Abstract
AIM
To describe the etiology, clinical characteristics, surgical options and surgical outcomes of isolated inferior oblique palsy (IOP).
METHODS
A retrospective review was performed on patients with isolated IOP who were seen between January 2010 and June 2017. The following clinical data were obtained from the patients' charts: visual acuity, ocular alignment, ocular motility, cyclotorsion, stereoacuity, Parks three-step test, surgical methods, surgical outcomes and complications. Surgical success was defined as horizontal deviation ≤10 prism diopters (PD) and a vertical deviation ≤5 PD in primary gaze at both near and distant vision as assessed at last follow-up.
RESULTS
The records from a total of 18 patients (8 males and 10 females) with an average age of 27.56y were included in this study. The right eye was affected in 11 patients, the left in 6 patients and both eyes in 1 patient. Twelve cases (66.7%) were congenital and 6 (33.3%) were acquired IOP. The 6 acquired cases involved 2 resulting from orbital trauma/surgery, 2 from midbrain microvascular ischemia, 1 from myasthenia gravis and 1 of unknown etiology. Strabismus surgery was performed in 13 cases. Surgical techniques included weakening of superior oblique and vertical rectus recession and resection. After a mean follow-up of 15.11mo, the corrected vertical deviation in primary position was 19.92±8.52 PD (P=0.000) and the corrected horizontal deviation was 14.31±12.68 PD (P=0.002). The surgical success rate was 61.5% and no surgical complications were present.
CONCLUSION
Isolated IOP represents a rare condition, with most cases (66.7%) involving a congenital basis. The acquired cases included vascular, orbital trauma/surgery and myasthenia gravis. Weakening of the ipsilateral superior oblique muscle and/or contralateral superior rectus recession often resulted in favorable surgical outcomes with a surgical success rate of 61.5%.
Keywords: inferior oblique palsy, strabismus surgery, ocular alignment, ocular motility
INTRODUCTION
Isolated inferior oblique palsy (IOP) is a rare clinical entity and the least frequent palsy among all those involving the 6 extraocular muscles. The main clinical features of isolated IOP consist of hypotropia and underelevation in adduction of the affected eye, along with absence of restriction on forced duction testing[1]–[2]. The inferior oblique muscle is innervated by the inferior branch of oculomotor nerve, the same nerve that is responsible for control of the inferior rectus (IR), medial rectus and pupillary sphincter. Few reports are available on isolated IOP, that is, those that lack involvement of any other extraocular muscles supplied by the oculomotor nerve. Unlike superior oblique palsy, isolated IOP palsy is inherently troubling[3]. Clinically, when a patient exhibits limitations of elevation in adduction, Brown syndrome is strongly suggested. However, Brown syndrome is characterized by a limitation of elevation in adduction which is similar to IOP, but a positive forced duction test which is different from IOP. In addition, lesions involving primary superior oblique overaction, skew deviation and heterotopic muscle pulley, appear symptomatically similar to IOP. Thus, clinicians may conclude that it is unlikely for only the inferior oblique muscle to be affected in these cases[3]–[4]. However, with use of high-resolution magnetic resonance imaging (MRI) it is possible to establish the existence of isolated IOP as a distinct subset of strabismus. In specific, these patients showed a reduction in size of their inferior oblique muscle, but normal sizes of their medial and IR muscles[5]. The possible etiology for this condition may be congenital or acquired. The acquired basis for this condition can result from damage of the inferior oblique nucleus or terminal branches of the oculomotor nerve serving only the inferior oblique, or direct inferior oblique muscle injury, such as orbital trauma, vascular lesion or myasthenia gravis[6]–[9]. Currently, much remains to be understood regarding the cause, clinical findings and surgical treatment of this intriguing condition. Therefore, it is imperative to recognize the clinical features of isolated IOP, differentiate this condition from other diseases, and then determine the proper surgical management of this rare and difficult disorder. In this article, we retrospectively reviewed isolated IOP cases and describe its etiology, clinical characteristics as well as surgical options and outcomes.
