Abstract
β-N-Acetylhexosaminidases are glycoside hydrolases (GHs) acting on N-acetylated carbohydrates and glycoproteins with the release of N-acetylhexosamines. Members of the family GH20 have been reported to catalyze the transfer of N-acetylglucosamine (GlcNAc) to an acceptor, i.e., the reverse of hydrolysis, thus representing an alternative to chemical oligosaccharide synthesis. Two putative GH20 β-N-acetylhexosaminidases, PhNah20A and PhNah20B, encoded by the marine bacterium Paraglaciecola hydrolytica S66T, are distantly related to previously characterized enzymes. Remarkably, PhNah20A was located by phylogenetic analysis outside clusters of other studied β-N-acetylhexosaminidases, in a unique position between bacterial and eukaryotic enzymes. We successfully produced recombinant PhNah20A showing optimum activity at pH 6.0 and 50 °C, hydrolysis of GlcNAc β-1,4 and β-1,3 linkages in chitobiose (GlcNAc)2 and GlcNAc-1,3-β-Gal-1,4-β-Glc (LNT2), a human milk oligosaccharide core structure. The kinetic parameters of PhNah20A for p-nitrophenyl-GlcNAc and p-nitrophenyl-GalNAc were highly similar: kcat/KM being 341 and 344 mM−1·s−1, respectively. PhNah20A was unstable in dilute solution, but retained full activity in the presence of 0.5% bovine serum albumin (BSA). PhNah20A catalyzed the formation of LNT2, the non-reducing trisaccharide β-Gal-1,4-β-Glc-1,1-β-GlcNAc, and in low amounts the β-1,2- or β-1,3-linked trisaccharide β-Gal-1,4(β-GlcNAc)-1,x-Glc by a transglycosylation of lactose using 2-methyl-(1,2-dideoxy-α-d-glucopyrano)-oxazoline (NAG-oxazoline) as the donor. PhNah20A is the first characterized member of a distinct subgroup within GH20 β-N-acetylhexosaminidases.
Keywords: N-acetylhexosamine specificity, glycoside hydrolase, GH20, phylogenetic analysis, transglycosylation, NAG-oxazoline, acceptor diversity, lacto-N-triose II, human milk oligosaccharides, NMR
1. Introduction
A new marine bacterial species Paraglaciecola hydrolytica S66T of the family Alteromonadaceae isolated from eelgrass (Zostera sp.) was shown by genome-sequencing [1] to encode 270 protein modules potentially acting on carbohydrates, 188 of which belong to enzyme families involved in degradation of carbohydrates [2,3]. The algal polysaccharides agar, agarose, alginate, porphyran or laminarin, but not carrageenans, fucoidan and ulvan, sustained the growth of P. hydrolytica as a sole carbon source, and the bacterium also grew on the plant polysaccharides: starch, amylopectin, amylose, xylan and pectin [2]. Overall, the large number of encoded carbohydrate-active enzymes (CAZymes) [4] and the flexibility with regard to carbon source indicates a very promising potential of the genome of P. hydrolytica for the discovery of enzymes with rare or not yet described activities.
Enzymes hydrolyzing glycosidic bonds with the release of N-acetylglucosamine (GlcNAc) are in focus since these carbohydrate residues occur in vital complex glycans, such as milk oligosaccharides and glycosphingolipids, for which there is a great demand [5]. Human milk oligosaccharides (HMOs) in particular are considered beneficial and needed for research and clinical trials within nutrition and as ingredients in functional foods and infant formulas [6,7,8]. HMOs are also regarded as emerging prebiotics or novel foods with positive health effects [9,10]. However, the chemical and enzymatic production of HMOs and their precursors or purification from natural sources are problematical [6,11,12], which creates bottlenecks for assessing the functional roles and applications of HMOs [13,14,15].
Lacto-N-triose II (LNT2, β-GlcNAc-1,3-β-Gal-1,4-Glc) is an HMO core structure in which N-acetylglucosamine is β-1,3-linked to lactose [6,16,17]. A few β-N-acetylhexosaminidases (β-NAHAs; EC 3.2.1.52) of the glycoside hydrolase family 20 (GH20) from bacteria, fungi and plants are reported to produce HMO-type GlcNAc-containing oligosaccharides with 1,3 linkages [15,18,19], as well as chitooligosaccharides and their analogs in transglycosylation reactions with the formation of 1,6 rather than 1,4 linkages [18,20,21,22]. In Nature, β-NAHAs from GH3, 20, 84, 109 and 116 [5,23,24] categorized in the CAZy database (www.cazy.org) [4] degrade N-acetylhexosamine-containing compounds by releasing GlcNAc and GalNAc from the non-reducing ends of N-acetylglucosides, N-acetylgalactosides, glycosphingolipids and glycoproteins [5,25,26,27]. Interestingly, these families display a variety of mechanisms, either retaining via a substrate-assisted mechanism (GH20 and GH84) [28,29] or a glycosyl-enzyme intermediate (GH3 and GH116) [30], or inverting via an oxidized form of nicotinamide adenine dinucleotide (NAD+)-depending mechanism (GH109) [31]. While being represented in five distinct GH families, the large majority of β-NAHAs belong to GH20.
N-acetylated oligo- and polysaccharides, e.g., chitooligosaccharides and chitin, are prevalent in marine organisms, thus crustaceans represent an abundant source of GlcNAc in marine environments. The National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/) currently has more than 112,000 predicted β-NAHAs, but out of the more than 200 characterized EC 3.2.1.52 enzymes (www.brenda-enzymes.org) [32], only a small number are of marine origin [21,26,33,34,35,36]. Accordingly, only six out of the 133 characterized GH20 β-NAHAs are from a marine organism (from www.cazy.org, 21st of November, 2019) even though a large number of sequences, also of marine origin, are annotated in genomes and metagenomes. These six characterized marine GH20 enzymes comprise Hex99 and Hex86 from Pseudoalteromonas piscicida (previously Alteromonas sp.) [21,35], Nag20A [36] and NagB [34] from the widespread Aeromonas hydrophila, chitobiase of Vibrio harveyi [37] and ExoI from Vibrio furnissii [33]. However, of these, only Hex99 from P. piscicida was examined for its ability to catalyze transglycosylation reactions [21].
Biochemical characteristics such as pH optimum (between pH 6.0‒7.0) and temperature optimum (37‒50 °C) of the six enzymes are rather similar. Moreover, based on KM and Vmax values, most of the enzymes have higher specific activity towards p-nitrophenyl-GlcNAc (pNPGlcNAc) compared to p-nitrophenyl-GalNAc (pNPGalNAc) [25,33,34,36].
It has not been possible to clearly distinguish between GH20 β-NAHAs from water-living and terrestrial organisms or from bacterial and eukaryotic organisms based solely on different functional features of the enzymes. The biochemical characteristics of GH20 β-NAHAs vary considerably, as it has been reviewed recently by Zhang et al. [25]. For example, pH optima of GH20 enzymes range from pH 3.0 for Hex of Streptomyces plicatus to pH 8.0 for Hex1 (from a metagenomic library) [18,25,38]. Affinities as given by KM values for pNPGlcNAc range from 53 µM for CfHex20 from Cellulomonas fimi [39] to 120 mM for BbhI of Bifidobacterium bifidum [40]. Murine cytosolic β-NAHA shows KM = 0.25 mM on pNPGalNAc, which it preferred over pNPGlcNAc [41]. Similarly, human plasma and pig brain β-NAHAs have a lower KM for pNPGalNAc of 0.17 mM and 0.2 mM, respectively [42,43]. Interestingly, salt-tolerant HJ5Nag from Microbacterium sp. has a high Vmax towards pNPGlcNAc of 3097 µmol·mg−1·min−1 [44]. One of the highest kcat and catalytic efficiency values reported towards pNPGlcNAc are for CfHex20 of C. fimi reaching 480 s−1 and 9000 mM−1·s−1, respectively [39]. Crystal structures are available for several terrestrial GH20 β-NAHAs, e.g., Hex1T from Paenibacillus sp. TS12 [45], StrH from Streptococcus pneumoniae [46], HexA from Streptomyces coelicolor [47], Hex from S. plicatus [38] and Am2301 from Akkermansia muciniphila [48], but not for any aquatic GH20 enzymes.
Here, the genome of P. hydrolytica S66T encoding 113 predicted glycoside hydrolases [1,3] was mined for β-NAHAs potentially acting on GlcNAc-containing compounds, e.g., chitooligosaccharides, which are abundant in the marine environment. Two putative GH20 encoding genes were identified in the genome, and one of the corresponding enzymes, PhNah20A, was produced recombinantly, characterized biochemically and moreover shown to catalyze transglycosylation using the GH20 reaction intermediate NAG-oxazoline [2-methyl-(1,2-dideoxy-α-d-glucopyrano)-oxazoline] as donor and lactose as well as a series of monosaccharides as acceptors.
2. Results and Discussion
2.1. Identification of Putative β-NAHAs in P. hydrolytica and Organization of Vicinal Genomic Regions
The marine bacterium P. hydrolytica degrades effectively many different polysaccharides [2] and its genome exhibits potential for the degradation of chitin and chitooligosaccharides. P. hydrolytica was grown in marine mineral medium supplemented with a mixture of chitooligosaccharides (GlcNAc)1–6 as the sole carbon source, which were hydrolyzed to GlcNAc (Supplementary Information 1, Figure S1A,B). P. hydrolytica, however, did not hydrolyze α-chitin from crab shells used to supplement the marine mineral medium, as neither GlcNAc nor chitooligosaccharides appeared during the incubation (Figure S1C). β-NAHA activity from P. hydrolytica was detected by a hydrolysis of the chromogenic 5-bromo-4-chloro-3-indolyl N-acetyl-β-d-glucosaminide (X-GlcNAc) on a complex marine agar medium (Figure S1D). These results indicated that the bacterium produced at least one β-NAHA which was active towards chitooligosaccharides.
The draft genome sequence of P. hydrolytica [1], deposited on the RAST server (http://rast.nmpdr.org/), encodes two putative GH20 β-NAHAs (EC 3.2.1.52) based on automatic annotation. Both genes were found in contig 11 of the P. hydrolytica whole genome shotgun sequence (NCBI accession: NZ_LSNE01000003.1). The protein sequence identity between full-length PhNah20A (WP_068373836.1) and PhNah20B (WP_082768773.1) was 23%.
Top hits of protein BLAST, showing up to 54% to PhNah20A and up to 49% sequence identity to PhNah20B, were GH20 β-NAHAs or chitobiases from phylogenetically closely related marine and soil bacteria belonging mostly to the same order as P. hydrolytica—Alteromonadales (Table S1). None of these proteins, encoded by genes from Paraglaciecola or related bacteria (Table S1), had been recombinantly produced or characterized.
