Abstract
Preclinical work suggests that GET73, a novel metabotropic glutamate receptor subtype 5 (mGluR5) negative allosteric modulator (NAM), may represent a novel pharmacological treatment for alcohol use disorder (AUD). Two independent experiments evaluated the effect of acutely administered GET 73 (0, 30, and 100 mg/kg, i.g.) on alcohol-induced hypolocomotion (N=72) and sedation/hypnosis (N=36) in rats. In healthy male volunteers (N=14), an open-label, randomized, crossover study was conducted to compare adverse events (AEs) and pharmacokinetic parameters, in two experiments in which GET 73 300-mg was administered, with and without alcohol, once and trice. In rats, when administered with alcohol-vehicle, 100 mg/kg, but not 30 mg/kg, GET 73 reduced spontaneous locomotor activity. When administered with alcohol, no dose of GET 73 altered either alcohol-induced hypolocomotion or sedation/hypnosis. In humans, both single and trice 300-mg GET 73 administrations were well tolerated, in the presence and absence of alcohol, with no differences in adverse events (AEs). There were no significant differences in relative bioavailability between administering 300-mg GET 73 in the presence or absence of alcohol.
Keywords: Rats, humans, mGluR5, alcohol, sedation/hypnosis safety, pharmacokinetics
Introduction
Alcohol use disorder (AUD) represents a worldwide medical and social problem with few approved pharmacotherapies (Haass-Koffler et al., 2014). The neurotransmitter glutamate plays key roles in the neurobiological mechanisms that lead to alcohol craving and excessive drinking (Gilpin and Koob, 2008).
Glutamate activates both metabotropic glutamate receptors (mGluRs), which are G-protein coupled receptors that mediate slow glutamate transmission, and ionotropic glutamate receptors (iGluRs) that mediate fast excitatory glutamate transmission. Blockade of glutamate transmission via both i/mGluRs reduces the rewarding effects of alcohol (Duncan and Lawrence, 2012; D’Souza, 2015). When tested in humans, iGluR antagonists may exhibit serious side effects (Olive, 2009). By contrast, mGluR ligands have a safer profile when tested in clinical trials for various medical conditions (Witkin et al., 2007; Spooren et al., 2003). In addition, preclinical work suggests that mGluRs represent a valid target for developing new pharmacotherapies for AUD (Duncan and Lawrence, 2012; Olive, 2009).
Pharmacological manipulations of mGluRs may be obtained through ligands acting at the orthosteric site or allosteric sites of the receptor. Allosteric modulators of mGluRs provide novel opportunities for drug discovery, potentially leading to drugs characterized by more specific effects and fewer side effects than other drugs affecting glutamate transmission (Conn et al., 2009). As regards to the mGluRs of Group I, mGluR5 negative allosteric modulators (NAMs) reduce drug intake, drug-associated reward and reinforcement, and relapse-related behaviors (Olive, 2009; Olive, 2010; Besheer et al., 2008; Mihov and Hasler, 2016).
GET73 (N-[4-(trifluoromethyl)benzyl]-4-methoxybutyramide) (PubChem SID: 329974174) is a novel chemical entity that seems to partially act like a mGluR5 antagonist or NAM (Beggiato et al., 2013; Ferraro et al., 2011). In selectively bred Sardinian alcohol-preferring (sP) rats, a validated rodent model of AUD (Bell et al., 2012; Colombo et al., 2006) GET73 reduces voluntary alcohol intake, alcohol deprivation effect and anxiety-like behaviors (Loche et al., 2012). Furthermore, GET73 shows a neuroprotective role against alcohol-induced neurotoxicity in primary cultures of rat hippocampal neurons (Tomasini et al., 2016). In addition, previous in vitro and in vivo work conducted in different preclinical experimental models demonstrated the safety of GET 73 in the central nervous system, cardiovascular, respiratory, and immune systems. Together, these results indicate that GET73 may represent a potentially safe and effective novel pharmacotherapy for AUD that should be investigated further.
The safety, tolerability and pharmacokinetics (PK) of GET 73 in humans was recently investigated in a double-blind, placebo-controlled first-in-man study conducted by single doses up to 600-mg and repeated ascending doses up to 450-mg twice a day (Haass-Koffler et al., 2017a; Haass-Koffler et al., 2017b). All adverse events (AEs) were mild to moderate in severity. Maximum plasma drug concentrations occurred between 0.5–2.05 hours after administration for both single and repeated doses. The PK parameters of 4-oxo-4-{[4-triflouromethyl)benzyl]amino}butanoic acid, the main metabolite of GET 73, were consistent with those of the parent drug. The safety, tolerability, and PK data of GET 73 in humans (Haass-Koffler et al., 2017a; Haass-Koffler et al., 2017b) led to further development of GET 73 as a potential treatment for AUD.
