Clopidogrel is a prodrug that requires bioactivation. CYP2C19 loss-of-function (LOF) alleles are common and impair clopidogrel active metabolite formation, platelet inhibition, and clinical effectiveness after percutaneous coronary intervention (PCI).1,2 In contrast, CYP2C19 polymorphisms do not alter prasugrel or ticagrelor effectiveness.2 Several institutions have implemented CYP2C19 genotyping into clinical care to guide selection of prasugrel or ticagrelor in CYP2C19 LOF allele carriers, and have demonstrated the clinical benefit of genotype-guided antiplatelet therapy over 12 months post-PCI.3–5
Approximately two-thirds of major adverse cardiovascular events (MACE) occur within 30 days following PCI. Retrospective genetic analyses have reported a significantly higher risk of MACE or stent thrombosis during the first 30 days post-PCI in CYP2C19 LOF allele carriers prescribed clopidogrel.1 Additionally, long-term use of prasugrel or ticagrelor is associated with increased bleeding risk and higher costs.2 Although CYP2C19-guided selection of antiplatelet therapy offers the potential to mitigate these risks, the impact of implementing a genotype-guided strategy on early clinical outcomes remains unknown. Therefore, we evaluated 30-day atherothrombotic and bleeding outcomes following PCI in a real-world cohort of CYP2C19-guided antiplatelet therapy.
The cohort included 1063 adults who underwent PCI and clinical CYP2C19 testing at the University of North Carolina-Chapel Hill from 2012–2014, which has been expanded since our recent publication.3 The study was approved by the Biomedical Institutional Review Board. The data that support the study findings are available on reasonable request.
The primary outcome was the net incidence of either a major adverse cardiovascular or cerebrovascular event (MACCE, defined as death, myocardial infarction, stent thrombosis, hospitalization for unstable angina, ischemic stroke, or transient ischemic attack) or clinically significant bleeding event (defined as a Global Use of Strategies to Open Occluded Arteries (GUSTO) moderate or severe/life-threatening bleed) within 30 days post-PCI. Data collection and analysis was completed, as previously described.3 Briefly, the relationship between CYP2C19 LOF carrier status, antiplatelet maintenance therapy (clopidogrel versus alternative) and time to occurrence of clinical outcomes was evaluated by Cox proportional hazards regression after adjusting for covariates that differed across CYP2C19-antiplatelet therapy groups or were associated with clinical outcome. The adjusted hazard ratio (HR) and 95% confidence intervals (CIs) for each comparison were calculated.
The mean age was 62±12 years, 67.7% were male, 19.2% were African-American, and 59.7% underwent PCI for an acute coronary syndrome (ACS) indication. CYP2C19 genotyping identified 329 (31.0%) patients that carried either one (303, 28.5%) or two (26, 2.4%) LOF alleles. Consistent with genotype-guided prescribing, prasugrel or ticagrelor was prescribed more frequently in CYP2C19 LOF allele carriers compared to patients without a LOF allele by day 30 (63.5% vs. 27.8%, respectively, P<0.001). Several clinical factors differed across CYP2C19-antiplatelet therapy groups (Figure A).
Figure. Baseline characteristics and 30-day cardiovascular and bleeding outcomes by CYP2C19 status and antiplatelet therapy.
