Abstract
AIM
To explore netrin-1 functions on corneal epithelium in vitro and in vivo.
METHODS
In vitro the human corneal epithelial (HCE) cells were treated with serum free DMEM-F12 basic media containing 0, 50, 100, 200, 300, 500, 800, and 1000 ng/mL of netrin-1, respectively. The cells viability was detected by cell counting kit-8 (CCK-8). The wound-healing assay was applied to assess the migration proficiency of HCE cells. Flow cytometry was used to analyze the cell-cycle distribution and apoptosis. In vivo, normal c57 (6wk) mice were demarcated with a trephine in the middle of the cornea to produce a 3-mm circular wound. Mice corneas were inflicted no epithelium with a 3-mm wound displayed, but remained the limbal epithelium intact. A blunt scalpel blade was used to remove the corneal epithelian cells, followed by topical netrin-1 application (200 ng/mL), and the group treated by PBS as control. The treated group was injected netrin-1 into the normal c57 mice inferior subconjunctival 4h before trauma. Mouse corneal inflammation and neovascularization were observed under slit lamp microscope. The apoptosis of corneal cells was determined by TUNEL staining.
RESLUTS
A concentration of 200 ng/mL netrin-1 enhanced 25% of the HCE viability. The relative migration rates were 76.3% and 100% in control and netrin-1 treated group after cultured 72h. Treated with netrin-1 (200 ng/mL) decreased the apoptosis of HCE cells, as well as decreased their percentage from 19.3%±0.57% to 12.7%±0.42% of the total. The remaining wound area was 1.22 mm2 in control group but 0.22 mm2 in the netrin-1 treated group. Exogenous Netrin-1 inhibits apoptosis of corneal epithelial cells of c57 mice. TUNEL-positive cells at the epithelial layer of the corneas of the control and netrin-1 treated c57 mice at 24h after wounding were 43.3% and 16.7% respectively.
CONCLUSION
Netrin-1 can reduce HCE apoptosis as well as promote its proliferation and migration. Topical application of netrin-1 promotes the injuryed cornea epithelial wound repair and inhibits apoptosis of corneal epithelial cells. These findings may offer potential therapies to repair the defects of corneal epithelial based on netrin-1.
Keywords: netrin-1, corneal epithelium, proliferation, apoptosis, migration, wound repair
INTRODUCTION
Corneal injuries greatly contribute to ocular morbidity and vision impairment. The transparency functionality of the cornea after injury greatly depends on optimal epithelial wound healing. Netrin-1 with functions in embryonic axon direction, which is also implicated in a number of multi-system diseases, which makes it a significant therapeutic target. Recently, it has been reported that epithelial cell function is also regulated by netrin-1. The epithelium lining the surface of the cornea is a barrier that protects the natural construction of the eye. Nonetheless, corneal epithelium is vulnerable to get injured since it is exposed to the external environment. Although the epithelium contains stem cells in the basal limbal epithelium that can mediate its regeneration[1], various inflammatory and autoimmune conditions, diabetes, grievous damage, or dry eye, can lead to a failure of wound healing[2]–[4]. In spite of its importance for proper wound healing and exclusion of pathogens, ocular inflammation can become persistent, and the resulting expression of pro-inflammatory cytokines can inhibit the healing procedure, causing persistent epithelium deficiency. Although there are many treatments for corneal epithelium deficiency, there is a quite high failure incidence, sometimes even leading to vision loss and corneal perforation.
Netrin-1 is an axon guidance protein binding to its receptors to exert its functions, UNC5 (A-D) or deleted in colorectal carcinoma (DCC)[5]–[6]. Netrin-1 is also existed non-nervous system that regulating adhesion, flexibility, proliferation, differentiation in numerous non-neuronal tissues[7]–[10], where it influences tissue morphogenesis through regulating cell migration and controlling intercellular or cell-matrix adhesion[5]. Corneal epithelium and stroma express netrin-1[11]–[13]. Netrin-1 is involved in the proliferation, invasion and apoptosis of cancer epithelial cells[14]–[15]. In vivo, netrin-1 is demonstrated to protect corneal epithelial cells (CECs) from apoptosis and facilitate cell survival in the alkali-burn model in rat eyes[11]. However, its underlying therapeutic efficacy on epithelial repair in other cornea injuries has not yet been explored.
In this study, netrin-1 functions on CECs were investigated in vitro and in vivo.
MATERIALS AND METHODS
Ethical Approval
Animal experiments were performed in accordance with the guidelines of the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research, and the animal experimental procedures were approved by the Experimental Animal Committee of Xiamen University. All rats were confirmed as being free of ocular diseases before the experiments.
Reagents
The recombinanted human netrin-1 protein was bought fromR&D Systems (China Co. Ltd.). The cell counting kit-8 (CCK-8) was bought from Dojindo (Kyushu, Japan).
