Abstract
Background: Carbon monoxide (CO) poisoning is an important public health issue around the world. Research indicates that many factors may be related to the rate of CO uptake and elimination in the human body. However, some factors related to CO uptake and elimination are considered controversial. Relatively little attention has been devoted to review and synthesis of factors affecting CO uptake and elimination. Purpose: This paper provides a critical scoping review of the factors and divides them into four aspects, including environmental, demographic, physiological and treatment factors. Methods: We searched the scientific databases for research that has proposed a mathematical equation as a synthesis of quantities related to CO poisoning, CO elimination, CO uptake, CO half-life, CO uptake and elimination and their relationships. After excluding the studies that did not meet the study criteria, there were 39 studies included in the review and the search was completed before 16 December 2019. Results and conclusion: This review discusses most of the factors that impact the rate of CO uptake and elimination. Several factors may be related to CO uptake and elimination, such as CO concentration, the duration of exposure to CO, age, sex, exercise, minute ventilation, alveolar ventilation, total haemoglobin mass and different treatments for CO poisoning. Although some potential factors were not included in the review, the findings are useful by presenting an overview for discussing factors affecting CO uptake and elimination and provide a starting point for further study regarding strategies for CO poisoning and the environmental standard of CO.
Keywords: carbon monoxide, CO uptake, CO elimination
1. Introduction
Exogenous carbon monoxide (CO) results from the incomplete combustion of carbon-containing molecules, and endogenous CO is formed within the body by metabolic processes [1]. CO is a neurotransmitter in the brain and peripheral autonomic nervous system but is also a poison in high enough quantities [2]. Here, we consider uptake to be due to breathing in exogenous CO, and excretion to include both exogenous and endogenous sources. CO is transported across the lungs into the bloodstream and binds preferentially to haemoglobin in the blood, forming carboxyhaemoglobin (COHb); the affinity of haemoglobin for CO is around 210 times greater than that for oxygen [3,4]. Inhaling excess CO can lead to a situation where there is inadequate oxygen transported by haemoglobin, and the human body will then suffer from hypoxia [3,5]. CO poisoning results in symptoms that range from headache to unconsciousness, depending on the dose.
Once exposure to exogenous CO ceases, the body’s mechanisms for excreting CO can return the COHb level to baseline. The typical baseline level of people unexposed to exogenous CO is around 0.8% COHb. For this process, many studies have shown that the half-life for COHb in the body is about 4 h [6].
CO enters the human body through the lungs, is transported via the blood system and enters the tissue/muscle system. Since the CO partial pressure is higher in the vascular system than in tissue, CO enters and can be stored in the tissue/muscle system. This CO transport process is reversible. If the partial pressure of CO is lower in the ambient environment than in the vascular system, then CO is released from the tissue to the blood and then to the lungs to be exhaled [7]. However, due to the stronger affinity of CO for Hb, there is a baseline COHb concentration in the blood.
Several factors are known to relate to CO uptake and excretion, including minute ventilation rate (VE), alveolar ventilation rate (VA), arterial oxygen tension, haem mass and haemoglobin mass. VE is the total rate of ventilation, and VA is the rate of the gas exchange via the alveolar surface during normal breathing. There is a relationship between VE and VA. The equation used is VA = VE − fVD, where f is the respiration rate (1/min), and VD is the dead space (mL) [1,8,9,10].
Haemoglobin is the main oxygen carrier in the human body. It contains a haem prosthetic group that has an iron atom, and it binds to oxygen to form oxyhaemoglobin. By this method, the haemoglobin takes the oxygen through the body [11]. In physiology, CO affects the oxygen–haemoglobin dissociation curve (ODC). Because CO has such a high affinity with haemoglobin, it decreases the blood oxygen concentration significantly [12].
Although several factors relating to CO uptake and elimination in the human body have been described, we did not find an overview of the situation worldwide. This review aimed to summarise the literature on factors that relate to CO uptake and elimination in the human body. Furthermore, we divided the factors into different dimensions to present a clear relationship between each factor. If we understand the factors that affect the rate of CO uptake and elimination, we will be able to predict the CO concentration in the human body and may be able to give suggestions for more effective treatment of CO poisoning.
In this paper, several factors are described that relate to the rate of CO uptake and elimination, which include environmental, demographic, physiological and treatment factors. The related factors contain different dimensions, from physical exposure to physiological metabolism.
2. Materials and Methods
2.1. Scope and Search Strategy
Scientific databases, including PubMed, EMBASE and Web of Science, were searched for studies. The search strategy used a combination of keywords related to carbon monoxide poisoning and elimination, carbon monoxide poisoning and uptake, carbon monoxide poisoning and half-life and carbon monoxide poisoning and equation. We also manually searched the references of every primary study and review article for further publications to make sure all relevant publications were included.
2.2. Inclusion and Exclusion Criteria
In the literature review, certain study inclusion and exclusion criteria were applied. Particularly, the review only included data-based studies on human subjects that appeared in peer-reviewed journals in the English language that had the full text available. Theses, dissertations or presentation abstracts that were not published in peer-reviewed journals were excluded. Also, the authors screened the titles and abstracts to exclude irrelevant publications.