In the current study, we assessed the PK profile of GET73 and of its main metabolite MET 2, when GET73 is co-administered with alcohol. It is critical to assess the safety and tolerability of GET73 when co-administered with alcohol, given the potentially serious consequences of drug-alcohol interactions (Haass-Koffler et al., 2017a; Haass-Koffler et al., 2017b), an approach consistent with recent guidelines from the Food and Drug Administration (FDA) (FDA, 2015) and with the European guidelines EMA/CHMP/EWP/20097/2008 Guidelines on the development of medicinal products for the treatment of alcohol dependence.
The primary objective of this study was to provide information on the safety and tolerability of GET 73 in rats and humans when co-administered with alcohol. Additional aims were to determine the effect of alcohol on the bioavailability of GET73 and of its major metabolite MET 2 in healthy volunteers.
Methods and materials
Rat study
The study was conducted at the Neuroscience Institute, National Research Council of Italy, Section Cagliari, Italy. All experimental procedures complied with the Italian law on the “Protection of animals used for experimental and other scientific reasons”. Two independent experiments were conducted, each one employing a separate set of rats.
Experiment 1: Locomotor activity
Male, adult Sprague-Dawley (SD) rats (Supplementary, Text S1) were divided into 6 groups (n=12 each), matched for body weight and treated at empty stomach with the described six drug combinations (Table 1). GET 73 and alcohol were administered intragastrically (i.g.). GET 73 was administered 30 minutes before alcohol. Five minutes after alcohol administration, rats were exposed to the motility cage for 60 minutes.
Table 1.
Rat Study: Doses of GET 73 and alcohol co-administered
| Experiment | Groups | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| 1 (n= 72) | GET 73 (mg/kg) | 0 | 30 | 100 | 0 | 30 | 100 |
| Alcohol (g/kg) | 0 | 0 | 0 | 2 | 2 | 2 | |
| 2 (n=36) | GET 73 (mg/kg) | 0 | 30 | 100 | - | - | - |
| Alcohol (g/kg) | 3 | 3 | 3 | - | - | - |
Experiment 2: Sedation/hypnosis
Male, adult SD rats were divided into 3 groups (n=12 each), GET 73 was administered i.g., Alcohol was administered intraperitoneally (i.p.) 30 minutes after GET 73. Groups were matched for body weight, and treated at empty stomach with three drug combinations (Table 1).
Statistical analysis
For Experiment 1, the measured variable was the total number of motility counts (photocell breaks; measure of horizontal, locomotory activity) recorded automatically by the apparatus over the 60-min session. Data were analyzed by a 2-way (GET 73 dose; alcohol dose) ANOVA, followed by the Newman-Keuls test for post hoc comparisons.
For Experiment 2, after alcohol administration, each rat was placed on its back once every 30s until the animal was unable to right itself within 60 seconds. The time lapse between alcohol administration and beginning of inability of the rat to right itself was measured as onset of loss of righting reflex (LORR). Subsequently, each rat was left undisturbed on its back until it spontaneously regained its righting reflex. Onset and duration of LORR were used as indexes of alcohol intoxication. They were expressed in minutes, respectively, and analyzed by separate 1-way ANOVAs.
Human study
The study was conducted at the Quotient Clinical, Nottingham, UK, between August and October 2011. Each participant provided written informed consent. The study was approved and conducted in accordance with the clinical protocol and with International Conference on Harmonisation Good Clinical Practice, Guidelines approved by the Committee for Medicinal Products for Human Use, the Medicines for Human Use (Clinical Trials) Regulations 2004, the Medicines for Human Use, Amendment Regulations 2006 and the Medicines for Human Use, Amendment Regulations 2008. The study was performed according to the ethical principles outlined in the World Medical Association Declaration of Helsinki. The trial was registered in EudraCT (2011–002354-31).
Design, medication and alcohol dosing selection
This was an open-label, counterbalanced, randomized, crossover study conducted in healthy male volunteers conducted in two independent experiments (3 and 4). Inclusion/exclusion criteria are described in (TextS2). In the development of medications for AUD, it is critical to assess the additive effects (behavioral, sedation) when the compound is administered with alcohol. It is also important to determine if alcohol may change the absorption and other PK parameters of GET 73. Finally, the pharmacokinetic half-life (t1/2) of GET 73 is 0.5–1.5 hour with tmax reached within 1 hour and alcohol fully absorbed into the bloodstream within 30 minutes (Haass-Koffler et al., 2017a; Haass-Koffler et al., 2017b). Repeated administration of GET 73 at extremely short intervals to achieve steady state would not be safe, neither would it be feasible for clinical use.