Cell Culture
Human corneal epithelial (HCE) cells transformed with Simian virus 40 (RIKEN Biosource Center, Tokyo, Japan), were passaged in Dulbecco's modified Eagle's medium (DMEM) comprising nutrient mixture F-12 (DMEM-F12; Invitrogen, Carlsbad, CA, USA), 6% heat-inactivated fetal bovine serum (FBS), recombinant human epidermal growth factor (7 ng/mL), bovine insulin (7 µg/mL), and 1% penicillin and streptomycin. The cell viability assay was conducted on HCE cells in 96-well culture plates at a density of 8000 cells/well. Once reaching 70% confluence, the culture supernatants were removed and serum free DMEM-F12 basic media containing 0, 50, 100, 200, 300, 500, 800, and 1000 ng/mL of netrin-1, respectively, were added, followed for an additional 72h of culture. For the entire rest of experiments, the HCE cells were plated into 6-well plates at 30×104 cells/well or into 24-well culture plates at 5×104 cells per well. The cells were cultured with the indicated concentrations of netrin-1 for 72h, after which the wound-healing assay was conducted to assess cell migration. Flow cytometry was used to analyze the cell-cycle distribution and apoptosis. The morphology and confluence were checked every day by directly observing the plates using an inverted phase-contrast microscope.
Cell Viability Assay
The CCK-8 was used to detect the cell viability on the basis of the instructions. The 5×103 HCE cells in 100 µL per well were allocated into 96-well plates in triplicate then incubated for 24h. Dosages of 0, 50, 100, 200, 300, 500, 800, 1000 ng/mL of netrin-1 were added to the medium, respectively, then subsequent addition of 10 µL CCK-8 solution (Dojindo, Kuma-moto, Japan) following 72h of culture, and incubation for 1h. Optical density (OD) was detected at 450 nm by a universal microplate reader (Bio-Tek, Winooski, USA), and plotted an OD graph against concentrations. Each mark denoted the average value of collected numerical reading, the results represent the means of at least 3 readings. Within 4h, OD at 570 nm were measured by a microplate reader (ELX800, BIO-TEK Corporation, USA).
Assess Migration by Wound-healing Assay
The wound-healing assay was applied to assess the migration proficiency of HCE cells[15]–[16]. The cells were seeded in duplicate at a density of 105/well onto 1% gelatin-coated 24-well plates (Corning, Netherlands). The cells were cultured to confluence, and a sterile 200-µL pipette tip was used to scratch open the cell layer in two perpendicular directions, creating linear, cross-stripe scrapes every 2 mm. PBS was used to wash the scratched monolayers in order to remove the dislodged cells, after which medium with 200 ng/mL netrin-1 was added for an additional 24h of culture. Assess the closure of the scratch uses an inverted phase contrast microscope at different time points.
Flow Cytometric Cell-cycle Analysis
HCE cells were grown to confluence in 35-mm dishes as described above, followed with the addition of 200 ng/mL netrin-1 and incubation for 72h at 37°C. Afterwards, 0.05% trypsin in PBS buffer was applied to detach the cells from the plate, pelleted (centrifugation for 5min at 300 g), washed for twice in PBS with 1% bovine serum albumin (BSA), resuspended in the same buffer and fixed with 70% ice-cold ethanol. The data were acquired using a FACS can flow cytometer (BD Bio-sciences, Franklin Lakes, USA).
Cell Apoptosis Analysis
HCE cells were plated in duplicate in 60-mm culture dishes at a density of 2×105. After reaching 70% to 80% confluence, the 200 ng/mL netrin-1 was added and persisted incubation for 72h at 37°C. An apoptosis assay kit with Hoechst 33342 and PI staining (Beyotime Institute of Biotechnology, Shanghai, China) was used to analyze apoptosis, in accordance with the instructions. In Brief, the HCE cells were trypsinized, washed in PBS two times, and resuspended in 100 µL of cell-staining buffer. Subsequently, 5 µL each of 10 µg/mL PI and Hoechst 33342 were added, followed by incubation in the dark at 4°C for 20min. The number of apoptotic cells was analyzed instantly after incubation with flow cytometer.
Corneal Epithelial Wound Debridement
Normal c57 (6wk) mice were anesthetized, and demarcated with a trephine in the middle of the cornea to produce a 3-mm circular wound. Mice corneas were inflicted no central epithelium and peripheral with a 3-mm wound displayed, but remained the limbal epithelium intact. A blunt scalpel blade was under a dissecting microscope to remove the CECs[17]–[19]. The scraped CECs were shock-frozen in liquid nitrogen, collected into a test tube and stored at -80°C. A bacitracin ophthalmic ointment was used to prevent infection after surgery. At 24h after the wounding intervention, a trephine of the same size was used to remove CECs from the original wound.
Subconjunctival Injection of Netrin-1
The 10 uL solution was injected each into the subconjunctival space superior and inferior quadrants of the cornea. PBS and 200 ng/mL netrin-1 was injected 4-6h before wounding.
