-2.06, I2=73.8%, Z=7.40, P<0.0001) or shorter follow-up (<12mo, MD=1.91, 95%CI: 1.25-2.57, I2=0, Z=5.65, P<0.0001). Similarly, when the pooled result was sub-grouped by different cannulations (Figure 8), significant medication decrease also could be achieved by different cannulations: microcatheter (MD=2.06, 95%CI: 1.38-2.75, I2=53.7%, Z=5.92, P<0.0001), suture (MD=1.51, 95%CI: 0.55-2.48, I2=74.5%, Z=3.07, P=0.002) and microcatheter/suture (MD=1.56, 95%CI: 1.12-2.00, I2=38.4%, Z=6.94, P<0.0001). The two subgroup analyses revealed longer follow-up (≥12mo; I2=73.8%, P=0.004) and suture (I2=74.5%, P=0.05) were two factors to cause the obvious heterogeneity.
Figure 6. Forest plot for medication decrease after GATT.
Figure 7. Subgroup analysis for medication decrease by different follow-ups.
Figure 8. Subgroup analysis for medication decrease by different cannulations.
Surgical Success Rate of GATT and Reoperation Rate
The combined surgical success rate was 85% (95%CI: 0.73-0.96, I2=79.3%). Although in some cases cannulations failed to pass 360 degrees in one direction, most of them could achieve circumferential trabeculotomy by threading microcatheter or suture in the opposite direction. In addition, the pooled reoperation rate was 20% (95%CI: 0.15-0.24, I2=30.0%).
Complications after GATT
Table 2 summarizes the complications that reported by these studies. Among the complications, hyphema was the most common one that encountered in all studies, and the pooled occurrence rate was 36.0% (95%CI: 0.23-0.49, I2=90.6%). Except for hyphema, transient hypotony and IOP spike were relatively common complications after GATT. Other sight-threatening complications reported in these studies were very rare, including corneal edema, Descemet's detachment, iritis, iridodialysis, vitreous hemorrhage, peripheral anterior synechia (PAS), goniosynechia, choroidal folds and cystoid macular edema (CME).
Table 2. Complications after GATT reported by the included studies.
| Author, year | Hyphema | TH | IOP spike | CE | DD | Iritis | Iridodialysis | VH | PAS | GS | CF | CME |
| Smith et al[18], 2018 | 1 (12.5) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Grover et al[14], 2018 | 37 (18.7) | NA | NA | 2 (1.0) | 1 (0.5) | 1 (0.5) | 1 (0.5) | NA | NA | NA | NA | NA |
| Rahmatnejad et al[11], 2017 | 27 (40.9) | NA | NA | NA | NA | 1 (1.5) | NA | NA | NA | NA | NA | NA |
| Grover et al[17], 2017 | 12 (34.3) | NA | NA | NA | NA | NA | NA | 1 (2.9) | NA | NA | NA | NA |
| Moster et al[19], 2017 | 15 (19.7) | 4 (5.3) | 18 (23.7) | NA | NA | 4 (5.3) | NA | NA | NA | NA | NA | NA |
| Seuthe et al[15], 2016 | 22 (25.0) | 4 (4.5) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Voykov et al[16], 2015 | 25 (80.6) | 2 (6.5) | 10 (32.3) | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Sato et al[12], 2015 | 8 (66.7) | NA | 3 (25.0) | NA | NA | NA | NA | NA | 3 (25.0) | 6 (50.0) | NA | NA |
| Grover et al[13], 2015 | 5 (35.7) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Grover et al[10], 2014 | 30 (28.3) | NA | 2 (1.9) | NA | NA | NA | NA | NA | NA | NA | 3 (2.8) | 1 (0.9) |
TH: Transient hypotony; CE: Corneal edema; DD: Descemet's detachment; VH: Vitreous hemorrhage; PAS: Peripheral anterior synechia; GS: Goniosynechia; CF: Choroidal folds; CME: Cystoid macular edema.
n (%)
DISCUSSION
In the present Meta-analysis, studies about treating OAG with GATT were systematically reviewed, and the data regarding IOP decrease, medication reduction, surgical success rate, reoperation rate and complications were Meta-analyzed. The combined data showed IOP and medication decreased significantly from the baselines. In addition, the pooled surgical success rate was 85%, and the reoperation rate was 20%. Although hyphema occurred in all studies, other sight-threatening complications were rarely reported in these studies.