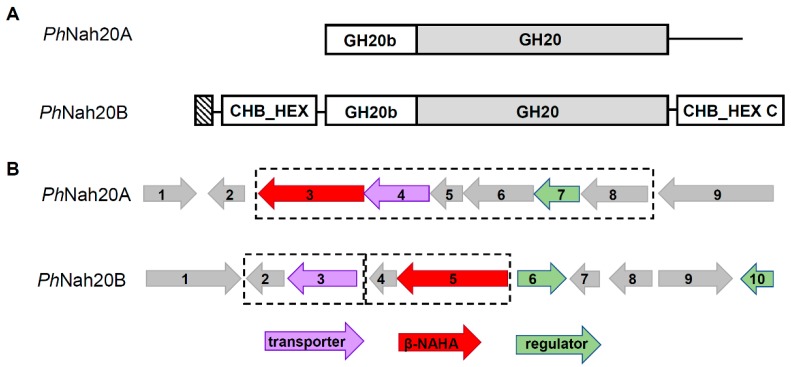
The closest relatives of PhNah20A are GH20 β-NAHAs from Bowmanella denitrificans and Lacimicrobium alkaliphilum with 53%‒54% sequence identity (Table S1). PhNah20A contains two domains, the GH20 catalytic (β/α)8-barrel domain (Pfam: PF00728) and the N-terminal GH20b domain (also referred to as GH20 domain 2; Pfam: PF02838) of a predicted zincin-like fold similar to zinc-dependent metalloproteases [49] consisting of four antiparallel β-strands and an α-helix [27,50]. These two domains are typical for GH20 enzymes [50], and importantly they constitute an active and stable minimum functional unit of GH20 enzymes, thus requiring both a catalytic GH20 and a GH20b domain [50]. PhNah20A has no predicted signal peptide sequence and most probably is not secreted, whereas a 28 residues N-terminal signal peptide was predicted for the hypothetical PhNah20B (Figure 1A). Therefore, during the growth of P. hydrolytica on chitooligosaccharides, PhNah20B probably performs the initial degradation of these substrates. PhNah20B, in addition to the GH20b and GH20 domains, contains a putative carbohydrate binding domain of the CHB_HEX superfamily (Pfam: PF03173) having a predicted β-sandwich structure similar to cellulose binding domains in cellulases [51], and a C-terminal CHB_HEX_C domain (Pfam: PF03174) of unknown function resembling an immunoglobulin-like fold [50,51]. A similar four-domain architecture was seen in the crystal structure of a chitobiase from S. marcescens [51], and has only been reported for bacterial GH20 enzymes [50,51]. Based on its protein sequence identity and domain architecture, PhNah20B resembles a biochemically uncharacterized GH20 chitobiase from Aliiglaciecola lipolytica and β-NAHAs from other phylogenetically close marine bacteria (Table S1). It can be concluded that one of the reasons for low sequence identity, i.e., 23%, between two putative GH20 enzymes of P. hydrolytica, was the different domain architecture of PhNah20A and PhNah20B (Figure 1A), as PhNah20B has two additional domains besides the GH20 catalytic domain and an N-terminal GH20b domain. The identity between the two proteins remained low when only the predicted GH20b and GH20 domain sequences were compared, as some regions are not aligning between proteins (Supplementary Information 2).
Figure 1.
Schematic domain architecture of P. hydrolytica PhNah20A and PhNah20B (A) and of genomic regions flanking the two putative β-N-acetylhexosaminidases (β-NAHAs) (red arrows) (B). (A) GH20 catalytic domains are gray and the N-terminal signal peptide is striped. (B) Predicted protein functions are color coded. The information was retrieved from the National Center for Biotechnology Information (NCBI) database (NZ_LSNE01000003.1), Uniprot and Pfam databases. The regions flanking PhNah20A (3): 1, LemA family protein; 2, hypothetical protein; 4, sodium:solute symporter, putative SLC5sbd family protein; 5, RidA (reactive intermediate/imine deaminase A) family protein; 6, d-aminoacylase; 7, MurR/RpiR family transcriptional regulator; 8, amino acid deaminase; 9, sodium/proton-translocating pyrophosphatase. The regions flanking PhNah20B (5): 1, TonB-dependent receptor; 2, DUF1624 domain-containing protein, putative acyltransferase; 3, glucose/galactose MFS transporter; 4, hypothetical protein, putative BadF-type ATPase; 6, LacI family DNA-binding transcriptional regulator; 7, dCTP deaminase; 8, iron–sulfur cluster carrier protein ApbC; 9, methionine-tRNA ligase; 10, TetR/AcrR family transcriptional regulator. Predicted operons are in dashed frames.
Genomic regions flanking the two annotated P. hydrolytica β-NAHAs, PhNah20A and PhNah20B, were examined for the presence of operons (Figure 1B), but were found not to be organized similarly to the operon responsible for chitobiose-utilization in Escherichia coli [52]. Surrounding putative genes, however, encoded proteins potentially participating in the modification of acetylated compounds, the transporter function and transcription regulation (Figure 1B; Table S2). Notably, a predicted operon of six genes that harbors PhNah20A (Figure 1B) included a putative amino acid deaminase, d-aminoacylase and the RidA (reactive intermediate/imine deaminase A) family protein, possibly associated with the processing of acylated compounds or amino acids [53]. A two-gene operon was predicted to harbor PhNah20B and a putative ATPase (Figure 1B, Table S2). Thus, GH20 β-NAHAs genes of P. hydrolytica were not situated adjacent to genes encoding proteins directly coupled to β-NAHA activity, but flanking genes may be important for regulation or substrate transport.
2.2. Phylogenetic Analysis of PhNah20A and PhNah20B
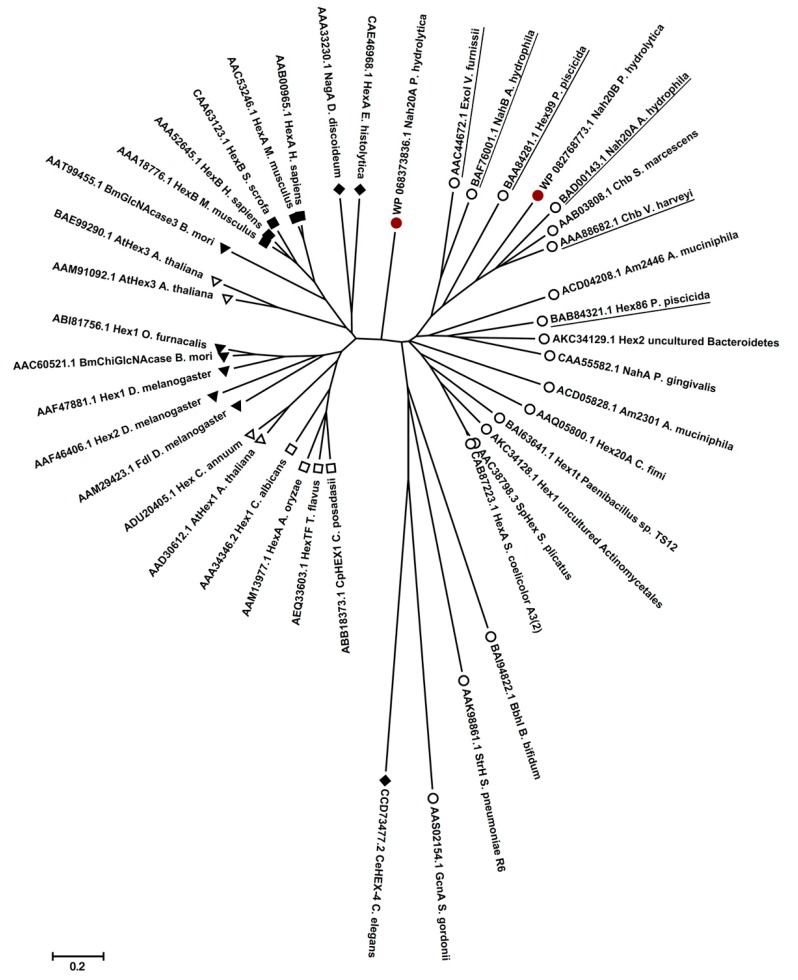
Sequences of PhNah20A, PhNah20B and 41 characterized GH20 enzymes were aligned (Supplementary Information 2). PhNah20A and PhNah20B shared a low sequence identity with the other GH20 enzymes (up to 34.1% for PhNah20A and 37.9% for PhNah20B) and only a few highly conserved regions were identified among these GH20 members (Supplementary Information 2). The closest homologs of PhNah20A were Hex2 of an uncultured Bacteroidetes (34.1% identity) and ExoI of the marine bacterium V. furnissii (33.1% identity). Remarkably, GH20 sequences from eukaryotes (human and mouse) were 31.3% and 30.9% identical and more similar to PhNah20A than most other included bacterial sequences. The PhNah20B sequence was most similar to chitobiases from S. marcescens (37.9% identity) and V. harveyi (36.4% identity). The evolutionary relationship illustrated by a radial phylogenetic tree (Figure 2; for bootstrap values see Figure S2) showed that bacterial GH20s segregate into three groups.
Figure 2.
Schematic phylogenetic tree of PhNah20A, PhNah20B (both marked with red circles) and 41 biochemically characterized GH20 (EC 3.2.1.52) enzymes. Evolutionary analyzes were conducted, and the tree was composed and visualized using MEGA v 7.0.26 [55]. Protein sequences were aligned with Clustal Omega and the BLOSUM62 protein weight matrix was used. Evolutionary relationships were calculated using the Neighbor-Joining method. Evolutionary distances were computed using the Poisson correction method. All positions containing gaps and missing data were eliminated, and there was in total 292 positions in the final dataset. The tree is in scale with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. Bacterial (○), fungal (□), plant (Δ), insect (▲) and mammal (■) sequences. Amoebae and C. elegans sequences are marked with a filled diamond (♦). Characterized GH20 enzymes from marine organisms are underlined.
PhNah20B clustered with β-NAHAs from water-living bacteria from the phylogenetically close species such as V. harveyi, P. piscicida and A. hydrophila. However, PhNah20A did not cluster with characterized bacterial β-NAHAs but seems to represent a new distinct group of GH20 enzymes situated between predominantly water-living bacteria and the eukaryotes (Figure 2).
NagA of the slime mold Dictyostelium discoideum which clusters not far from PhNah20A (Figure 2), is a lysosomal enzyme that maintains the size of pseudoplasmodia [54], and shares 28.5% sequence identity with PhNah20A. According to the BLAST analysis, PhNah20A has higher sequence identity to biochemically uncharacterized β-NAHAs from phylogenetically close marine bacteria (Table S1). Additionally, protein sequences with 44–47% identity to PhNah20A were found in compost, hydrothermal vent and marine sediment metagenomes (Table S1) highlighting unexplored resources harbouring a new group of β-NAHAs.
According to the literature, substrate specificities and biochemical features (e.g., pH and temperature optima) are reported for 41 β-NAHAs of GH20 [4,32] mostly from terrestrial organisms. The few enzymes being from marine bacteria comprise ExoI and chitobiase from Vibrio sp. [33,37], Hex99 and Hex86 from P. piscicida [21,35] and Nag20A and NagB from A. hydrophila [34,36]. The limited knowledge on GH20 from marine organisms motivated the present characterisation of β-NAHA from P. hydrolytica S66T.
2.3. Cloning and Production of β-NAHA
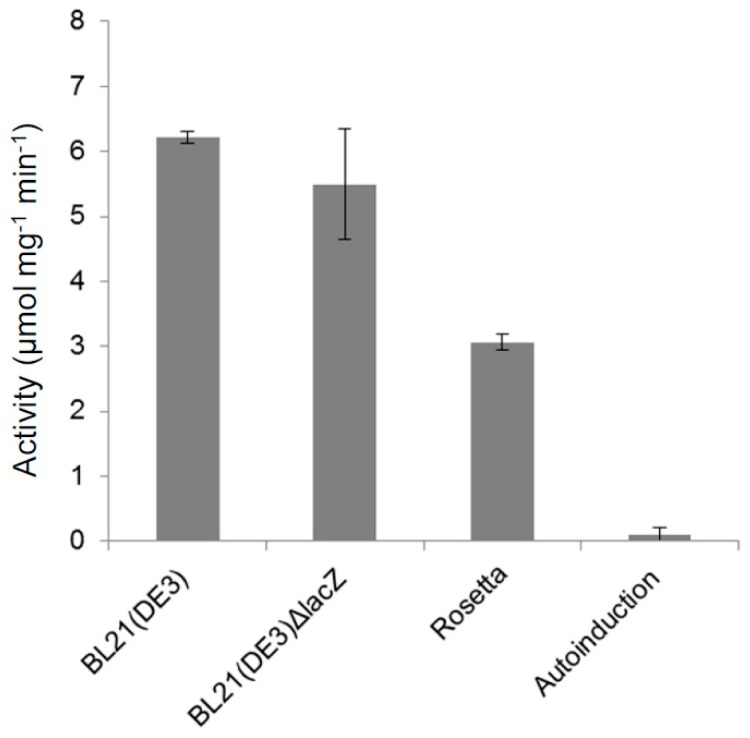
From the two candidate β-NAHA genes (Figure 1A), only recombinant PhNah20A was successfully produced in E. coli (Figure 3). PhNah20B cloned without the N-terminal signal peptide (Figure S3) was not obtained despite expression attempts in three E. coli strains [BL21(DE3), BL21(DE3)ΔlacZ and Rosetta], using different induction methods: isopropyl thio-β-d-galactoside (IPTG)-induction in lysogeny broth (LB) or auto-induction. PhNah20B was not found in the insoluble fraction by analyzing whole cells from IPTG-induced cultures (Figure S4). The yield of PhNah20A was modest, probably due to a low expression level. Using different strains and induction strategies resulted in the highest β-NAHA activity of 6 µmol p-nitrophenol released per min and per mg protein in the E. coli cell lysate for IPTG-induced BL21(DE3) transformants in LB medium (Figure 3).