Behavioral data however, suggest that GET 73 may last longer and support an 8 hour dosing interval (Ottani et al., 2007; Loche et al., 2012; Tacchi et al., 2008). Simultaneous administration of alcohol and GET 73 at 8 hour allowed investigations of drug-alcohol pharmacodynamics (PD) effect, drug/metabolite accumulation and change in PK. All doses of GET 73 were administered after an overnight fasting, with 240-mL of water, and within 15 minutes of receiving the soda/±alcohol (12 g). GET 73 doses and alcohol administration rationale are further described in TextS3 and additional information on experimental procedures are provided in TextS4. The assessments’ schedule is in TableS1 and the timing of medication administration, PK blood samples, electrocardiogram (ECG) and vital signs measurements are in TableS2–S3.
Experiment 3 (300-mg GET 73 once in the absence/presence of alcohol)
Fourteen healthy male volunteers were enrolled in the study to receive a single 300-mg dose of GET 73 either with soda without alcohol (intervention NALC) or with soda and alcohol (intervention ALC) in a randomized-counterbalanced order (Table 3). Following completion of this period, an interim pharmacokinetics (PK) and safety evaluation determined the dose selected for the next experiment.
Table 3.
Human Study: Experiments and Intervention procedures
| Experiment | 300-mg GET 73 | intervention a | standard mixer | alcohol (g) |
|---|---|---|---|---|
| 3 | single dose | NALC | 240-mL soda | 0 |
| ALC | 12 | |||
| 4 | trice a day | NALC | 240-mL soda | 0 |
| ALC | 12b |
NALC: soda with No Alcohol, ALC: soda with Alcohol;
Given three times, total 36 g
Experiment 4 (300-mg GET 73 trice in the absence/presence of alcohol)
Eleven participants from Experiment 3 received 300-mg of GET 73 administered trice either with NALC or ALC intervention in a randomized-counterbalanced order (Table 3).
Statistical analysis
For GET 73 and the major metabolite MET 2, following natural logarithmic transformation, Cmax, AUClast and AUCi∞ were subjected to analysis of variance (ANOVA) to assess for relative bioavailability comparing intervention NALC versus ALC. Participants were randomized in a counterbalanced design to control for order effects and a mixed model was used including terms for treatment received, period and sequence fitted as fixed effects and subject within sequence fitted as a random effect.
For GET 73 and MET 2, we calculated the geometric mean (geometric coefficient of variation [CV%]) values of key PK parameters, tmax was analyzed using a non-parametric Friedman X2-squared test (untransformed data) at the 5% level of significance to compare intervention NALC versus ALC. The Cochran-Mantel-Haenszel test statistic and the corresponding p-value for comparing the means were calculated. Statistical analysis was performed after all participants had their follow-up visit and the study database had been locked. Data management and statistical analysis was performed by Data Magik Ltd (Laburnum House, East Grimstead, Salisbury, Wiltshire, SP5 3RT, UK). All the statistical procedures described below were performed by the package SAS® (v.9.13.) and graphs were prepared using Graphpad (v.5)
Results
Rat study
Experiment 1: Locomotor activity
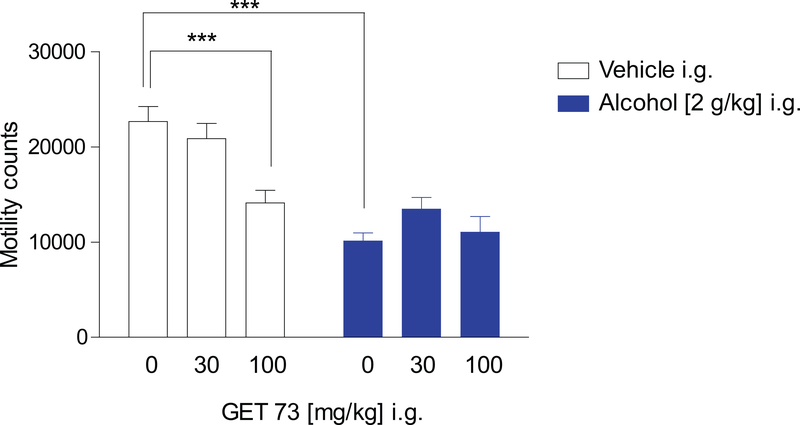
There was a main effect of alcohol [F1,66=46.23, P<0.0001], a main effect of GET 73 [F2,66=6.27, P<0.005], and a GET 73 x alcohol interaction [F2,66=5.86, P<0.005] on the number of motility counts. Post-hoc analyses indicated that administration of GET 73 alone (with alcohol vehicle) reduced the number of motility counts at the dose of 100 mg/kg (40% reduction compared to control rats, P<0.0005), but not at the dose of 30 mg/kg (Figure 1). Administration of alcohol alone (with GET 73 vehicle) resulted in a reduction of the number of motility counts (55% compared to control rats, P<0.0005) (Figure 1). The combination of alcohol and both doses of GET 73 did not alter the number of motility counts compared to rats treated with alcohol alone (P>0.05) (Figure 1).
