Abstract
AIM
To evaluate the efficacy and safety of combined intra-arterial chemotherapy (IAC) and intravitreal melphalan (IVM) for the treatment of advanced unilateral retinoblastoma.
METHODS
This retrospective study involved 30 consecutive eyes from 30 Chinese patients with advanced unilateral retinoblastoma. All patients were initially treated with IAC combined with IVM. The clinical status and complications were recorded at each visit.
RESULTS
The International Intraocular Retinoblastoma Classification groups were D in 23 eyes and E in 7 eyes. All eyes showed severe cloud vitreous seeds at the first visit. The mean number of IAC cycles and intravitreal injections was 3.2 (range, 3-4) and 6 (range, 1-14), respectively. The median follow-up time was 29mo (range, 7-36mo). Treatment success with regression of the retinal tumor and vitreous seeds was achieved in 29 of 30 eyes (96.7%). Globe salvage was attained in 93.3% (28/30) eyes, and enucleation (n=2) was performed due to neovascular glaucoma and persistent vitreous hemorrhage. Complications included retinal pigment epithelium (RPE) atrophy (n=13; 43%), mild lens opacity (n=7; 23%), vitreous hemorrhage (n=5; 17%) and rhegmatogenous retinal detachment (n=1; 3%). No extraocular tumor extension or metastasis occurred.
CONCLUSION
Combined IAC and IVM is effective and safe for the treatment of advanced unilateral retinoblastoma.
Keywords: intra-arterial chemotherapy, intravitreal chemotherapy, melphalan, retinoblastoma, advanced stage, unilateral disease
INTRODUCTION
Retinoblastoma is the most common primary intraocular malignancy in children. Except for enucleation and external beam radiotherapy, other treatment modalities are available for retinoblastoma. Intravenous chemotherapy is an effective way for the treatment of early-stage retinoblastoma (groups A, B, and C) that achieves globe salvage rates from 90% to 100%. However, many advanced retinoblastoma (groups D and E) still require enucleation with intravenous chemotherapy alone[1]–[2]. Additionally, intravenous chemotherapy is associated with many systemic side effects, such as hearing loss and secondary acute myeloid leukemia[3]–[4].
Intra-arterial chemotherapy (IAC) was introduced as a salvage therapy for advanced retinoblastoma patients who failed intravenous chemotherapy. Subsequently, many centers attempted to use IAC as the initial treatment for advanced retinoblastoma and achieved great success in preventing enucleation[5]–[8]. However, the most challenging aspect in advanced retinoblastoma is the control of vitreous seeds, which are partially responsive to IAC due to their location in the vitreous without a blood supply.
In recent years, intravitreal chemotherapy has been widely employed for vitreous seeds control. Many centers worldwide have demonstrated that intravitreal chemotherapy is safe and effective for the treatment of vitreous disease[9]–[14]. Melphalan is the most commonly used chemotherapy agent, and a dosage of 20 to 30 µg is ideal for vitreous seeds control with minimal toxicity[9]–[12].
In the present study, we evaluated the efficacy and safety of combined IAC and intravitreal melphalan (IVM) as the initial treatment for advanced unilateral retinoblastoma in Chinese patients.
SUBJECTS AND METHODS
Ethical Approval
This is a retrospective, nonrandomized, noncomparative study. The study adhered to the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. Written informed consent was obtained from the guardian of each patient.
Inclusion criteria were patients presenting advanced intraocular retinoblastoma in a single eye without any previous treatment. Advanced retinoblastoma was defined as International Intraocular Retinoblastoma Classification (IIRC)[15] groups D and E. Exclusion criteria were eyes that displayed extraocular tumor extension or those presented at risk for metastatic disease, such as anterior chamber seeds, uveal or optic nerve invasion. In total, 30 consecutive eyes from 30 Chinese patients with advanced unilateral retinoblastoma, who were treated at Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine between January 2014 and July 2017, were included. All patients were initially treated with IAC combined with IVM.