Figure 3.
β-NAHA activity in µmol per min per mg of total protein in the lysates of three different Escherichia coli (E. coli) strains harboring PhNah20A grown in lysogeny broth (LB) induced by isopropyl thio-β-d-galactoside (IPTG) or in the auto-induction medium ZYM-5052 [56] (30 h). E. coli BL21(DE3) transformants carrying the full-length PhNah20A gene in an pURI3TEV expression vector were used in the auto-induction experiment. Values are given as the average of three independent experiments and the standard deviation (SD) is shown.
Previously, an increased expression of GH20 β-NAHAs from a metagenome [18] was achieved in E. coli strains BL21(DE3), Turner, C41 or C43 grown in an auto-induction medium ZYM-5052 [56], but this medium gave a very low yield of PhNah20A (Figure 3) and failed to lead to PhNah20B production.
Up to 2 mg of PhNah20A was purified in two chromatographic steps from one liter of E. coli BL21(DE3) culture (see Section 3.4). Expression of truncated PhNah20A and PhNah20B, containing only the catalytic and not the GH20b domain (see Figure S3), did not result in protein production which is in agreement with previous findings that GH20b is essential for enzyme production and activity [50]. Attempts to produce PhNah20B without the CHB_HEX domains (Figure S3) also gave no detected protein or β-NAHA activity.
2.4. Characterization of PhNah20A
2.4.1. Enzyme Stability
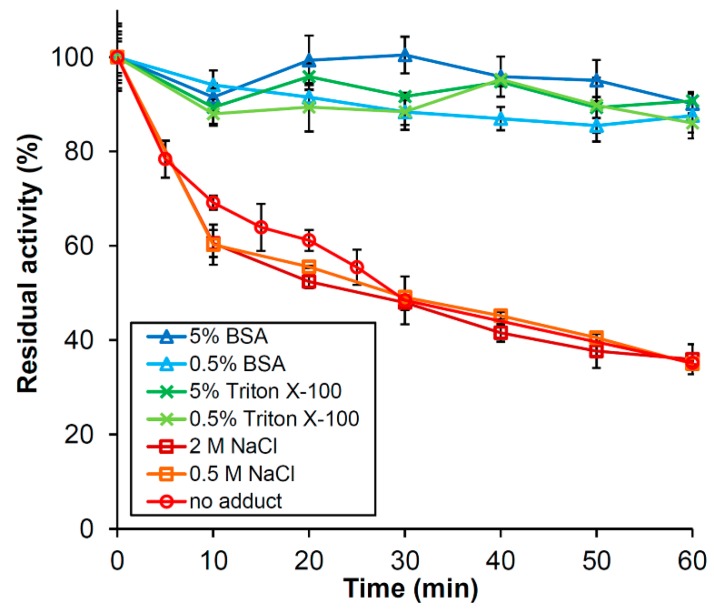
The activity of PhNah20A decreased immediately after dilution to the low concentration of 5 µg·mL−1, even when kept on ice (Figure 4). By contrast, 1 mg·mL−1 PhNah20A retained activity at least four months at 4 °C in 50 mM sodium phosphate pH 7.0, 0.3 M NaCl and 0.02% NaN3. The presence of 0.5% BSA or 0.5% Triton X-100 efficiently stabilized PhNah20A at 5 µg·mL−1 and pH 6.0 (see Figure 5A), whereas 0.5 and 2 M NaCl had no effect (Figure 4). This behavior and the absence of a signal peptide suggest PhNah20A is an intracellular enzyme. Without a stabilizing agent, 5 µg·mL−1 PhNah20A was completely inactivated within 5 min at 50 °C, while 50% and 3% activity were retained after 20 min and 4 h, respectively, in 0.5% BSA (Figure S5), and activity was fully retained after 4 d at 37 °C. β-NAHAs from E. coli [57], Prunus serotina [58], Bos taurus [59], Hordeum vulgare [60] and Streptomyces plicatus [61] were similarly found to lose activity by dilution. BSA has been identified as an activating compound to some β-NAHAs, e.g., from Mus musculus [41] and human plasma [42]. Notably, Hex, the commercial S. plicatus β-NAHA, is produced as a fusion with maltose-binding protein to secure stability and the Hex reaction mixture contained 0.3% of BSA to maintain activity [38].
Figure 4.
Effect of bovine serum albumin (BSA), Triton X-100, and NaCl on the stability of 5 µg·mL−1 PhNah20A on ice. The retained activity was measured at 37 °C in McIlvaine buffer, pH 6.0, using 2 mM pNPGlcNAc as the substrate. PhNah20A, BSA, NaCl and Triton X-100 were further 10 times diluted in the activity assay done at 0.5 µg·mL−1 PhNah20A, 0.05% or 0.5% BSA, 0.05% or 0.5% Triton X-100, 0.05 or 0.2 M NaCl.
Figure 5.
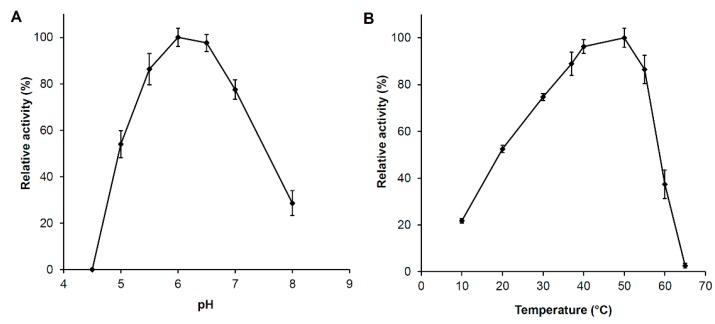
PhNah20A pH and temperature optima. The effect of pH (A) at 37 °C and the temperature at pH 6.0 (B) on initial rates of the hydrolysis of 2 mM pNPGlcNAc are both expressed as relative activity (%) from optimal activity values.
2.4.2. pH and Temperature Optima
PhNah20A was most active at pH 5.0–7.5 with a maximum around pH 6.0 (Figure 5A) and a temperature optimum at 50 °C (Figure 5B). The pH optimum of PhNah20A is highly similar to numerous characterized GH20 β-NAHAs [25], e.g., from Microbacterium sp. [44], Paenibacillus sp. [45] and A. hydrophila [34]. Some fungal β-NAHAs have more acidic pH optima (pH 4–5) [62,63,64]. Similarly high temperature optima as for PhNah20A were found for chitinases from Salinivibrio costicola [65], β-NAHAs from Serratia marcescens [66], A. hydrophila [36] as well as Penicillium oxalicum [27]. PhNah20A, however, when diluted in buffer lost activity completely within 5 min at 50 °C in the absence of stabilizers (Figure S5), emphasizing the importance of an environment with high protein concentration for the stability of PhNah20A. The optimal growth temperature of P. hydrolytica was 20–25 °C [2], but the temperature optimum for PhNah20A activity was much higher, which is a common phenomenon reported for other bacterial GH20 enzymes [27,66].
2.4.3. Substrate Specificity and Kinetic Parameters of PhNah20A
PhNah20A hydrolyzed N,N′-diacetylchitobiose [chitobiose, (GlcNAc)2, β-GlcNAc-1,4-GlcNAc] and lacto-N-triose II (β-GlcNAc-1,3-β-Gal-1,4-Glc, LNT2) with the release of GlcNAc. Chitobiose was a poor substrate and 1 U·mL−1 (11.6 µg·mL−1) PhNah20A converted only 25% of 200 mM chitobiose in 20 h at pH 6.0 as analyzed by high-performance anion exchange chromatography with pulsed amperometric detector (HPAEC-PAD). Similarly, only trace amounts of GlcNAc were released from chitobiose by the GH20 BbhI from B. bifidum [40]. The action on LNT2 motivated assaying for transglycosylation activity (see Section 2.5), i.e., the ability to catalyze the reverse reaction of hydrolysis and in particular to produce HMOs, as described for the BbhI from B. bifidum [15,40].
Kinetic parameters for PhNah20A hydrolyzing pNPGlcNAc and pNPGalNAc (Table 1) were very similar, kcat being slightly higher on pNPGalNAc. This identified PhNah20A as an N-acetylhexosaminidase rather than either an N-acetylglucosaminidase or an N-acetylgalactosaminidase. Most β-NAHAs, especially bacterial GH20 enzymes, prefer pNPGlcNAc (Table 1) and are referred to as N-acetylglucosaminidases. For instance, S. marcescens β-NAHA showed only 28.1% activity on pNPGalNAc compared to pNPGlcNAc [66]. Similarly, HexA from the ameba E. histolytica had 38% activity on pNPGalNAc compared to pNPGlcNAc [67]. Nag20A from A. hydrophila had very similar KM for pNPGlcNAc and pNPGalNAc, but Vmax for pNPGalNAc was only 13% of Vmax for pNPGlcNAc [36]. Nag20B, also from A. hydrophila, showed about 20 times higher KM for pNPGlcNAc and pNPGalNAc [34] than PhNah20A. Other differences include V. furnissii ExoI showing 3.6 times lower KM towards pNPGlcNAc than pNPGalNAc [33]. Similarly, Hex1 and Hex2 from a metagenomic library showed very poor activity for pNPGalNAc [18]. On the other hand, β-NAHAs of human and mouse prefer pNPGalNAc as a substrate over pNPGlcNAc and have a high affinity towards it (KM of 0.17 and 0.25 mM, respectively) [41,42]. Another eukaryotic β-NAHA from D. discoideum showed equal affinity (KM of 1.5 mM) for both substrates [68], thus resembling more PhNah20A and some other bacterial enzymes (Table 1). Interestingly, BbhI had very high KM of 120 mM for pNPGlcNAc (Table 1), but much lower KM of 0.36 mM for LNT2 [40].
Table 1.
Kinetic parameters of PhNah20A and β-NAHAs from the literature on pNPGlcNAc and pNPGalNAc. Sm—S. marcescens; Ah—A. hydrophila; Bb—B. bifidum; Cf—C. fimi; Vf—V. furnissii; Eh—E. histolytica; Tr—Trichoderma reesei.