2.3. Search Results and Study Characteristics
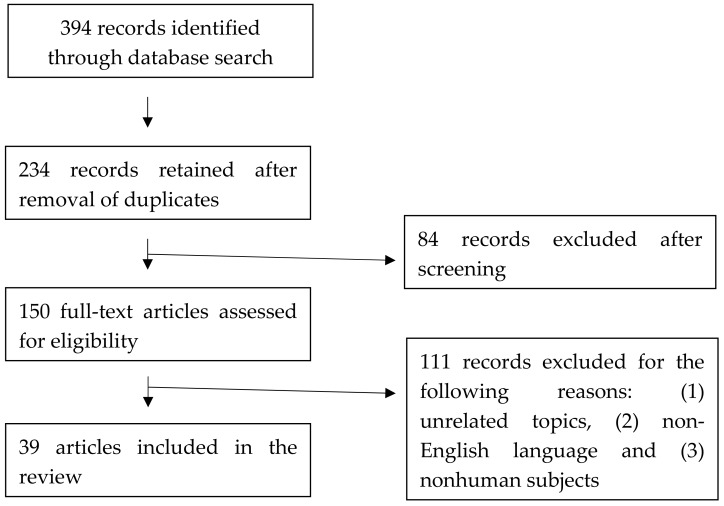
The initial search identified 394 studies by the keywords. The references from the papers were checked to see if there were papers that needed to be considered. After deleting duplicative papers and screening the titles and abstracts, 39 studies met the criteria (Figure 1). We identified 39 studies published since 1945 and the search was completed on 16 December 2019.
Figure 1.
Summary of review process.
3. Results and Discussion
These 39 studies were divided into four aspects, including environmental, demographic, physiological and treatment factors.
3.1. Environmental Factors
When measuring the rate of CO uptake and elimination, the first point to consider is the dose of CO to which the subjects are exposed. There are several environmental factors related to CO exposure, including CO concentration in the ambient air, the duration of CO exposure, the oxygen concentration in the ambient air and altitude.
3.1.1. CO Concentration in Ambient Air and Duration
From the literature, the main factor that may relate to the rate of CO uptake and elimination is ambient CO concentration. In Forbes et al.’s study, the authors obtained more than 100 observations from seven healthy male laboratory workers. When the concentration of CO increased in the inspired air, the rate of CO uptake would also rise [13]. Moreover, Peterson and Stewart created an experiment for 22 subjects. Two subjects (subjects 21 and 32) breathed in 200 ppm CO, and two other subjects (subjects 1 and 12) breathed in 100 ppm CO. As a result, for subjects who breathed in 200 ppm CO, their COHb reached 10% in around 60 min. However, for the subjects who breathed in 100 ppm CO, their COHb reached 10% by 200 min later [8].
The duration of CO exposure affects CO uptake and elimination. In a multicompartment model, researchers tried to predict the CO washout time from different durations. Bruce and Bruce matched the simulation model with the measured data from Benignus et al.’s study and found that the model predicted COHb concentration well [14,15]. They simulated the same dose of CO through two different scenarios of CO exposure. One was exposed to 10,000 ppm CO for 5 min, and the other was exposed to 1250 ppm CO for 40 min. The result for the elimination time of the long duration was slower than for the short duration [15].
3.1.2. Oxygen Concentration in Ambient Air
In Forbes et al.’s study, the authors made subjects breathe CO in the air environment and also in a pure oxygen environment. Then, they compared the rate of CO uptake of the subjects. The ratio of CO uptake rate in the pure oxygen environment compared with in air was around 0.77 during rest and 0.62 during hard work. The reason is that there would be much more oxygen competing with CO if the subjects breathe CO in oxygen than in air. Therefore, the CO uptake will also be slower in oxygen than in air [13].
3.1.3. Altitude
Some researchers found that altitude may be a factor that governs the rate of CO uptake and elimination [13,16]. Collier and Goldsmith modified the Coburn–Forster–Kane (CFK) equation by adding altitude as a factor affecting CO uptake and elimination [16]. For example, the partial pressure of oxygen decreases when the altitude increases. Therefore, when people breathe the same amount of CO, it may cause a higher CO concentration at higher altitudes than at sea level. The reason may be due to the lower partial pressure of oxygen at high altitudes, which means there is less oxygen to compete with CO and the COHb half-life increases. Moreover, altitude may also affect the ODC to the left, which increases haemoglobin’s affinity to bind to oxygen [16]. However, Forbes et al. recorded the CO uptake of three subjects at sea level, 16,000 ft and 40,000 ft. The results showed that the CO uptake rate increased by increasing the altitude due to higher VE [13].
All the factors described above are mainly divided into three parts, namely, the CO concentration, the duration of CO exposure and the partial pressure of CO. When people are exposed to high concentrations of CO or high partial pressure of CO, the rate of CO uptake increases. However, when considering the duration of CO exposure, even though subjects are exposed to a lower concentration of CO, they have a longer elimination time if the exposure time is increased. Both the CO concentration and duration of CO exposure are critical environmental factors related to the rate of CO uptake by, and washout from, the human body.
3.2. Demographic Factors
In many disease-related studies, we could find demographic factors that may be relevant to the disease [17,18]. In some studies, age and sex were reported to relate to the rate of CO uptake and elimination [19,20].
3.2.1. Age
In Klasner et al.’s study, the authors focused on CO poisoning in the paediatric population. Compared with previous studies, they found that children had a shorter COHb half-life than adults. For 26 children, the mean half-life of COHb was 44.0 min on 100% oxygen at 1 atm. However, the half-life of COHb in adults was around 80 min in the same situation. The authors assumed that the reason for this was the difference in minute ventilation between children and adults. Although children have a smaller tidal volume than adults, they have faster respiratory rates, which leads to an increase in their VE [19,21].
Moreover, there are still several factors that may change with age, including the volume of haem, blood volume, muscle myoglobin mass and lung function. Therefore, further studies need to be done to understand the age effects.
3.2.2. Sex
Tracing back to Pace et al.’s study, they found a sex-related difference in the half-life of COHb. The half-life of COHb elimination by breathing 100% oxygen at 2.5 atmosphere absolute (ATA) was 22.3 min for men and 15.1 min for women. However, the authors did not explain the reason for the sex-related difference [22]. Although some studies showed a sex-related difference, there were still other studies that found no sex-related difference in the rate of CO uptake and elimination [9,23]. In a large natural experiment, 184 people were exposed to CO in a public high school for around 2.5 h. The researchers gave questionnaires to the victims and analysed the data. They found no differences between ages, sexes and smokers and nonsmokers [23]. Moreover, Weaver et al. found that sex did not have a significant influence on half-life [9].