GATT is a delicate surgical technique that invented by Grover et al[10] in 2014 to lower IOP for patients with OAG. Different to AECT, all the procedures of GATT were manipulated in the anterior chamber without violating the conjunctiva and sclera. The effectiveness of GATT to decrease IOP should be equivalent to AECT theoretically, because both the techniques decrease IOP through 360-degree trabeculotomy. However, our pooled IOP decrease (9.81 mm Hg, 95%CI: 7.98-11.63) was less than the decrease (13.1 mm Hg) that reported by Chin et al[20] who carried out AECT to treat adult patients with OAG. In addition, IOP decrease after AECT in congenital glaucomas that reported by Girkin et al[8] also showed better results which reduced from 33.8±6.3 mm Hg preoperatively to 18.3±3.5 mm Hg. We assume certain aqueous outflow through the scleral flap that made by AECT may account for more IOP decrease. Although GATT seemed to be inferior to AECT in reducing IOP, it is undeniable that the technique is a promising anti-glaucoma surgery when considering its nature of sparing conjunctiva and sclera, which will not cause scarring of the conjunctiva and compromise the integrity of the globe. Thus, GATT may become the first-line treatment for OAG since filtration surgeries could be done after GATT failed to control the IOP.
Although most patients with OAG after GATT still need anti-glaucoma medications to achieve their target IOPs, the significant decrease of the medications after GATT has been shown by our pooled results. In this study, the combined medication decrease was 1.68 (95%CI: 1.31-2.04) that was also lower than the decrease (2.3) after ab externo circumferential trabeculotomy at postoperative month 12[20].
In this study, the pooled surgical success rate of GATT was 85% which seemed to be better than those of AECT[5]–[7]. Some authors suppose that GATT was easier to manipulate, which may account for the higher surgical success rate[12]. Different to AECT, the critical steps of GATT, such as goniotomy and cannulation threading, are visualized with the help of gonioscopy, so the risk of insertion into the anterior chamber or subchoroidal space is avoided. Some authors postulated that the abnormally developed collector system, collapse of Schlemm's canal and discontinuity of the canal that caused by prior incisional glaucoma surgeries might be the causes for the failed cases[11],[18]. However, Grover et al[17] found most eyes (88.6%) that have undergone trabeculectomy or glaucoma drainage device previously could be still achieved at least 300° trabeculotomy, suggesting GATT was also applicable for the failed cases after filtration surgeries.
Several studies have proven that GATT was a safe procedure to lower IOP, because sight-threatening complications rarely occurred[10]–[19]. The most common complication was hyphema that observed in all the studies, and our pooled occurrence rate was as high as 36.0% (95%CI: 0.23-0.49). Hyphema is mainly caused by the reflux of episcleral veins that is also the successful indication for the surgery[12]. Although hyphema is unavoidable for GATT to some extent, most of the hyphema could be absorbed within one month after the surgery, and Rahmatnejad et al[11] found that hyphema was not related to postoperative IOP spike and surgical failure. To reduce the chance of postoperative hyphema, Grover et al[10],[13],[17] suggested that 25% anterior chamber should be filled with viscoelastic substances at the end of the surgery and the head also should be elevated 30° for the first 1-2wk.