Apoptosis Detection Assay
In order to identify and quantify the cell apoptosis, the cornea was embedded in OCT compound, cross sectioned, and underwent terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) staining (Dead End Fluorometric TUNEL system, Promega, Shanghai, China), conforming to the instruction. The 4′-6-diamidino-2-phenylindole (DAPI) was applied to stain the nucleus, and apoptosis were observed using a laser confocal microscope (Fluoview 1000, Olympus, Tokyo, Japan). The cell nucleus and apoptotic cells were calculated in 3 sections of individual sample.
Statistical Analysis
One-way analysis of variance (ANOVA, GraphPad Software, La Jolla, CA, USA) was administered to evaluate the data of CCK-8 assay, migration, cell-cycle and apoptosis, a post hoc analysis Tukey test or a Student's t-test were applied to analyze the differences between groups. P<0.05 was deemed to be statistically significant.
RESULTS
Netrin-1 Affects HCE Viability in a Concentration Dependence
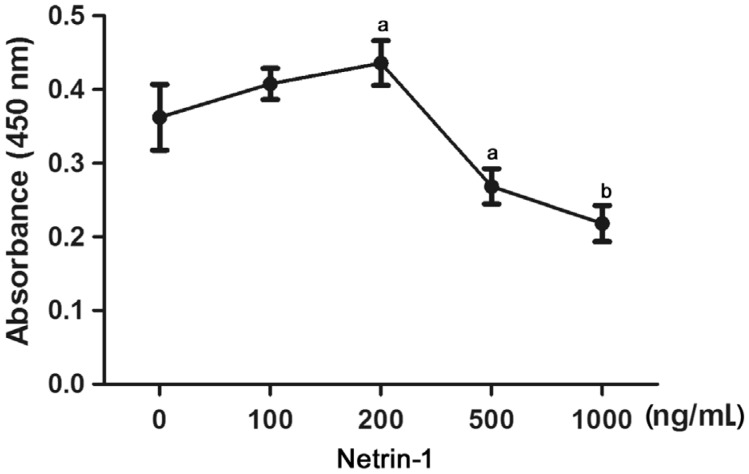
The HCE cultured cells were exposed to netrin-1 at different concentrations to evaluate its effects on cell viability. A concentration of 200 ng/mL enhanced 25% of the HCE viability, but higher concentrations significantly suppressed it in a concentration dependent manner from 500-1000 ng/mL (Figure 1), suggesting that the viability-promoting effect of netrin-1 follows an optimal concentration curve.
Figure 1. Effect of netrin-1 on the viability of cultured HCE cells.
Different concentrations of netrin-1 (0, 50, 100, 200, 300, 500, 800, and 1000 ng/mL) were treated for 72h, and then detected by CCK-8 assay. Datas were presented as mean±SD; n=3; aP<0.05, bP<0.01.
Netrin-1 Effects HCE Proliferation
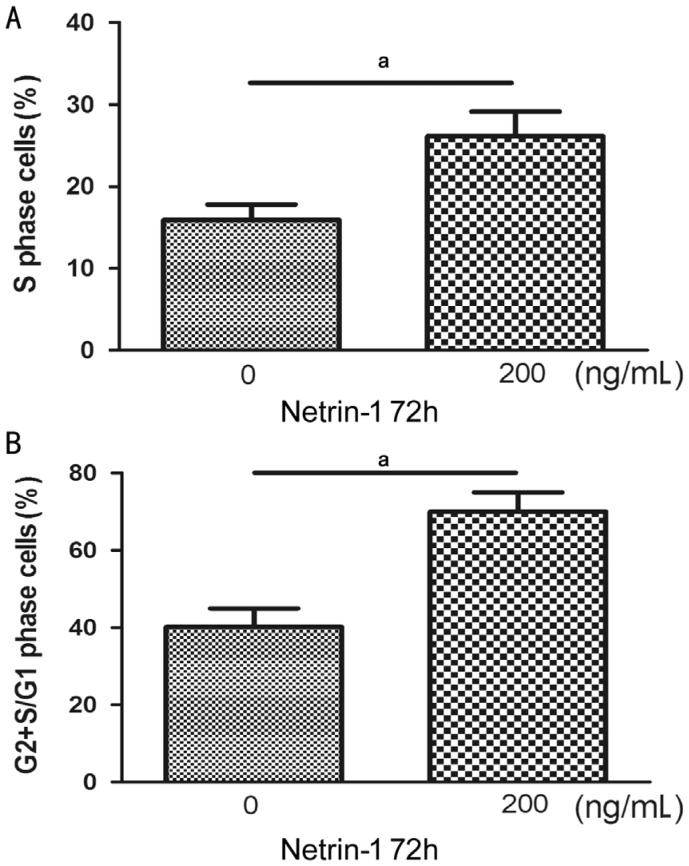
Flow cytometry was applied to evaluate the cell cycle effects of netrin-1. After 72h, an increased percentage of the cells were disposed by netrin-1 with the concentration 200 ng/mL at the S phase. Moreover, the phase of ratio G2+S to phase G1 cells was also added (Figure 2).