SUBJECTS AND METHODS
Ethical Approval
The study was performed in accordance with the 1964 Declaration of Helsinki. Informed consent was obtained from all patients.
The medical records of 18 patients diagnosed with IOP at the Eye Hospital of the Zhongshan Ophthalmic Center, of Sun Yat-sen University, China, from January 2010 to June 2017 were retrospectively reviewed. Data on best corrected visual acuity, cycloplegic refraction, intraocular pressure, anterior segment, fundi, ocular alignment and ocular motility were collected from all patients. Ocular alignment was assessed with use of the cover/uncover and alternate prism cover test at distance (6 m) in primary and cardinal gaze positions. Stereoacuity at distance was measured by random-dot stereograms and at near by Titmus stereograms. The Parks three-step test was employed to analyze vertical rotator muscular palsy in each patient. Vertical deviations were also measured in both right and left head tilts. When the difference of vertical deviation was greater than 4 prism diopters (PD), the head tilt test was considered positive. Objective and subjective torsion were assessed by fundus photography and double Maddox rod testing, respectively. All patients with acquired isolated IOP got a MRI or computed tomography (CT) scan.
The diagnostic criteria for isolated IOP in this case series was based on: 1) presence of hypotropia and underelevation in adduction of the affected eye; 2) absence of restriction in forced duction testing; 3) vertical deviations increased on gaze to the unaffected side; 4) incyclotorsion in one or both eyes; 5) increased hypotropia in contralateral head tilt; 6) superior oblique overaction of the involved eye and potential presence of an A pattern strabismus; and 7) a head tilt toward the paralytic side, face rotation to the unaffected side and an elevated chin. Among the 7 diagnostic criteria for isolated IOP, the first 5 criteria were required in order to make the diagnosis in our patients.
Our patients received either conservative or surgical treatments. Patients with acquired IOP, such as those caused by blepharoplasty or trauma of the lower eyelid/orbit and midbrain vascular lesions were required to be observed for at least 6-12mo before strabismus surgery, with prisms being used for correcting diplopia. Surgery was necessary in patients with congenital IOP, and one with acquired IOP who showed little recovery after more than 6mo of observation. In this study, we use both graded (lengthening) and nongraded (tenotomy and tenectomy) procedures to weaken the superior oblique. As for superior oblique tendon lengthening, a suture is placed between the cut ends of the tendon. When the amount of superior oblique overaction is +1, +2, +3 and +4 respectively, a tendon lengthening of 4, 5, 6, 7 mm is used accordingly. If the patient does not have binocular vision and is not necessary to titrate the surgical effect, superior oblique tenotomy or tenectomy is used. Whether tenotomy or tenectomy is chosen depends on doctor's surgical habit. When the patient does have binocular vision and is necessary to titrate the surgical effect, superior oblique lengthening is used.
If hypotropia in primary position ranged from 15-30 PD and significant hypotropia was present in the field of inferior oblique, only contralateral superior rectus (SR) recession was performed. If hypotropia in primary position was less than 15 PD, along with significant incyclotorsion and superior oblique overaction, a weakening of the ipsilateral superior oblique was performed. However, if binocular single vision was present, we usually choose not to weaken the superior oblique. If hypotropia in primary position was greater than 30 PD, a significant hypotropia was also observed in the superior oblique field. In such cases, a combination of contralateral SR recession and IR resection (R&R procedure) were performed. If hypotropia in primary position was more than 30 PD and severe amblyopia was present in the affected eye, a combination of ipsilateral IR recession and SR resection was executed. One patient (case 4) received a superior oblique tendon transposition. For this case, the superior oblique tendon was isolated nasally and severed near the SR, a 10 mm anterior portion of the tendon was excised and the tendon was then sutured to the sclera above the insertion of medial rectus using 6-0 absorbable suture.