Zavorsky et al. did find a sex-related difference for the half-life of CO elimination and revealed the factors behind this effect. The results showed that women had a shorter half-life of CO elimination than men. The factors found to influence the rate of CO elimination were VA and total haemoglobin mass [20].
3.2.3. Smoking
When someone smokes a cigarette, the smoker is likely to be exposed to CO concentrations of around 400–500 ppm and experience a higher COHb concentration than a nonsmoker. The COHb concentration is usually less than 5% in nonsmokers and more than 5% in smokers [24]. Another study also showed that COHb is different between smokers and nonsmokers in London. Smokers have COHb levels of around 5%–8% compared with nonsmokers, who have COHb levels of about 1%–3% [25]. This is in contrast with the study of Burney et al. where no differences were observed [23].
3.2.4. Exercise
The level of exercise or activity of subjects may have some influence on the rate of CO uptake and elimination. In Forbes et al.’s study, the rate of CO uptake in the subjects was higher during hard work than during rest [13]. Filley et al. also found that the rate of CO uptake was different between subjects at rest and exercise. When the subjects increased the level of exercise, the minute ventilation and the rate of CO uptake also rose [26]. However, the rate of CO uptake was not significantly different between either a low (~45 W) or moderate (~90 W) power output measured by a cycle ergometer in an experiment involving 29–37-year-old subjects [27].
Demographic factors, such as age, sex and exercise, are related to the rate of CO uptake and elimination. However, the physiological factors of minute ventilation, alveolar ventilation and total haemoglobin mass likely explain the demographic observations.
3.3. Physiological Factors
When people breathe in CO, the CO gas enters the lungs and then transfers via the alveoli into the vascular system. Through the blood circulation, most of the CO binds to haemoglobin and is transferred from the arterial to the venous blood. Besides the blood, some of the CO also crosses into the tissue and binds to it, leading to the formation of carboxymyoglobin [7]. Consequently, like lung and cardiovascular functions, muscle function may play a role in CO circulation in the human body and it is related to the rate of CO uptake and elimination. In Penney’s book, he stated that the two main physiological factors that affect the rate of CO uptake and elimination are the ventilation and diffusion rates of CO [28].
3.3.1. Lung Function
Ventilation Rate
Many studies have discovered that the ventilation rate may affect the rate of CO uptake and elimination [1,13,19,26,29,30]. When people breathe at a high ventilation rate, they tend to absorb more CO into the lungs and blood. However, a high ventilation rate can also exhale more CO than a low ventilation rate over the same duration [1,13]. In a study by Zavorsky et al. (2014), the results showed that men have a more prolonged washout time of CO than women, and the authors tried to explain the result. After they tested different factors in the subjects, they found that the alveolar ventilation and total haemoglobin mass may be the reasons that explain the difference in the CO half-life. When people have increased alveolar ventilation, the CO elimination time decreases [20].
However, in Bruce and Bruce’s model (2006), they found that the half-life of COHb has a higher correlation (r = 0.714) with Vb/VAwo (blood volume/ventilation during washout) than ventilation alone. Because the CO is exhaled directly from the lungs and carried by the blood, the limiting factor may be this ratio [15].
Diffusion Capacity of CO (DLCO)
Between the alveolar and pulmonary capillaries, gas passes the pulmonary membrane by simple diffusion. The diffusion capacity “is the ability of the lungs to transfer gas from inhaled air to the red blood cells in pulmonary capillaries” [26]. The diffusion capacity is affected by molecular species, body size, rate of work, temperature and pressure [8]. The DLCO is widely used to test patients’ lung function in hospitals nowadays [31]. The mean values for DLCO were found to be 28.05 ± 5.07 mL/min/mmHg for men and 20.79 ± 4.03 mL/min/mmHg for women [32]. The CFK equation, an equation for the study of the endogenous production of CO, CO distribution, CO uptake and elimination, contains the pulmonary diffusing capacity as a factor that may affect the rate of CO uptake and elimination. When the diffusion capacity is higher, it means that CO has a great ability to pass through the membrane, and the rate of the CO uptake and elimination is increased [1,33,34]. However, in Filley et al.’s study, the authors found that the ventilation rate may play a more important role in the rate of CO uptake and elimination [26].
Chronic Obstructive Pulmonary Disease (COPD)
COPD is defined as an obstruction of the airways that makes it hard to breathe. COHb levels were found to be significantly higher in COPD patients compared with the normal population [35,36]. Some COPD patients have a lower diffusing capacity for CO in the lungs [37,38,39]. In Crowley et al.’s study, their data suggested that the half-life of COHb is around 6.5 h in COPD patients compared with healthy subjects, who have a COHb half-life of about 2–5 h [40]. Therefore, COPD patients may have a slower rate of CO elimination than healthy people due to the lower gas exchange and poor respiratory mechanics. However, Crowley et al. explained that there was no dramatic difference of COHb half-life between COPD patients and normal subjects, so it might be the sedentary life of COPD patients that causes the longer COHb half-life [40].
3.3.2. Cardiovascular Function
Blood Volume
When CO enters the vascular system, most of the CO combines with haemoglobin as COHb. At the end of CO exposure, most of the CO stays in the blood. Consequently, the blood volume may be an important factor that relates to CO uptake and elimination. In Pugh’s study, the average blood volume was around 78 mL/kg [41]. In the CFK equation, blood volume is one of the factors affecting the rate of CO uptake and elimination [1]. Furthermore, in Bruce and Bruce’s study, their model predicted that if people have a large blood volume, they carry more CO in the body and have an increased rate of CO uptake and elimination [15].
Haemoglobin Mass
Haemoglobin is the crucial factor that determines the maximum amount of oxygen uptake. The average haemoglobin mass is about 11.6 g/kg [41]. However, when compared with oxygen, CO has around 210 times greater affinity for haemoglobin [4]. In Zavorsky et al.’s study, the authors suggested that the total haemoglobin mass affects the rate of CO uptake and elimination [20]. However, the effects of total haemoglobin mass on the rate of CO uptake and elimination require further investigation.