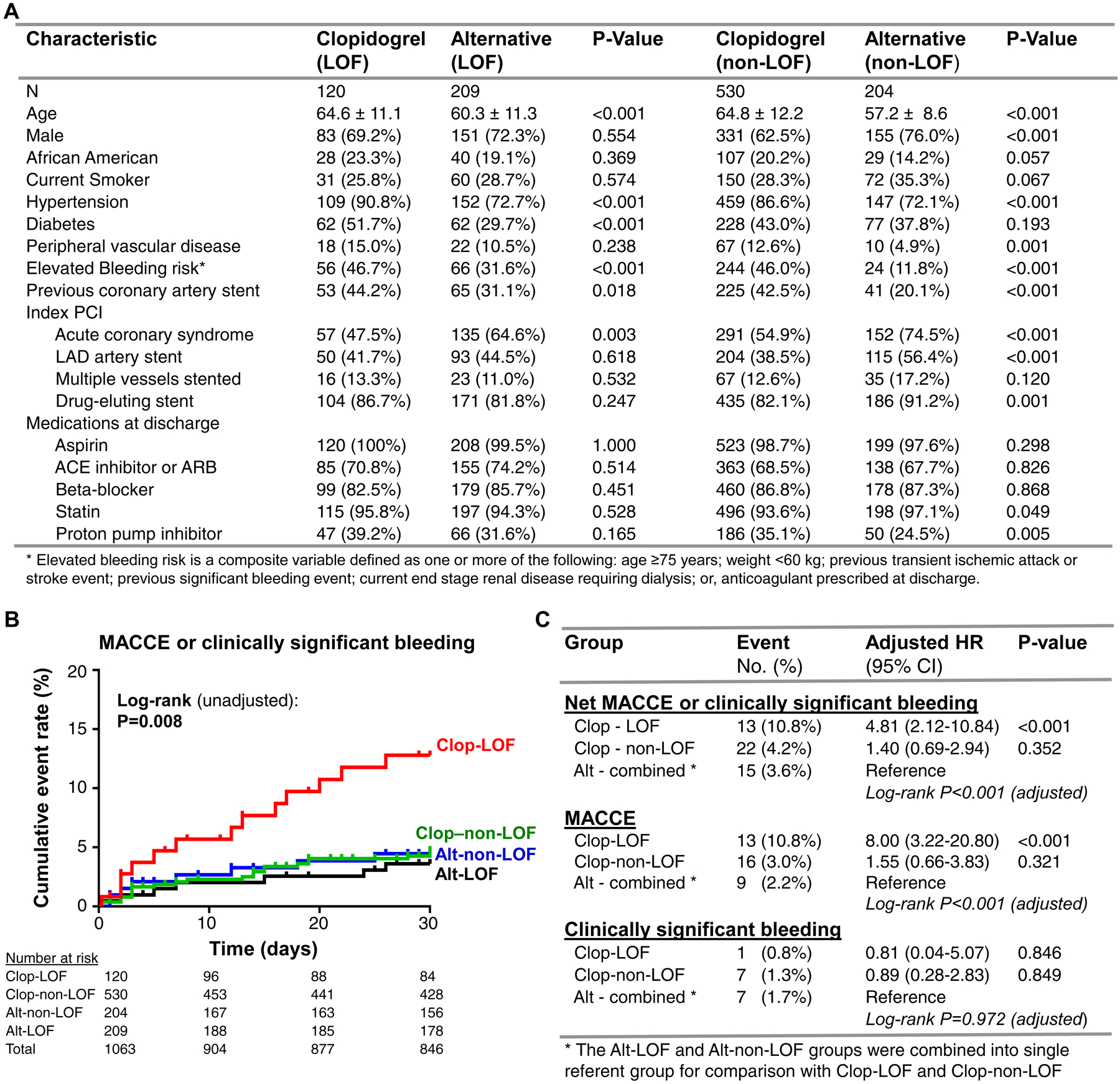
A. Baseline characteristics are presented across selected maintenance therapy (clopidogrel versus alternative [prasugrel or ticagrelor]) after stratifying by CYP2C19 loss-of-function (LOF) allele status. P-value corresponds to comparison between antiplatelet therapy groups after stratifying by CYP2C19 status using Chi-square or Fisher’s exact test. B. Kaplan-Meier curve describing 30-day cumulative event rate for the net composite of either MACCE or clinically significant bleeding across the four CYP2C19 genotype and antiplatelet therapy strata: LOF allele carriers prescribed clopidogrel (Clop-LOF), LOF allele carriers prescribed alternative therapy (Alt-LOF), patients without a LOF allele prescribed clopidogrel (Clop-non-LOF), patients without a LOF allele prescribed alternative therapy (Alt-non-LOF). The unadjusted log-rank P-value for outcomes across the four groups is provided. C. Summary of the primary (net MACCE or clinically significant bleeding) and secondary (MACCE, clinically significant bleeding) outcomes at 30 days by CYP2C19 status and antiplatelet maintenance therapy. The number (percentage) in each group that experienced the event, and the adjusted HR, 95% CI and P-value for the Cox proportional hazards regression analysis, is provided. *LOF and non-LOF allele carrier’s prescribed alternative (prasugrel or ticagrelor) therapy were combined into a single referent group for comparison. The adjusted log-rank P-value across the three CYP2C19-antiplatelet groups is provided.