Of the included studies, either illuminated microcatheters or sutures (5-0 or 10-0 suture) were used to thread the Schlemm's canal and cleave it, but the different cannulations also showed varied capacities for IOP decrease. Our pooled results demonstrated that the IOP decreases achieved by using microcatheter and suture were 11.33 mm Hg (95%CI: 8.97-13.69) and 7.57 mm Hg (95%CI: 3.85-11.30), respectively. We assume the larger diameter of the microcatheter may lead to a wider opening of the canal which would facilitate the aqueous outflow through the collector system. On the other hand, sutures seem to be more accessible and cheaper (about US$20) than the microcatheter (US$700 to US$1000)[14], although the latter one showed better effectiveness to decrease IOP. The two cannulations with different costs provide us options to choose based on certain clinical settings.
To date, some authors have utilized trabectome (Neomedix Corporation, Tustin, CA, USA) instead of microvitreoretinal blade to initiate goniotomy[12],[18]. Although trabectome would increase the costs of the surgery, its advantages for GATT have been recommended by the authors. First, the special-designed tip of the trabectome enables an appropriate cutting depth to avoid damaging the outer canal wall. Second, initiation of cutting the inner canal wall by trabectome ablation proved to be easier, and the wider opening would facilitate insertion of suture afterward. Lastly, trabectome ablation could also be the backup procedure to decrease the IOP in case of failing to perform GATT[12],[18].
Honestly, among these included studies, some variances regarding manipulation, follow-up, baseline IOP and primary diseases could invalidate our pooled results to some extent. In addition, most of the included studies were retrospective case serials which had no randomization and control group. More importantly, as a newly-emerged technique, the authors may be prone to choose simple patients to complete their initial cases, which may also bias the validity of the results. Thus, randomized, controlled studies with large sample size should be conducted before definite conclusions could be drawn.
In conclusion, GATT could effectively lower IOP and decrease medications for patients with OAG. Moreover, the procedure appears to be a safe and promising treatment for OAG due to its minimally-invasive and conjunctiva-sparing nature.
Acknowledgments
Conflicts of Interest: Guo CY, None; Qi XH, None; Qi JM, None.
REFERENCES
- 1.Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL, Tube versus Trabeculectomy Study Group Treatment outcomes in the Tube Versus Trabeculectomy (TVT) Study after five years of follow-up. Am J Ophthalmol. 2012;153(5):789–803.e2. doi: 10.1016/j.ajo.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bettin P, Di Matteo F. Glaucoma: present challenges and future trends. Ophthalmic Res. 2013;50(4):197–208. doi: 10.1159/000348736. [DOI] [PubMed] [Google Scholar]
- 3.Drake M. Complications of glaucoma filtration surgery. Int Ophthalmol Clin. 1992;32(4):115–130. doi: 10.1097/00004397-199223000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Harms H, Dannheim R. Epicritical consideration of 300 cases of trabeculotomy ‘ab externo’. Trans Ophthalmol Soc U K. 1970;89:491–499. [PubMed] [Google Scholar]
- 5.Lim ME, Neely DE, Wang J, Haider KM, Smith HA, Plager DA. Comparison of 360-degree versus traditional trabeculotomy in pediatric glaucoma. J AAPOS. 2015;19(2):145–149. doi: 10.1016/j.jaapos.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Beck AD, Lynch MG. 360 degrees trabeculotomy for primary congenital glaucoma. Arch Ophthalmol. 1995;113(9):1200–1202. doi: 10.1001/archopht.1995.01100090126034. [DOI] [PubMed] [Google Scholar]