Diffusion Rate of CO Flux from Blood to Muscle Compartment
The blood-to-muscle diffusion coefficient (Dmco) refers to the diffusion rate of the CO entering the muscle compartment. In a multicompartment model, Bruce and Bruce (2006) set the Dmco to zero and tried to determine how it would influence the half-life for CO washout. When the Dmco was set to zero, the half-life increased. Therefore, this means that no CO entered the muscle compartment, and all the CO decreased by exhalation from the lungs [15]. Moreover, in 2008, the authors tested the model with experimental data, including human and animal data [14,23,42,43]. They found their model could fit well with the experimental data when changing the Dmco in different conditions [44].
Muscle Mass
Not only can haemoglobin bind to CO in muscle cells, but myoglobin also contains haem, to which CO can bind. Muscle tissue can take up CO over a prolonged period, even after the end of exposure. For a young adult male, the muscle compartment may account for about 41% of the total body mass [45]. In their study, Möller and Sylvén assumed that every gram wet weight of muscle would contain about 4.7 mg of myoglobin [46]. Take a 70 kg man, for example, who may, approximately, have 135 g of myoglobin. Each myoglobin molecule contains a haem molecule that could bind up to 178 mL of CO. Therefore, the muscle compartment could be an essential place to store CO and increase the half-life of CO elimination. Although muscle may be a factor, it is less critical for the half-life of COHb. The reason is that the volume of CO removed from muscle is less than the volume of CO removed from the blood [15].
Anaemia
Anaemia refers to a low haemoglobin level or low red blood cell count in the blood or increased destruction of red blood cells. In Woehlck et al.’s study (2001), the authors predicted that patients would have more severe CO poisoning according to the haematocrit level. They explained that patients with low haemoglobin tend to have a higher COHb concentration than people with normal haemoglobin after exposure to CO. When the subjects breathed in the same CO concentration, the rate of COHb increased more rapidly in the subjects with a lower haematocrit level than a higher haematocrit level [47].
Among physiological factors, besides ventilation rate and diffusion capacity (which have been emphasised for a long time), there are still many factors that need to be considered. For example, our review indicates that blood volume, total haemoglobin mass, muscle mass and disease may influence the rate of CO uptake and elimination. However, the physiology of the human body is known to be complicated. Some factors may have a relationship with other factors. Isolating the role of any specific factor will require careful study.
3.4. Treatment Factors
The most common treatment for CO poisoning is breathing 100% oxygen as soon as possible. Moreover, high-pressure oxygen or hyperbaric oxygen (HBO) therapy also has been used in several countries as a solution for CO poisoning. The rate of CO elimination may relate to the atmospheric pressure or percentage of oxygen. Higher atmospheric pressure and percentage of oxygen result in a faster CO elimination rate [19,29].
3.4.1. 100% Oxygen
Weaver et al. (2000) conducted a study to understand which factors may influence the COHb half-life. Through their retrospective chart review from 1985 to 1995, they showed that the half-life of COHb decreases with the increase of arterial oxygen tension. As a result, they found that the half-life of COHb was around 74 min for patients treated with 100% oxygen at atmospheric pressure, which was shorter than for patients only breathing in air [9].
There are several methods to provide 100% oxygen to patients, such as a rebreathing reserve mask, high-flow nasal cannula (HFNC) oxygen and oxygen therapy with continuous positive airway pressure (CPAP) [48,49,50,51]. In Kim et al.’s (2019) study, HFNC did not reduce the CO half-life compared with a rebreathing reserve mask [49]. When comparing normobaric oxygen therapy with 1 h of CPAP therapy, Bal et al. discovered that patients receiving CPAP therapy had a shorter CO half-life than those receiving normobaric oxygen therapy. The authors assumed that CPAP therapy increases the gas exchange area and improves ventilation due to the positive pressure going into the alveoli [50].
3.4.2. HBO Therapy
Treating CO-poisoned patients with HBO therapy is still controversial [6,52,53]. There are different policies in different countries. However, Pace et al. found that high-pressure oxygen could increase CO elimination in CO-poisoned patients [22]. In Ernst and Zibrak’s study, they found that the half-life of COHb would be approximately 4 h on air, 1.5 h on oxygen and less than 20 min during HBO therapy [54].
3.4.3. Carbogen
Carbogen is a mixture of carbon dioxide and oxygen gas. Usually, CO2 is set at 5%–10% in O2 [55,56]. When patients breathe in carbogen, their brain CO2 sensor detects that more CO2 is stored in the body, and as a result, the brain sends a signal to increase alveolar ventilation, thus decreasing the half-life of COHb [56,57].
The 100% oxygen and HBO therapies are based on the theory that the alveolar partial pressures of oxygen would be affected by the inhaled partial pressure of oxygen. When increasing the partial pressure of oxygen, there is more oxygen that can compete with CO to bind with haemoglobin. Then, the rate of CO elimination would be raised. However, using HBO therapy for CO-poisoned patients is still controversial. Although HBO therapy is not recommended for CO-poisoned patients in the United Kingdom, it is a treatment for CO-poisoned patients that is widely used in Taiwan.
4. Conclusions
In the literature review, some environmental, demographic, physiological and treatment factors were found to have an impact on the rate of CO uptake and elimination (Table 1 and Table 2). Among environmental factors, the rate of CO uptake increases by raising the CO concentration or reducing the oxygen concentration of the inhaled gas. Moreover, the altitude can alter the rate of CO uptake due to the different partial pressure of oxygen at different altitudes. The duration of CO exposure is an important factor. If people were exposed to CO for a long time, even if the concentration of CO were low, it would also have an adverse impact and reduce the rate of CO elimination. That is why attention is needed not only for acute CO poisoning but also chronic CO poisoning, which is often ignored.