Figure 2. The effects of netrin-1 on HCE proliferation analyzed by flow cytometric.
Cultured HCE for 72h in no-serum medium with or without netrin-1. The cells stained with propidium iodide (PI). The cell proliferation rate was expressed as the G2+S/G1 percentage and the ratio of cells at the S phase (aP<0.01). A: Ratio of cells at the S-phase; B: G2+S/G1 ratio.
Netrin-1 Effects Human Corneal Epithelial Apoptosis
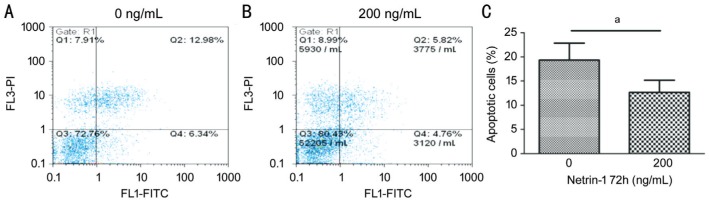
The cells were starved overnight to induce apoptosis and handled with netrin-1 (200 ng/mL) to investigate its effect on cell apoptosis. Representative flow cytometry dot plots of annexin V/PI staining were presented in Figure 3A. Treated with netrin-1 (200 ng/mL) decreased the apoptotic HCE cells, as well as decreased their percentage from 19.3%±0.57% to 12.7%±0.42% of the total (Figure 3B). These results demonstrate that treatment with 200 ng/mL netrin-1 can indeed prevent apoptosis to a certain degree (Figure 3C).
Figure 3. Netrin-1 effects HCE cells apoptosis.
A-B: Measured the cells apoptosis with flow cytometry in conjunction with annexin V-FITC/PI double staining. The values represent the mean percentages of apoptotic cells (aP<0.05). C. Quantization of the numbers of apoptotic cells. Every value represents the mean±SD, n=3. aP<0.05.
Netrin-1 Effects Human Corneal Epithelial Migration
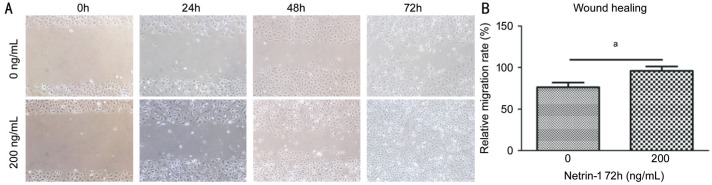
After verifying that netrin-1 may influence proliferation, we used a scratch-wound model to investigate the migration propensity of HCE cells. The wounds without netrin-1 did not heal within 72h, with an average wound gap of 23.7% remaining. By contrast, treatment with netrin-1 (200 ng/mL) led to wound closure within this timeframe. Accordingly, the migration rates after 72h were 76.3% of control group and 100% of netrin-1-treated group (Figures 4). Netrin-1 therefore has a concentration dependence on the HCE cells proliferation, migration. Netrin-1 promoted the HCE cells proliferation and migration, decreased their rate of apoptosis at 200 ng/mL.
Figure 4. Netrin-1 effects HCE migration.
HCE were treatment with netrin-1 (200 ng/mL) in medium no-serum for 24h. A: Results at 0, 24, 48, and 72h separately after insert removal; B: Data statistics as ratio of the cell wound healing (mean±SD). n=3. aP<0.05.
Netrin-1 from Exogenous Accelerate Epithelial Wound Healing in Normal c57 Mice Corneas
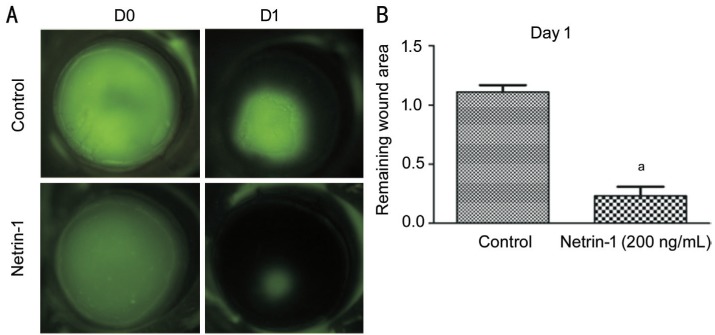
After confirming the positive effects of netrin-1 on the proliferation and migration of HCE cells, we studied the effect of external netrin-1 addition on delayed closure of epithelial wounds in the corneas of normal c57 mice. The remaining wound area was 1.22 mm2 in control group but 0.22 mm2 in the netrin-1 treated group. And an injection of a 200 ng/mL netrin-1 solution 4h before epithelial debridement markedly accelerated wound closure (Figure 5).
Figure 5. Topical application of netrin-1 promotes the injuryed cornea epithelial wound repair.