| Enzyme | Substrate | KM (mM) | Vmax (µmoL·mg−1·min−1) | kcat (s−1) | kcat/KM (mM−1·s−1) |
|---|---|---|---|---|---|
| PhNah20A | pNPGlcNAc | 0.43 ± 0.07 | 93.7 ± 5.0 | 146.8 | 341 |
| pNPGalNAc | 0.56 ± 0.11 | 123.0 ± 7.0 | 192.7 | 344 | |
| SmChb 1 | pNPGlcNAc | 56.7 ± 4.3 | NI | 111.0 | 1.95 |
| AhNag20A 2 | pNPGlcNAc | 0.52 | 115 | NI | NI |
| pNPGalNAc | 0.5 | 7.6 | NI | NI | |
| AhNagB 2 | pNPGlcNAc | 8.57 | 25 | NI | NI |
| pNPGalNAc | 11.1 | 11 | NI | NI | |
| BbhI of Bb 3 | pNPGlcNAc | 120.0 ± 0.2 | NI | 213 | 178 |
| pNPGalNAc | NA | NA | NA | NA | |
| CfHex20 4 | pNPGlcNAc | 0.053 | NI | 482.3 | 9090 |
| pNPGalNAc | 0.066 | NI | 129.1 | 1950 | |
| VfExoI 5 | pNPGlcNAc | 0.09 | 270 | NI | NI |
| pNPGalNAc | 0.33 | 130 | NI | NI | |
| Hex2 6 | pNPGlcNAc | 0.48 | NI | 60.0 ± 1.7 | NI |
| EhHexA 7 | pNPGlcNAc | 0.1 | 3.8 | NI | NI |
| TrNag1 | pNPGlcNAc | 69.4 ± 4.0 | NI | NI | 1023 ± 23 |
2.5. Transglycosylation by PhNah20A
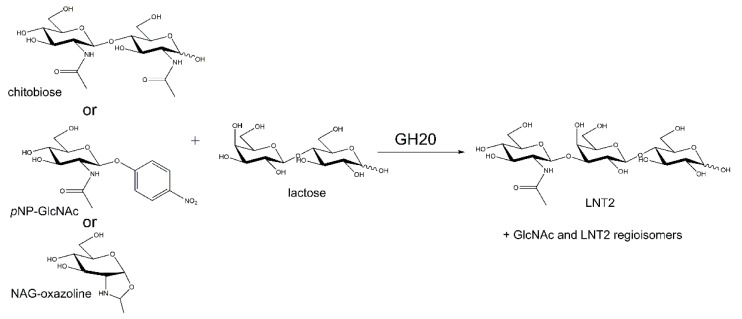
There are a few reports on LNT2 formation by GH20 catalyzed transglycosylation with (GlcNAc)2 or pNPGlcNAc as donors and lactose as the acceptor [15,18,64] (see Figure 6). Hydrolysis of LNT2 by PhNah20A, an HMO core structure [70], warranted the investigation of the transglycosylation with (GlcNAc)2 and the GH20 reaction intermediate NAG-oxazoline [2-methyl-(1,2-dideoxy-α-d-glucopyrano)-oxazoline] [71] as a donor and lactose as an acceptor (Figure 7A). We here also demonstrated transglycosylation by the commercial GH20 N-acetylglucosaminidase from S. plicatus (SpHex) [38] (see Figure S6), which has not been previously reported. Notably, the protein sequence identity between SpHex and a bacterial transglycosylating enzyme Hex1 isolated from a metagenome [18] was as high as 53.6%.
Figure 6.
Scheme of transglycosylation reactions catalyzed by GH20 enzymes showing three different possible donors and lactose as an acceptor.
Figure 7.
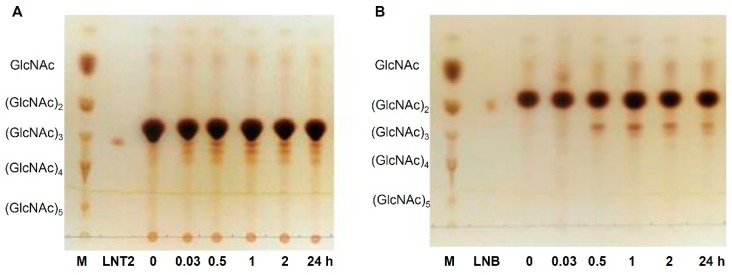
Time course of transglycosylation by PhNah20A (10 U·mL−1) with 100 mM NAG-oxazoline as donor and either 200 mM lactose (A) or d-galactose (B) as acceptor (see Section 3.8 for details). Chitooligosaccharides (M), lacto-N-triose II (LNT2) and lacto-N-biose (LNB) were used as references.
Transglycosylation by β-NAHAs has been rarely investigated, and in one case there is a report on a bacterial GH20 enzyme for which no transglycosylation was detected [72], indicating that not all GH20 enzymes have the ability to transglycosylate. A GH20 chitobiase Hex99 from the Alteromonas sp. strain O-7 (currently classified as P. piscicida) of the order Alteromonadales formed β-GlcNAc-1,6-GlcNAc from (GlcNAc)2 by transglycosylation. It is to date the only marine GH20 enzyme reported to produce GlcNAc-containing oligosaccharides [21]. Notably, P. piscicida belongs to the same bacterial order as P. hydrolytica. Hex99 has a unique substrate specificity, as it hydrolyzed only chitobiose and pNP(GlcNAc)2, but neither other chitooligosaccharides nor pNPGlcNAc.
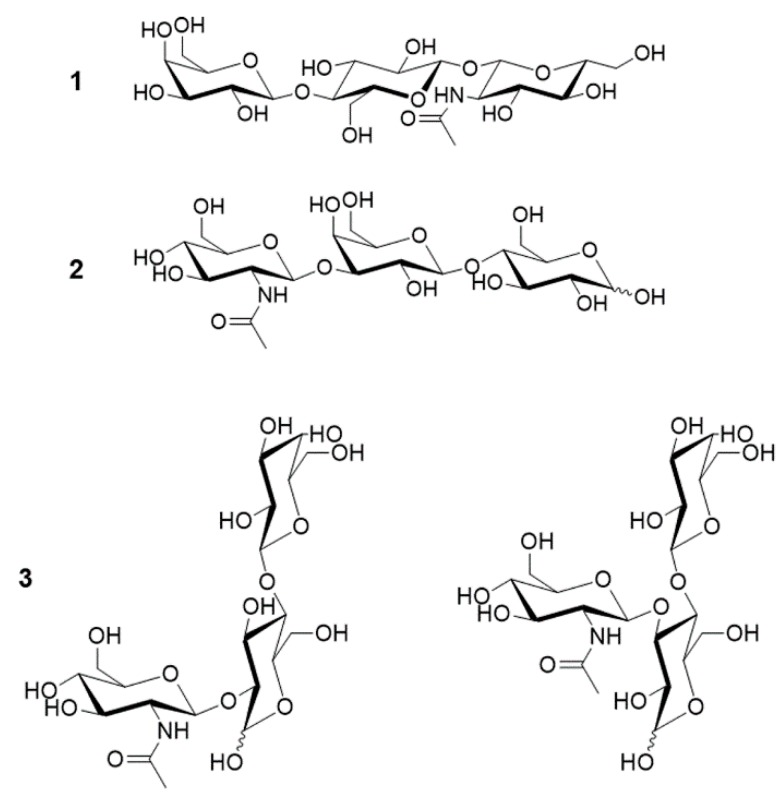
PhNah20A transglycosylated lactose with NAG-oxazoline as the donor (Figure 7A), resulting in three trisaccharides (Figure 8). 2, purified by gel permeation chromatography (GPC) (Figure S7) migrated similarly to LNT2 in thin-layer chromatography (TLC), and nuclear magnetic resonance (NMR) spectroscopy confirmed the product structure (Figure S9). 1 was determined to be a non-reducing trisaccharide, β-Gal-1,4-β-Glc-1,1-β-GlcNAc (Figure 8 and Figure S8; Tables S3 and S4), once reported as a transglycosylation product of a β-NAHA from Aspergillus flavofurcatis CCF 3061 [73]. For full NMR assignment as well as all measurable 3JH,H coupling constants of 1, see Tables S3 and S4. The 1,1-linkage was supported by heteronuclear multiple-bond correlation spectroscopy (HMBC) and rotating frame nuclear Overhauser effect spectroscopy (ROESY) correlations between the two anomeric positions as well as by lack of a reducing end. Lastly, the β-configuration was determined of the anomeric positions using the 3JH,H coupling constants between the anomeric proton and the neighboring proton (Table S4). A third trisaccharide (3) was detected, but not fully characterized due to low abundance. Based on chemical shifts of 3 (Figure S10), however, it seemed unlikely that the galactose moiety in lactose acted as an acceptor, as none of the corresponding chemical shifts were affected. Consequently, most probably the glucose moiety was the acceptor. As O6 was determined to be unsubstituted and glucose was the reducing end residue, therefore either β-Gal-(β-GlcNAc)-1,2-Glc or β-Gal-(β-GlcNAc)-1,3-Glc was produced (Figure S10).
Figure 8.
Structures of transglycosylation products determined by nuclear magnetic resonance (NMR). The three detected regioisomers are β-Gal-1,4-β-Glc-1,1-β-GlcNAc (1), β-GlcNAc-1,3-β-Gal-1,4-Glc, LNT2 (2) and β-Gal-1,4-(β-GlcNAc)-1,x-Glc (3), (x = 2, 3). Both possible structures of 3 are shown.
Several examples exist in Nature of β-Gal-(β-GlcNAc)-1,2-Glc and β-GlcNAc-1,3-Glc being part of polysaccharide backbones, such as the O-antigens (O-polysaccharides) of lipopolysaccharides from Gram-negative bacteria, i.e., Proteus sp., Hafnia alvei and Citrobacter werkmanii [74,75,76].
The overall transglycosylation yield for trisaccharides was estimated from the high-performance anion exchange chromatography with pulsed amperometric detector (HPAEC-PAD) chromatogram to 3.8% obtained with 200 mM acceptor and 100 mM donor. Since other trisaccharides were formed, no further optimization of transglycosylation conditions were pursued, even though LNT2 was the major product. Notably, the three trisaccharides were not completely separated by gel permeation chromatography (GPC) (Figure S7), but thin-layer chromatography (TLC) and HPAEC-PAD analysis (Figures S7 and S11) showed products consistent with trisaccharides 1 and 3 (Figure 8).
The acceptor specificity of PhNah20A was explored using d-galactose, d-glucose, 2-deoxy-d-glucose or l-fucose as an acceptor and NAG-oxazoline as a donor. These monosaccharides all proved to be transglycosylated (Figure 7B and Figure S12) with the similar velocity and transglycosylation products being detected in the most cases already after 0.03 h (2 min) incubation. Therefore, 2 h incubation was sufficient to assess the transglycosylation ability of PhNah20A (Figure 7 and Figure S12). PhNah20A thus showed unusual promiscuity towards acceptor molecules, but due to the low yields and formation of several products as seen by TLC (Figure 7, Figure S12), purification and NMR analysis were not pursued. Remarkably, however, the ability to transglycosylate a wide range of acceptors has very rarely been reported for GH20 enzymes [18] and perhaps is associated with the marine origin and the unique phylogenetic relation of PhNah20A. S. marcescens Chb (see Section 2.2) is able to transglycosylate several alcohols, albeit sugar alcohols were not effective acceptors [66]. Some bacterial and fungal β-NAHAs can use lactose as their acceptor [15,18,64], and two Hex enzymes from uncultured bacteria were reported to transfer GlcNAc to d-glucose, d-galactose, sucrose and maltose [18].
3. Materials and Methods
3.1. Materials
LNT2 was purchased from Elicityl Oligotech (Crolles, France). Lactose, pNPGalNAc and 5-bromo-4-chloro-3-indolyl N-acetyl-β-d-glucosaminide (X-GlcNAc) were from Carbosynth (San Diego, CA, USA), N,N′-diacetylchitobiose [(GlcNAc)2] from Omicron Biochemicals (South Bend, IN, USA), and N,N′,N″-triacetylchitotriose [(GlcNAc)3] and pNPGlcNAc from Megazyme (Bray, Co. Wicklow, Ireland). A mixture of chitooligosaccharides, (GlcNAc)1–6, was from Koyo Chemicals (Osaka, Japan). All other chemicals were purchased from Sigma-Aldrich (Merck, Darmstadt, Germany) and used without further purification. S. plicatus β-NAHA in fusion with maltose-binding protein was purchased from New England Biolabs (Ipswich, MA, USA).
3.2. Bacterial Strains and Media
Paraglaciecola hydrolytica (type strain S66T) [1,2] was grown at 23 °C in Difco Marine Broth 2216 (BD, Franklin Lakes, NJ, USA) or on Marine Broth supplemented with 15 g·L−1 agar. X-GlcNAc was added to the marine agar medium to 20 mg·L−1. Hydrolytic activity was assessed in 5 mL marine mineral medium [77] supplemented with chitooligosaccharides (5 g·L−1) or α-chitin (2 g·L−1) at 23 °C. E. coli DH5α was used for molecular cloning, E. coli BL21(DE3), BL21(DE3)ΔlacZ [78] and Rosetta (Novagen, Merck, Darmstadt, Germany) for gene expression and E. coli BL21(DE3) for recombinant protein production. E. coli was grown in Lysogeny Broth (LB; MoBio Laboratories, Carlsbad, CA, USA) or on LB agar plates at 37 °C. Media were supplemented with 100 mg·L−1 ampicillin for selection. Auto-induction medium ZYM-5052 was prepared as described [56]. Liquid cultures were aerated on a shaker (160 rpm).
