Abstract
Immunotherapy has become a promising cancer therapy, improving the prognosis of patients with many different types of cancer and offering the possibility for long-term cancer remission. Nevertheless, some patients do not respond to these treatments and immunotherapy has shown some limitations, such as immune system resistance or limited bioavailability of the drug. Therefore, new strategies that include the use of nanoparticles (NPs) are emerging to enhance the efficacy of immunotherapies. NPs present very different pharmacokinetic and pharmacodynamic properties compared with free drugs and enable the use of lower doses of immune-stimulating molecules, minimizing their side effects. However, NPs face issues concerning stability in physiological conditions, protein corona (PC) formation, and accumulation in the target tissue. PC formation changes the physicochemical and biological properties of the NPs and in consequence their therapeutic effect. This review summarizes the recent advances in the study of the effects of PC formation in NP-based immunotherapy. PC formation has complex effects on immunotherapy since it can diminish (“immune blinding”) or enhance the immune response in an uncontrolled manner (“immune reactivity”). Here, future perspectives of the field including the latest advances towards the use of personalized protein corona in cancer immunotherapy are also discussed.
Keywords: cancer immunotherapy, nanoparticles, protein corona, personalized protein corona, nanomedicine, immune response, immune blinding, immune reactivity, checkpoint inhibitors, personalized medicine
1. Introduction
Immunotherapy has attracted special attention in recent years as a novel cancer therapy, achieving durable and long-term responses in patients through the use of monoclonal antibodies or adoptive cell therapy [1,2]. However, immunotherapeutic resistance undermines the efficacy of these types of treatments [3]. Cancer vaccines based on antigen-specific immune responses against tumor-associated antigens present potential advantages in combination with other immune therapeutics [4,5].
Nanoparticles (NPs) are ideal candidates as platforms for developing novel cancer nano-vaccines due to their excellent physical and chemical properties, good colloidal stability, low toxicity, good biocompatibility, and due to the possibility of simultaneously loading adjuvants and antigens [6,7]. Despite these advantages, NPs frequently present colloidal stability problems under physiological conditions, poor circulation time in the bloodstream, and unwanted interactions with cells of the reticuloendothelial system such as macrophages [8]. However, one of the biggest limitations for nanoparticle-based therapies is the formation of the protein corona (PC) on the nanoparticle surface [9,10]. PC can change the physicochemical properties of the nano-formulations, and hence the desired therapeutic effect. This issue is especially important in the case of immunotherapy, where the recognition of the molecules on the surface of NPs is essential to trigger an immune response. In this review, we will summarize the latest research on PC and its effects on the immune-related treatment modulation.
The immune system is composed of different immune-organs and immune cells, which in communication with other non-immunological organs and cells are able to protect humans against foreign bodies or microorganisms. This protection is performed in parallel with the maintenance of tolerance towards self-antigens, contrary to for example in autoimmunity diseases or allergies in which this tolerance gets compromised [11,12,13]. The immune system generates two types of immune responses: the innate and the adaptive immune response. The first involves dendritic cells (DCs), natural killers (NKs), and macrophages, among other cells, and it is responsible for the very first barrier that pathogens meet. DCs and macrophages reside in many different tissues and when a foreign body is recognized by the pattern recognition receptors (PRRs), they trigger an inflammation response which serves as an alert to other immune cells (innate and adaptive cells). The innate immune cells that migrate to the inflammation area enhance the alert signal, and the adaptive immune cells (lymphocytes) start a specific response against the foreign body. The main differences between the two immune responses are the response time and the specificity, the latter being a unique feature of the adaptive immune response [14,15]. Sometimes, the immune system itself can cause pathologies by losing the control over the small auto-reactive population of cells normally present in the body, overreacting against the body itself, and leading to autoimmune diseases [16]. Another disorder, which is not considered a disease, is allergy. In the case of allergy, the presence of an antigen that should not be immunogenic provokes a strong immune response [17]. What is more, immune disorders can be also caused by a clever pathogen such as human immunodeficiency virus (HIV), that misleads the immune system and provokes an immunodeficiency [18].
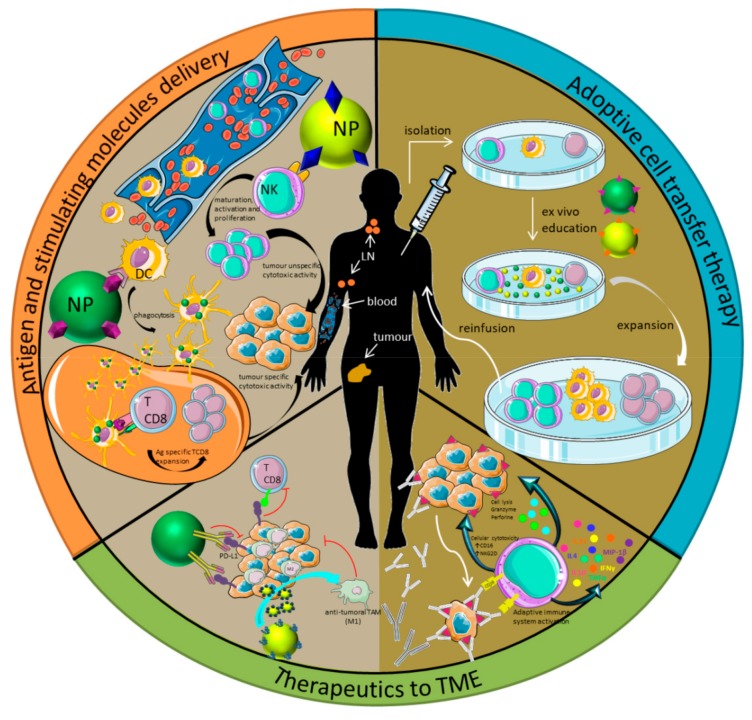
Immunotherapy focuses on exploiting the immune system of the patient to treat a disease. Immunotherapy can be applied for two main purposes, inhibition or enhancement of the immune system, depending on the desired effect. In terms of therapy, the most general classification is the division between active or passive immunotherapy (Figure 1) [19]. This classification depends on how the therapy stimulates the immune system. Active immunotherapy includes treatments aimed at priming the immune response against antigens. In this category are included vaccines and checkpoint inhibitors for example, since they are only immune active with the collaboration of the host immune system. In contrast, passive immunotherapy involves an intrinsic immune response, mediated by the administration of immune stimulating molecules, like cytokines or antibodies. This is the case of adoptive transfer therapy and monoclonal therapeutic antibodies (mAb), among others [20].
Figure 1.
Classification of immunotherapy strategies. Active (pale brown on the left) vs. passive immunotherapy (dark brown on the right). The three main immunotherapy strategies used in cancer treatment are shown in the figure separately: (1) Antigen and stimulating molecule delivery (orange); (2) Adoptive transfer therapy (blue); and (3) Therapeutics to the tumor microenvironment (TME) (green). Figure adapted from [20,27]. NP: nanoparticle; NK: natural killer; DC: dendritic cell.
Although cancer is not considered an immune disease, the pathology emerges from failures in the immune response. In a normal situation, cancer cells should be eliminated from the body through the immune response. However, in cancer pathology, these aberrant cells (cancer cells) confuse the immune system and cause immune-resistance by promoting immunosuppressive signals. This immune escape results in the tumor progression [21,22]. Considering this effect, treating cancer in combination with immune-stimulating therapies arises as a promising therapeutic strategy. This strategy was applied for first time in the late state of nineteenth-century when William B. Coley treated a patient who had an inoperable sarcoma and Streptococcus sp. He observed how the tumor volume decreased as a consequence of the immune response boosted by the bacterial infection [23]. During the last two decades, immunotherapies have been improved, not only as proof of concept at the pre-clinical stage but also in clinical research, which has led to ongoing clinical trials (NCT01898793, NCT03068819, and NCT02782546) [24]. In depth, several new treatments have been approved in the last decade (Table 1) and James P. Allison and Tasuku Honjo were awarded the Nobel Prize in Physiology or Medicine 2018 for their work in the field of cancer immunotherapy and the discovery of cancer therapy by inhibition of negative immune regulation [25,26].
Table 1.