Table 1.
The factors related to CO uptake.
| Field | Factor | Results | Experiment | Control | Reference |
|---|---|---|---|---|---|
| Environment | CO concentration increase | CO uptake rate increase | Range: 0.01%–0.2% CO | Forbes et al. (1945) | |
| Range: 0–523 CO ppm | Peterson and Stewart (1970) | ||||
| Range: 8.7–1000 CO ppm | Peterson and Stewart (1975) | ||||
| Duration of exposure longer | CO uptake amount increase | Range: 0–270 min | Forbes et al. (1945) | ||
| Range: 15–480 min | Peterson and Stewart (1970) | ||||
| Range: 0–1440 min (50 CO ppm) | Benignus et al. (1994) | ||||
| O2 concentration increase | CO uptake rate decrease | Oxygen | Air | Forbes et al. (1945) | |
| Altitude increase | CO uptake rate increase | 16,000 ft; 40,000 ft |
0 ft | Forbes et al. (1945) | |
| Exercise increase | CO uptake rate increase | Hard work | Rest | Forbes et al. (1945) | |
| CO uptake rate increase | Light exercise; moderate exercise | Resting | Filley et al. (1954) | ||
| No difference | Moderate exercise | Low exercise | Tikuisis et al. (1992) | ||
| Physiology | Ventilation rate increase | CO uptake rate increase | Range: 6–30 L/min | Forbes et al. (1945) | |
| CO uptake rate increase | Range: 5.8–105 L/min | Filley et al. (1954) | |||
| Diffusion capacity of CO (DLCO) increase | CO uptake rate increase | 36.3 cm3/min/mmHg | 16.9 cm3/min/mmHg | Filley et al. (1954) | |
| CO uptake rate increase | Range: 5–30 mL/min/torr | Bruce and Bruce (2003) | |||
| CO uptake rate increase | - | - | Gosselin et al. (2009) | ||
| Blood volume increase | CO uptake rate increase | - | - | Coburn et al. (1965) | |
| Diffusion rate of CO flux from blood to muscle compartment in crease | CO uptake rate increase | Range: 0–100 mL/min/torr | Bruce et al. (2008) | ||
| Muscle mass | Less important | - | - | Bruce and Bruce (2006) | |
| Anaemia | CO uptake rate increase | Haematocrits of 18% and 30% | Haematocrits of 42% and 60% | Woehlck et al. (2001) | |
Note: 1 torr = 1 mmHg, a unit of pressure based on an absolute scale; 1 cm3 = 1 mL.
Table 2.
The factors related to CO elimination.
| Field | Factor | Results | Experiment | Control | Reference |
|---|---|---|---|---|---|
| Environment | CO concentration increase | CO half-life longer | 200.8 CO ppm for 60 min | 51.6 CO ppm for 60 min | Peterson and Stewart (1970) |
| Duration of exposure longer | CO half-life longer | 1250 CO ppm for 40 min | 10,000 CO ppm for 5 min | Bruce and Bruce (2006) | |
| (same CO dose in two groups) | |||||
| O2 concentration increase | CO half-life shorter | 100% oxygen | - | Weaver et al. (2000) | |
| 2.5 atm, 100% oxygen (HBO) | - | Pace et al. (1950) | |||
| Demography | Age increase | No difference | Range: 9–86 years old | Burney et al. (1982) | |
| >40 years old | <40 years old | Weaver et al. (2000) | |||
| CO half-life shorter | 4–12 years old | - | Klasner et al. (1998) | ||
| Sex | No difference | Female | Male | Burney et al. (1982) | |
| Female | Male | Weaver et al. (2000) | |||
| CO half-life shorter | Female | Male | Pace et al. (1950) | ||
| Female | Male; | Zavorsky et al. (2014) | |||
| Smoking | No difference | Smokers | Nonsmokers | Burney et al. (1982) | |
| Physiology | Ventilation rate increase | CO half-life shorter | Range: 4–10 L/min | Coburn et al. (1965) | |
| 15 and 30 L/min | 3 and 6 L/min | Selvakumar et al. (1993) | |||
| Range: 5–20 L/min | Kreck et al. (2001) | ||||
| Range: 4–40 L/min | Zavorsky et al. (2014) | ||||
| Chronic obstructive pulmonary disease (COPD) | No difference/CO half-life slightly longer | COPD patients | Normal subjects | Crowley et al. (1989) | |
| Blood volume increase | CO half-life shorter | - | - | Coburn et al. (1965) | |
| Range: 0.3–0.7 (Vb/VAwo) | Bruce and Bruce (2006) | ||||
| Haemoglobin mass increase | CO half-life longer | Male | Female | Zavorsky et al. (2014) | |
| Diffusion rate of CO flux from blood to muscle compartment increase | CO half-life shorter | Range: 0–2 mL/min/torr | Bruce et al. (2003) | ||
| Muscle mass | Less important | - | - | Bruce and Bruce (2006) | |
| Anaemia | CO half-life shorter | Anaemia | Polycythaemia | Zavorsky et al. (2014) | |
| Treatment | 100% oxygen | CO half-life shorter | 100% oxygen | - | Weaver et al. (2000) |
| High-flow nasal cannula (HFNC) | No difference | 100% oxygenwith high flow | 100% oxygen | Kim et al. (2019) | |
| Continuous positive airway pressure (CPAP) | CO half-life shorter | 100% oxygenwith positive pressure | 100% oxygen | Bal et al. (2019)Caglar et al. (2019) | |
| Hyperbaric oxygen (HBO) therapy | CO half-life shorter | 2.5 atm, 100% oxygen | - | Pace et al. (1950) | |
| 3 atmosphere absolute (ATA), 100% oxygen | 1 ATA, 100% oxygen | Peterson and Stewart (1970) | |||
| Carbogen | CO half-life shorter | Hyperventilation(6% CO2 in O2) | Without isocapnia | Sein Anand et al. (2017) | |
Note: 1 torr = 1 mmHg, a unit of pressure based on an absolute scale.