A: Micrographs of fluorescein-stained injured corneas 24h after epithelial wounded. Green areas show epithelial defects, the less area indicates faster repairment or closure of the epithelium traumaed. B: The mean areas of fluorescein-stained areas were calculated using Image J software. The areas were notably smaller in the netrin-1-treated corneas at 24h. The data represent means±SD. Representative data from two independent experiments are shown, with five mice per group. aP<0.001 vs control.
Exogenous Netrin-1 Inhibits Apoptosis of Corneal Epithelial Cells of c57 Mice
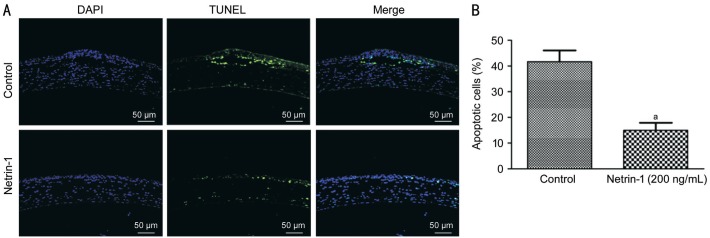
We investigated the epithelial cells apoptotic in control and netrin-1 treated corneas. Figure 6 shows TUNEL-positive cells at the epithelial layer of the corneas of the control and netrin-1 treated c57 mice at 24h after wounding were 43.3% and 16.7% respectively. Exogenous netrin-1 inhibited apoptosis of epithelial cells in the corneas of c57 mice. By contrast, there were no TUNEL-positive epithelial cells in the uninjured or healing corneas of normal c57 mice.
Figure 6. TUNEL staining for apoptosis in netrin-1 treated corneal epithelium.
Corneal cryostat control and netrin-1 treated sections corneas of C57 mice were evaluated using the TUNEL assay 24h after wounding. The merged TUNEL and DAPI counterstaining of nuclei is shown by representative images from two independent experiments. Three to five corneas per condition. aP<0.01 vs control.
DISCUSSION
Improper wound healing may result in an irreversible impairment on corneal transparency and loss of vision. In recent years, varying factors have been shown to exert pro-epithelial effects on the cornea[20]–[23]. Here, we demonstrated that netrin-1 promotes the human CECs proliferation and migration in vitro. In addition, in vivo topical injection of netrin-1 significantly improved corneal epithelial repair in mice, which illustrated by a higher rate of wound closure and reduced apoptosis of CECs in a sterile wound model. In non-neural systems, it has been shown that netrin-1 can affect the mammary gland development[24], regulate the formation of lung[24]–[25], control the apoptosis of intestinal cells[26], promote the morphogenesis in the vascular system[27], and facilitate migration of leukocytes[28], contributing to inflammation[29]–[30], cell migration and adhesion in human hepatocellular carcinoma[31], tumor progression and angiogenesis[8].
Lu et al[32] found netrin-1 can inhibit capillary endothelial tip cells migration regression of mediated by its receptor UNC5B. Paradoxically, it has been reported migration and proliferation of cultured human umbilical vein endothelial cells (HUVECs) stimulated by netrin-1 promotes angiogenesis independent of netrin-1 receptor family members[33]. Netrin-1 is a guidance cue to control the axonal growth cones and neurons, attractive or repellent depends on it binds receptors[34]–[36]. DCC receptor mediated attraction[37], whereas the UNC5 family mediated repulsion, alone or together with DCC family receptors to mediated repulsion[32]. Netrin-1 guides axon pathfinding and regulate neuron cells proliferation, also involved in the angiogenesis. Yang et al[38], demonstrated netrin-1 regulated angiogenesis by a dual role. It can produce facilitative or inhibitory effect depending upon its concentrations. Moreover, in HUVECs, UNC5B was the only subtype detected of netrin-1 receptor. In vascular endothelial cells to knockdown UNC5B target, using a specific siRNA, lead to a significant cell proliferation and migration along with loss of the inhibitory effect, regardless of concentration. The study revealed that netrin-1 maybe as a dual-function regulator of angiogenesis.
In the research, we found netrin-1 and nertin-4 have inhibitory effects on ocular neovascularization[39]–[41]. We also found that netrin-1 had a proliferative effect on corneal epithelium in vivo[11]. In the current study, we explored netrin-1 affects on corneal epithelium and we observed that in low concentrations it increases the activity of HCE, while in high concentrations it reduces the activity of HCE. Low concentrations of netrin-1 could promote the HCE proliferation and migration. In the animal study, we found that subconjunctival injection of 200 ng/mL netrin-1 could significantly accelerate the healing of corneal wounds. The possible causes of dose dependent effect depended on the binding receptors. In our later study, we will continue to study the receptors in depth.