Recently studied nanoformulations for immunotherapy. Some nano-formulations are approved by Food and Drug Administration (FDA) and others are in clinical trials [28].
| Compound Name | Formulation Description | Chemotherapy | Immunotherapy Type | Route of Immunization | Clinical Trials | Approved by the FDA | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Immunotherapy | Ferumoxytol (Ferahem®) |
IONP | No | Active | TME→ M2-like macrophages to M1-like | Yes, for anemia and kidney diseases | [29] | |
| eCPMV | VLP of cowpea mosaic virus | No | Active | Neutrophil activation in the TME | - | - | [30] | |
| RNA-LPX (Lipoplex®) | RNA-loaded liposomes | No | Active | DC maturation, Tcell response, inflammatory response | Phase I (2016) | [31] | ||
| PTX-LDE | Paclitaxel-loaded lipid core NPs | Yes | Active | DC maturation [32] | Phase II (2017) | [33] | ||
| MRX34 | miRNA-34a-loaded liposome | No | Passive | Downregulation of immune evasion tumor genes | Phase I (2016) | [34] | ||
| nab-Paclitaxel (Abraxane®) | Paclitaxel-loaded albumin NPs | Yes | Not applied right now | DC maturation | Phase III (2017) | Yes, for cancer treatment | [35,36] | |
| aCD47@CaCO3 | Anti CD47-loaded CaCO3 NP in fibrinogen solution | No | Active | After surgery, and with the addition of thrombin, aCD47@CaCO3 forms a immunotherapeutic gel in situ in the TME | - | - | [37] | |
| Sipuleucel-T (Provenge®) |
ex vivo DCs | No | Active | Vaccine | Yes, for prostate cancer | [38] | ||
| Blinatumomab (Blincyto®) |
Bi-specific T cell engager (BiTE). Specific to CD19 and CD3. | No | Passive | BiTE targeting CD19 (malignant B cell) and CD3 (T cell) and cytotoxicity effect against B cells. | Yes, for Philadelphia negative Acute lymphocytic leukaemia (ALL) | [39] | ||
| Talimogene Laherparepvec (T-VEC) | Injectable modified herpes virus | No | Active | Vaccine | Yes, for advanced melanoma | [40] | ||
| No immunotherapy | BIND-014 | Docetaxel-loaded Poly-Lactic Acid (PLA) NP and Prostate-Specific Membrane Antigen (PSMA) in the surface | Yes | No | - | Phase II(2018) | No | [41,42] |
| SPIO | Super paramagnetic iron oxide NPs | No. Only for imaging | - | - | Yes for imaging | [43] | ||
| Doxil® | Dox-loaded liposome | Yes | No | - | - | Yes | [44] | |
| Marqibo | Vincristine-loaded liposome | Yes | No | - | - | Yes, for Ph negative ALL | [45] | |
| Ontak® | Protein NPs | Yes | No | - | - | Yes, for cutaneous T cell lymphoma | [46] | |
In addition to the general classification of immunotherapies in active or passive therapies, there are three main strategies in which immunotherapies could be grouped (Figure 1), at least in cancer immunotherapy. The first strategy is the use of nanoparticles (NPs) as delivery systems for antigens or stimulating molecules (Figure 1, orange section). NPs could act as nanocarriers for stimulating molecules, which will be transported to target specific receptors and trigger the immune system activation or inhibition. Moreover, if the transported molecule is an antigen they are known as nano-vaccines. In this case, the objective of the strategy is to reach the lymph nodes (LNs) to target the T lymphocytes and generate a specific cytotoxic response against the tumor [47,48]. The second strategy is the adoptive cell transfer therapy (Figure 1, blue section), in which the immune cells are isolated from the patient, educated ex vivo [49,50,51], and reinfused into the patient. Finally, the third strategy is the delivery of therapeutics to the tumor microenvironment, by the direct infusion of the therapeutic agents into the tumor area (Figure 1, green section). One example is the use of checkpoint inhibitors that recognize programmed death-ligand 1 (PD-L1) or cytotoxic T lymphocyte antigen 4 (CTLA-4) ligands on the cell membrane of tumor cells and block the inhibition mediated by those checkpoint inhibitors that prevent T cells from killing cancer cells [52,53]. Other promising strategies are based on the polarization of the tumor-associated macrophages (TAMs) from M2 to M1, which present anti-tumor properties [29,54]. These strategies present some limitations including (1) the determination of the more effective small molecules in TAM polarization, (2) the difficulty in preferentially delivering small molecules to TAMs in vivo, and (3) reaching sub-optimal therapeutic efficacy. Rodell et al. described the polarization of TAMs from M2 to M1 in multiple tumor models in mice [55].Using a monotherapy based on an agonist of the toll-like receptors (TLRs)loaded in β-cyclodextrin nanoparticles they altered the functional orientation of the tumor immune microenvironment towards an M1 phenotype, leading to a controlled tumor growth and the protection of the animals against tumor re-challenge. In addition, using this nano-formulation in combination with the immune checkpoint inhibitor anti-PD-1, they also observed improved immunotherapy response rates in a tumor model resistant to anti-PD-1 therapy [55]. More recently, this strategy in combination with CD47 antagonists has been considered to inhibit cancer recurrence and metastasis effectively. Chen et al. have developed an in situ formed immunotherapeutic bioresponsive gel that controls both local tumor recurrence after surgery and development of distant tumors. In this work, calcium carbonate nanoparticles pre-loaded with the anti-CD47 antibody were encapsulated in a fibrin gel and scavenge H+ in the surgical wound, allowing polarization of tumor-associated macrophages to the M1-like phenotype. The released anti-CD47 antibody blocks the ‘don’t eat me’ signal in cancer cells, thereby increasing phagocytosis of cancer cells by macrophages. In this way, macrophages can promote effective antigen presentation and initiate T cell mediated immune responses that control tumor growth [37].
Over the last years, immunotherapy has become the main promise in cancer treatment. However, there are still some challenges related to the successful use of immunotherapy in cancer. One of these limitations is primary or acquired resistance. Cancer cells may change the expression levels of their membrane proteins, for example, the programmed death-ligand 1 (PD-L1). The binding of the PD-L1 to its receptor PD-1 (present in the surface of immune cells) involves the delivery of inhibitory signals that function as a brake for immune responses. Therefore, through the over-expression of PD-L1, cancer cells send immune inhibitory signals through PD-L1/PD-1 complex favoring immune escape and accelerating tumor progression. This is the mechanism by which cancer tissues limit the host immune response via up-regulation of the abovementioned PD-L1 and its binding to the programmed death-1 (PD-1) receptor on antigen-specific CD8+ T cells (termed adaptive immune resistance) [56,57]. Therapies targeting these inhibitory receptors, for example PD-1 receptor, have shown outstanding rates of durable clinical responses in patients with different cancer types [58]. Another limitation of cancer immunotherapy is the difficulty of reaching the tumor location away from the injection route. To overcome this limitation, the treatment dose is generally increased to achieve an efficient drug dose in the tumor [59]. However, this dose increase could trigger undesired side effects harmful to the patients. This limitation makes NPs ideal candidates for the delivery of cancer immunotherapy since they follow different pharmacokinetics and pharmacodynamics compared to free drugs [60,61].
Over the last two decades, NPs have been widely explored for their use in biomedical applications. Nevertheless, immunotherapy was not the first biomedical use of NPs. A few years ago, editors from Science named immunotherapy as the “Breakthrough of the year” [1]. After this fact, NPs started being used as immunotherapeutic agents in addition to chemotherapeutic agents. Moreover, some anticancer treatments based on NP formulations have been approved by the FDA, and are currently in clinical trials (Table 1). NPs have shown great promise in the treatment of cancer as cancer nano-immunotherapies. However, these systems face issues concerning stability in physiological media, PC formation, and accumulation in the target tissue. Among several important issues, the formation of a PC around the NPs in the presence of biological fluids plays an important role, mainly in changing the physicochemical properties of the nano-formulations, with consequent diminished in the therapeutic efficacy of nanomedicines. Furthermore, the modification of the surface of the particles is patient-specific and the formation of a PC may have additional undesired effects on the performance of the NPs including loss of efficacy of targeting moieties, undesired flagging by the complement, unspecific uptake by immune cells, and immunotoxicity.
2. Formation of Protein Corona: Effect on NP-Based Immunotherapy
In the last years NPs have been widely explored for their use in biomedical applications. In this context, it is important to understand the interactions occurring at the interface between NPs and biological fluids to predict the fate of injected NPs. It is commonly accepted that the interaction of the NPs and biological fluids is a consequence of several factors.NP size, shape, charge, or coating agents are critical [62,63,64,65,66,67,68,69], but the characteristics of the biological fluids are also very important (ionic strength, protein concentration, pH, and temperature) [70]. Once NPs are exposed to biological fluids, they interact with active biomolecules (mostly proteins, but also sugars, nucleic acids, and lipids) and PC is formed around them by the unspecific absorption of proteins on the surface of the NPs. This effect gives the NPs, upon protein corona formation, a different biological identity compared to bare NPs. The physicochemical properties of the bare NPs such as size, surface charge, surface composition, and functionality, change due to the PC formation. Therefore, the characterization of the properties of NPs after their exposure to a biological fluid has become mandatory for two purposes, to understand how these new characteristics affect the behavior of the nano-formulation in vivo and to design strategies to avoid the PC formation. In this context, Zhou et al. disclosed that the dynamic structure of nanoparticle surfaces can affect the protein adsorption kinetics and thus the interaction between nanoparticles/adsorbed proteins and cells [71].