- 7.Mendicino ME, Lynch MG, Drack A, Beck AD, Harbin T, Pollard Z, Vela MA, Lynn MJ. Long-term surgical and visual outcomes in primary congenital glaucoma: 360 degrees trabeculotomy versus goniotomy. J AAPOS. 2000;4(4):205–210. doi: 10.1067/mpa.2000.106201. [DOI] [PubMed] [Google Scholar]
- 8.Girkin CA, Marchase N, Cogen MS. Circumferential trabeculotomy with an illuminated microcatheter in congenital glaucomas. J Glaucoma. 2012;21(3):160–163. doi: 10.1097/IJG.0b013e31822af350. [DOI] [PubMed] [Google Scholar]
- 9.Sarkisian SR., Jr An illuminated microcatheter for 360-degree trabeculotomy [corrected] in congenital glaucoma: a retrospective case series. J AAPOS. 2010;14(5):412–416. doi: 10.1016/j.jaapos.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Grover DS, Godfrey DG, Smith O, Feuer WJ, Montes de Oca I, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014;121(4):855–861. doi: 10.1016/j.ophtha.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Rahmatnejad K, Pruzan NL, Amanullah S, Shaukat BA, Resende AF, Waisbourd M, Zhan T, Moster MR. Surgical outcomes of gonioscopy-assisted transluminal trabeculotomy (GATT) in patients with open-angle glaucoma. J Glaucoma. 2017;26(12):1137–1143. doi: 10.1097/IJG.0000000000000802. [DOI] [PubMed] [Google Scholar]
- 12.Sato T, Hirata A, Mizoguchi T. Prospective, noncomparative, nonrandomized case study of short-term outcomes of 360° suture trabeculotomy ab interno in patients with open-angle glaucoma. Clin Ophthalmol. 2015;9:63–68. doi: 10.2147/OPTH.S75739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grover DS, Smith O, Fellman RL, Godfrey DG, Butler MR, Montes de Oca I, Feuer WJ. Gonioscopy assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy for the treatment of primary congenital glaucoma and juvenile open angle glaucoma. Br J Ophthalmol. 2015;99(8):1092–1096. doi: 10.1136/bjophthalmol-2014-306269. [DOI] [PubMed] [Google Scholar]
- 14.Grover DS, Smith O, Fellman RL, Godfrey DG, Gupta A, Montes de Oca I, Feuer WJ. Gonioscopy-assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy: 24 months follow-up. J Glaucoma. 2018;27(5):393–401. doi: 10.1097/IJG.0000000000000956. [DOI] [PubMed] [Google Scholar]
- 15.Seuthe AM, Januschowski K, Szurman P. Micro-invasive 360-degree suture trabeculotomy after successful canaloplasty - one year results. Graefes Arch Clin Exp Ophthalmol. 2016;254(1):155–159. doi: 10.1007/s00417-015-3192-y. [DOI] [PubMed] [Google Scholar]
- 16.Voykov B, Szurman P, Dimopoulos S, Ziemssen F, Alnahrawy O. Micro-invasive suture trabeculotomy after canaloplasty: preliminary results. Clin Exp Ophthalmol. 2015;43(5):409–414. doi: 10.1111/ceo.12482. [DOI] [PubMed] [Google Scholar]
- 17.Grover DS, Godfrey DG, Smith O, Shi W, Feuer WJ, Fellman RL. Outcomes of gonioscopy-assisted transluminal trabeculotomy (GATT) in eyes with prior incisional glaucoma surgery. J Glaucoma. 2017;26(1):41–45. doi: 10.1097/IJG.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 18.Smith BL, Ellyson AC, Kim WI. Trabectome-initiated gonioscopy-assisted transluminal trabeculotomy. Mil Med. 2018;183(suppl_1):146–149. doi: 10.1093/milmed/usx174. [DOI] [PubMed] [Google Scholar]
- 19.Moster M, Rahmatnejad K, Amanullah S, Waisbourd M, Resende A. The outcomes of gonioscopy-assisted transluminal trabeculotomy (GATT) in open-angle glaucoma. Invest Ophthalmol Vis Sci. 2017;58(8):4988. doi: 10.1097/IJG.0000000000000802. [DOI] [PubMed] [Google Scholar]
- 20.Chin S, Nitta T, Shinmei Y, Aoyagi M, Nitta A, Ohno S, Ishida S, Yoshida K. Reduction of intraocular pressure using a modified 360-degree suture trabeculotomy technique in primary and secondary open-angle glaucoma: a pilot study. J Glaucoma. 2012;21(6):401–407. doi: 10.1097/IJG.0b013e318218240c. [DOI] [PubMed] [Google Scholar]