Surgical success was defined as a horizontal deviation of ≤10 PD and a vertical deviation of ≤5 PD in primary gaze at both near and distance as determined at their last follow-up visit. All patients were followed for a minimum period of 6mo. Paired t-tests were for statistical analyses of the data in this study as achieved with use of SPSS version 18.0 for Windows (SPSS, Chicago, IL). A P value of <0.05 was required for results to be considered statistically significant.
RESULTS
A total of 18 patients (8 males and 10 females) were included in this study. The right eye was affected in 11 patients, the left in 6 patients and both eyes in 1 patient. Mean±SD age of the patients was 27.56±19.84y (range: 4-64y). Among the 18 cases, 12 (66.7%) were congenital and 6 (33.3%) involved acquired isolated IOP. Of the 6 cases with acquired IOP, 1 was due to orbital trauma, 1 from orbital surgery, 2 involved midbrain vascular lesions (microvascular ischemia), 1 due to myasthenia gravis and 1 with no obvious etiology.
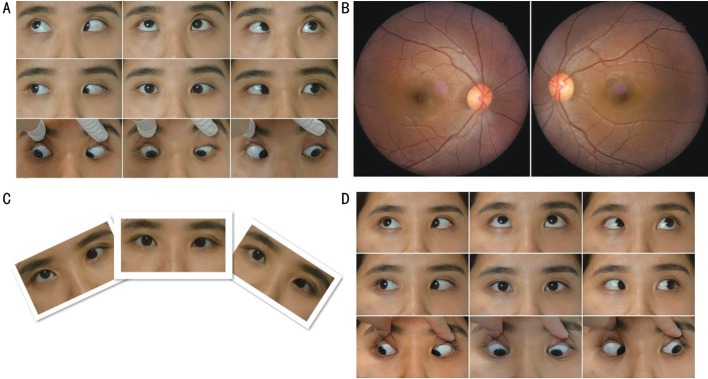
All cases showed hypotropia and underelevation in adduction of the paralytic eye, along with absence of restriction on forced duction testing and ocular incyclotorsion as assessed by fundus photography (Figure 1). A compensatory head posture with a head tilt toward the paralytic side, elevated chin and rotation of the face to the unaffected side was observed in 11 cases. Mean vertical deviation in primary position was 19.22±12.48 PD (range: 2-40 PD). Fifteen cases demonstrated a horizontal deviation, of which, 9 were exotropia (2 patients with A exotropia) and 6 were esotropia (2 patients with A esotropia). Mean horizontal deviation was 14.22±12.36 PD (range: esotropia 40 PD-exotropia 30 PD). Two cases showed bilateral dissociated vertical deviation (DVD), with even the paralytic eye showing drift after covering. Four cases had severe strabismic amblyopia.
Figure 1. Clinical photograph of case 1.
A: A 9-gaze eye position photograph in a 26-year-old female with congenital left IOP presenting with underelevation in adduction, 20 PD esotropia and 15 PD hypotropia of the left eye in the primary position. The difference of esotropia in upward and downward gaze of 25° was 14 PD (A pattern esotropia). B: Fundus photograph showing incyclotorsion in her right eye. C: Although head position appeared normal, with a head tilt test she demonstrated an increased vertical deviation when her head was tilted to the right side. D: Orthophoria was present in the primary position and her A pattern esotropia dissipated at 12mo after tenotomy of the superior oblique and a 3 mm medial rectus recession in the left eye.