Recently, the scientific community has been moving from the mere evaluation of the impact of the PC on the physicochemical properties of NPs to the evaluation of the impact on their behavior in physiological systems. Furthermore, a large number of studies have provided much insight into the layer thickness and composition of the PC, and the adsorption kinetics under different experimental setups. Many techniques have been used to measure the absorption of proteins around the NPs such as UV-visible spectroscopy (UV/Vis), dynamic light scattering (DLS), transmission electron microscopy (TEM), and fluorescence correlation spectroscopy (FCS) [72,73,74]. Another non-optical method that allows for the measurement of PC formation in complex media such as blood is 19F diffusion measured by nuclear magnetic resonance (NMR) [75]. In addition, different techniques such as surface plasmon resonance (SPR), isothermal titration calorimetry (ITC), differential centrifugal sedimentation (DCS), and quartz crystal microbalance (QCM) have been used to quantify the affinities of proteins for NPs [76,77,78,79,80]. Nevertheless, liquid chromatography–mass spectrometry (LC-MS) is probably the most powerful tool to identify proteins present in the protein corona [81,82].
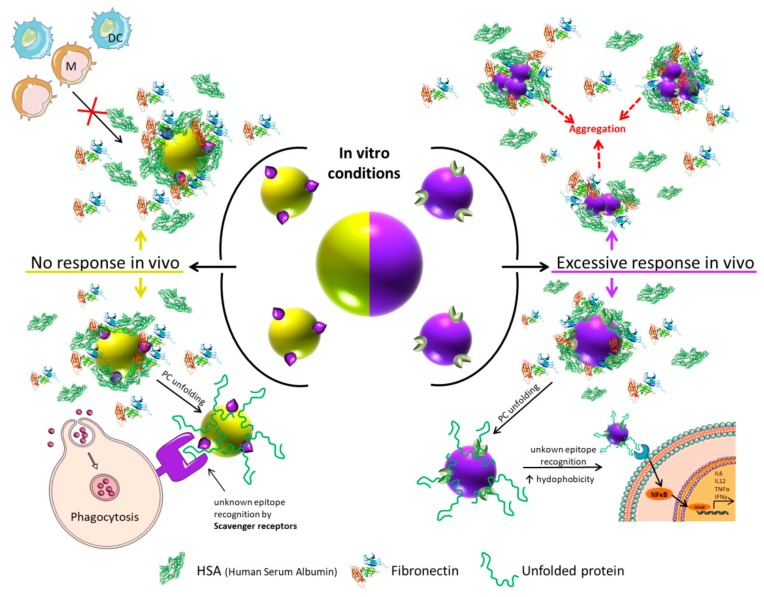
PC formation is especially relevant in the field of the immunotherapy, since in order to trigger an immune response it is essential the interaction between antigens or other molecules and their receptors. In this sense, has been demonstrated that the PC can have a dual role in biomolecular recognition (Figure 2). In some cases, the PC hides the antigen/molecule on the NPs surface, thereby inhibiting the interaction with its specific receptor (this immune-escape process could be defined as “immune-blinding”); and in other cases the PC contains proteins that act as ligands for receptors on specific immune cells and triggers undesirable immune responses (immune reactivity) [83,84]. Additionally, PC can provoke the phagocytosis by monocytes and macrophages [83] and in consequence could avoid the recognition of the stimulating molecules exposed in the surface of the NPs.
Figure 2.
Nanoparticle-based immunotherapy failure because of protein corona (PC) formation. No-response (left) vs. excessive or uncontrolled response (right). Top left panel: Immune cells are not able to recognize the molecules on the surface of the NPs because the PC covers the NPs partially or totally. Bottom left: NP phagocytosis by macrophages because of the denaturalization of the proteins (in green) on the surface of the NPs. Top right: Aggregation of NPs triggers toxic effects by strange-body recognition by immune system. Bottom right: Nuclear Factor κB (NF-κB) translocation to the nucleus because of the recognition of denatured proteins on the surface of the NPs.
2.1. Immune-Blinding as a Consequence of PC Formation
As shown in Figure 2, the immune-blinding could be promoted by two main mechanisms. On the one hand, PC may fully or partially covers the antigens/molecules present on the surface of the NPs, and in consequence, the specific stimulation will be low or fail and consequently the immune response will not occur. Shanehsazzadeh et al. described a good example on how PC can induce immune-blinding on a nano-formulation in vivo. The uptake of NPs functionalized with anti-mucin1 protein (anti-MUC-1) antibody was nine times higher in MUC-1-positive cells than in the MUC-1 negative cells in vitro. However, in the in vivo mice model, the antibody-functionalized NPs showed higher distribution in blood and muscle than in tumor. The conclusion of this work was that the PC covered the targeting molecules and in consequence, the tumor uptake in vivo was reduced [85]. Conversely, other NP formulations based on the virus-like particle (VLP)-functionalized anti-MUC-1 antibody displayed higher tumor accumulation and also higher metastatic protection in mice than non-functionalized NPs [86]. These differences in vivo evidence the importance of the NP composition in the possible immune-blinding effect promoted by the PC, as was also shown in a study in which a differential PC formation was observed depending on the PEGylation grade of NPs [87]. In the case of nano-vaccine based therapies, the PC also plays a critical role in the uptake of the nano-formulation by dendritic cells, which is a critical step for an effective therapeutic response [62,88].
On the other hand, the blinding effect can be due to the homeostatic function of immune cells. Macrophages have so-called scavenger receptors that recognize biological patterns on strange bodies [89]. Sometimes, the structure of the proteins that formed the PC is altered during the PC formation on the surface of the NPs [90], and in consequence usually unexposed epitopes are presented to the immune system (Figure 3a). Macrophages could recognize these epitopes and phagocyte the NP–PC complexes through the scavenger receptors (Figure 2) [91,92]. This situation could be solved changing the physicochemical properties of the NPs and in consequence reducing the uptake of the NPs by macrophages [93].
Figure 3.
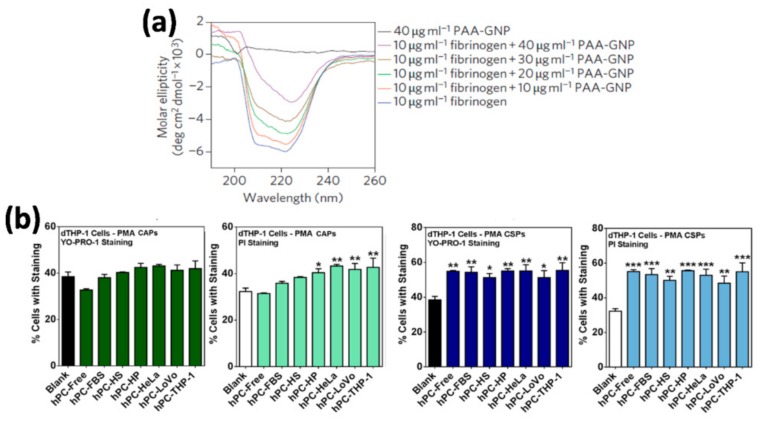
(a) Protein denaturalization on the surface of NPs. Secondary structure of the fibrinogen protein measured by circular dichroism after being incubated with different concentrations of 5-nm poly(acrylic acid)-coated gold nanoparticles (PAA–GNPs).Figure from [94]. (b) Effect of protein corona formation and composition on early apoptosis and cell death responses in THP-1 cells, a human Leukemic monocytes cell line. Poly (metacrylic acid) hollow particles (PMA CAPs) (in green) and PMA cores-shell particles (PMA CSPs) (in blue) were exposed to various environments (different media, serum free control, fetal bovine serum (FBS), human serum (HS), human plasma (HP), and culture media with three different cell lines HeLa, LoVo, and THP-1) to acquire a hard protein corona (hPC). The early apoptosis (YO-PRO-1 staining) and cell death (PI staining) were measured by flow cytometry. Data are shown as the mean ± standard error of at least four independent experiments, with at least 10,000 cells analyzed in each experiment. * p< 0.05, ** p< 0.01, *** p< 0.001, versus the column of “Blank” (indicating untreated cells) (one-way ANOVA Dunnett’s multiple comparison test). Figure from [95].