The netrin-1's receptors UNC5C and DCC could induce cell apoptosis without netrin ligand, as well as inhibit apoptosis while bound with ligand[42], but the signaling pathway involved is not clear. It has been observed that lessen netrin-1 expression is related to the growth retardation of the developing embryo. Netrin-1 can increase the human embryonic microvascular endothelial cells survival capability and inhibit cells apoptotic[43]. In a tumor study, it was suggested that UNC5B was a direct transcriptional target of p53, which mediates p53-dependent apoptosis[44]. In addition, the combination of netrin-1 and UNC5B could completely inhibit the apoptosis induced by p53. The expression levels of UNC5A, B and C are down regulated in malignant tumors in humans, including those of colorectal cancer, lung cancer and renal tumors, suggesting that UNC5 receptor plays the role of tumor suppressor[45]–[46]. Thus, DCC and UNC5 might serve as the tumor suppressors to induce apoptotic without netrin-1 ligand. However, with the existence of netrin-1, the receptors promoted tumor formation by inhibiting apoptosis. In this research, we found netrin-1 prominently reduced apoptosis of HCE. We also found netrin-1 can significantly suppress the apoptosis of epithelial cells in vivo.
Taken together, the data indicated that topical application netrin-1 markedly promoted corneal epithelium wound repairment by increasing cells proliferation and migration, as well as suppressing the apoptosis of epithelial cells. The data implied new roles of netrin-1 in corneal regeneration, offering a foundation for the improvement of innovative therapeutic approaches for persistent corneal epithelial defects based on netrin-1. In our later study, we will study the effects of netrin-1 on corneal nerves.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81300729; No.81160118; No.81460092; No.81660158); Natural Science Foundation of Fujian Province (No.2015J05170).
Conflicts of Interest: Han Y, None; Jiang N, None; Su T, None; Yang QC, None; Yan CC, None; Ye L, None; Yuan Q, None; Zhu PW, None; Li W, None; Liu ZG, None; Shao Y, None.
REFERENCES
- 1.Thoft RA, Wiley LA, Sundarraj N. The multipotential cells of the limbus. Eye (Lond) 1989;3(Pt 2):109–113. doi: 10.1038/eye.1989.17. [DOI] [PubMed] [Google Scholar]
- 2.Katzman LR, Jeng BH. Management strategies for persistent epithelial defects of the cornea. Saudi J Ophthalmol. 2014;28(3):168–172. doi: 10.1016/j.sjopt.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayashi Y, Toshida H, Matsuzaki Y, Matsui A, Ohta T. Persistent corneal epithelial defect responding to rebamipide ophthalmic solution in a patient with diabetes. Int Med Case Rep J. 2016;9:113–116. doi: 10.2147/IMCRJ.S103299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziaei M, Greene C, Green CR. Wound healing in the eye: therapeutic prospects. Adv Drug Deliv Rev. 2018;126:162–176. doi: 10.1016/j.addr.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Moore SW, Tessier-Lavigne M, Kennedy TE. Netrins and their receptors. Adv Exp Med Biol. 2007;621:17–31. doi: 10.1007/978-0-387-76715-4_2. [DOI] [PubMed] [Google Scholar]
- 6.Rajasekharan S, Kennedy TE. The netrin protein family. Genome Biol. 2009;10(9):239. doi: 10.1186/gb-2009-10-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paradisi A, Mehlen P. Netrin-1, a missing link between chronic inflammation and tumor progression. Cell Cycle. 2010;9(7):1253–1262. doi: 10.4161/cc.9.7.11072. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu A, Nakayama H, Wang P, König C, Akino T, Sandlund J, Coma S, Italiano JE, Jr, Mammoto A, Bielenberg DR, Klagsbrun M. Netrin-1 promotes glioblastoma cell invasiveness and angiogenesis by multiple pathways including activation of RhoA, cathepsin B, and cAMP-response element-binding protein. J Biol Chem. 2013;288(4):2210–2222. doi: 10.1074/jbc.M112.397398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazelin L, Bernet A, Bonod-Bidaud C, Pays L, Arnaud S, Gespach C, Bredesen DE, Scoazec JY, Mehlen P. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431(7004):80–84. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- 10.Gu CH, Giraudo E. The role of semaphorins and their receptors in vascular development and cancer. Exp Cell Res. 2013;319(9):1306–1316. doi: 10.1016/j.yexcr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han Y, Shao Y, Lin ZR, Qu YL, Wang H, Zhou YP, Chen WS, Chen YX, Chen WL, Hu FR, Li W, Liu ZG. Netrin-1 simultaneously suppresses corneal inflammation and neovascularization. Invest Ophthalmol Vis Sci. 2012;53(3):1285–1295. doi: 10.1167/iovs.11-8722. [DOI] [PubMed] [Google Scholar]