IAC was performed under general anesthesia with systemic intravenous heparinization. After femoral artery puncture, a 5-French (F) catheter was passed into the ipsilateral internal carotid artery and flushed continuously with heparinized saline. Then, a 1.5-F catheter was placed into the ostium of the ophthalmic artery for superselective IAC. After confirming the correct positioning with an injection of contrast agent, each chemotherapy agent (diluted in 20 mL of saline) was superselectively infused into the ophthalmic artery for 10min. The chemotherapy agents included carboplatin, topotecan and melphalan. According to Gobin et al[16], the drug dosage was based on the patient's age, weight, angioanatomy, etc. All patients received 3-4 cycles of superselective IAC at monthly intervals.
Intravitreal chemotherapy was performed according to the standard treatment method[17]. Before each intravitreal injection, anterior chamber paracentesis was performed using a 27-gauge needle to create a transient hypotony to prevent reflux. A volume of 0.1 mL of aqueous humor was aspirated and sent for cytopathological analysis. The injection site was 2.5-3.0 mm from the limbus depending on patient's age and was carefully chosen to avoid contact with the tumor, seeds, or detached retina. A 30-gauge needle mounted on a tuberculin syringe was placed perpendicularly through the scleral entry, and 30 µg of melphalan in 0.1 mL of solution was slowly and continuously injected into the vitreous cavity within 5s. Three cycles of freeze and thaw were applied at the injection site while withdrawing the needle. Then, the eyeball was carefully jiggled to disperse the drug. Intravitreal chemotherapy was performed at 1 to 2wk intervals during the monthly administration of IAC. Repeated intravitreal injections were performed until complete vitreous seeds control (regression of vitreous seeds) was achieved.
Patients were examined every 2 to 4wk under anesthesia using RetCam III (Clarity, Pleasanton, CA, USA), indirect ophthalmoscope, B-scan ultrasonography and fluorescein fundus angiography (FFA). The clinical status and complications were recorded at each visit. Focal consolidation treatment for the retinal tumor, such as laser coagulation and cryotherapy, was applied if necessary. Treatment success was defined as complete regression of the retinal tumor and vitreous seeds without recurrence. Recurrent disease was defined as regrowth of inactive tumor or seeds requiring additional treatment. If treatment success was achieved, follow-up was extended at one month, three months, and then every six months thereafter. Additionally, all patients were followed up with contrast-enhanced MRI of orbit/brain every six months for systemic monitoring.
RESULTS
Thirty eyes from thirty Chinese patients with advanced unilateral retinoblastoma (17 boys and 13 girls) were included in this study. The median age at diagnosis was 30mo (range, 12-84mo). The affected eyes were classified (IIRC) as group D (n=23; 77%) and group E (n=7; 23%). All eyes showed severe vitreous seeds classified as clouds based on their morphologic features[18].
No eyes received any previous treatment, and all were initially treated with IAC combined with IVM in our center. The detailed information of each patient is listed in Table 1. IAC was performed in three cycles for 24 eyes (80%) and in four cycles for 6 eyes (20%). The mean number of IAC cycles was 3.2 (range, 3-4). The mean number of intravitreal injections was 6 (range, 1-14). Three patients achieved complete vitreous seeds control with a single injection. The median time to regression of vitreous seeds was 4mo (range, 2-8mo). In addition, cytopathological examination of the aqueous humor was negative for malignant cells in each patient.
Table 1. Treatment summary of 30 eyes receiving combined IAC and IVM.