2.2. Immune-Response or Immune-Reactivity as a Consequence of PC Formation
The difference between a controlled and an uncontrolled immune response, which could be called immune-reactivity, is not always clear. As immunotherapy enhances the very complex immune system, this stimulation has to be carefully designed. In nano-immunotherapy it is essential to consider that the contact of NPs with a biological fluid will most probably provoke the formation of a protein corona on the NPs surface that could trigger an excessive immune activity, resulting mostly in inflammation [93]. This phenomenon is commonly related to the excessive production of the pro-inflammatory cytokines such as TNFα, INFγ, IL-6, and IL-12 and the exacerbated inflammatory response associated to high levels of these cytokines that could destroy healthy cells and tissues. In this context, Dai et al. observed differences in pro-inflammatory cytokine secretion and immune cell apoptosis when studying the interaction of NP–PC complexes, formed in various biologically relevant environments, with macrophages. They observed that the NP–PC complexes either increased or mitigated the secretion of a specific cytokine, depending on the environment where the protein corona was formed (Figure 3b) [95]. On the other hand, although it has been demonstrated that the PC could trigger pro-inflammatory responses and cell death, some works suggest that PC could also have protective properties. Escamilla-Rivera et al. studied the role of the PC as potential protector for Reactive Oxygen Species (ROS)-induced cytotoxicity and pro-inflammatory response in macrophages exposed to iron oxide NPs. They observed that the reduction in Iron Oxide Nano-Particle (IONP) cytotoxicity can be attributed to the PC shielding against ROS generation and pro-inflammatory response in macrophages [96].
The formation of protein corona on the NP surface does not always trigger an excessive immune activity per se, as has been previously described. Sometimes, the excessive immune activity could be associated with the presence of unfolded proteins in the NP–PC complexes. In the same way that macrophages can recognize some unfolded proteins present in NP–PC complexes through the scavenger receptors and phagocyte them, other specific receptors can recognize unfolded proteins present in NP–PC complexes and trigger an exacerbate inflammation response. Deng et al. described that negatively charged poly(acrylic acid) (PLA)-conjugated gold NPs bind to and induce unfolding of fibrinogen, which promotes interaction with the integrin receptor, Mac-The activation of Mac-1 receptor increases the NF-κB signaling pathway, resulting in the release of inflammatory cytokines [94]. However, not all NPs that bind to fibrinogen showed this effect, which illustrates the influence of the physicochemical properties of the NPs on the cellular innate immune response. The role of the physicochemical properties of the materials, such us surface chemistry and wettability, on the formation of PC in human serum and the subsequent effects on the cellular innate immune response have been also investigated. Visalakshan et al. demonstrated that the amount and identity of proteins adsorbed on the surface of the different materials were strongly influenced by surface chemistry and wettability, which led to a distinct immune response from macrophages. Hydrophilic surfaces mostly adsorbed dysopsonin and albumin, which induced a greater expression of anti-inflammatory cytokines by macrophages. In contrast, hydrophobic surfaces mostly adsorbed Immunoglobulin G2 (IgG2) type opsonin, which caused increased production of pro-inflammatory signaling molecules [97]. Therefore the identity of the adsorbed proteins on the surface of the NPs has also an important role in triggering excessive immune responses. The administration of any nanomaterial to animals or humans results in the adsorption of proteins onto the nanomaterial surface and the subsequent complement activation. The complement pathway activation is translated into an exacerbate inflammation response. This effect may be related to the observations that the presence in the PC of the third component of the complement protein (C3) affects the immune cell recognition of nanomedicines [98,99,100]. To avoid the nanomaterial-induced complement activation, many researchers have used highly biocompatible materials such as zwitterionic polymers as well as hydrophilic nanoparticles which decrease the protein adsorption [101], and biomaterials already wrapped with “self” proteins such as CD200 [102]. More recently, cell membrane coatings have emerged as a new class of coatings that enable the camouflage of NPs for evading immune clearance and lessen the complement activation by nanoparticles [10,103,104]. For example, Fan et al. developed a coating based on red blood cell (RBC) membranes that was able to camouflage the particles from the immune system and significantly reduced the number of infiltrating neutrophils [105].
These works and other many studies map out relationships between the physicochemical properties of the NPs and other materials, the protein corona formation, and subsequent cellular innate immune responses [106]. The potential outcomes of these studies can guide the development of new nanomaterials to modulate serum protein adsorption and to avoid undesirable innate immune responses.
3. Future Perspectives
Nanoparticles have shown great promise in the treatment of cancer and as synthetic nano-vaccines. However, these systems face issues concerning stability under physiological conditions, protein corona formation, and accumulation in the target tissue. One of the biggest limitations for nanoparticle-based therapies is the formation of a protein corona (PC) on the nanoparticle surface and its effect on their biological performance. Therefore, it is fundamental to include systematic studies on NP–PC complexes and to define the biological identity of the final nano-formulation in the field of nano-therapy [107,108]. These studies will be useful in order to predict the physicochemical changes, such as protein absorption, of the NPs that could affect NP-based immunotherapies causing “immune blinding” or immune reactivity.
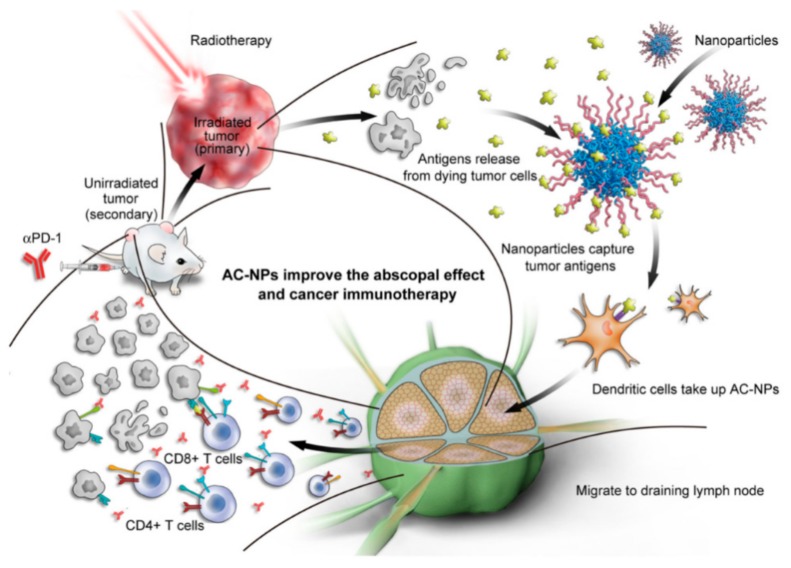
Even though protein adsorption and PC formation appear to be two of the biggest limitations for nanoparticle-based therapies, there is a possibility to revert this situation to a positive scenario. This is the case of the personalized protein corona (PPC) [109,110,111]. Recently, it has been shown that the protein corona is strongly affected for example by specific diseases in patients from which the plasma is obtained. Therefore, the same nanomaterial incubated with plasma proteins from patients with different pathologies generates protein coronas with different compositions, giving rise to the concept of personalized protein corona [109]. In recent years, the PPC has become a very interesting approach to screen protein biomarkers in plasma for early diagnosis and treatment of different diseases [102,112]. Specially, the PPC could be revolutionary in the field of cancer immunotherapy. In this sense, one of the best examples of NP–PPC based cancer immunotherapy was designed taking into account a key clinical approach to improve cancer immunotherapy: the combination of radiotherapy with checkpoint inhibitors. This combinatory therapy produces an immune stimulation by radiation-induced pro-inflammatory protein production and increases the exposure of immune cells to cancer-specific antigens that are released following radiotherapy-induced cancer cell death [113,114,115,116]. Therefore, NPs could be used to capture tumor-derived protein antigens (TDPAs) released during radiotherapy and transporting them to antigen-presenting cells (APCs), thereby promoting anti-cancer immunity (Figure 4) [117].
Figure 4.
The protein corona in cancer immunotherapy. Schematic description of an NP–PPC based therapy mechanism in which radiotherapy induces the release of tumor-derived protein antigens (TDPAs) that are integrated in the PC and promote the antigen presentation and the anti-cancer immune activation. This approach is applied in combination with checkpoint inhibitor therapy. Figure from [117]. PPC: personalized protein corona. AC-NPs refers to Antigen Capture-Nano Particles.
In addition to the PPC, PC could also be modified to modulate the NP–PC biological properties. In this context, Wan et al. demonstrated that the post-modification (de-glycosylation) of the NP–PC complexes had a critical role in the improvement of NP-cell interaction and in the cellular uptake. The de-glycosylation of the NP–PC complexes enhanced cell membrane adhesion to two types of THP-1 differentiated macrophages (polarized to M1 and M2), leading to an increase in NPs uptake [118].