The MRI and CT scan showed no inferior oblique muscle atrophy in the five cases with acquired IOP. All patients with acquired IOP received conservative treatment after consulting with the neurology department. Oral drugs such as vitamin B and neurotrophic medicines were recommended for 3 to 6mo and they usually took effect after 1 to 2mo. In 4/5 of these acquired IOP cases, diplopia, abnormal head position (AHP) and incyclotropia were eliminated and eye movement returned to normal. We didn't rule out the possibility that some cases might improve spontaneously. The remaining 1 case, whose acquired IOP was complicated due to removal of an orbital neurilemmoma, continued under observation. The stereoacuity measurements both before and after treatment was normal in 2 cases, and the stereoacuity in other 3 cases was not available. Strabismus surgery under general anesthesia was performed in 13 cases. Surgical techniques included weakening of the superior oblique, IR recession and/or SR resection in the paralytic eye, and SR recession and/or IR resection in the unaffected eye. In addition, 7 cases, with significant horizontal deviation, underwent horizontal muscle surgery. Among all patients, 10 patients got only 1 surgery, 2 patients got 2 surgeries and 1 patient underwent 3 surgeries. The mean number of surgeries required was 1.31 in this study. After a mean follow-up of 15.11±6.44mo (range: 6-24mo), mean corrected vertical deviation in primary position was 19.92±8.52 PD (P=0.000) and mean corrected horizontal deviation was 14.31±12.68 PD (P=0.002). Surgical success rate was 61.5% (8/13), the rate of undercorrection was 38.5%, and there was no overcorrection in any of our cases. The A pattern in all 4 cases dissipated after surgery (Figure 1). Among the 6 patients with AHP, the AHP was greatly improved in 5 patients and disappeared in 1 patient after surgery. Twelve out of 13 cases did not have diplopia before surgery. After surgery, all 13 cases had no diplopia. Eight cases did not have stereoacuity both before and after surgery. The stereoacuity measurement was not available in 2 cases. In the other 3 cases, the stereoacuity was 40, 100 and 400s respectively before surgery and 40, 80 and 200s respectively after surgery. The average degrees of incyclotropia were 10.46 before surgery and 3.85 after surgery. The incyclotropia difference before and after surgery was significant (P<0.05). No superior oblique paresis developed in any of the cases who underwent weakening of superior oblique (Figure 1), nor were any surgical complications present in these patients. The demographic data, etiology, clinical characteristics, surgical methods and final outcomes are summarized in Tables 1 and 2.
Table 1. Clinical data of 18 cases of isolated IOP.
| Case | Age/sex/eye | Etiology | Pre-surgical deviation (PD) (vertical, horizontal) | Other diagnosis | Surgical methods | Post-surgical deviation (PD) (vertical, horizontal) | Follow up (mo) |
| 1 | 26/F/L | Congenital | RHT 15, ET 20 | Strabismic amblyopia (L), a esotropia | Tenotomy of SO and MR rec 3 mm of the left eye | RHT 2, ET 3 | 12 |
| 2 | 22/F/L | Congenital | RHT 20, XT 30 | Strabismic amblyopia (L), a exotropia | Tenotomy of SO, LR rec 12 mm and IR rec 5 mm of the left eye | RHT 8, XT 10 | 24 |
| 3 | 29/F/R | Congenital | LHT 30, XT 22 | DVD (B) | Tenotomy of right SO, SR rec 10 mm and LR rec 10 mm of the left eye | LHT 5, XT 8 | 18 |
| 4 | 20/M/L | Congenital | RHT 25, XT 25 | Congenital ptosis (L), strabismic amblyopia (L), a exotropia | LR rec 10 mm and SO tendon transposition of the left eye | RHT 5, ET 2 | 12 |
| 5 | 21/F/R | Congenital | LHT 40, ET 35 | A esotropia, strabismic amblyopia (R) | Tenotomy of SO and SR res 5 mm+IR rec 5 mm of the right eye, MR rec 5 mm+LR res 6 mm of the right eye | LHT 10, ET 2 | 24 |