| Case No. | IIRC group | IAC drugs (C, T, M; mg) and cycles | No. of IVM injection | Time to regression of VS (mo) | Outcome of retinal tumor and VS | Complications | Follow-up (mo) |
| 1 | D | (40,1,4); 4 | 4 | 4 | Regress | RPE atrophy | 33 |
| 2 | D | (60,1,6); 3 | 6 | 2 | Regress | VH | 34 |
| 3 | D | (40,1,4); 3 | 6 | 5 | Regress | None | 31 |
| 4 | D | (40,1,4); 3 | 9 | 5 | Regress | RPE atrophy | 29 |
| 5 | D | (40,1,4); 3 | 3 | 4 | Regress | None | 22 |
| 6 | D | (40,1,4); 3 | 7 | 5 | Regress | None | 14 |
| 7 | D | (40,1,4); 3 | 3 | 2 | Regress | RPE atrophy | 30 |
| 8 | D | (40,1,4); 3 | 4 | 4 | Regress | None | 16 |
| 9 | D | (40,1,4); 3 | 4 | 2 | Regress | None | 35 |
| 10 | D | (40,1,4); 3 | 4 | 4 | Regress | RPE/optic atrophy, lens opacity | 29 |
| 11 | E | (40,1,4); 3 | 9 | 6 | Regress | RPE/optic atrophy, lens opacity | 24 |
| 12 | E | (40,1,4); 4 | 5 | 4 | Regress | RPE/optic atrophy | 33 |
| 13 | E | (40,1,4); 3 | 11 | 5 | Regress | VH, NVG | 21 |
| 14 | D | (40,1,4); 4 | 11 | 6 | Regress | None | 30 |
| 15 | E | (40,1,4); 4 | 6 | 6 | Regress | RPE atrophy, lens opacity, VH | 27 |
| 16 | D | (50,1,5); 3 | 14 | 6 | Regress | RPE atrophy, lens opacity | 36 |
| 17 | D | (40,1,4); 3 | 3 | 2 | Regress | RPE atrophy | 30 |
| 18 | D | (40,1,4); 4 | 6 | 7 | Regress | RPE/optic atrophy | 31 |
| 19 | D | (40,1,4); 3 | 3 | 2 | Regress | RPE atrophy | 30 |
| 20 | E | (40,1,4); 3 | 8 | 8 | Regress | Lens opacity | 20 |
| 21 | D | (40,1,4); 3 | 4 | 4 | Regress | RRD | 17 |
| 22 | E | (50,1,5); 3 | 7 | 4 | Recur (retinal tumor) | RPE atrophy, lens opacity | 15 |
| 23 | D | (50,1,5); 3 | 6 | 3 | Regress | RPE atrophy | 15 |
| 24 | D | (60,1,6); 3 | 11 | 5 | Regress | Lens opacity, VH | 29 |
| 25 | D | (40,1,4); 3 | 1 | 2 | Regress | None | 29 |
| 26 | D | (40,1,4); 3 | 3 | 2 | Regress | None | 28 |
| 27 | D | (40,1,4); 3 | 6 | 3 | Regress | None | 34 |
| 28 | E | (40,1,4); 3 | 1 | 2 | Regress | None | 28 |
| 29 | D | (30,1,3); 3 | 1 | 3 | Regress | None | 27 |
| 30 | D | (/,2,5); 4 | 9 | 5 | Regress | VH, NVG | 35 |
IIRC: International Intraocular Retinoblastoma Classification; C: Carboplatin; T: Topotecan; M: Melphalan; VS: Vitreous seeds; Regress: Regression; Recur: Recurrent; RPE: Retinal pigment epithelium; VH: Vitreous hemorrhage; NVG: Neovascular glaucoma; RRD: Rhegmatogenous retinal detachment.
The patients were followed up for 27mo on average (median, 29mo; range, 7-36mo). Focal consolidation treatment was performed in 12 patients. Treatment success (regression of both the retinal tumor and vitreous seeds) was achieved in 29 of 30 eyes (96.7%; Figure 1). Recurrence was observed in one patient (case 22), which occurred in retinal tumor 10mo after the initial treatment and was controlled with an additional IAC. Globe salvage was attained in 28 of 30 eyes (93.3%). Enucleation was performed in two eyes due to neovascular glaucoma (NVG) and persistent vitreous hemorrhage. The pathologic report showed no active retinoblastoma with extensive calcification, and the resection margins and optic nerve were tumor-free.
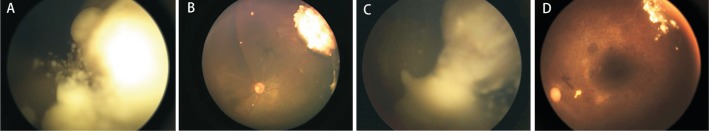
Figure 1. Advanced unilateral retinoblastoma treated with IAC combined with IVM.
A: A 19-month-old boy with group D retinoblastoma with cloud vitreous seeds (case 3); B: Following 3 cycles of IAC (carboplatin, 40 mg; topotecan, 1 mg; melphalan, 4 mg) and 6 injections of IVM (30 µg), complete regression of the retinal tumor and vitreous seeds; C: A 4-year-old boy with group D retinoblastoma with cloud vitreous seeds (case 16); D: Following 3 cycles of IAC (carboplatin, 50 mg; topotecan, 1 mg; melphalan, 5 mg) and 14 injections of IVM (30 µg), complete regression of the retinal tumor and vitreous seeds.