Within the context of the recent developments in cancer immunotherapy research and in the impact of protein corona formation on the efficacy of nanotherapies, a few aspects can be highlighted to establish the path for the future developments. The complexity of the effect of protein corona formation in nanomedicine and in particular in the context of nano-immunotherapy reveals the need of detailed fundamental studies on the protein corona formation and the interaction of protein corona with the immune system. Recently, big research efforts have been made toward developing “stealth” nanomaterials to avoid the formation of the protein corona. However, these approaches had shown limited success, and there is still room for future surface engineering advances to minimize the formation of the protein corona.
An alternative ground-breaking path is the use of the protein corona to endow biological functions to the nanoparticles by fine tuning and modifying the protein corona composition. The encouraging preliminary studies on the use of personalized protein corona in cancer immunotherapy described above establish the basis for this research direction. Therefore, the next frontier in nano-immunotherapy is the design of advanced nanomaterials with the personalized protein corona, in which the previous drawbacks related to the formation of the protein corona are turned into advantages. This approach is framed in the broader context of the recent boost of the personalized medicine.
Author Contributions
All the authors have contributed to the conceptualization, writing, and editing of the review. All authors have read and agreed to the published version of the manuscript.
Funding
Authors acknowledge the Maria de Maeztu Units of Excellence Program from the Spanish State Research Agency—Grant No. MDM-2017-0720 (CIC biomaGUNE). Authors also acknowledge the Basque Government, Health Department (RIS3-2019222005).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Couzin-Frankel J. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 2.Sharpe A.H. Introduction to checkpoint inhibitors and cancer immunotherapy. Immunol. Rev. 2017;276:5–8. doi: 10.1111/imr.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P., Hu-Lieskovan S., Wargo J.A., Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeng H., Terabe M., Berzofsky J.A. Cancer vaccines: Translation from mice to human clinical trials. Curr. Opin. Immunol. 2018;51:111–122. doi: 10.1016/j.coi.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vilgelm A.E., Johnson D.B., Richmond A. Combinatorial approach to cancer immunotherapy: Strength in numbers. J. Leukoc. Biol. 2016;100:275–290. doi: 10.1189/jlb.5RI0116-013RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith D.M., Simon J.K., Baker J.R. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irvine D.J., Hanson M.C., Rakhra K., Tokatlian T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem. Rev. 2015;115:11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grabowski N., Hillaireau H., Vergnaud J., Tsapis N., Pallardy M., Kerdine-Römer S., Fattal E. Surface coating mediates the toxicity of polymeric nanoparticles towards human-like macrophages. Int. J. Pharm. 2015;482:75–83. doi: 10.1016/j.ijpharm.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 9.Corbo C., Molinaro R., Parodi A., Toledano Furman N.E., Salvatore F., Tasciotti E. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine. 2016;11:81–100. doi: 10.2217/nnm.15.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang R.H., Kroll A.V., Gao W., Zhang L. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018;30:1–34. doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munro N. Immunology and immunotherapy in critical care: An overview. AACN Adv. Crit. Care. 2019;30:113–125. doi: 10.4037/aacnacc2019415. [DOI] [PubMed] [Google Scholar]
- 12.Gensollen T., Blumberg R.S. Correlation between early-life regulation of the immune system by microbiota and allergy development. J. Allergy Clin. Immunol. 2017;139:1084–1091. doi: 10.1016/j.jaci.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gershwin L.J. Current and Newly Emerging Autoimmune Diseases. Vet. Clin. North Am. Small Anim. Pract. 2018;48:323–338. doi: 10.1016/j.cvsm.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Ferro K., Peuß R., Yang W., Rosenstiel P., Schulenburg H., Kurtz J. Experimental evolution of immunological specificity. Proc. Natl. Acad. Sci. USA. 2019;116:20598–20604. doi: 10.1073/pnas.1904828116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Natoli G., Ostuni R. Adaptation and memory in immune responses. Nat. Immunol. 2019;20:783–792. doi: 10.1038/s41590-019-0399-9. [DOI] [PubMed] [Google Scholar]
- 16.Wahren-Herlenius M., Dörner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet. 2013;382:819–831. doi: 10.1016/S0140-6736(13)60954-X. [DOI] [PubMed] [Google Scholar]
- 17.Moriyama T. Diversity of food allergy. J. Nutr. Sci. Vitaminol. 2015;61:S106–S108. doi: 10.3177/jnsv.61.S106. [DOI] [PubMed] [Google Scholar]
- 18.Vitallé J., Terrén I., Gamboa-Urquijo L., Orrantia A., Tarancón-Díez L., Genebat M., Ruiz-Mateos E., Leal M., García-Obregón S., Zenarruzabeitia O., et al. Altered expression of CD300a inhibitory receptor on CD4+ T cells from human immunodeficiency virus-1-infected patients: Association with disease progression markers. Front. Immunol. 2018;9:1–13. doi: 10.3389/fimmu.2018.01709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lesterhuis W.J., Haanen J.B.A.G., Punt C.J.A. Cancer immunotherapy-revisited. Nat. Rev. Drug Discov. 2011;10:591–600. doi: 10.1038/nrd3500. [DOI] [PubMed] [Google Scholar]
- 20.Galluzzi L., Vacchelli E., Bravo-San Pedro J.-M., Buqué A., Senovilla L., Baracco E.E., Bloy N., Castoldi F., Abastado J.-P., Agostinis P., et al. Classification of current anticancer immunotherapies. Oncotarget. 2014;5:12472–12508. doi: 10.18632/oncotarget.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAllister S.S., Weinberg R.A. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat. Cell Biol. 2014;16:717–727. doi: 10.1038/ncb3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen S.R., Schmid M.C. Macrophages as Key Drivers of Cancer Progression and Metastasis. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/9624760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coley W.B. The Treatment of Inoperable Sarcoma by Bacterial Toxins (the Mixed Toxins of the Streptococcus erysipelas and the Bacillus prodigiosus) Proc. R. Soc. Med. 1909;3:1–48. doi: 10.1177/003591571000301601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulder A., Willey S., Schappe T., Berrien-Elliott M.M., Schneider S.E., Oh S.T., Wagner J.A., Yu L., Claas F., Neal C.C., et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016;8:357. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 26.Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C., Ye Y., Hu Q., Bellotti A., Gu Z. Tailoring Biomaterials for Cancer Immunotherapy: Emerging Trends and Future Outlook. Adv. Mater. 2017;29:1–24. doi: 10.1002/adma.201606036. [DOI] [PubMed] [Google Scholar]
- 28.Sahakyan N., Haddad A., Richardson S., Forcha-Etieundem V., Christopher L., Alharbi H., Campbell R. Personalized Nanoparticles for Cancer Therapy: A Call for Greater Precision. Anticancer. Agents Med. Chem. 2017;17:1033–1039. doi: 10.2174/1871520617666170102150730. [DOI] [PubMed] [Google Scholar]
- 29.Zanganeh S., Hutter G., Spitler R., Lenkov O., Mahmoudi M., Shaw A., Pajarinen J.S., Nejadnik H., Goodman S., Coussens L.M., et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lizotte P.H., Wen A.M., Sheen M.R., Fields J., Rojanasopondist P., Steinmetz N.F., Fiering S. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 2016;11:295–303. doi: 10.1038/nnano.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C., Meng M., Fritz D., Vascotto F., Hefesha H., et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 32.Pfannenstiel L.W., Lam S.S.K., Emens L.A., Jaffee E.M., Armstrong T.D. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell. Immunol. 2010;263:79–87. doi: 10.1016/j.cellimm.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graziani S.R., Vital C.G., Morikawa A.T., Van Eyll B.M., Fernandes Junior H.J., Kalil Filho R., Maranhão R.C. Phase II study of paclitaxel associated with lipid core nanoparticles (LDE) as third-line treatment of patients with epithelial ovarian carcinoma. Med. Oncol. 2017;34:151. doi: 10.1007/s12032-017-1009-z. [DOI] [PubMed] [Google Scholar]