Demographic factors, such as age, sex, smoking and exercise, are not direct factors relating to the rate of CO uptake and elimination. Included in these factors may be physiological factors, such as minute ventilation, alveolar ventilation and total haemoglobin mass, which are direct factors affecting the rate of CO uptake and elimination. Other physiological factors, including muscle mass and diffusion capacity, can also influence the rate of CO uptake and elimination. Consider the treatments, for example: the three treatments for CO poisoning increase the rate of CO elimination by raising the partial pressure of oxygen and the ventilation rate. Therefore, these treatments are based on the physiological reactions that have been tested in past studies.
This review discussed most of the factors that impact the rate of CO uptake and elimination. Information remains limited and there are numerous other potentially important factors that could influence CO update and elimination, such as genetics, disease, vulnerable groups, children, the elderly, weight and so forth. Thus, there may be different treatment strategies for groups with different characteristics. Further studies focused on this field may find better ways to increase the rate of CO elimination in CO-poisoned patients.
Author Contributions
K.-T.P., B.C. and G.S.L. are responsible for the conception and design of the study. K.-T.P. reviewed the papers and drafted the manuscript. K.-T.P., B.C. and G.S.L. discussed and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was conducted as part of K-TP’s PhD studies at UCL supported by a grant from the Taiwanese government.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Coburn R.F., Forster R.E., Kane P.B. Considerations of the physiological variables that determine the blood carboxyhemoglobin concentration in man. J. Clin. Investig. 1965;44:1899–1910. doi: 10.1172/JCI105296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barañano D.E., Snyder S.H. Neural roles for heme oxygenase: Contrasts to nitric oxide synthase. Proc. Natl. Acad. Sci. USA. 2001;98:10996–11002. doi: 10.1073/pnas.191351298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolan M.C. Carbon monoxide poisoning. CMAJ. 1985;133:392–399. [PMC free article] [PubMed] [Google Scholar]
- 4.Blumenthal I. Carbon monoxide poisoning. J. R. Soc. Med. 2001;94:270–272. doi: 10.1177/014107680109400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kao L.W., Nanagas K.A. Carbon monoxide poisoning. Emerg. Med. Clin. North. Am. 2004;22:985–1018. doi: 10.1016/j.emc.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Weaver L.K., Hopkins R.O., Chan K.J., Churchill S., Elliott C.G., Clemmer T.P., Orme J.F., Jr., Thomas F.O., Morris A.H. Hyperbaric oxygen for acute carbon monoxide poisoning. N. Engl. J. Med. 2002;347:1057–1067. doi: 10.1056/NEJMoa013121. [DOI] [PubMed] [Google Scholar]
- 7.Erupaka K., Bruce E.N., Bruce M.C. Prediction of Extravascular Burden of Carbon Monoxide (CO) in the Human Heart. Ann. Biomed. Eng. 2010;38:403–438. doi: 10.1007/s10439-009-9814-y. [DOI] [PubMed] [Google Scholar]
- 8.Peterson J.E., Stewart R.D. Predicting the carboxyhemoglobin levels resulting from carbon monoxide exposures. J. Appl. Physiol. 1975;39:633. doi: 10.1152/jappl.1975.39.4.633. [DOI] [PubMed] [Google Scholar]
- 9.Weaver L.K., Howe S., Hopkins R., Chan K.J. Carboxyhemoglobin half-life in carbon monoxide-poisoned patients treated with 100% oxygen at atmospheric pressure. Chest. 2000;117:801–808. doi: 10.1378/chest.117.3.801. [DOI] [PubMed] [Google Scholar]
- 10.Brochu P., Brodeur J., Krishnan K. Derivation of cardiac output and alveolar ventilation rate based on energy expenditure measurements in healthy males and females. J. Appl. Toxicol. 2012;32:564–580. doi: 10.1002/jat.1651. [DOI] [PubMed] [Google Scholar]
- 11.Hsia C.C.W. Respiratory Function of Hemoglobin. N. Engl. J. Med. 1998;338:239–248. doi: 10.1056/NEJM199801223380407. [DOI] [PubMed] [Google Scholar]
- 12.Morgan T.J. The oxyhaemoglobin dissociation curve in critical illness. Crit. Care Resusc. 1999;1:93–100. [PubMed] [Google Scholar]
- 13.Forbes W.H., Sargent F., Roughton F.J.W. The rate of carbon monoxide uptake by normal men. Am. J. Physiol. 1945;143:594–608. doi: 10.1152/ajplegacy.1945.143.4.594. [DOI] [Google Scholar]
- 14.Benignus V.A., Hazucha M.J., Smith M.V., Bromberg P.A. Prediction of carboxyhemoglobin formation due to transient exposure to carbon monoxide. J. Appl. Physiol. 1994;76:1739. doi: 10.1152/jappl.1994.76.4.1739. [DOI] [PubMed] [Google Scholar]
- 15.Bruce M.C., Bruce E.N. Analysis of factors that influence rates of carbon monoxide uptake, distribution, and washout from blood and extravascular tissues using a multicompartment model. J. Appl. Physiol. 2006;100:1171. doi: 10.1152/japplphysiol.00512.2005. [DOI] [PubMed] [Google Scholar]
- 16.Collier C.R., Goldsmith J.R. Interactions of carbon monoxide and hemoglobin at high altitude. Atmos. Environ. 1983;17:723–728. doi: 10.1016/0004-6981(83)90419-5. [DOI] [Google Scholar]