| 6 | 24/F/R | Congenital | LHT 40, ET 7 | No | Tenectomy 3 mm of right SO and left SR rec 9 mm | LHT 7, ET 6 | 12 |
| 7 | 5/M/R | Congenital | LHT 25, XT 12 | No | Tenectomy 3 mm of right SO | LHT 6, XT 10 | 18 |
| 8 | 15/M/R | Congenital | LHT 12, ET 40 | No | Tenotomy of SO, MR rec 4 mm and LR res 8 mm of the right eye | LHT 4, ET 3 | 24 |
| 9 | 5/M/R | Congenital | LHT 25, ET 16 | Accommodative esotropia (B); DVD (B) | Tenotomy of right SO; left SR rec 6 mm; right SR rec 7.5 mm | RHT 2, ET 6 | 18 |
| 10 | 9/F/R | Congenital | LHT 10, XT 18 | No | Lengthening 6 mm of SO and LR rec 5 mm of the right eye | RHT 2, ET 2 | 12 |
| 11 | 4/M/R | Congenital | LHT 15, XT 8 | No | Lengthening 6mm of right SO; left IR res 4 mm | LHT 2, XT 5 | 18 |
| 12 | 54/M/L | Orbital fracture | RHT 20, XT 10 | Post-surgical correction of orbital floor fracture | Right SR rec 7 mm | RHT 5, XT 8 | 12 |
| 13 | 42/M/R | Congenital | LHT 40, 0 | No | Left SR rec 5 mm and IR res 4 mm | LHT 8, 0 | 18 |
| 14 | 7/F/R | Unknown | LHT 5, XT 2 | No | Drug therapy such as vitamin B and Methylcobalamin | 0, 0 | 6 |
| 15 | 54/F/R | Vascular | LHT 3, 0 | No | Drug therapy such as vitamin B and Methylcobalamin | 0, 0 | 6 |
| 16 | 60/F/B | Vascular | LHT 2, 0 | No | Drug therapy such as vitamin B and Methylcobalamin | 0, 0 | 8 |
| 17 | 64/F/L | Orbital surgery | RHT 12, XT 4 | After surgical removal of left orbital neurilemmoma | Observation | RHT 15, XT 10 | 24 |
| 18 | 35/M/L | MG | RHT 7, ET 7 | MG | Drug therapy by nerve physician | RHT 2, ET 4 | 6 |
IOP: Inferior oblique palsy; F: Female; M: Male; R: Right eye; L: Left eye; B: Both eyes; PD: Prism diopter; ET: Esotropia; XT: Exotropia; RHT: Right hypertropia; LHT: Left hypertropia; DVD: Dissociated vertical deviation; MG: Myasthenia gravis; SO: Superior oblique; SR: Superior rectus; IR: Inferior rectus; MR: Medial rectus; LR: lateral rectus; rec: Recession; res: Resection.
Table 2. Other pre- and post surgical data of 18 cases of isolated IOP.
| Case | Age/sex/eye | Presurgical AHP | Presurgical diplopia | Presurgical incyclotorsion | Presurgical stereoacuity (arc sec) | Postsurgical AHP | Postsurgical diplopia | Postsurgical incyclotorsion | Postsurgical stereoacuity (arc sec) |
| 1 | 26/F/L | No | No | 10 | No | No | No | 2 | No |
| 2 | 22/F/L | No | No | 12 | No | No | No | 5 | No |
| 3 | 29/F/R | Head titled toward the right side, chin elevated and face turned to the left | No | 9 | No | Greatly improved | No | 2 | No |
| 4 | 20/M/L | No | No | 10 | No | No | No | 0 | No |
| 5 | 21/F/R | No | No | 20 | No | No | No | 11 | No |
| 6 | 24/F/R | No | No | 5 | 40 | No | No | 1 | 40 |
| 7 | 5/M/R | Head titled toward the right side | No | 4 | 100 | No | No | 1 | 80 |
| 8 | 15/M/R | No | No | 12 | No | No | No | 2 | No |
| 9 | 5/M/R | No | No | 16 | No | No | No | 10 | No |
| 10 | 9/F/R | Head titled toward the right side, chin elevated and face turned to the left | No | 13 | N/A | Greatly improved | No | 5 | N/A |
| 11 | 4/M/R | Head titled toward the right side, chin elevated and face turned to the left | No | 11 | No | Greatly improved | No | 5 | No |
| 12 | 54/M/L | Head titled toward the left side and face turned to the right | Yes | 5 | 400 | Greatly improved | No | 0 | 200 |
| 13 | 42/M/R | Head titled toward the right side, chin elevated and face turned to the left | No | 9 | N/A | Greatly improved | No | 6 | N/A |
| 14 | 7/F/R | Head titled toward the right side, chin elevated and face turned to the left | Yes | 4 | 40 | No | No | 0 | 40 |
| 15 | 54/F/R | Head titled toward the right side, chin elevated and face turned to the left | Yes | 5 | N/A | No | No | 0 | N/A |
| 16 | 60/F/B | Chin elevated | Yes | 6 | N/A | No | No | 0 | N/A |