- 34.Beg M.S., Brenner A.J., Sachdev J., Borad M., Kang Y.K., Stoudemire J., Smith S., Bader A.G., Kim S., Hong D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest. New Drugs. 2017;35:180–188. doi: 10.1007/s10637-016-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martín M., Chacón J.I., Antón A., Plazaola A., García-Martínez E., Seguí M.A., Sánchez-Rovira P., Palacios J., Calvo L., Esteban C., et al. Neoadjuvant Therapy with Weekly Nanoparticle Albumin-Bound Paclitaxel for Luminal Early Breast Cancer Patients: Results from the NABRAX Study (GEICAM/2011-02), a Multicenter, Non-Randomized, Phase II Trial, with a Companion Biomarker Analysis. Oncologist. 2017;22:1301–1308. doi: 10.1634/theoncologist.2017-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Zeng J., Huang M., An J., Bai P., Wu L., Zhang R. A phase 2 study of nanoparticle albumin-bound paclitaxel plus nedaplatin for patients with advanced, recurrent, or metastatic cervical carcinoma. Cancer. 2017;123:420–425. doi: 10.1002/cncr.30328. [DOI] [PubMed] [Google Scholar]
- 37.Chen Q., Wang C., Zhang X., Chen G., Hu Q., Li H., Wang J., Wen D., Zhang Y., Lu Y., et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019;14:89–97. doi: 10.1038/s41565-018-0319-4. [DOI] [PubMed] [Google Scholar]
- 38.Higano C.S., Armstrong A.J., Sartor A.O., Vogelzang N.J., Kantoff P.W., McLeod D.G., Pieczonka C.M., Penson D.F., Shore N.D., Vacirca J., et al. Real-world outcomes of sipuleucel-T treatment in PROCEED, a prospective registry of men with metastatic castration-resistant prostate cancer. Cancer. 2019;152:4172–4180. doi: 10.1002/cncr.32445. [DOI] [Google Scholar]
- 39.Dufner V., Sayehli C.M., Chatterjee M., Hummel H.D., Gelbrich G., Bargou R.C., Goebeler M.-E. Long-term outcome of patients with relapsed/refractory B-cell non-Hodgkin lymphoma treated with blinatumomab. Blood Adv. 2019;3:2491–2498. doi: 10.1182/bloodadvances.2019000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y., Fang Z., Li R., Huang X., Liu Q. Design of Outer Membrane Vesicles as Cancer Vaccines: A New Toolkit for Cancer Therapy. Cancers. 2019;11:1314. doi: 10.3390/cancers11091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Von Hoff D.D., Mita M.M., Ramanathan R.K., Weiss G.J., Mita A.C., Lorusso P.M., Burris H.A., Hart L.L., Low S.C., Parsons D.M., et al. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin. Cancer Res. 2016;22:3157–3163. doi: 10.1158/1078-0432.CCR-15-2548. [DOI] [PubMed] [Google Scholar]
- 42.Autio K.A., Dreicer R., Anderson J., Garcia J.A., Alva A., Hart L.L., Milowsky M.I., Posadas E.M., Ryan C.J., Graf R.P., et al. Safety and Efficacy of BIND-014, a Docetaxel Nanoparticle Targeting Prostate-Specific Membrane Antigen for Patients with Metastatic Castration-Resistant Prostate Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4:1344–1351. doi: 10.1001/jamaoncol.2018.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karakatsanis A., Olofsson H., Stålberg P., Bergkvist L., Abdsaleh S., Wärnberg F. Simplifying Logistics and Avoiding the Unnecessary in Patients With Breast Cancer Undergoing Sentinel Node Biopsy. A Prospective Feasibility Trial of the Preoperative Injection of Super Paramagnetic Iron Oxide Nanoparticles. Scand. J. Surg. 2018;107:130–137. doi: 10.1177/1457496917738867. [DOI] [PubMed] [Google Scholar]
- 44.Barenholz Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J., Chen Y., Li X., Liang X., Luo X. The influence of different long-circulating materials on the pharmacokinetics of liposomal vincristine sulfate. Int. J. Nanomedicine. 2016;11:4187–4197. doi: 10.2147/IJN.S109547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z., Zheng Q., Zhang H., Bronson R.T., Madsen J.C., Sachs D.H., Huang C.A., Wang Z. Ontak-like human IL-2 fusion toxin. J. Immunol. Methods. 2017;448:51–58. doi: 10.1016/j.jim.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cobaleda-Siles M., Henriksen-Lacey M., De Angulo A.R., Bernecker A., Vallejo V.G., Szczupak B., Llop J., Pastor G., Plaza-Garcia S., Jauregui-Osoro M., et al. An iron oxide nanocarrier for dsRNA to target lymph nodes and strongly activate cells of the immune system. Small. 2014;10:5054–5067. doi: 10.1002/smll.201470156. [DOI] [PubMed] [Google Scholar]
- 48.Ruiz-De-Angulo A., Zabaleta A., Gómez-Vallejo V., Llop J., Mareque-Rivas J.C. Microdosed lipid-coated 67Ga-magnetite enhances antigen-specific immunity by image tracked delivery of antigen and cpg to lymph nodes. ACS Nano. 2016;10:1602–1618. doi: 10.1021/acsnano.5b07253. [DOI] [PubMed] [Google Scholar]
- 49.Houot R., Schultz L.M., Marabelle A., Kohrt H. T-cell based immunotherapy: Adoptive cell transfer and checkpoint inhibition. Cancer Immunol. Res. 2015;3:1115–1122. doi: 10.1158/2326-6066.CIR-15-0190. [DOI] [PubMed] [Google Scholar]
- 50.Khalil D.N., Smith E.L., Brentjens R.J., Wolchok J.D. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016;13:273–290. doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen L.T., Saibil S.D., Sotov V., Le M.X., Khoja L., Ghazarian D., Bonilla L., Majeed H., Hogg D., Joshua A.M., et al. Phase II clinical trial of adoptive cell therapy for patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and low-dose interleukin-2. Cancer Immunol. Immunother. 2019;68:773–785. doi: 10.1007/s00262-019-02307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bianchini G., Pusztai L., Pienkowski T., Im Y.H., Bianchi G.V., Tseng L.M., Liu M.C., Lluch A., Galeota E., Magazzù D., et al. Immune modulation of pathologic complete response after neoadjuvant HER2-directed therapies in the NeoSphere trial. Ann. Oncol. 2015;26:2429–2436. doi: 10.1093/annonc/mdv395. [DOI] [PubMed] [Google Scholar]
- 53.Varricchi G., Marone G., Mercurio V., Galdiero M.R., Bonaduce D., Tocchetti C.G. Immune Checkpoint Inhibitors and Cardiac Toxicity: An Emerging Issue. Curr. Med. Chem. 2018;25:1327–1339. doi: 10.2174/0929867324666170407125017. [DOI] [PubMed] [Google Scholar]
- 54.Chen X., Zhu X., Ma L., Lin A., Gong Y., Yuan G., Liu J. Core-shell structure QRu-PLGA-RES-DS nanocomposite with photothermal response induced M2 macrophage polarization for therapy rheumatoid arthritis. Nanoscale. 2019;11:18209–18223. doi: 10.1039/C9NR05922A. [DOI] [PubMed] [Google Scholar]
- 55.Rodell C.B., Arlauckas S.P., Cuccarese M.F., Garris C.S., Li R., Ahmed M.S., Kohler R.H., Pittet M.J., Weissleder R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018;2:578–588. doi: 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spranger S., Spaapen R.M., Zha Y., Williams J., Meng Y., Ha T.T., Gajewski T.F. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tumeh P.C., Harview C.L., Yearley J.H., Shintaku I.P., Taylor E.J.M., Robert L., Chmielowski B., Spasic M., Henry G., Ciobanu V., et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang D., Baldwin P., Leal A.S., Carapellucci S., Sridhar S., Liby K.T. A nano-liposome formulation of the PARP inhibitor Talazoparib enhances treatment efficacy and modulates immune cell populations in mammary tumors of BRCA-deficient mice. Theranostics. 2019;9:6224–6238. doi: 10.7150/thno.36281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roma-Rodrigues C., Pombo I., Raposo L., Pedrosa P., Fernandes A.R., Baptista P.V. Nanotheranostics Targeting the Tumor Microenvironment. Front. Bioeng. Biotechnol. 2019;7:1–18. doi: 10.3389/fbioe.2019.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li S., Huang L. Pharmacokinetics and Biodistribution of Nanoparticles. Mol. Pharm. 2008;5:496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 62.Saikia J., Yazdimamaghani M., Hadipour Moghaddam S.P., Ghandehari H. Differential Protein Adsorption and Cellular Uptake of Silica Nanoparticles Based on Size and Porosity. ACS Appl. Mater. Interfaces. 2016;8:34820–34832. doi: 10.1021/acsami.6b09950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glancy D., Zhang Y., Wu J.L.Y., Ouyang B., Ohta S., Chan W.C.W. Characterizing the protein corona of sub-10 nm nanoparticles. J. Control. Release. 2019;304:102–110. doi: 10.1016/j.jconrel.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 64.Franqui L.S., De Farias M.A., Portugal R.V., Costa C.A.R., Domingues R.R., Souza Filho A.G., Coluci V.R., Leme A.F.P., Martinez D.S.T. Interaction of graphene oxide with cell culture medium: Evaluating the fetal bovine serum protein corona formation towards in vitro nanotoxicity assessment and nanobiointeractions. Mater. Sci. Eng. C. 2019;100:363–377. doi: 10.1016/j.msec.2019.02.066. [DOI] [PubMed] [Google Scholar]