3.3. Molecular Cloning and Plasmids
P. hydrolytica genomic DNA was purified using the Gentra Puregene Yeast/Bact kit B (Qiagen, Venlo, The Netherlands) and plasmid DNA was isolated using the GeneJET Plasmid Miniprep kit (Thermo Fisher Scientific, Waltham, MA, USA). DNA content was determined on NanoDrop Lite (Thermo Fisher Scientific, Waltham, MA, USA). Two putative P. hydrolytica β-NAHA-encoding genes were amplified from genomic DNA by Phusion high-fidelity polymerase (Thermo Fisher Scientific, Waltham, MA, USA) using specific primers (Table S5). Genes were cloned as full-length or truncated variants (see Figure S3) into the pURI3TEV vector by PCR cloning [79].
DNA sequencing (Eurofins Genomics, Ebersberg, Germany) verified that cloned sequences matched the sequences in the P. hydrolytica genome. Plasmids were transformed into E. coli DH5α or BL21(DE3) by electroporation.
3.4. Recombinant Protein Production and Purification
For initial expression analysis E. coli BL21(DE3) harboring PhNah20A in pURI3TEV grown in 20 mL LB medium at 37 °C until OD600nm ≈ 0.5 was induced by 0.5 mM isopropyl thio-β-d-galactoside (IPTG), and incubated at 22 °C. Aliquots (10 µL) were mixed at 0, 4, 20 h with 4 µL SDS-PAGE sample buffer, heated (10 min, 80 °C) to lyse cells and denature proteins, centrifuged (12,000× g, 1 min, RT) and analyzed on pre-cast SDS-polyacrylamide gels according to the manufacturers’ instructions (NuPAGE, Thermo Fisher Scientific, Waltham, MA, USA) in an XCell SureLock mini-cell electrophoresis system (Thermo Fisher Scientific, USA). Gels were stained by Coomassie Brilliant Blue G-250. Cell lysates were prepared from cell pellets after IPTG-induction by suspension in 0.4 mL 50 mM sodium phosphate pH 7.0, added 0.4 mL BugBuster protein extraction reagent (Merck, Darmstadt, Germany), approx. 100 U Benzonase nuclease (Merck, Darmstadt, Germany), and centrifuged (12,000× g, 20 min, 4 °C).
For enzyme preparation E. coli BL21(DE3) harboring PhNah20A in pURI3TEV was grown in 1 L LB medium at 37 °C to OD600nm ≈ 0.5, induced by 0.5 mM IPTG, and incubated (20 h, 22 °C). Cells collected by centrifugation (10,000× g, 15 min, 4 °C) were resuspended in 50 mL lysis buffer (50 mM sodium phosphate, pH 7.0, 0.3 M NaCl, 20 mM imidazole containing 250 U Benzonase nuclease), disrupted (Cell Pressure Homogenizer, Stansted, UK) and centrifuged to remove debris (25,000× g, 20 min, 4 °C). The supernatant was filtered (0.45 µm sterile polyvinylidene fluoride (PVDF) membrane filter, Millex-HV, Merck, Darmstadt, Germany) and PhNah20A purified by Ni2+-affinity chromatography (HisTrapHP, GE Healthcare, Uppsala, Sweden) followed by size-exclusion chromatography (HiLoad 16/60 Superdex 200 pg; ÄKTA Avant chromatography system, GE Healthcare, Uppsala, Sweden) in 50 mM sodium phosphate, pH 7.0, 0.3 M NaCl at a flow rate of 2 mL·min−1. Eluate was analyzed by SDS-PAGE and fractions containing PhNah20A were pooled, concentrated (Amicon ultra-15 30K centrifugal filter device, Merck, Darmstadt, Germany), and had added to them 0.02% Na-azide, and then were stored in the above-mentioned buffer at 4 °C. Protein concentration was determined by the Pierce Coomassie (Bradford) Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) for cell lysates and NanoDrop Lite (Themo Fisher Scientific, Waltham, MA, USA) for purified protein using the calculated ε280 = 136,835 M−1·cm−1 (ExPasy server; https://web.expasy.org/protparam/). After spectrophotometric determination of the concentration of PhNah20A, bovine serum albumin (BSA) was added to 0.5% of final concentration for storage.
3.5. Activity Assays
PhNah20A activity was routinely determined on 2 mM pNPGlcNAc at 37 °C in two-fold diluted McIlvaine buffer pH 6.0 (63 mM Na2HPO4; 18 mM citric acid), containing 0.05% BSA. The reaction (total volume 500 µL) was performed in McIlvaine buffer, pH 6.0 (250 µl), 100 µl milliQ water and 100 µL of substrate was added. The reaction was initiated by adding 50 µL of PhNah20A (prepared immediately before use in McIlvaine buffer, pH 6.0, 0.5% BSA, and kept on ice) to the reaction mixture yielding a final concentration of 0.3–5 µg·mL−1. The reaction was stopped typically after 2–5 min by 250 µL 1 M Na2CO3 and the product was measured spectrophotometrically at 400 nm (Ultrospec 3100 pro UV/Visible spectrophotometer, GE Healthcare, Uppsala, Sweden) using pNP (ε400 = 18,000 M−1·cm−1) as the standard. One U of activity was defined as the amount of enzyme releasing 1 µmol pNP per min from 2 mM pNPGlcNAc. pH activity optimum was determined for PhNah20A in McIlvaine buffers (pH 4.0–8.0) at 37 °C towards 2 mM pNPGlcNAc and the temperature optimum was determined from the initial rates of pNP release at temperatures in the range 10–65 °C at pH 6.0.
To determine the hydrolysis by PhNah20A 200 mM (GlcNAc)2 was incubated with 1 U·mL−1 (11.6 µg·mL−1) or 5 mM LNT2 with 10 U·mL−1 (116 µg·mL−1) in 50 mM sodium phosphate, pH 6.0, 0.5% BSA, at 37 °C for 20 h. The release of GlcNAc was monitored by high-performance anion exchange chromatography with pulsed amperometric detector (HPAEC-PAD) for (GlcNAc)2 and by thin-layer chromatography (TLC) for LNT2 (see Section 3.9).
3.6. Kinetics
PhNah20A (final concentration 0.3–1.2 µg·mL−1) was added to initiate the hydrolysis of 0.05–2 mM pNPGlcNAc (six concentrations) and 0.1–2 mM pNPGalNAc (five concentrations) in 500 μL two-fold diluted McIlvaine buffer pH 6.0, 0.05% BSA at 37 °C. The reaction was stopped at suitable time points by the addition of 250 μL 1 M Na2CO3 and quantified spectrophotometrically as above. Initial rates calculated from pNP formation versus time were plotted against substrate concentration and fitted to the Michaelis-Menten equation using OriginPro 2015 (OriginLab, Northampton, MA, USA) to obtain kcat and KM. The kcat/KM values were either calculated or determined from rates of hydrolysis at low substrate concentration.
3.7. Synthesis of NAG-Oxazoline
NAG-oxazoline [2-methyl-(1,2-dideoxy-α-d-glucopyrano)-oxazoline] was synthesized and purified as described previously [71]. Briefly, 2 g GlcNAc (9 mmol) was dissolved in 20 mL acetic anhydride, then we added 10 mL pyridine and stirred overnight at room temperature (RT). After extraction by dichloromethane (DCM) and successive washings (Na2CO3, H2O, H2SO4, H2O) the organic layer was dried and evaporated. Trimethylsilyl trifluoromethanesulfonate (0.8 mL) was added to 1.5 g peracetylated glucosamine dissolved in 1,2-dichloroethane and stirred at 50 °C until completion of the reaction (about 4 h). Trimethylamine was added (2 mL) followed by 50 mL DCM, washed with cold water, dried and evaporated. The product was purified by flash chromatography (cyclohexane: 1% triethylamine in ethyl acetate 100:0 to 40:60). Peracetylated oxazole (300 mg) in 10 mL anhydrous methanol at 0 °C was added 15 μL 5.3 M sodium methanolate in methanol and stirred at RT until the reaction was completed (about 3 h). The resulting NAG-oxazoline was dried and used without further purification.
3.8. Transglycosylation
Reaction mixtures for transglycosylation contained either 100 mM NAG-oxazoline (from 1 M stock in 50 mM sodium borate, pH 9.3) or 100 mM (GlcNAc)2 donor, and as acceptor 200 mM lactose, d-galactose, d-glucose, 2-deoxy-d-glucose or l-fucose; 1 or 10 U·mL−1 (11.6 or 116 µg·mL−1) PhNah20A or 10 U·mL−1 S. plicatus β-NAHA in 50 mM sodium phosphate, pH 8.0, 0.5% BSA, at 37 °C. The reaction volume was typically 20 µL for TLC analysis and 250 µL for product yield and structure determination. Slightly basic conditions were required as NAG-oxazoline is not stable at neutral or acidic pH [71]. Reactions were stopped at various time points by heating (5 min, 90 °C), cooled to RT and centrifuged (12,000× g, 1 min, 4 °C). Samples were diluted four- and 150-fold in milliQ water for TLC and HPAEC-PAD (see Section 3.9), respectively. For the reaction mixtures for the analysis of transglycosylation products after gel permeation chromatography (GPC), containing 10 U·mL−1 PhNah20A, 100 mM NAG-oxazoline and 200 mM lactose in 50 mM sodium phosphate pH 8.0, 0.5% BSA were incubated 2 h at 37 °C followed by heating (5 min, 90 °C). To the sample was added three volumes of sterile milliQ water, and the enzyme was removed (Amicon Ultra 0.5 mL centrifugal device, Mw cut-off 30 kDa; Merck, Darmstadt, Germany) followed by filtration (0.45 µm filters; Millex-HV, Merck, Darmstadt, Germany) prior to GPC.
3.9. Chromatographic Methods
Reaction mixtures containing 15–30 µg carbohydrate were spotted onto TLC plates (Silica Gel 60 F254 plates; Merck, Darmstadt, Germany) developed twice in chloroform:acetic acid:water (6:7:1; v:v:v) [80,81] or n-butanol: ethanol: water (5:3:2; v:v:v) [82]. Carbohydrates were visualized with orcinol (0.5% 5-methyl resorcinol and 10% H2SO4 in ethanol) or aniline dye (1.2% aniline hydrochloride and 1.2% diphenylamine in acidic methanol).
Oligosaccharides were also separated by HPAEC-PAD at 22 °C (Dionex CarboPac PA1 column, 250 × 4 mm with 50 × 4 mm Guard, Thermo Fisher Scientific, Waltham, MA, USA) using an ICS-5000 system (Thermo Fisher Scientific, Waltham, MA, USA) equipped with AS autosampler and pulsed amperometric detector (carbohydrate four-potential waveform, sampling rate 2 Hz) with a gold electrode (Au) and an Ag/AgCl reference electrode.
The elution was done with (A) water; (B) 1 M NaOH; (C) 200 mM NaOH + 800 mM NaOAc isocratically using 7.5% B in A (25 min) followed by 100% C (1 min) and column re-equilibration (9 min) at 7.5% B in A at 1.0 mL·min−1. Oligosaccharides in water (10 µL) containing 9 µM l-fucose as standard were injected by autosampler kept at 5 °C. LNT2, glucose, galactose, lactose, GlcNAc, (GlcNAc)2 and chitooligosaccharides were used as standards for calibration. Reaction mixtures (0.5 mL) containing approximately 10 mg oligosaccharides were separated by GPC (Bio-Gel P-2, Bio-Rad Laboratories, Hercules, CA, USA; 16 × 900 mm XK16/100 mounted on an ÄKTAprime plus chromatography system, GE Healthcare, Sweden), eluted by degassed milliQ water at flow rate of 0.1 mL·min−1 at RT and pressure limit set to 0.3 MPa. Reducing sugar in collected fractions (2 mL) were quantified by the Nelson-Somogyi method [83] using glucose and GlcNAc as standards. Fractions containing trisaccharides were dried (SpeedVac, Thermo Fisher Scientific, Waltham, MA, USA) at 50 °C, dissolved in 50 µL milliQ water and subjected to TLC for the preliminary identification of transglycosylation products. For NMR analysis, identical trisaccharide-containing fractions from two GPC runs were pooled, dried (SpeedVac) and dissolved in 0.5 mL D2O (Sigma-Aldrich, USA). Each fraction contained a major component and trace amounts of one or two other products.