| 17 | 64/F/L | Head titled toward the left side, chin elevated and face turned to the right | Yes | 4 | N/A | The same as before | Yes | 6 | N/A |
| 18 | 35/M/L | Head titled toward the left side, chin elevated and face turned to the right | Yes | 3 | 40 | No | No | 0 | 40 |
IOP: Inferior oblique palsy; AHP: Abnormal head position; N/A: Not available.
Besides the change in vertical deviation and surgical success rate, we also discussed the other effects of surgery, such as cyclotorsion, diplopia, anomalous head position, and stereoacuity. These are all important clinical data for considerationas.
DISCUSSION
Control of the inferior oblique muscle is dominated by the oculomotor nerve (CN III), which divides into a superior and inferior branch after entering the orbit. The superior branch supplies the SR and levator palpebrae superioris muscles while the inferior branch supplies the medial rectus, inferior rectus and inferior oblique muscles. A nerve injury producing paresis limited to the inferior oblique would be located at the level of the oculomotor nucleus or at nerve fascicles where oculomotor neurons remain relatively separated. As a result, this could involve the site of an isolated injury from occlusion of a perforating terminal artery[3],[7]. Congenital IOP can be attributable to damage resulting from early embryologic trauma that partially spares the Edinger-Westphal nucleus[4]. Other possible etiologies included direct/isolated damage to the inferior oblique itself, often seen in patients following orbital or palpebral plastic surgery[6],[9] and myasthenia gravis, which can produce a selective impairment limited to the inferior oblique muscle. For example, Almog et al[8] reported that 63.3% of their patients with myasthenia gravis showed IOP, and 7.5% of these presented with an isolated IOP. In this study, congenital isolated IOP was the most frequent condition, accounting for 66.7% of the cases, while the remaining 33.3% consisted of acquired IOP involving midbrain microvascular lesions, orbital trauma, orbital surgery and myasthenia gravis. No inferior oblique muscle atrophy was found in the cases with acquired IOP through MRI or CT scan. This may be related to the short duration from the onset of the disease to the patients' first visit to our hospital. Overall, our findings are in accordance with other reports indicating that isolated IOP represents a benign entity without other significant neurological signs or symptoms, with the palsy etiology often being attributable to a congenital, traumatic or vascular basis[6]–[11].
As an intriguing disease, isolated IOP should be distinguished from Brown syndrome which present a positive forced duction test and restriction in elevation of the adducted eye during both ocular version and ocular duction[12]. And it is important to distinguish IOP from other disorders such as[1]–[2]: 1) Skew deviation—often associated with lesions in the brainstem, cerebellum or vestibular system, this condition presents as incyclotorsion of the hypertropia eye and excyclotorsion of the hypotropia eye[3],[13]; 2) Contralateral superior oblique palsy—a superior oblique palsy with excyclotorsion in the eye and increased vertical deviation on gaze to the unaffected side, distinguished from isolated IOP in that the eye shows incyclotorsion and decreased vertical deviation on ipsilateral gaze; 3) IR palsy in the contralateral eye—a contralateral IR palsy with a maximal vertical deviation in lateral and downward gazes of the affected eye along with increased vertical deviation when the head is tilted to the unaffected side, distinguished from isolated IOP in that the maximal vertical deviation was present in medial and upward gazes of the affected eye and a decreased vertical deviation following ipsilateral head tilt.