- 65.Hühn D., Kantner K., Geidel C., Brandholt S., De Cock I., Soenen S.J.H., Riveragil P., Montenegro J.M., Braeckmans K., Müllen K., et al. Polymer-coated nanoparticles interacting with proteins and cells: Focusing on the sign of the net charge. ACS Nano. 2013;7:3253–3263. doi: 10.1021/nn3059295. [DOI] [PubMed] [Google Scholar]
- 66.Lai W., Wang Q., Li L., Hu Z., Chen J., Fang Q. Interaction of gold and silver nanoparticles with human plasma: Analysis of protein corona reveals specific binding patterns. Colloids Surfaces B Biointerfaces. 2017;152:317–325. doi: 10.1016/j.colsurfb.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 67.Giulimondi F., Digiacomo L., Pozzi D., Palchetti S., Vulpis E., Capriotti A.L., Chiozzi R.Z., Laganà A., Amenitsch H., Masuelli L., et al. Interplay of protein corona and immune cells controls blood residency of liposomes. Nat. Commun. 2019;10:3686. doi: 10.1038/s41467-019-11642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Partikel K., Korte R., Stein N.C., Mulac D., Herrmann F.C., Humpf H.U., Langer K. Effect of nanoparticle size and PEGylation on the protein corona of PLGA nanoparticles. Eur. J. Pharm. Biopharm. 2019;141:70–80. doi: 10.1016/j.ejpb.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 69.Aires A., Cabrera D., Alonso-Pardo L.C., Cortajarena A.L., Teran F.J. Elucidation of the physicochemical properties ruling the colloidal stability of iron oxide nanoparticles under physiological conditions. ChemNanoMat. 2017;3:183–189. doi: 10.1002/cnma.201600333. [DOI] [Google Scholar]
- 70.Ekdahl K.N., Fromell K., Mohlin C., Teramura Y., Nilsson B. A human whole-blood model to study the activation of innate immunity system triggered by nanoparticles as a demonstrator for toxicity. Sci. Technol. Adv. Mater. 2019;20:688–698. doi: 10.1080/14686996.2019.1625721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou H., Fan Z., Li P.Y., Deng J., Arhontoulis D.C., Li C.Y., Bowne W.B., Cheng H. Dense and Dynamic Polyethylene Glycol Shells Cloak Nanoparticles from Uptake by Liver Endothelial Cells for Long Blood Circulation. ACS Nano. 2018;12:10130–10141. doi: 10.1021/acsnano.8b04947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kokkinopoulou M., Simon J., Landfester K., Mailänder V., Lieberwirth I. Visualization of the protein corona: Towards a biomolecular understanding of nanoparticle-cell-interactions. Nanoscale. 2017;9:8858–8870. doi: 10.1039/C7NR02977B. [DOI] [PubMed] [Google Scholar]
- 73.Martinez-Moro M., Di Silvio D., Moya S.E. Fluorescence correlation spectroscopy as a tool for the study of the intracellular dynamics and biological fate of protein corona. Biophys. Chem. 2019;253:106218. doi: 10.1016/j.bpc.2019.106218. [DOI] [PubMed] [Google Scholar]
- 74.Carrillo-Carrion C., Carril M., Parak W.J. Techniques for the experimental investigation of the protein corona. Curr. Opin. Biotechnol. 2017;46:106–113. doi: 10.1016/j.copbio.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 75.Carril M., Padro D., Del Pino P., Carrillo-Carrion C., Gallego M., Parak W.J. In situ detection of the protein corona in complex environments. Nat. Commun. 2017;8:1542. doi: 10.1038/s41467-017-01826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lindman S., Lynch I., Thulin E., Nilsson H., Dawson K.A., Linse S. Systematic investigation of the thermodynamics of HSA adsorption to N-iso-propylacrylamide/N-tert-butylacrylamide copolymer nanoparticles. Effects of particle size and hydrophobicity. Nano Lett. 2007;7:914–920. doi: 10.1021/nl062743+. [DOI] [PubMed] [Google Scholar]
- 77.Baier G., Costa C., Zeller A., Baumann D., Sayer C., Araujo P.H.H., Mailänder V., Musyanovych A., Landfester K. BSA Adsorption on Differently Charged Polystyrene Nanoparticles using Isothermal Titration Calorimetry and the Influence on Cellular Uptake. Macromol. Biosci. 2011;11:628–638. doi: 10.1002/mabi.201000395. [DOI] [PubMed] [Google Scholar]
- 78.Bousquet Y., Swart P.J., Schmitt-Colin N., Velge-Roussel F., Kuipers M.E., Meijer D.K., Breton P. Molecular Mechanisms of the Adsorption of a Model Protein (Human Serum Albumin) on Poly(Methylidene Malonate 2.1.2) Nanoparticles.pdf. Pharm. Res. 1999;16:141–147. doi: 10.1023/A:1018843401077. [DOI] [PubMed] [Google Scholar]
- 79.Milani S., Baldelli Bombelli F., Pitek A.S., Dawson K.A., Rädler J. Reversible versus irreversible binding of transferrin to polystyrene nanoparticles: Soft and hard corona. ACS Nano. 2012;6:2532–2541. doi: 10.1021/nn204951s. [DOI] [PubMed] [Google Scholar]
- 80.Jiang X., Weise S., Hafner M., Röcker C., Zhang F., Parak W.J., Nienhaus G.U. Quantitative analysis of the protein corona on FePt nanoparticles formed by transferrin binding. J. R. Soc. Interface. 2010;7 doi: 10.1098/rsif.2009.0272.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Partikel K., Korte R., Mulac D., Humpf H.U., Langer K. Serum type and concentration both affect the protein-corona composition of PLGA nanoparticles. Beilstein J. Nanotechnol. 2019;10:1002–1015. doi: 10.3762/bjnano.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gossmann R., Fahrländer E., Hummel M., Mulac D., Brockmeyer J., Langer K. Comparative examination of adsorption of serum proteins on HSA- and PLGA-based nanoparticles using SDS-PAGE and LC-MS. Eur. J. Pharm. Biopharm. 2015;93:80–87. doi: 10.1016/j.ejpb.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 83.Kah J.C.Y., Wong K.Y., Neoh K.G., Song J.H., Fu J.W.P., Mhaisalkar S., Olivo M., Sheppard C.J.R. Critical parameters in the pegylation of gold nanoshells for biomedical applications: An in vitro macrophage study. J. Drug Target. 2009;17:181–193. doi: 10.1080/10611860802582442. [DOI] [PubMed] [Google Scholar]
- 84.Barbero F., Russo L., Vitali M., Piella J., Salvo I., Borrajo M.L., Busquets-Fité M., Grandori R., Bastús N.G., Casals E., et al. Formation of the Protein Corona: The Interface between Nanoparticles and the Immune System. Semin. Immunol. 2017;34:52–60. doi: 10.1016/j.smim.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 85.Shanehsazzadeh S., Gruettner C., Lahooti A., Mahmoudi M., Allen B.J., Ghavami M., Daha F.J., Oghabian M.A. Monoclonal antibody conjugated magnetic nanoparticles could target MUC-1-positive cells in vitro but not in vivo. Contrast Media Mol. Imaging. 2015;10:225–236. doi: 10.1002/cmmi.1627. [DOI] [PubMed] [Google Scholar]
- 86.Wu X., McKay C., Pett C., Yu J., Schorlemer M., Ramadan S., Lang S., Behren S., Westerlind U., Finn M.G., et al. Synthesis and Immunological Evaluation of Disaccharide Bearing MUC-1 Glycopeptide Conjugates with Virus-Like Particles. ACS Chem. Biol. 2019;14:2176–2184. doi: 10.1021/acschembio.9b00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bargheer D., Nielsen J., Gébel G., Heine M., Salmen S.C., Stauber R., Weller H., Heeren J., Nielsen P. The fate of a designed protein corona on nanoparticles in vitro and in vivo. Beilstein J. Nanotechnol. 2015;6:36–46. doi: 10.3762/bjnano.6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Monteiro-Riviere N.A., Riviere J.E., Choi K. Biocorona formation on gold nanoparticles modulates human proximal tubule kidney cell uptake, cytotoxicity and gene expression. Toxicol. Vitr. 2017;42:150–160. doi: 10.1016/j.tiv.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 89.PrabhuDas M.R., Baldwin C.L., Bollyky P.L., Bowdish D.M.E., Drickamer K., Febbraio M., Herz J., Kobzik L., Krieger M., Loike J., et al. A Consensus Definitive Classification of Scavenger Receptors and Their Roles in Health and Disease. J. Immunol. 2017;198:3775–3789. doi: 10.4049/jimmunol.1700373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fleischer C.C., Payne C.K. Nanoparticle-cell interactions: Molecular structure of the protein corona and cellular outcomes. Acc. Chem. Res. 2014;47:2651–2659. doi: 10.1021/ar500190q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fleischer C.C., Payne C.K. Secondary structure of corona proteins determines the cell surface receptors used by nanoparticles. J. Phys. Chem. B. 2014;118:14017–14026. doi: 10.1021/jp502624n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mortimer G.M., Butcher N.J., Musumeci A.W., Deng Z.J., Martin D.J., Minchin R.F. Cryptic epitopes of albumin determine mononuclear phagocyte system clearance of nanomaterials. ACS Nano. 2014;8:3357–3366. doi: 10.1021/nn405830g. [DOI] [PubMed] [Google Scholar]