Retinal pigment epithelium (RPE) atrophy occurred in 13 eyes (43%), and 4 of these 13 eyes were accompanied by optic atrophy. FFA in the eyes with RPE atrophy showed window defect hyperfluorescence and chorioretinal nonperfusion (Figure 2). Seven eyes (17%) developed mild lens opacity that did not affect fundus examination. Vitreous hemorrhage occurred in 5 eyes (17%). Spontaneous clearance of vitreous hemorrhage was observed in 3 of 5 eyes. The other two eyes (cases 13 and 30) showed persistent dense vitreous hemorrhage and developed NVG, which received enucleation. Rhegmatogenous retinal detachment (RRD) occurred in one eye (3%), which received scleral bucking surgery. No severe systemic complications were observed, and no patient had extraocular tumor extension or metastasis during the follow-up period.
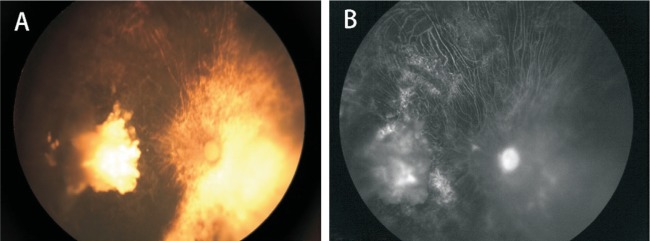
Figure 2. RPE atrophy.
Complete regression of tumor and prominent RPE/optic atrophy after combined treatment in case 12.
DISCUSSION
This study reports combined IAC and IVM as the initial treatment for 30 Chinese patients with advanced unilateral retinoblastoma. It showed that combined treatment was effective and safe for the treatment of advanced unilateral retinoblastoma.
In the present study, combined treatment achieved a high control of both the retinal tumor and vitreous seeds (29 of 30 eyes). There was only one recurrent case after the initial treatment. IAC has been reported to be effective for the treatment of advanced unilateral retinoblastoma[8],[19]–[20]. Abramson et al[20] reported that the enucleation rate of advanced unilateral retinoblastoma decreased from over 95% to 7.4% with the use of IAC. Shields et al[19] demonstrated that IAC achieved a significantly higher globe salvage rate than intravenous chemotherapy for the treatment of group D unilateral retinoblastoma (91% vs 48%). In addition to remarkable effectiveness of IAC, high globe salvage rate should also be attributed to intravitreal chemotherapy due to its contribution in eliminating vitreous seeds, especially the cloud vitreous seeds seen in our study. Cloud vitreous seeds have been recognized as the most intractable type of vitreous seeds[21]. In a recent study investigating the treatment of advanced retinoblastoma with cloud vitreous seeds, the authors found that IAC plus intravitreal chemotherapy was associated with a shorter time to regression of vitreous seeds (5.6 vs 14.6mo), a lower recurrence rate (5.9% vs 32.9%), and a higher globe salvage rate (100% vs 83.3%) compared with IAC alone[22]. Another study from Shields et al[23] also demonstrated that IAC plus intravitreal chemotherapy as primary treatment improved globe salvage in eyes with advanced retinoblastoma. IAC combined with IVM appears to be more efficient and effective than IAC alone in the management of advanced unilateral retinoblastoma, especially when accompanied by severe vitreous disease.
Globe salvage rate was achieved in 93.3% (28/30) in the present study. In two cases, which were manifested as NVG and persistent vitreous hemorrhage, enucleation was performed for fear of loss of tumor control. However, the pathologic reports showed no active retinoblastoma, and the resection margins and optic nerve were free from tumor. Persistent vitreous hemorrhage and NVG might be linked to neovascularization secondary to chorioretinal ischemia after IAC[24]–[27]. Munier et al[25] reported that intravitreal anti-VEGF injections contributed to globe salvage by treating neovascularization after conservative treatment of retinoblastoma. However, anti-VEGF agents should be cautiously used in pediatric patients due to underlying systemic side effects. In our opinion, the occurrence of neovascularization does not mean treatment failure; however, we recommend early enucleation for fear of neovascularization concomitant with active retinoblastoma, especially in unilateral disease without potential visual function.