Surgical management of IOP is not simple. In our study, the main approaches used in these surgeries included weakening of the ipsilateral superior oblique muscle and contralateral SR recession, similar to the surgical methods of Kutschke and Scott[10] and Pollard[11]. The postoperative mean corrected vertical deviation was 19.92±8.52 PD (P=0.000) and mean corrected horizontal deviation was 14.31±12.68 PD (P=0.002). We reported a success rate of 61.5% (8/13), which was slightly lower than that of the 71% success rate of Kutschke and Scott[10]. Pollard[11] reported a surgical success rate of 100% in the 20 of the 25 patients that underwent surgery. However, the presurgical vertical deviation in primary position in most of their cases was less than 5 PD. Unfortunately, majority of our patients did not have diplopia and stereoacurity both before and after surgery due to their congenital nature and old age at surgery. However, the incyclotropia of 10.46 before surgery was reduced to 3.85 after surgery (P<0.05).
It was reported that following superior oblique tenotomy or tenectomy, approximately 50% of the patients show a potential for development of consecutive iatrogenic superior oblique paresis. This possible outcome is believed to result from an uncontrolled separation between the severed ends of the tendon[10],[12],[14]. However, with use of a superior oblique tendon suture spacer, an effective and safe option for correcting inferior oblique paresis without developing iatrogenic superior oblique paresis is achievable[15]. In our study, no evidence of superior oblique paresis was observed following either free tenotomy/tenectomy of the superior oblique muscle or lengthening of the superior oblique tendon (essentially the same effect as accomplished with use of the superior oblique tendon suture spacer). It may be due to the preoperative presence of a large vertical deviation and apparent underaction of the inferior oblique within the same eye. Of note in our paper was the finding that a superior oblique tendon transposition alone (case 4) corrected a large vertical deviation, although the downward motility of this patient was moderately affected after surgery.
There exist limitations in our study. First, it was a retrospective case review and subject to measurement and interpretation errors. The sample size was relatively small, but achieving large sample for this rare entity is difficult. Secondly, some of the patients with Brown syndrome might be misdiagnosed as isolated IOP because the forced duction test is subjective. Another limitation was the arbitrary nature of the definition of surgical success. Indeed, many adults will be symptomatic with a hypertropia of 3 or even 2 PD, and reversal of the hypertropia (from right to left hypertropia, or vice versa) is more likely to be symptomatic. However, most of our patients were congenital IOP and did not have normal binocular vision before surgery. After surgery, they were not symptomatic. Finally, the individualized surgical methods employed in this paper preclude direct comparisons of success rates among the different surgical procedures.
In conclusion, isolated IOP is a rare clinical entity. Most of the patients we identified involved a congenital basis, accounting for 66.7% of our cases. The remaining 33.3% consisted of acquired cases involving vascular, orbital trauma/surgery and myasthenia gravis. Weakening of the ipsilateral superior oblique muscle and/or contralateral SR recession often resulted in favorable surgical outcomes, with a surgical success rate of 61.5% at our hospital. No superior oblique paresis was observed in any of the cases who underwent weakening of superior oblique, as accomplished by either tenotomy or lengthening of superior oblique tendon.
Acknowledgments
Foundations: Supported by the Nature Science Foundation of China (No.81670885); the Science and Technology Program of Guangdong Province, China (No.2013B020400003); Science and Technology Program of Guangzhou, China (No.15570001).
Conflicts of Interest: Wu XF, None; Yan JH, None.