- 12.Yan W, Han P, Zhou ZZ, Tu W, Liao JZ, Li PY, Liu M, Tian DA, Fu Y. Netrin-1 induces epithelial-mesenchymal transition and promotes hepatocellular carcinoma invasiveness. Dig Dis Sci. 2014;59(6):1213–1221. doi: 10.1007/s10620-013-3016-z. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YY, Chen P, Di GH, Qi X, Zhou QJ, Gao H. Netrin-1 promotes diabetic corneal wound healing through molecular mechanisms mediated via the adenosine 2B receptor. Sci Rep. 2018;8(1):5994. doi: 10.1038/s41598-018-24506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Xiao M, Guo FC. The role of Sox6 and Netrin-1 in ovarian cancer cell growth, invasiveness, and angiogenesis. Tumour Biol. 2017;39(5):1010428317705508. doi: 10.1177/1010428317705508. [DOI] [PubMed] [Google Scholar]
- 15.Bijman MN, van Nieuw Amerongen GP, Laurens N, van Hinsbergh VW, Boven E. Microtubule-targeting agents inhibit angiogenesis at subtoxic concentrations, a process associated with inhibition of Rac1 and Cdc42 activity and changes in the endothelial cytoskeleton. Mol Cancer Ther. 2006;5(9):2348–2357. doi: 10.1158/1535-7163.MCT-06-0242. [DOI] [PubMed] [Google Scholar]
- 16.Bijnsdorp IV, Vrijland K, Vroling L, Fukushima M, Peters GJ. Increased migration by stimulation of thymidine phosphorylase in endothelial cells of different origin. Nucleosides Nucleotides Nucleic Acids. 2010;29(4-6):482–487. doi: 10.1080/15257771003730201. [DOI] [PubMed] [Google Scholar]
- 17.Rittié L, Hutcheon AEK, Zieske JD. Mouse models of corneal scarring. Methods Mol Biol. 2017;1627:117–122. doi: 10.1007/978-1-4939-7113-8_8. [DOI] [PubMed] [Google Scholar]
- 18.Wang F, Gao N, Yin J, Yu FS. Reduced innervation and delayed re-innervation after epithelial wounding in type 2 diabetic Goto-Kakizaki rats. Am J Pathol. 2012;181(6):2058–2066. doi: 10.1016/j.ajpath.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin J, Huang J, Chen C, Gao N, Wang F, Yu FS. Corneal complications in streptozocin-induced type I diabetic rats. Invest Ophthalmol Vis Sci. 2011;52(9):6589–6596. doi: 10.1167/iovs.11-7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res. 2015;49:17–45. doi: 10.1016/j.preteyeres.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong WY, Yoo HY, Kim CW. Neuregulin-1 accelerates corneal epithelial wound healing. Growth Factors. 2017;35(6):225–233. doi: 10.1080/08977194.2018.1436055. [DOI] [PubMed] [Google Scholar]
- 22.Miyagi H, Thomasy SM, Russell P, Murphy CJ. The role of hepatocyte growth factor in corneal wound healing. Exp Eye Res. 2018;166:49–55. doi: 10.1016/j.exer.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strizzi L, Mancino M, Bianco C, Raafat A, Gonzales M, Booth BW, Watanabe K, Nagaoka T, Mack DL, Howard B, Callahan R, Smith GH, Salomon DS. Netrin-1 can affect morphogenesis and differentiation of the mouse mammary gland. J Cell Physiol. 2008;216(3):824–834. doi: 10.1002/jcp.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu YR, Stein E, Oliver T, Li Y, Brunken WJ, Koch M, Tessier-Lavigne M, Hogan BL. Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr Biol. 2004;14(10):897–905. doi: 10.1016/j.cub.2004.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun HX, Zhu YY, Pan HY, Chen XS, Balestrini JL, Lam TT, Kanyo JE, Eichmann A, Gulati M, Fares WH, Bai HW, Feghali-Bostwick CA, Gan Y, Peng XY, Moore MW, White ES, Sava P, Gonzalez AL, Cheng YW, Niklason LE, Herzog EL. Netrin-1 regulates fibrocyte accumulation in the decellularized fibrotic sclerodermatous lung microenvironment and in bleomycin-induced pulmonary fibrosis. Arthritis & Rheumatology (Hoboken, N.J.) 2016;68(5):1251–1261. doi: 10.1002/art.39575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko SY, Dass CR, Nurgali K. Netrin-1 in the developing enteric nervous system and colorectal cancer. Trends Mol Med. 2012;18(9):544–554. doi: 10.1016/j.molmed.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Aherne CM, Collins CB, Eltzschig HK. Netrin-1 guides inflammatory cell migration to control mucosal immune responses during intestinal inflammation. Tissue Barriers. 2013;1(2):e24957. doi: 10.4161/tisb.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Layne K, Ferro A, Passacquale G. Netrin-1 as a novel therapeutic target in cardiovascular disease: to activate or inhibit? Cardiovasc Res. 2015;107(4):410–419. doi: 10.1093/cvr/cvv201. [DOI] [PubMed] [Google Scholar]