3.10. Nuclear Magnetic Resonance (NMR)
All NMR spectra were recorded on an 800 MHz Bruker Avance III (799.75 MHz for 1H and 201.10 MHz for 13C) equipped with a 5 mm TCI cryoprobe. Acetone was used as internal reference (2.22 ppm and 30.89 ppm for 1H and 13C, respectively). The following experiments were used for the structure elucidation: 1H with presaturation, double quantum filtered correlation spectroscopy (DQF-COSY), rotating frame nuclear Overhauser effect spectroscopy (ROESY), heteronuclear single-quantum correlation spectroscopy (HSQC), heteronuclear single-quantum correlation spectroscopy-total correlation spectroscopy (HSQC-TOCSY) and heteronuclear multiple-bond correlation spectroscopy (HMBC) all performed using standard Bruker pulse sequences. LNT2 and lactose were used as reference compounds. Structural elucidation was carried out by first identifying all 1H and corresponding 13C chemical shifts using 1H with presaturation and HSQC. Subsequently, the different signals belonging to each position in each monosaccharide were determined, primarily using DQF-COSY and HSQC-TOCSY, and finally the connections between the monosaccharides were determined using HMBC and ROESY, as well as comparing chemical shifts to reference compounds.
3.11. In Silico Methods
The draft genome sequence of P. hydrolytica S66T [1] annotated on the RAST server (http://rast.nmpdr.org/) [84] was mined on 20 March 2016, to identify putative β-NAHAs. Visualization of the RAST-annotated proteins was done on the SEED Viewer v 2.0 (www.theSEED.org).
Protein sequences of characterized β-NAHAs were retrieved from UniprotKB (https://www.uniprot.org/) on 10 February 2019. Nucleotide BLAST and protein BLAST tools (https://blast.ncbi.nlm.nih.gov/Blast.cgi) were used in 10 February 2019 and 3 January 2020 for identity analysis of nucleotide and protein sequences, respectively. Multiple sequence alignments were carried out with Clustal Omega v 2.1 (https://www.ebi.ac.uk/Tools/msa/clustalo/) and visualized by BioEdit v 7.0.5.3 (https://www.softpedia.com/get/Science-CAD/BioEdit.shtml).
The phylogenetic tree was constructed and visualized using MEGA v 7.0.26 (https://megasoftware.net/) [55].
N-terminal signal peptide prediction was done by SignalP v 4.1 with sensitive default cut-off values (http://www.cbs.dtu.dk/services/SignalP/) [85]. Promoter locations were predicted by SoftBerry tool BPROM (http://www.softberry.com/berry.phtml?topic=bprom&group=programs&subgroup=gfindb).
4. Conclusions
The genome of the marine bacterium P. hydrolytica S66T encodes two putative GH20 β-N-acetylhexosaminidase (EC 3.2.1.52) having protein sequences that differed remarkably from earlier characterized β-NAHAs (≤30% identity). PhNah20A was positioned on a phylogenetic tree between β-NAHAs of water-associated bacteria, i.e., Vibrio furnissii and Aeromonas hydrophila, and unicellular eukaryotes (amobae). PhNah20A, produced in E. coli, was unstable if diluted, but was stabilized by BSA or Triton X-100. PhNah20A is a genuine β-NAHA with essentially the same catalytic efficiency for pNPGlcNAc and pNPGalNAc, and thus differs from most of the previously studied bacterial β-NAHAs, which prefer pNPGlcNAc as a substrate while some eukaryotic GH20 prefer pNPGalNAc. PhNah20A also hydrolyzed LNT2, a core structure of human milk oligosaccharides, and showed biosynthetic activity (transglycosylation) which is a poorly studied aspect of GH20 β-NAHAs, especially from eukaryotes and water-living prokaryotes. PhNah20A was able to form LTN2 by transglycosylation using NAG-oxazoline as a donor and lactose as an acceptor, LNT2, β-Gal-1,4-β-Glc-1,1-β-GlcNAc and β-Gal-1,4-(β-GlcNAc)-1,2/3-Glc being identified by NMR as main transglycosylation products. Several monosaccharides were also recognized as acceptors by PhNah20A. To date, based on pH and temperature optima, kinetic parameters or stability characteristics alone, no clear distinction can be made between eukaryotic versus prokaryotic or terrestrial versus aquatic GH20 β-NAHAs. However, this may be due to the very limited number of characterized β-NAHAs of salt or fresh water origin. PhNah20A is the first characterized member of a distinct group of GH20 β-NAHAs located phylogenetically between eukaryotic and prokaryotic enzymes.
Acknowledgments
Karina Jansen (Technical University of Denmark) is thanked for general technical assistance, Pernille K. Bech and Mikkel Schultz-Johansen (University of Copenhagen) for providing the P. hydrolytica strain, Corinna Schiano di Cola for preparing autoinduction medium and Tiina Alamäe (University of Tartu) for fruitful discussions.
Abbreviations
| BSA | bovine serum albumin |
| DCM | dichloromethane |
| GH | glycoside hydrolase |
| GlcNAc | N-acetylglucosamine |
| (GlcNAc)2 | N,N’-diacetylchitobiose, chitobiose |
| GPC | gel permeation chromatography |
| HMOs | human milk oligosaccharides |
| HPAEC-PAD | high-performance anion exchange chromatography with pulsed amperometric detector |
| IPTG | isopropyl thio-β-d-galactoside |
| LNT2 | lacto-N-triose II |
| NAG-oxazoline | 2-methyl-(1,2-dideoxy-α-d-glucopyrano)-oxazoline |
| β-NAHA | β-N-acetylhexosaminidases |
| NCBI | National Center for Biotechnology Information |
| NMR | nuclear magnetic resonance |
| pNPGlcNAc | p-nitrophenyl-GlcNAc |
| pNPGalNAc | p-nitrophenyl-GalNAc |
| TLC | thin layer chromatography |
| X-GlcNAc | 5-bromo-4-chloro-3-indolyl N-acetyl-β-d-glucosaminide |
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/2/417/s1. The following materials are available online: Supplementary Information 1 containing Figure S1. TLC analysis of growth media of P. hydrolytica (A, B, C) and growth phenotype on marine agar medium with X-GlcNAc (5-bromo-4-chloro-3-indolyl N-acetyl-β-d-glucosaminide) (D); Figure S2. Phylogenetic tree with bootstrap test (1000 replicates) of PhNah20A, PhNah20B (both marked with red circles) and 41 biochemically characterised GH20 (EC 3.2.1.52) enzymes; Figure S3. Schematic domain architecture of full-length and truncated variants of PhNah20A and PhNah20B; Figure S4. IPTG-induced E. coli transformants growing in LB analysed by SDS-PAGE; Figure S5. Inactivation of 5 µg·mL−1 PhNah20A at 50 °C and pH 6.0 in the presence of 0.5% BSA; Figure S6. Time course of transglycosylation by S. plicatus Hex (10 U·mL−1) with 100 mM NAG-oxazoline as donor and 200 mM lactose as acceptor; Figure S7. TLC of trisaccharide-containing fractions of the PhNah20A reaction (2 h; Figure 7A) separated by gel-permeation chromatography; Figure S8. HSCQ spectrum of the chromatographic fraction 50 (see Figure S7) containing over 80% of compound 1; Figure S9. HSCQ spectrum of the chromatographic fraction 51 (see Figure S7) containing over 75% of 2 (LNT2); Figure S10. HSCQ spectrum of the chromatographic fraction 53 (see Figure S7) containing primarily 3; Figure S11. Extraction of HPAEC-PAD analysis of transglycosylation products by PhNah20A (10 U·mL−1) reacting 2 h with 100 mM NAG-oxazoline as donor and 200 mM lactose as acceptor (blue line); Figure S12. Time course of transglycosylation by PhNah20A (10 U·mL−1) with 100 mM NAG-oxazoline as donor and 200 mM D-glucose (A), 2-deoxy-d-glucose (B) or L-fucose (C) as acceptor; Table S1. BLAST analysis of putative β-NAHAs (EC 3.2.1.52) from P. hydrolytica. Table S2. Information on proteins flanking identified β-NAHAs (presented in Figure 1B); Table S3. NMR assignment of 1. The methyl of the GlcNAc acetyl group was at 2.090 ppm for 1H and 22.81 ppm for 13C and the carbonyl of the acetyl was at 176.06 ppm 13C; Table S4. 3H-H coupling constants for 1 measured through DQF-COSY; Table S5. PCR primers to isolate full-length β-NAHA encoding genes and indicated truncated variants. Underlined sequences are priming with pURI3-TEV expression vector; Supplementary Information 2 containing multiple sequence alignment.