In the present study, RPE atrophy was not uncommon and occurred in 13 of 30 eyes (43%). The mechanism of RPE damage remains unclear but may be associated with ischemic vasculopathy after IAC or direct cytotoxicity of chemotherapy drugs. FFA was reported to be effective for evaluating retinal ischemia after IAC[25]–[26]. We performed FFA in these eyes, which showed typical chorioretinal nonperfusion and window defect in the area of RPE loss. Moreover, RPE atrophy was related to cytotoxicity of chemotherapy drugs. It has been confirmed that a high concentration of chemotherapy drugs accumulated in the RPE and choroid (pigmented tissues) after IAC[28]. We speculate that the high occurrence of RPE atrophy was linked to ethnic differences; thus, more deeply pigmented eyes (such as those of Chinese patients) may absorb more chemotherapy drugs, resulting in more RPE toxicity. It implied that the dosage of chemotherapy drugs can be reduced to minimize toxicity in Chinese patients; however, the treatment effect on retinoblastoma should be ensured.
Although combined IAC and IVM achieved a high globe salvage rate in patients with advanced unilateral retinoblastoma, patient survival should never be compromised. In view of our observation, no severe systemic complications occurred, and no patient had extraocular tumor extension or metastasis during the follow-up period. A report from four of the largest IAC centers worldwide demonstrated the safety of IAC and found that its use did not increase metastasis-related death[29].
There are some limitations in the present study. First, our study was retrospective and nonrandomized. However, the rarity of the disease makes prospective randomized studies difficult to establish. Second, this study lacked a comparison between IAC alone and IAC plus intravitreal chemotherapy. All eyes in our study showed severe cloud vitreous disease, which seemed impossible to achieve tumor control with IAC alone. Our study investigating combined treatment can serve as a useful reference for the further studies. Finally, we did not perform electroretinogram to assess ocular toxicity and to predict visual function. In view of relatively high occurrence of RPE atrophy in our study, the electroretinogram results are likely to be unsatisfied.
In conclusion, our study confirmed that combined IAC and IVM is effective and safe for the treatment of advanced unilateral retinoblastoma in Chinese patients. Further studies involving larger samples and a longer follow-up are needed to evaluate the true benefit of combined treatment.
Acknowledgments
Foundations: Supported by Shanghai Science and Technology Committee (No.17411952900); Shanghai Shen Kang Hospital Development Center (No.16CR4017A).
Conflicts of Interest: Liang TY, None; Zhu XY, None; Hua XM, None; Ji XD, None; Zhao PQ, None.
REFERENCES
- 1.Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, Shields JA. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113(12):2276–2280. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Shields CL, Ramasubramanian A, Thangappan A, Hartzell K, Leahey A, Meadows AT, Shields JA. Chemoreduction for group E retinoblastoma: comparison of chemoreduction alone versus chemoreduction plus low-dose external radiotherapy in 76 eyes. Ophthalmology. 2009;116(3):544–551.e1. doi: 10.1016/j.ophtha.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Zage PE, Reitman AJ, Seshadri R, Weinstein JL, Mets MB, Zeid JL, Greenwald MJ, Strauss LC, Goldman S. Outcomes of a two-drug chemotherapy regimen for intraocular retinoblastoma. Pediatr Blood Cancer. 2008;50(3):567–572. doi: 10.1002/pbc.21301. [DOI] [PubMed] [Google Scholar]