- 93.Lartigue L., Wilhelm C., Servais J., Factor C., Dencausse A., Bacri J.C., Luciani N., Gazeau F. Nanomagnetic sensing of blood plasma protein interactions with iron oxide nanoparticles: Impact on macrophage uptake. ACS Nano. 2012;6:2665–2678. doi: 10.1021/nn300060u. [DOI] [PubMed] [Google Scholar]
- 94.Deng Z.J., Liang M., Monteiro M., Toth I., Minchin R.F. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat. Nanotechnol. 2011;6:39–44. doi: 10.1038/nnano.2010.250. [DOI] [PubMed] [Google Scholar]
- 95.Dai Q., Guo J., Yan Y., Ang C.S., Bertleff-Zieschang N., Caruso F. Cell-Conditioned Protein Coronas on Engineered Particles Influence Immune Responses. Biomacromolecules. 2017;18:431–439. doi: 10.1021/acs.biomac.6b01545. [DOI] [PubMed] [Google Scholar]
- 96.Escamilla-Rivera V., Uribe-Ramírez M., González-Pozos S., Lozano O., Lucas S., De Vizcaya-Ruiz A. Protein corona acts as a protective shield against Fe3O4-PEG inflammation and ROS-induced toxicity in human macrophages. Toxicol. Lett. 2016;240:172–184. doi: 10.1016/j.toxlet.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 97.Visalakshan R.M., MacGregor M.N., Sasidharan S., Ghazaryan A., Mierczynska-Vasilev A.M., Morsbach S., Mailänder V., Landfester K., Hayball J.D., Vasilev K. Biomaterial Surface Hydrophobicity-Mediated Serum Protein Adsorption and Immune Responses. ACS Appl. Mater. Interfaces. 2019;11:27615–27623. doi: 10.1021/acsami.9b09900. [DOI] [PubMed] [Google Scholar]
- 98.Wang G., Griffin J.I., Inturi S., Brenneman B., Banda N.K., Michael Holers V., Moghimi S.M., Simberg D. In vitro and in vivo differences in murine third complement component (C3) opsonization and macrophage/leukocyte responses to antibody-functionalized iron oxide nanoworms. Front. Immunol. 2017;8:1–9. doi: 10.3389/fimmu.2017.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen F., Wang G., Griffin J.I., Brenneman B., Banda N.K., Holers V.M., Backos D.S., Wu L., Moghimi S.M., Simberg D. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat. Nanotechnol. 2017;12:387–393. doi: 10.1038/nnano.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bertrand N., Grenier P., Mahmoudi M., Lima E.M., Appel E.A., Dormont F., Lim J.M., Karnik R., Langer R., Farokhzad O.C. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 2017;8:777. doi: 10.1038/s41467-017-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jackson M.A., Werfel T.A., Curvino E.J., Yu F., Kavanaugh T.E., Sarett S.M., Dockery M.D., Kilchrist K.V., Jackson A.N., Giorgio T.D., et al. Zwitterionic Nanocarrier Surface Chemistry Improves siRNA Tumor Delivery and Silencing Activity Relative to Polyethylene Glycol. ACS Nano. 2017;11:5680–5696. doi: 10.1021/acsnano.7b01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu Y.-Q., Qu H., Sfyroera G., Tzekou A., Kay B.K., Nilsson B., Nilsson Ekdahl K., Ricklin D., Lambris J.D. Protection of Nonself Surfaces from Complement Attack by Factor H-Binding Peptides: Implications for Therapeutic Medicine. J. Immunol. 2011;186:4269–4277. doi: 10.4049/jimmunol.1003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gao M., Liang C., Song X., Chen Q., Jin Q., Wang C., Liu Z. Erythrocyte-membrane-enveloped perfluorocarbon as nanoscale artificial red blood cells to relieve tumor hypoxia and enhance cancer radiotherapy. Adv. Mater. 2017;29:1–7. doi: 10.1002/adma.201701429. [DOI] [PubMed] [Google Scholar]
- 104.Li P.Y., Fan Z., Cheng H. Cell Membrane Bioconjugation and Membrane-Derived Nanomaterials for Immunotherapy. Bioconjug. Chem. 2018;29:624–634. doi: 10.1021/acs.bioconjchem.7b00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fan Z., Li P.Y., Deng J., Bady S.C., Cheng H. Cell membrane coating for reducing nanoparticle-induced inflammatory responses to scaffold constructs. Nano Res. 2018;11:5573–5583. doi: 10.1007/s12274-018-2084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Figueiredo Borgognoni C., Kim J.H., Zucolotto V., Fuchs H., Riehemann K. Human macrophage responses to metal-oxide nanoparticles: A review. Artif. Cells, Nanomedicine Biotechnol. 2018;46:694–703. doi: 10.1080/21691401.2018.1468767. [DOI] [PubMed] [Google Scholar]
- 107.Lundqvist M., Augustsson C., Lilja M., Lundkvist K., Dahlbäck B., Linse S., Cedervall T. The nanoparticle protein corona formed in human blood or human blood fractions. PLoS ONE. 2017;12:1–15. doi: 10.1371/journal.pone.0175871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bros M., Nuhn L., Simon J., Moll L., Mailänder V., Landfester K., Grabbe S. The protein corona as a confounding variable of nanoparticle-mediated targeted vaccine delivery. Front. Immunol. 2018;9:1–10. doi: 10.3389/fimmu.2018.01760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Corbo C., Molinaro R., Tabatabaei M., Farokhzad O.C., Mahmoudi M. Personalized protein corona on nanoparticles and its clinical implications. Biomater. Sci. 2017;5:378–387. doi: 10.1039/C6BM00921B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lazarovits J., Chen Y.Y., Song F., Ngo W., Tavares A.J., Zhang Y.N., Audet J., Tang B., Lin Q., Cruz Tleugabulova M., et al. Synthesis of Patient-Specific Nanomaterials. Nano Lett. 2019;19:116–123. doi: 10.1021/acs.nanolett.8b03434. [DOI] [PubMed] [Google Scholar]
- 111.Aguado B.A., Grim J.C., Rosales A.M., Watson-Capps J.J., Anseth K.S. Engineering precision biomaterials for personalized medicine. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aam8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ren J., Cai R., Wang J., Daniyal M., Baimanov D., Liu Y., Yin D., Liu Y., Miao Q., Zhao Y., et al. Precision Nanomedicine Development Based on Specific Opsonization of Human Cancer Patient-Personalized Protein Coronas. Nano Lett. 2019;19:4692–4701. doi: 10.1021/acs.nanolett.9b01774. [DOI] [PubMed] [Google Scholar]
- 113.Sharabi A.B., Lim M., DeWeese T.L., Drake C.G. Radiation and checkpoint blockade immunotherapy: Radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–e509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 114.Chan J.K., Horwood N., Nanchahal J., Chan J.K., Roth J., Oppenheim J.J., Tracey K.J., Vogl T., Feldmann M., Horwood N., et al. Alarmins: Awaiting a clinical response. J. Clin. Investig. 2012;122:2711–2719. doi: 10.1172/JCI62423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Curtin J.F., Liu N., Candolfi M., Xiong W., Assi H., Yagiz K., Edwards M.R., Michelsen K.S., Kroeger K.M., Liu C., et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Apetoh L., Ghiringhelli F., Tesniere A., Obeid M., Ortiz C., Criollo A., Mignot G., Maiuri M.C., Ullrich E., Saulnier P., et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 117.Min Y., Roche K.C., Tian S., Eblan M.J., Mckinnon K.P., Caster J.M., Chai S., Herring L.E., Zhang L., Zhang T., et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy HHS Public Access Author manuscript. Nat. Nanotechnol. 2017;12:877–882. doi: 10.1038/nnano.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wan S., Kelly P.M., Mahon E., Stöckmann H., Rudd P.M., Caruso F., Dawson K.A., Yan Y., Monopoli M.P. The “sweet” Side of the protein corona: Effects of glycosylation on nanoparticle-cell interactions. ACS Nano. 2015;9:2157–2166. doi: 10.1021/nn506060q. [DOI] [PubMed] [Google Scholar]