REFERENCES
- 1.Hilton AF. The diagnosis and management of isolated cyclo vertical muscle palsy. Aust J Ophthalmol. 1982;10(1):31–40. [PubMed] [Google Scholar]
- 2.Santiago AP, Rosenbaum AL. Inferior oblique palsy: diagnosis and management. In: Rosenbaum AL., Santiago AP., editors. Clinical Strabismus Management: Principles and Surgical Techniques. Philadelphia (PA): WB Saunders; 1999. pp. 230–236. [Google Scholar]
- 3.Donahue SP, Lavin PJM, Mohney B, Hamed L. Skew deviation and inferior oblique palsy. Am J Ophthalmol. 2001;132(5):751–756. doi: 10.1016/s0002-9394(01)01234-x. [DOI] [PubMed] [Google Scholar]
- 4.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic muscle pulleys or oblique muscle dysfunction? J Am Assoc Pediatr Ophthalmol Strabismus. 1998;2(1):17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 5.Ela-Dalman N, Velez FG, Demer JL, Rosenbaum AL. High-resolution magnetic resonance imaging demonstrates reduced inferior oblique muscle size in isolated inferior oblique palsy. J AAPOS. 2008;12(6):602–607. doi: 10.1016/j.jaapos.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiedemann LM, Lefebvre DR, Wan MJ, Dagi LR. Iatrogenic inferior oblique palsy: intentional disinsertion during transcaruncular approach to orbital fracture repair. J AAPOS. 2014;18(5):511–514. doi: 10.1016/j.jaapos.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Castro O, Johnson LN, Mamourian AC. Isolated inferior oblique paresis from brain-stem infarction. Perspective on oculomotor fascicular organization in the ventral midbrain tegmentum. Arch Neurol. 1990;47(2):235–237. doi: 10.1001/archneur.1990.00530020149032. [DOI] [PubMed] [Google Scholar]
- 8.Almog Y, Ben-David M, Nemet AY. Inferior oblique muscle paresis as a sign of myasthenia gravis. J Clin Neurosci. 2016;25:50–53. doi: 10.1016/j.jocn.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Kakizaki H, Zako M, Iwaki M, Mito H, Katori N. Incarceration of the inferior oblique muscle branch of the oculomotor nerve in two cases of orbital floor trapdoor fracture. Jpn J Ophthalmol. 2005;49(3):246–252. doi: 10.1007/s10384-004-0184-6. [DOI] [PubMed] [Google Scholar]
- 10.Kutschke PJ, Scott WE. Postoperative results in inferior oblique palsy. J Pediatr Ophthalmol Strabismus. 1996;33(2):72–78. doi: 10.3928/0191-3913-19960301-03. [DOI] [PubMed] [Google Scholar]
- 11.Pollard ZF. Diagnosis and treatment of inferior oblique palsy. J Pediatr Ophthalmol Strabismus. 1993;30(1):15–18. doi: 10.3928/0191-3913-19930101-05. [DOI] [PubMed] [Google Scholar]
- 12.Olivier P, von Noorden GK. Results of superior oblique tenectomy in inferior oblique paresis. Arch Ophthalmol. 1982;100(4):581–584. doi: 10.1001/archopht.1982.01030030583005. [DOI] [PubMed] [Google Scholar]
- 13.Brodsky MC, Donahue SP, Vaphiades M, Brandt T. Skew deviation revisited. Surv Ophthalmol. 2006;51(2):105–128. doi: 10.1016/j.survophthal.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Scott WE, Nankin SJ. Isolated inferior oblique paresis. Arch Ophthalmol. 1977;95(9):1586–1593. doi: 10.1001/archopht.1977.04450090108009. [DOI] [PubMed] [Google Scholar]
- 15.Fard MA, Ameri A, Anvari F, Jafari AK, Yazdian Z. Adjustable superior oblique tendon spacer with application of nonabsorbable suture for treatment of isolated inferior oblique paresis. Eur J Ophthalmol. 2010;20(4):659–663. doi: 10.1177/112067211002000402. [DOI] [PubMed] [Google Scholar]