- 4.Gombos DS, Hungerford J, Abramson DH, Kingston J, Chantada G, Dunkel IJ, Antoneli CB, Greenwald M, Haik BG, Leal CA, Medina-Sanson A, Schefler AC, Veerakul G, Wieland R, Bornfeld N, Wilson MW, Yu CB. Secondary acute myelogenous leukemia in patients with retinoblastoma: is chemotherapy a factor? Ophthalmology. 2007;114(7):1378–1383. doi: 10.1016/j.ophtha.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 5.Tuncer S, Sencer S, Kebudi R, Tanyıldız B, Cebeci Z, Aydın K. Superselective intra-arterial chemotherapy in the primary management of advanced intra-ocular retinoblastoma: first 4-year experience from a single institution in Turkey. Acta Ophthalmol. 2016;94(7):e644–e651. doi: 10.1111/aos.13077. [DOI] [PubMed] [Google Scholar]
- 6.Shields CL, Bianciotto CG, Jabbour P, Ramasubramanian A, Lally SE, Griffin GC, Rosenwasser R, Shields JA. Intra-arterial chemotherapy for retinoblastoma: report No. 1, control of retinal tumors, subretinal seeds, and vitreous seeds. Arch Ophthalmol. 2011;129(11):1399–1406. doi: 10.1001/archophthalmol.2011.150. [DOI] [PubMed] [Google Scholar]
- 7.Abramson DH, Fabius AW, Francis JH, Marr BP, Dunkel IJ, Brodie SE, Escuder A, Gobin YP. Ophthalmic artery chemosurgery for eyes with advanced retinoblastoma. Ophthalmic Genet. 2017;38(1):16–21. doi: 10.1080/13816810.2016.1244695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munier FL, Mosimann P, Puccinelli F, Gaillard MC, Stathopoulos C, Houghton S, Bergin C, Beck-Popovic M. First-line intra-arterial versus intravenous chemotherapy in unilateral sporadic group D retinoblastoma: evidence of better visual outcomes, ocular survival and shorter time to success with intra-arterial delivery from retrospective review of 20y of treatment. Br J Ophthalmol. 2017;101(8):1086–1093. doi: 10.1136/bjophthalmol-2016-309298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. 2012;130(10):1268–1271. doi: 10.1001/archophthalmol.2012.1983. [DOI] [PubMed] [Google Scholar]
- 10.Munier FL, Gaillard MC, Balmer A, Soliman S, Podilsky G, Moulin AP, Beck-Popovic M. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012;96(8):1078–1083. doi: 10.1136/bjophthalmol-2011-301450. [DOI] [PubMed] [Google Scholar]
- 11.Shields CL, Manjandavida FP, Arepalli S, Kaliki S, Lally SE, Shields JA. Intravitreal melphalan for persistent or recurrent retinoblastoma vitreous seeds: preliminary results. JAMA Ophthalmol. 2014;132(3):319–325. doi: 10.1001/jamaophthalmol.2013.7666. [DOI] [PubMed] [Google Scholar]
- 12.Tuncer S, Balcı Ö, Tanyıldız B, Kebudi R, Shields CL. Intravitreal lower-dose (20 µg) melphalan for persistent or recurrent retinoblastoma vitreous seeds. Ophthalmic Surg Lasers Imaging Retina. 2015;46(9):942–948. doi: 10.3928/23258160-20151008-07. [DOI] [PubMed] [Google Scholar]
- 13.Ji XD, Hua PY, Li J, Li JK, Zhao JY, Zhao PQ. Intravitreal melphalan for vitreous seeds: initial experience in China. J Ophthalmol. 2016;2016:4387286. doi: 10.1155/2016/4387286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis JH, Brodie SE, Marr B, Zabor EC, Mondesire-Crump I, Abramson DH. Efficacy and toxicity of intravitreous chemotherapy for retinoblastoma: four-year experience. Ophthalmology. 2017;124(4):488–495. doi: 10.1016/j.ophtha.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linn Murphree A. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am. 2005;18(1):41–53. viii. doi: 10.1016/j.ohc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Gobin YP, Cloughesy TF, Chow KL, Duckwiler GR, Sayre JW, Milanese K, Viñuela F. Intraarterial chemotherapy for brain tumors by using a spatial dose fractionation algorithm and pulsatile delivery. Radiology. 2001;218(3):724–732. doi: 10.1148/radiology.218.3.r01mr41724. [DOI] [PubMed] [Google Scholar]
- 17.Munier FL, Soliman S, Moulin AP, Gaillard MC, Balmer A, Beck-Popovic M. Profiling safety of intravitreal injections for retinoblastoma using an anti-reflux procedure and sterilisation of the needle track. Br J Ophthalmol. 2012;96(8):1084–1087. doi: 10.1136/bjophthalmol-2011-301016. [DOI] [PubMed] [Google Scholar]