Author Contributions
Conceptualization, P.S., T.V. and B.S.; methodology, T.V., C.K., A.L. and L.H.P.; validation, T.V., D.T., C.K. and L.H.P.; formal analysis, T.V., C.K., A.L. and D.T.; investigation, T.V., C.K., D.T., A.L. and L.H.P.; resources, B.S., J.Ø.D., L.H.P., C.A.-M., D.T. and P.S.; data curation, T.V., B.S.; writing—original draft preparation, T.V., D.T., C.K., P.S. and B.S.; writing—review and editing, T.V., D.T., C.K., P.S. and B.S.; visualization, T.V. and D.T.; supervision, P.S., J.Ø.D., L.H.P. and B.S.; project administration, P.S., T.V., B.S.; funding acquisition, P.S., D.T., T.V. and B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Innovation Fund Denmark to the project “OliGram. Design and gram scale enzymatic synthesis of human milk oligosaccharides”, grant number 1308-00014B having P.S. as PI. D.T. is grateful to the Novo Nordisk Foundation for a postdoctoral fellowship (grant NNF17OC0025660). The APC was funded by University of Tartu Feasibility Fund grant PLTMRARENG13 to T.V. and the NNF17OC0025660 grant to D.T.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyzes, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Schultz-Johansen M., Glaring M.A., Bech P.K., Stougaard P. Draft genome sequence of a novel marine bacterium, Paraglaciecola sp. strain S66, with hydrolytic activity against seaweed. Genome Announc. 2016;4:e00304-16. doi: 10.1128/genomeA.00304-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bech P.K., Schultz-Johansen M., Glaring M.A., Barbeyron T., Czjzek M., Stougaard P. Paraglaciecola hydrolytica sp. nov., a bacterium with hydrolytic activity against multiple seaweed-derived polysaccharides. Int. J. Syst. Evol. Microbiol. 2017;67:2242–2247. doi: 10.1099/ijsem.0.001933. [DOI] [PubMed] [Google Scholar]
- 3.Schultz-Johansen M., Bech P., Hennessy R., Glaring M., Barbeyron T., Czjzek M., Stougaard P. A novel enzyme portfolio for red algal polysaccharide degradation in the marine bacterium Paraglaciecola hydrolytica S66T encoded in a sizeable polysaccharide. Front. Microbiol. 2018;9:839. doi: 10.3389/fmicb.2018.00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lombard V., Golaconda Ramulu H., Drula E., Coutinho P.M., Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:490–495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slámová K., Bojarová P. Engineered N-acetylhexosamine-active enzymes in glycoscience. Biochim. Biophys. Acta. 2017;1861:2070–2087. doi: 10.1016/j.bbagen.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Bode L. The functional biology of human milk oligosaccharides. Early Hum. Dev. 2015;91:619–622. doi: 10.1016/j.earlhumdev.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Kunz C., Kuntz S., Rudloff S. Bioactivity of human milk oligosaccharides. In: Moreno J.F., Sanz M.L., editors. Food Oligosaccharides: Production, Analysis and Bioactivity. John Wiley & Sons Ltd.; Hoboken, NJ, USA: 2014. pp. 1–20. [Google Scholar]
- 8.Barile D., Rastall R.A. Human milk and related oligosaccharides as prebiotics. Curr. Opin. Biotechnol. 2013;24:214–219. doi: 10.1016/j.copbio.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Verspreet J., Damen B., Broekaert W.F., Verbeke K., Delcour J.A., Courtin C.M. A critical look at prebiotics within the dietary fiber concept. Annu. Rev. Food Sci. Technol. 2016;7:167–190. doi: 10.1146/annurev-food-081315-032749. [DOI] [PubMed] [Google Scholar]
- 10.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 11.Boltje T.J., Buskas T., Boons G.J. Opportunities and challenges in synthetic oligosaccharide and glycoconjugate research. Nat. Chem. 2009;1:611–622. doi: 10.1038/nchem.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeuner B., Teze D., Muschiol J., Meyer A.S. Synthesis of human milk oligosaccharides: Protein engineering strategies for improved enzymatic transglycosylation. Molecules. 2019;24:2033. doi: 10.3390/molecules24112033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeuner B., Jers C., Mikkelsen J.D., Meyer A.S. Methods for improving enzymatic trans-glycosylation for synthesis of human milk oligosaccharide biomimetics. J. Agric. Food Chem. 2014;62:9615–9631. doi: 10.1021/jf502619p. [DOI] [PubMed] [Google Scholar]
- 14.Jamek S.B., Nyffenegger C., Muschiol J., Holck J., Meyer A.S., Mikkelsen J.D. Characterization of two novel bacterial type A exo-chitobiose hydrolases having C-terminal 5/12-type carbohydrate-binding modules. Appl. Microbiol. Biotechnol. 2017;101:4533–4546. doi: 10.1007/s00253-017-8198-4. [DOI] [PubMed] [Google Scholar]
- 15.Chen X., Xu L., Jin L., Sun B., Gu G., Lu L., Xiao M. Efficient and regioselective synthesis of β-GalNAc/GlcNAc-lactose by a bifunctional transglycosylating β-N-acetylhexosaminidase from Bifidobacterium bifidum. Appl. Environ. Microbiol. 2016;82:5642–5652. doi: 10.1128/AEM.01325-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumgärtner F., Conrad J., Sprenger G.A., Albermann C. Synthesis of the human milk oligosaccharide lacto-N-tetraose in metabolically engineered, plasmid-free E. coli. ChemBioChem. 2014;15:1896–1900. doi: 10.1002/cbic.201402070. [DOI] [PubMed] [Google Scholar]
- 17.Wang M., Li M., Wu S., Lebrilla C.B., Chapkin R.S., Ivanov I., Donovan S.M. Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed. J. Pediatr. Gastroenterol. Nutr. 2015;60:825–833. doi: 10.1097/MPG.0000000000000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyffenegger C., Nordvang R.T., Zeuner B., Łężyk M., Difilippo E., Logtenberg M.J., Schols H.A., Meyer A.S., Mikkelsen J.D. Backbone structures in human milk oligosaccharides: Trans-glycosylation by metagenomic β-N-acetylhexosaminidases. Appl. Microbiol. Biotechnol. 2015;99:7997–8009. doi: 10.1007/s00253-015-6550-0. [DOI] [PubMed] [Google Scholar]
- 19.Murata T., Tashiro A., Itoh T., Usui T. Enzymic synthesis of 3′-O- and 6′-O-N-acetylglucosaminyl-N-acetyllactosaminide glycosides catalyzed by β-N-acetyl-d-hexosaminidase from Nocardia orientalis. Biochim. Biophys. Acta. 1997;1335:326–334. doi: 10.1016/S0304-4165(96)00152-3. [DOI] [PubMed] [Google Scholar]
- 20.Slámová K., Gažák R., Bojarová P., Kulik N., Ettrich R., Pelantová H., Sedmera P., Křen V. 4-deoxy-substrates for β-N-acetylhexosaminidases: How to make use of their loose specificity. Glycobiology. 2010;20:1002–1009. doi: 10.1093/glycob/cwq058. [DOI] [PubMed] [Google Scholar]
- 21.Tsujibo H., Kondo N., Tanaka K., Miyamoto K., Baba N., Inamori Y. Molecular analysis of the gene encoding a novel transglycosylative enzyme from Alteromonas sp. strain O-7 and its physiological role in the chitinolytic system. J. Bacteriol. 1999;181:5461–5466. doi: 10.1128/JB.181.17.5461-5466.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakshmanan T., Loganathan D. Enzymatic synthesis of N-glycoprotein linkage region disaccharide mimetics using β-N-acetylhexosaminidases from Aspergillus oryzae and Vigna radiata. Tetrahedron Asymmetry. 2005;16:255–260. doi: 10.1016/j.tetasy.2004.11.016. [DOI] [Google Scholar]
- 23.Beier S., Bertilsson S. Bacterial chitin degradation-mechanisms and ecophysiological strategies. Front. Microbiol. 2013;4:149. doi: 10.3389/fmicb.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slámová K., Bojarová P., Petrásková L., Křen V. β-N-Acetylhexosaminidase: What’s in a name...? Biotechnol. Adv. 2010;28:682–693. doi: 10.1016/j.biotechadv.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Zhang R., Zhou J., Song Z., Huang Z. Enzymatic properties of beta-N-acetylglucosaminidases. Appl. Microbiol. Biotechnol. 2018;102:93–103. doi: 10.1007/s00253-017-8624-7. [DOI] [PubMed] [Google Scholar]
- 26.Choi K.H., Seo J.Y., Park K.M., Park C.S., Cha J. Characterization of glycosyl hydrolase family 3β-N-acetylglucosaminidases from Thermotoga maritima and Thermotoga neapolitana. J. Biosci. Bioeng. 2009;108:455–459. doi: 10.1016/j.jbiosc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Ryšlavá H., Kalendová A., Doubnerová V., Skočdopol P., Kumar V., Kukačka Z., Pompach P., Vaněk O., Slámová K., Bojarová P., et al. Enzymatic characterization and molecular modeling of an evolutionarily interesting fungal β-N-acetylhexosaminidase. FEBS J. 2011;278:2469–2484. doi: 10.1111/j.1742-4658.2011.08173.x. [DOI] [PubMed] [Google Scholar]
- 28.Piszkiewicz D., Bruice T.C., Glycoside H., III Intramolecular acetamido group participation in the specific acid catalyzed hydrolysis of methyl 2-acetamido-2-deoxy-β-d-glucopyranoside. J. Am. Chem. Soc. 1968;378:5844–5848. doi: 10.1021/ja01023a032. [DOI] [Google Scholar]
- 29.Knapp S., Vocadlo D., Gao Z., Kirk B., Lou J., Withers S.G. NAG-thiazoline, an N-acetyl-β-hexosaminidase inhibitor that implicates acetamido participation. J. Am. Chem. Soc. 1996;118:6804–6805. doi: 10.1021/ja960826u. [DOI] [Google Scholar]
- 30.Ferrara M.C., Cobucci-Ponzano B., Carpentieri A., Henrissat B., Rossi M., Amoresano A., Moracci M. The identification and molecular characterization of the first archaeal bifunctional exo-β-glucosidase/N-acetyl-β-glucosaminidase demonstrate that family GH116 is made of three functionally distinct subfamilies. Biochim. Biophys. Acta. 2014;1840:367–377. doi: 10.1016/j.bbagen.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Teze D., Shuoker B., Chaberski E.K., Kunstmann R.S., Fredslund F., Peters G.H.J., Karlsson E.N., Welner D.H., Abou Hachem M. The catalytic acid-base in GH109 resides in a conserved GGHGG loop and allows for comparable α-retaining and β-inverting activity in an N-acetylgalactosaminidase from Akkermansia muciniphila. ChemRxiv. 2019:preprint. [Google Scholar]
- 32.Placzek S., Schomburg I., Chang A., Jeske L., Ulbrich M., Tillack J., Schomburg D. BRENDA in 2017: New perspectives and new tools in BRENDA. Nucleic Acids Res. 2017;45:D380–D388. doi: 10.1093/nar/gkw952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keyhani N.O., Roseman S. The chitin catabolic cascade in the marine bacterium Vibrio furnissii. J. Biol. Chem. 1996;271:33425–33432. doi: 10.1074/jbc.271.52.33425. [DOI] [PubMed] [Google Scholar]
- 34.Lan X., Zhang X., Kodaira R., Zhou Z., Shimosaka M. Gene cloning, expression, and characterization of a second β-N-acetylglucosaminidase from the chitinolytic bacterium Aeromonas hydrophila strain SUWA-9. Biosci. Biotechnol. Biochem. 2008;72:492–498. doi: 10.1271/bbb.70573. [DOI] [PubMed] [Google Scholar]
- 35.Tsujibo H., Miyamoto K., Yoshimura M., Takata M., Miyamoto J., Inamori Y. Molecular cloning of the gene encoding a novel β-N-acetylhexosaminidase from a marine bacterium, Alteromonas sp. strain O-7, and characterization of the cloned enzyme. Biosci. Biotechnol. Biochem. 2002;66:471–475. doi: 10.1271/bbb.66.471. [DOI] [PubMed] [Google Scholar]
- 36.Lan X., Ozawa N., Nishiwaki N., Kodaira R., Okazaki M., Shimosaka M. Purification, cloning, and sequence analysis of β-N-acetylglucosaminidase from the chitinolytic bacterium Aeromonas hydrophila strain SUWA-9. Biosci. Biotechnol. Biochem. 2004;68:1082–1090. doi: 10.1271/bbb.68.1082. [DOI] [PubMed] [Google Scholar]
- 37.Soto-Gil R.W., Zyskind J.W. N,N’-Diacetylchitobiase of Vibrio harveyi. Primary structure, processing, and evolutionary relationships. J. Biol. Chem. 1989;264:14778–14783. [PubMed] [Google Scholar]
- 38.Mark B.L., Wasney G.A., Salo T.J., Khan A.R., Cao Z., Robbins P.W., James M.N., Triggs-Raine B.L. Structural and functional characterization of Streptomyces plicatus beta-N-acetylhexosaminidase by comparative molecular modeling and site-directed mutagenesis. J. Biol. Chem. 1998;273:19618–19624. doi: 10.1074/jbc.273.31.19618. [DOI] [PubMed] [Google Scholar]
- 39.Mayer C., Vocadlo D.J., Mah M., Rupitz K., Stoll D., Warren R.A.J., Withers S.G. Characterization of a β-N-acetylhexosaminidase and a β-N-acetylglucosaminidase/β-glucosidase from Cellulomonas fimi. FEBS J. 2006;273:2929–2941. doi: 10.1111/j.1742-4658.2006.05308.x. [DOI] [PubMed] [Google Scholar]
- 40.Miwa M., Horimoto T., Kiyohara M., Katayama T., Kitaoka M., Ashida H., Yamamoto K. Cooperation of β-galactosidase and β-N-acetylhexosaminidase from bifidobacteria in assimilation of human milk oligosaccharides with type 2 structure. Glycobiology. 2010;20:1402–1409. doi: 10.1093/glycob/cwq101. [DOI] [PubMed] [Google Scholar]