- 17.Bueno V., Sant’Anna O.A., Lord J.M. Ageing and myeloid-derived suppressor cells: Possible involvement in immunosenescence and age-related disease. Age. 2014;36:9729. doi: 10.1007/s11357-014-9729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aziz A., Hansen H.S., Sechtem U., Prescott E., Ong P. Sex-Related Differences in Vasomotor Function in Patients With Angina and Unobstructed Coronary Arteries. J. Am. Coll. Cardiol. 2017;70:2349–2358. doi: 10.1016/j.jacc.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Klasner A.E., Smith S.R., Thompson M.W., Scalzo A.J. Carbon monoxide mass exposure in a pediatric population. Acad. Emerg. Med. 1998;5:992–996. doi: 10.1111/j.1553-2712.1998.tb02778.x. [DOI] [PubMed] [Google Scholar]
- 20.Zavorsky G.S., Tesler J., Rucker J., Fedorko L., Duffin J., Fisher J.A. Rates of carbon monoxide elimination in males and females. Physiol. Rep. 2014;2:e12237. doi: 10.14814/phy2.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson J.E., Stewart R.D. Absorption and Elimination of Carbon Monoxide by Inactive Young Men. Arch. Environ. Health Int. J. 1970;21:165–171. doi: 10.1080/00039896.1970.10667215. [DOI] [PubMed] [Google Scholar]
- 22.Pace N., Strajman E., Walker E.L. Acceleration of Carbon Monoxide Elimination in Man by High Pressure Oxygen. Science. 1950;111:652. doi: 10.1126/science.111.2894.652. [DOI] [PubMed] [Google Scholar]
- 23.Burney R.E., Wu S.-C., Nemiroff M.J. Mass carbon monoxide poisoning: Clinical effects and results of treatment in 184 victims. Ann. Emerg. Med. 1982;11:394–399. doi: 10.1016/S0196-0644(82)80033-4. [DOI] [PubMed] [Google Scholar]
- 24.Marshall M.D., Kales S.N., Christiani D.C., Goldman R.H. Are reference intervals for carboxyhemoglobin appropriate? A survey of Boston area laboratories. Clin. Chem. 1995;41:1434. doi: 10.1093/clinchem/41.10.1434. [DOI] [PubMed] [Google Scholar]
- 25.Castleden C.M., Cole P.V. Carboxyhaemoglobin levels of smokers and non-smokers working in the City of London. Br. J. Ind. Med. 1975;32:115–118. doi: 10.1136/oem.32.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filley G.F., MacIntosh D.J., Wright G.W. Carbon monoxide uptake and pulmonary diffusing capacity in normal subjects at rest and during exercise. J. Clin. Investig. 1954;33:530–539. doi: 10.1172/JCI102923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tikuisis P., Kane D.M., McLellan T.M., Buick F., Fairburn S.M. Rate of formation of carboxyhemoglobin in exercising humans exposed to carbon monoxide. J. Appl. Physiol. 1992;72:1311. doi: 10.1152/jappl.1992.72.4.1311. [DOI] [PubMed] [Google Scholar]
- 28.Penney D.G. Carbon Monoxide. 1st ed. CRC Press; Boca Raton, FL, USA: 1996. [Google Scholar]
- 29.Selvakumar S., Sharan M., Singh M.P. A Mathematical Model for the Elimination of Carbon Monoxide in Humans. J. Theor. Biol. 1993;162:321–336. doi: 10.1006/jtbi.1993.1090. [DOI] [PubMed] [Google Scholar]
- 30.Kreck T., Shade E., Lamm W., McKinney S., Hlastala M. Isocapnic Hyperventilation Increases Carbon Monoxide Elimination and Oxygen Delivery. Am. J. Respir. Crit. Care Med. 2001;163:458–462. doi: 10.1164/ajrccm.163.2.2003039. [DOI] [PubMed] [Google Scholar]
- 31.Bowley N.B., Hughes J.M.B., Steiner R.E. The chest X-ray in pulmonary capillary haemorrhage: Correlation with carbon monoxide uptake. Clin. Radiol. 1979;30:413–417. doi: 10.1016/S0009-9260(79)80221-4. [DOI] [PubMed] [Google Scholar]
- 32.Park J.O., Choi I.S., Park K.O. Normal Predicted Values of Single-Breath Diffusing Capacity of the Lung in Healthy Nonsmoking Adults. Korean J. Intern. Med. 1986;1:178–184. doi: 10.3904/kjim.1986.1.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruce E.N., Bruce M.C. A multicompartment model of carboxyhemoglobin and carboxymyoglobin responses to inhalation of carbon monoxide. J. Appl. Physiol. 2003;95:1235. doi: 10.1152/japplphysiol.00217.2003. [DOI] [PubMed] [Google Scholar]
- 34.Gosselin N.H., Brunet R.C., Carrier G. Determination of carboxyhaemoglobin in humans following low-level exposures to carbon monoxide. Inhal. Toxicol. 2009;21:1077–1091. doi: 10.3109/08958370902744848. [DOI] [PubMed] [Google Scholar]
- 35.Özgür Dogan N., Corbacioglu S., Bildik F., Kilicaslan I., Gunaydın G., Günaydın G.P., Cevik Y., Ülker V., Hakoglu O., Gökcen E. Determining the diagnostic value of endogenous carbon monoxide in Chronic Obstructive Pulmonary Disease exacerbations. J. Pak. Med. Assoc. 2014;64:1037–1041. [PubMed] [Google Scholar]
- 36.Nambu A., Zach J., Schroeder J., Jin G.Y., Kim S.S., Kim Y.-I.L., Schnell C., Bowler R., Lynch D.A. Relationships between diffusing capacity for carbon monoxide (DLCO), and quantitative computed tomography measurements and visual assessment for chronic obstructive pulmonary disease. Eur. J. Radiol. 2015;84:980–985. doi: 10.1016/j.ejrad.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Sandek K., Bratel T., Lagerstrand L. Effects on diffusing capacity and ventilation–perfusion relationships of budesonide inhalations for 2 months in chronic obstructive pulmonary disease (COPD) Respir. Med. 2001;95:676–684. doi: 10.1053/rmed.2001.1124. [DOI] [PubMed] [Google Scholar]