- 18.Munier FL. Classification and management of seeds in retinoblastoma. Ellsworth lecture Ghent August 24th 2013. Ophthalmic Genet. 2014;35(4):193–207. doi: 10.3109/13816810.2014.973045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shields CL, Jorge R, Say EA, Magrath G, Alset A, Caywood E, Leahey AM, Jabbour P, Shields JA. Unilateral retinoblastoma managed with intravenous chemotherapy versus intra-arterial chemotherapy. Outcomes based on the international classification of retinoblastoma. Asia Pac J Ophthalmol (Phila) 2016;5(2):97–103. doi: 10.1097/APO.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 20.Abramson DH, Fabius AW, Issa R, Francis JH, Marr BP, Dunkel IJ, Gobin YP. Advanced unilateral retinoblastoma: the impact of ophthalmic artery chemosurgery on enucleation rate and patient survival at MSKCC. PLoS One. 2015;10(12):e0145436. doi: 10.1371/journal.pone.0145436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis JH, Abramson DH, Gaillard MC, Marr BP, Beck-Popovic M, Munier FL. The classification of vitreous seeds in retinoblastoma and response to intravitreal melphalan. Ophthalmology. 2015;122(6):1173–1179. doi: 10.1016/j.ophtha.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Francis JH, Iyer S, Gobin YP, Brodie SE, Abramson DH. Retinoblastoma vitreous seed clouds (class 3): a comparison of treatment with ophthalmic artery chemosurgery with or without intravitreous and periocular chemotherapy. Ophthalmology. 2017;124(10):1548–1555. doi: 10.1016/j.ophtha.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Shields CL, Alset AE, Say EA, Caywood E, Jabbour P, Shields JA. Retinoblastoma control with primary intra-arterial chemotherapy: outcomes before and during the intravitreal chemotherapy era. J Pediatr Ophthalmol Strabismus. 2016;53(5):275–284. doi: 10.3928/01913913-20160719-04. [DOI] [PubMed] [Google Scholar]
- 24.Shields CL, Manjandavida FP, Lally SE, Pieretti G, Arepalli SA, Caywood EH, Jabbour P, Shields JA. Intra-arterial chemotherapy for retinoblastoma in 70 eyes: outcomes based on the international classification of retinoblastoma. Ophthalmology. 2014;121(7):1453–1460. doi: 10.1016/j.ophtha.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 25.Munier FL, Beck-Popovic M, Balmer A, Gaillard MC, Bovey E, Binaghi S. Occurrence of sectoral choroidal occlusive vasculopathy and retinal arteriolar embolization after superselective ophthalmic artery chemotherapy for advanced intraocular retinoblastoma. Retina. 2011;31(3):566–573. doi: 10.1097/IAE.0b013e318203c101. [DOI] [PubMed] [Google Scholar]
- 26.Bianciotto C, Shields CL, Iturralde JC, Sarici A, Jabbour P, Shields JA. Fluorescein angiographic findings after intra-arterial chemotherapy for retinoblastoma. Ophthalmology. 2012;119(4):843–849. doi: 10.1016/j.ophtha.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 27.Stathopoulos C, Gaillard MC, Moulin A, Puccinelli F, Beck-Popovic M, Munier FL. Intravitreal anti-vascular endothelial growth factor for the management of neovascularization in retinoblastoma after intravenous and/or intraarterial chemotherapy: long-term outcomes in a series of 35 eyes. Retina. 2019;39(12):2273–2282. doi: 10.1097/IAE.0000000000002339. [DOI] [PubMed] [Google Scholar]
- 28.Schaiquevich P, Buitrago E, Taich P, Torbidoni A, Ceciliano A, Fandino A, Asprea M, Requejo F, Abramson DH, Bramuglia GF, Chantada GL. Pharmacokinetic analysis of melphalan after superselective ophthalmic artery infusion in preclinical models and retinoblastoma patients. Invest Ophthalmol Vis Sci. 2012;53(7):4205–4212. doi: 10.1167/iovs.12-9501. [DOI] [PubMed] [Google Scholar]
- 29.Abramson DH, Shields CL, Munier FL, Chantada GL. Treatment of retinoblastoma in 2015: agreement and disagreement. JAMA Ophthalmol. 2015;133(11):1341–1347. doi: 10.1001/jamaophthalmol.2015.3108. [DOI] [PubMed] [Google Scholar]