Overall, 38 (3.6%) and 15 (1.4%) patients experienced a MACCE or bleeding event by day 30, respectively. The risk of MACCE or bleeding was significantly associated with CYP2C19 genotype and the prescribed antiplatelet therapy (Figure B). CYP2C19 LOF allele carriers receiving clopidogrel exhibited a significantly higher net risk of MACCE or bleeding over 30 days compared to use of alternative therapy (adjusted HR 4.81, 95% CI 2.12–10.84, P<0.001). This association was driven by a higher risk of MACCE, since bleeding event rates were not significantly different across groups (Figure C). In contrast, no significant difference in risk of MACCE or bleeding was observed in clopidogrel-treated patients without a LOF allele versus those treated with alternative therapy.
The adverse cardiovascular outcomes conferred by clopidogrel use in CYP2C19 LOF allele carriers over the first 30 days post-PCI are consistent with recent data from a multicenter pragmatic study, and our single-center analysis, demonstrating that use of prasugrel or ticagrelor in CYP2C19 LOF allele carriers is associated with lower risk of major atherothrombotic events over 12 months post-PCI.3,5 Taken together, these results suggest that use of CYP2C19 genotype testing to guide antiplatelet therapy selection improves clinical outcomes and will likely lower 30-day readmission rates after PCI in a real-world setting. These data also illustrate the importance of treating CYP2C19 LOF allele carriers with prasugrel or ticagrelor during the early post-PCI period, and that this strategy is not associated with increased bleeding risk. Use of prasugrel or ticagrelor early after PCI when risk for atherothrombotic events is greatest, followed by genotype-guided de-escalation to clopidogrel in patients without a LOF allele may offer a practical solution to overcome barriers, such as delayed return and interpretation of genotype results and the higher costs of prasugrel and ticagrelor, and maximize the effectiveness of antiplatelet therapy post-PCI. However, the clinical impact of a CYP2C19-guided de-escalation strategy remains unknown.
This study was limited by the observational design. Because genotype-guided therapy was not randomized, we cannot attribute causality to the observed association between genotype, antiplatelet therapy, and clinical outcome. Although covariate-adjusted analyses were conducted to lessen the impact of potential confounding factors, residual confounding may remain. Additionally, our single-center results may not be generalizable to other settings. Prospective validation in larger, multi-center populations is needed.
Acknowledgements:
The authors gratefully acknowledge the UNC Cardiac Catheterization Laboratory and the UNC Molecular Genetics Laboratory staff for their important contributions.
Footnotes
Disclosures:
None
References
- 1.Mega JL, et al. Reduced-Function CYP2C19 Genotype and Risk of Adverse Clinical Outcomes Among Patients Treated With Clopidogrel Predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott SA, et al. ; Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C19 Genotype and Clopidogrel Therapy: 2013 Update. Clin Pharmacol Ther. 2013;94:317–323. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CR, et al. Clinical Outcomes and Sustainability of Using CYP2C19 Genotype–Guided Antiplatelet Therapy After Percutaneous Coronary Intervention. Circ Genom Precis Med. 2018;11:e002069. doi: 10.1161/CIRCGEN.117.002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Empey PE, et al. Multisite Investigation of Strategies for the Implementation of CYP2C19 Genotype-Guided Antiplatelet Therapy. Clin Pharmacol Ther. 2018;104:664–674. doi: 10.1002/cpt.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavallari LH, et al. ; IGNITE Network. Multisite Investigation of Outcomes With Implementation of CYP2C19 Genotype-Guided Antiplatelet Therapy After Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2018;11:181–191. doi: 10.1016/j.jcin.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