- 38.Murphy T.F., Sethi S. Chronic Obstructive Pulmonary Disease. Drugs Aging. 2002;19:761–775. doi: 10.2165/00002512-200219100-00005. [DOI] [PubMed] [Google Scholar]
- 39.O’Donnell D.E., Laveneziana P., Webb K., Neder J.A. Chronic Obstructive Pulmonary Disease: Clinical Integrative Physiology. Clin. Chest Med. 2014;35:51–69. doi: 10.1016/j.ccm.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Crowley T.J., Andrews A.E., Cheney J., Zerbe G., Petty T.L. Carbon monoxide assessment of smoking in chronic obstructive pulmonary disease. Addict. Behav. 1989;14:493–502. doi: 10.1016/0306-4603(89)90069-5. [DOI] [PubMed] [Google Scholar]
- 41.Pugh L.G.C.E. Blood volume changes in outdoor exercise of 8-10 hour duration. J. Physiol. 1969;200:345–351. doi: 10.1113/jphysiol.1969.sp008697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roughton F.J.W., Root W.S. The fate of co in the body during recovery from mild carbon monoxide poisoning in man. Am. J. Physiol. Leg. Content. 1945;145:239. doi: 10.1152/ajplegacy.1945.145.2.239. [DOI] [PubMed] [Google Scholar]
- 43.Prommer N., Schmidt W. Loss of CO from the intravascular bed and its impact on the optimised CO-rebreathing method. Eur. J. Appl. Physiol. 2007;100:383–391. doi: 10.1007/s00421-007-0439-2. [DOI] [PubMed] [Google Scholar]
- 44.Bruce E.N., Bruce M.C., Erupaka K. Prediction of the rate of uptake of carbon monoxide from blood by extravascular tissues. Respir. Physiol. Neurobiol. 2008;161:142–159. doi: 10.1016/j.resp.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee R.C., Wang Z., Heo M., Ross R., Janssen I., Heymsfield S.B. Total-body skeletal muscle mass: Development and cross-validation of anthropometric prediction models. Am. J. Clin. Nutr. 2000;72:796–803. doi: 10.1093/ajcn/72.3.796. [DOI] [PubMed] [Google Scholar]
- 46.Möller P., Sylvén C. Myoglobin in human skeletal muscle. Scand. J. Clin. Lab. Investig. 1981;41:479–482. doi: 10.3109/00365518109090486. [DOI] [PubMed] [Google Scholar]
- 47.Woehlck H.J., Mei D., Dunning 3rd M.B., Ruiz F. Mathematical Modeling of Carbon Monoxide Exposures from Anesthetic Breakdown Effect of Subject Size, Hematocrit, Fraction of Inspired Oxygen, and Quantity of Carbon Monoxide. Anesthesiology. 2001;94:457–460. doi: 10.1097/00000542-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Ozturan I.U., Yaka E., Suner S., Ozbek A.E., Alyesil C., Dogan N.O., Yilmaz S., Pekdemir M. Determination of carboxyhemoglobin half-life in patients with carbon monoxide toxicity treated with high flow nasal cannula oxygen therapy. Clin. Toxicol. 2019;57:617–623. doi: 10.1080/15563650.2018.1540046. [DOI] [PubMed] [Google Scholar]
- 49.Kim Y.-M., Shin H.-J., Choi D.-w., Kim J.-M., Lee S.-W., Jeong S.-H., Kim H. Comparison of high-flow nasal cannula oxygen therapy and conventional reserve-bag oxygen therapy in carbon monoxide intoxication: A pilot study. Am. J. Emerg. Med. 2019 doi: 10.1016/j.ajem.2019.158451. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 50.Bal U., Sönmez B.M., Inan S., Iscanli M.D., Yilmaz F. The efficiency of continuous positive airway Press. therapy in carbon monoxide poisoining in the emergency department. Eur. J. Emerg. Med. 2019 doi: 10.1097/MEJ.0000000000000647. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 51.Caglar B., Serin S., Yilmaz G., Torun A., Parlak I. The Impact of Treatment with Continuous Positive Airway Pressure on Acute Carbon Monoxide Poisoning. Prehospital Disaster Med. 2019;34:588–591. doi: 10.1017/S1049023X19005028. [DOI] [PubMed] [Google Scholar]
- 52.Buckley N.A., Juurlink D.N., Isbister G., Bennett M.H., Lavonas E.J. Hyperbaric oxygen for carbon monoxide poisoning. Cochrane Database Syst. Rev. 2011;4:CD002041. doi: 10.1002/14651858.CD002041.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pan K.-T., Shen C.-H., Lin F.-G., Chou Y.-C., Croxford B., Leonardi G., Huang K.-L. Prognostic factors of carbon monoxide poisoning in Taiwan: A retrospective observational study. BMJ Open. 2019;9:e031135. doi: 10.1136/bmjopen-2019-031135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ernst A., Zibrak J.D. Carbon monoxide poisoning. N. Engl. J. Med. 1998;339:1603–1608. doi: 10.1056/NEJM199811263392206. [DOI] [PubMed] [Google Scholar]
- 55.Douglas T.A., Lawson D.D., Ledingham I.M., Norman J.N., Sharp G.R., Smith G. Carbogen in Experimental Carbon-monoxide Poisoning. Br. Med. J. 1961;2:1673. doi: 10.1136/bmj.2.5268.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sein Anand J., Schetz D., Waldman W., Wiśniewski M. Hyperventilation with Maintenance of Isocapnia. An Old New Method in Carbon Monoxide Intoxication. PLoS ONE. 2017;12:e0170621. doi: 10.1371/journal.pone.0170621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fisher J.A., Iscoe S., Fedorko L., Duffin J. Rapid elimination of CO through the lungs: Coming full circle 100 years on. Exp. Physiol. 2011;96:1262–1269. doi: 10.1113/expphysiol.2011.059428. [DOI] [PMC free article] [PubMed] [Google Scholar]