- 41.Gutternigg M., Rendić D., Voglauer R., Iskratsch T., Wilson I.B.H. Mammalian cells contain a second nucleocytoplasmic hexosaminidase. Biochem. J. 2009;419:83–90. doi: 10.1042/BJ20081630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verpoorte J.A. Isolation and characterization of the major beta-N-acetyl-d-glucosaminidase from human plasma. Biochemistry. 1974;13:793–799. doi: 10.1021/bi00701a023. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Alonso J., Reglero A., Cabezas J.A. Purification and properties of β-N-acetylhexosaminidase a from pig brain. Int. J. Biochem. 1990;22:645–651. doi: 10.1016/0020-711X(90)90043-3. [DOI] [PubMed] [Google Scholar]
- 44.Zhou J., Song Z., Zhang R., Liu R., Wu Q., Li J., Tang X., Xu B., Ding J., Han N., et al. Distinctive molecular and biochemical characteristics of a glycoside hydrolase family 20 β-N-acetylglucosaminidase and salt tolerance. BMC Biotechnol. 2017;17:37. doi: 10.1186/s12896-017-0358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sumida T., Ishii R., Yanagisawa T., Yokoyama S., Ito M. Molecular coning and crystal structural analysis of a novel β-N-acetylhexosaminidase from Paenibacillus sp. TS12 capable of degrading glycosphingolipids. J. Mol. Biol. 2009;392:87–99. doi: 10.1016/j.jmb.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 46.Jiang Y.-L., Yu W.-L., Zhang J.-W., Frolet C., Di Guilmi A.-M., Zhou C.-Z., Vernet T., Chen Y. Structural basis for the substrate specificity of a novel β-N-acetylhexosaminidase StrH protein from Streptococcus pneumoniae R6. J. Biol. Chem. 2011;286:43004–43012. doi: 10.1074/jbc.M111.256578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thi N.N., Offen W.A., Shareck F., Davies G.J., Doucet N. Structure and activity of the Streptomyces coelicolor A3(2) β-N-acetylhexosaminidase provides further insight into GH20 family catalysis and inhibition. Biochemistry. 2014;53:1789–1800. doi: 10.1021/bi401697j. [DOI] [PubMed] [Google Scholar]
- 48.Chen X., Wang J., Liu M., Yang W., Wang Y., Tang R., Zhang M. Crystallographic evidence for substrate-assisted catalysis of β-N-acetylhexosaminidas from Akkermansia muciniphila. Biochem. Biophys. Res. Commun. 2019;511:833–839. doi: 10.1016/j.bbrc.2019.02.074. [DOI] [PubMed] [Google Scholar]
- 49.Lenart A., Dudkiewicz M., Grynberg M., Pawłowski K. CLCAs—A family of metalloproteases of intriguing phylogenetic distribution and with cases of substituted catalytic sites. PLoS ONE. 2013;8:e62272. doi: 10.1371/journal.pone.0062272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Val-Cid C., Biarnés X., Faijes M., Planas A. Structural-functional analysis reveals a specific domain organization in family GH20 hexosaminidases. PLoS ONE. 2015;10:e0128075. doi: 10.1371/journal.pone.0128075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tews I., Perrakis A., Wilson K.S., Oppenheim A., Dauter Z., Vorgias C.E., Tews I. Bacterial chitobiase structure provides insight into catalytic mechanism and the basis of Tay–Sachs disease. Nat. Struct. Biol. 1996;3:638–648. doi: 10.1038/nsb0796-638. [DOI] [PubMed] [Google Scholar]
- 52.Verma S.C., Mahadevan S. The ChbG gene of the chitobiose (chb) operon of Escherichia coli encodes a chitooligosaccharide deacetylase. J. Bacteriol. 2012;194:4959–4971. doi: 10.1128/JB.00533-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang W., Xi H., Bi Q., Hu Y., Zhang Y., Ni M. Cloning, expression and characterization of D-aminoacylase from Achromobacter xylosoxidans subsp. denitrificans ATCC 15173. Microbiol. Res. 2013;168:360–366. doi: 10.1016/j.micres.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Graham T.R., Zassenhaus H.P., Kaplan A. Molecular cloning of the cDNA which encodes β-N-acetylhexosaminidase A from Dictyostelium discoideum. Complete amino acid sequence and homology with the human sequence. J. Biol. Chem. 1988;263:16823–16829. [PubMed] [Google Scholar]
- 55.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Studier F.W. Protein production by auto-induction in high-density shaking cultures. Protein Expr. Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 57.Yem D.W., Wu H.C. Purification and properties of beta-N-acetylglucosaminidase from Escherichia coli. J. Bacteriol. 1976;125:324–331. doi: 10.1128/JB.125.1.324-331.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poulton J.E., Thomas M.A., Ottwell K.K., McCormick S.J. Partial purification and characterization of a β-N-acetylhexosaminidase from black cherry (Prunus serotina EHRH.) seeds. Plant Sci. 1985;42:107–114. doi: 10.1016/0168-9452(85)90150-5. [DOI] [Google Scholar]
- 59.Vehpoorte J.A. Purification of two β-N-acetyl-d-glucosaminidases from beef spleen. J. Biol. Chem. 1972;247:4787–4793. [PubMed] [Google Scholar]
- 60.Mitchell E.D., Houston W.C., Latimer S.B. Purification and properties of a β-N-acetylaminoglucohydrolase from malted barley. Phytochemistry. 1976;15:1869–1871. doi: 10.1016/S0031-9422(00)88833-0. [DOI] [Google Scholar]
- 61.Trimble R.B., Evans G., Maley F. Purification and properties of endo-beta-N-acetylglucosaminidase L from Streptomyces plicatus. J. Biol. Chem. 1979;254:9708–9713. [PubMed] [Google Scholar]
- 62.Lisboa De Marco J., Valadares-Inglis M.C., Felix C.R. Purification and characterization of an N-acetylglucosaminidase produced by a Trichoderma harzianum strain which controls Crinipellis perniciosa. Appl. Microbiol. Biotechnol. 2004;64:70–75. doi: 10.1007/s00253-003-1490-5. [DOI] [PubMed] [Google Scholar]
- 63.Chen F., Chen X.-Z., Qin L.-N., Tao Y., Dong Z.-Y. Characterization and homologous overexpression of an N-acetylglucosaminidase Nag1 from Trichoderma reesei. Biochem. Biophys. Res. Commun. 2015;459:184–188. doi: 10.1016/j.bbrc.2014.12.066. [DOI] [PubMed] [Google Scholar]
- 64.Matsuo I., Kim S., Yamamoto Y., Ajisaka K., Maruyama J., Nakajima H., Kitamoto K. Cloning and overexpression of beta-N-acetylglucosaminidase encoding gene nagA from Aspergillus oryzae and enzyme-catalyzed synthesis of human milk oligosaccharide. Biosci. Biotechnol. Biochem. 2003;67:646–650. doi: 10.1271/bbb.67.646. [DOI] [PubMed] [Google Scholar]
- 65.Aunpad R., Rice D.W., Sedelnikova S., Panbangred W. Biochemical characterisation of two forms of halo- and thermo-tolerant chitinase C of Salinivibrio costicola expressed in Escherichia coli. Ann. Microbiol. 2007;57:249–257. doi: 10.1007/BF03175215. [DOI] [Google Scholar]
- 66.Kurakake M., Goto T., Ashiki K., Suenaga Y., Komaki T. Synthesis of new glycosides by transglycosylation of N-acetylhexosaminidase from Serratia marcescens YS-1. J. Agric. Food Chem. 2003;51:1701–1705. doi: 10.1021/jf020965x. [DOI] [PubMed] [Google Scholar]
- 67.Flockenhaus B., Kieß M., Müller M.C.M., Leippe M., Scholze H., Riekenberg S., Vahrmann A. The β-N-acetylhexosaminidase of Entamoeba histolytica is composed of two homologous chains and has been localized to cytoplasmic granules. Mol. Biochem. Parasitol. 2004;138:217–225. doi: 10.1016/j.molbiopara.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Dimond R.L., Loomis W.F. Vegetative isozyme of N-acetylglucosaminidase in Dictyostelium discoideum. J. Biol. Chem. 1974;249:5628–5632. [PubMed] [Google Scholar]
- 69.Drouillard S., Armand S., Davies J.G., Vorgias E.C., Henrissat B. Serratia marcescens chitobiase is a retaining glycosidase utilizing substrate acetamido group participation. Biochem. J. 1997;328:945–949. doi: 10.1042/bj3280945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kobata A. Structures and application of oligosaccharides in human milk. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010;86:731–747. doi: 10.2183/pjab.86.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.André-Miral C., Koné F.M., Solleux C., Grandjean C., Dion M., Tran V., Tellier C. De novo design of a trans-β-N-acetylglucosaminidase activity from a GH1 β-glycosidase by mechanism engineering. Glycobiology. 2015;25:394–402. doi: 10.1093/glycob/cwu121. [DOI] [PubMed] [Google Scholar]
- 72.Nguyen H.A., Nguyen T., K Křen V., Eijsink V.G.H., Haltrich D., Peterbauer C.K. Heterologous expression and characterization of an N-acetyl-β-d-hexosaminidase from Lactococcus lactis ssp. lactis IL1403. J. Agric. Food Chem. 2012;60:3275–3281. doi: 10.1021/jf204915e. [DOI] [PubMed] [Google Scholar]
- 73.Rauvolfová J., Kuzma M., Weignerová L., Fialová P., Přikrylová V., Pišvejcová A., Macková M., Křen V. β-N-Acetylhexosaminidase-catalysed synthesis of non-reducing oligosaccharides. J. Mol. Catal. B Enzym. 2004;29:233–239. doi: 10.1016/j.molcatb.2003.10.008. [DOI] [Google Scholar]
- 74.Sidorczyk Z., Senchenkova S.N., Perepelov A.V., Kondakova A.N., Kaca W., Rozalski A., Knirel Y.A. Structure and serology of O-antigens as the basis for classification of Proteus strains. Innate Immun. 2010;17:70–96. doi: 10.1177/1753425909360668. [DOI] [PubMed] [Google Scholar]
- 75.Katzenellenbogen E., Kocharova N.A., Korzeniowska-Kowal A., Bogulska M., Rybka J., Gamian A., Kachala V.V., Shashkov A.S., Knirel Y.A. Structure of the glycerol phosphate-containing O-specific polysaccharide and serological studies on the lipopolysaccharides of Citrobacter werkmanii PCM 1548 and PCM 1549 (serogroup O14) FEMS Immunol. Med. Microbiol. 2008;54:255–262. doi: 10.1111/j.1574-695X.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- 76.Katzenellenbogen E., Kocharova N.A., Zatonsky G.V., Shashkov A.S., Bogulska M., Knirel Y.A. Structures of the biological repeating units in the O-chain polysaccharides of Hafnia alvei strains having a typical lipopolysaccharide outer core region. FEMS Immunol. Med. Microbiol. 2005;45:269–278. doi: 10.1016/j.femsim.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 77.Thomas F., Barbeyron T., Michel G. Evaluation of reference genes for real-time quantitative PCR in the marine flavobacterium Zobellia galactanivorans. J. Microbiol. Methods. 2011;84:61–66. doi: 10.1016/j.mimet.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 78.Ashida H., Miyake A., Kiyohara M., Yoshida E., Kumagai H., Yamamoto K. Two distinct α-L -fucosidases from Bifidobacterium bifidum are essential for the utilization of fucosylated milk oligosaccharides and glycoconjugates. Glycobiology. 2009;19:1010–1017. doi: 10.1093/glycob/cwp082. [DOI] [PubMed] [Google Scholar]
- 79.Curiel J.A., De Las Rivas B., Mancheño J.M., Muñoz R. The pURI family of expression vectors: A versatile set of ligation independent cloning plasmids for producing recombinant His-fusion proteins. Protein Expr. Purif. 2011;76:44–53. doi: 10.1016/j.pep.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 80.Stingele F., Newell J.W., Neeser J.R. Unraveling the function of glycosyltransferases in Streptococcus thermophilus Sfi6. J. Bacteriol. 1999;181:6354–6360. doi: 10.1128/JB.181.20.6354-6360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Viigand K., Visnapuu T., Mardo K., Aasamets A., Alamäe T. Maltase protein of Ogataea (Hansenula) polymorpha is a counterpart to the resurrected ancestor protein ancMALS of yeast maltases and isomaltases. Yeast. 2016;33:415–432. doi: 10.1002/yea.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reiffová K., Nemcová R. Thin-layer chromatography analysis of fructooligosaccharides in biological samples. J. Chromatogr. A. 2006;1110:214–221. doi: 10.1016/j.chroma.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 83.McCleary B.V., McGeough P. A Comparison of polysaccharide substrates and reducing sugar methods for the measurement of endo-1,4-β-xylanase. Appl. Biochem. Biotechnol. 2015;177:1152–1163. doi: 10.1007/s12010-015-1803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Overbeek R., Olson R., Pusch G.D., Olsen G.J., Davis J.J., Disz T., Edwards R.A., Gerdes S., Parrello B., Shukla M., et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST) Nucleic Acids Res. 2014;42:206–214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petersen T.N., Brunak S., Von Heijne G., Nielsen H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.