- 29.Tadagavadi RK, Wang WW, Ramesh G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. J Immunol. 2010;185(6):3750–3758. doi: 10.4049/jimmunol.1000435. [DOI] [PubMed] [Google Scholar]
- 30.Ranganathan P, Mohamed R, Jayakumar C, Ramesh G. Guidance cue netrin-1 and the regulation of inflammation in acute and chronic kidney disease. Mediators Inflamm. 2014;2014:525891. doi: 10.1155/2014/525891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han P, Fu Y, Liu JM, Wang YW, He JY, Gong J, Li MK, Tan QH, Li DX, Luo YX, Han J, Liu JQ, Tu W, Wang Y, Tian DA, Yan W. Netrin-1 promotes cell migration and invasion by down-regulation of BVES expression in human hepatocellular carcinoma. Am J Cancer Res. 2015;5(4):1396–1409. [PMC free article] [PubMed] [Google Scholar]
- 32.Lu XW, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Bréant C, Claes F, De Smet F, Thomas JL, Autiero M, Carmeliet P, Tessier-Lavigne M, Eichmann A. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432(7014):179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 33.Park KW, Crouse D, Lee M, Karnik SK, Sorensen LK, Murphy KJ, Kuo CJ, Li DY. The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci U S A. 2004;101(46):16210–16215. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colamarino SA, Tessier-Lavigne M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell. 1995;81:621–629. doi: 10.1016/0092-8674(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 35.Yee KT, Simon HH, Tessier-Lavigne M, O'Leary DM. Extension of long leading processes and neuronal migration in the mammalian brain directed by the chemoattractant netrin-1. Neuron. 1999;24(3):607–622. doi: 10.1016/s0896-6273(00)81116-2. [DOI] [PubMed] [Google Scholar]
- 36.Alcántara S, Ruiz M, De Castro F, Soriano E, Sotelo C. Netrin 1 Acts as an attractive or as a repulsive cue for distinct migrating neurons during the development of the cerebellar system. Development. 2000;127(7):1359–1372. doi: 10.1242/dev.127.7.1359. [DOI] [PubMed] [Google Scholar]
- 37.Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, Simons J, Bronson RT, Gordon JI, Tessier-Lavigne M, Weinberg RA. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386(6627):796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Zou L, Wang Y, Xu KS, Zhang JX, Zhang JH. Axon guidance cue Netrin-1 has dual function in angiogenesis. Cancer Biol Ther. 2007;6(5):743–748. doi: 10.4161/cbt.6.5.3976. [DOI] [PubMed] [Google Scholar]
- 39.Yu Y, Zou J, Han Y, Quyang L, He H, Hu P, Shao Y, Tu P. Effects of intravitreal injection of netrin-1 in retinal neovascularization of streptozotocin-induced diabetic rats. Drug Des Devel Ther. 2015;9:6363–6377. doi: 10.2147/DDDT.S93166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han Y, Shao Y, Liu TT, Li SM, Li W, Liu ZG. Therapeutic effects of topical netrin-4 in a corneal acute inflammatory model. Int J Ophthalmol. 2015;8(2):228–233. doi: 10.3980/j.issn.2222-3959.2015.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han Y, Shao Y, Liu TT, Qu YL, Li W, Liu ZG. Therapeutic effects of topical netrin-4 inhibits corneal neovascularization in alkali-burn rats. PLoS One. 2015;10(4):e0122951. doi: 10.1371/journal.pone.0122951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grandin M, Meier M, Delcros JG, Nikodemus D, Reuten R, Patel TR, Goldschneider D, Orriss G, Krahn N, Boussouar A, Abes R, Dean Y, Neves D, Bernet A, Depil S, Schneiders F, Poole K, Dante R, Koch M, Mehlen P, Stetefeld J. Structural decoding of the netrin-1/UNC5 interaction and its therapeutical implications in cancers. Cancer Cell. 2016;29(2):173–185. doi: 10.1016/j.ccell.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Qian-hua W, Shao-ping Z, Jian-wen Z, Yun Y, Li Z. Reduced expression of netrin-1 is associated with fetal growth restriction. Mol Cell Biochem. 2011;350(1-2):81–87. doi: 10.1007/s11010-010-0684-2. [DOI] [PubMed] [Google Scholar]
- 44.Lee M, Kang H, Jang SW. CoCl2 induces PC12 cells apoptosis through p53 stability and regulating UNC5B. Brain Res Bull. 2013;96:19–27. doi: 10.1016/j.brainresbull.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Arakawa H. Netrin-1 and its receptors in tumorigenesis. Nat Rev Cancer. 2004;4(12):978–987. doi: 10.1038/nrc1504. [DOI] [PubMed] [Google Scholar]
- 46.Klagsbrun M, Eichmann A. A role for axon guidance receptors and ligands in blood vessel development and tumor angiogenesis. Cytokine Growth Factor Rev. 2005;16(4-5):535–548. doi: 10.1016/j.cytogfr.2005.05.002. [DOI] [PubMed] [Google Scholar]