FIGURE 1– Rats: Experiment 1: locomotor activity.
Effect of the acute administration of GET 73 and alcohol on the number of counts (photocell breaks; index of locomotor activity) in Sprague-Dawley rats exposed to an automated motility cage for 60 minutes (n = 12 per group), ***P<0.0005. Results are reported as the Mean ± S.E.M.
Experiment 2: Sedation/hypnosis
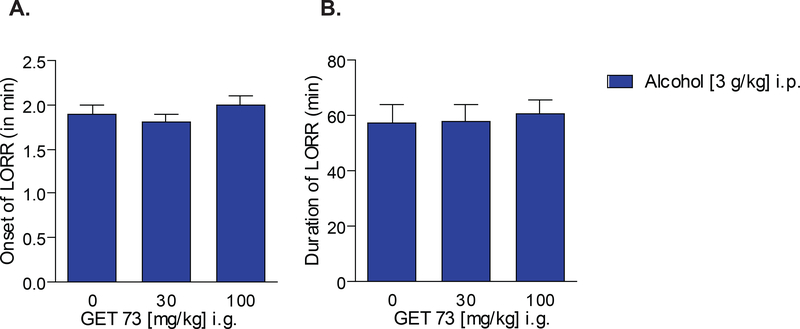
There was no significant effect of treatment with GET 73 on onset [F2,33=1.06, P>0.05] (Figure 2A) and duration [F2,33=0.07, P>0.05] (Figure 2B) of alcohol-induced LORR.
FIGURE 2– Rats: Experiment 2: sedation/hypnosis.
Effect of the acute administration of GET 73 and alcohol on onset (left panel) and duration (right panel) of loss of righting reflex (LORR in the y-axis legend; both measures of alcohol induced sedation/hypnosis) in Sprague-Dawley rats (n = 12 per group). Results are reported as the Mean ± S.E.M.
Human study
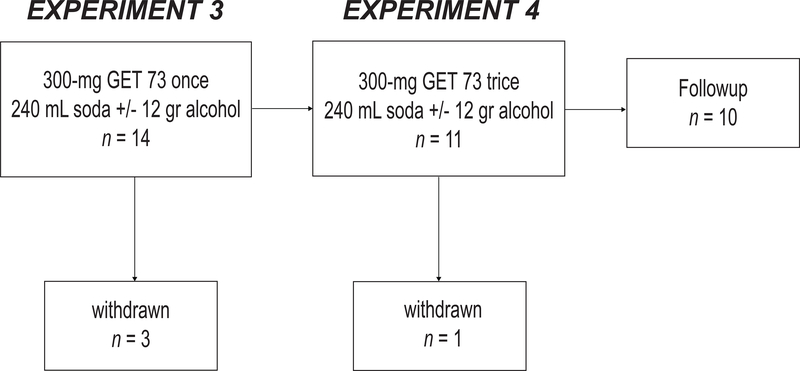
Fourteen healthy male volunteers were randomized and ten completed the study. Their demographic characteristics are in Table 2 and the experiments and intervention procedures are in Table 3. The flowchart of the study is in Figure 3.
Table 2.
Human Study: Demographic of the healthy male volunteers (N = 14)
| Age (years) | 36.1 (13.3) |
| Height (cm) | 177.05 (7.80) |
| Weight (kg) | 83.24 (17.13) |
| BMI (kg/m2) | 26.64 (4.83) |
| Race (White/%) | 14 (100) |
Results are reported as Mean (SD); BMI: Body Mass Index
FIGURE 3– Humans: Study flowcharts.
Fourteen healthy male volunteers (10 completers) were enrolled in the study to receive a single 300-mg dose of GET 73 (Experiment 3) and then trice 300-mg dose of GET 73 (Experiment 4) either with intervention NALC (soda, no alcohol) or intervention ALC (soda + alcohol) in a randomized counterbalanced order. In Experiment 3, one participant was withdrawn after dosing with intervention NAL, two participants were withdrawn after dosing with intervention ALC. In Experiment 4, 11 participants received 300-mg of GET 73 trice either with intervention NALC (soda, no alcohol) or intervention ALC (soda + alcohol), in a randomized counterbalanced order, 1 participant was withdrawn after dosing with intervention NALC and none after intervention ALC.
Study population
In Experiment 3, one participant was withdrawn after dosing with intervention NALC (not available for the subsequent dosing dates), two participants were withdrawn after dosing with intervention ALC (one reported a skin rash and one was withdrawn due to a protocol violation consisting of allocation to the incorrect dose condition). In Experiment 2, one participant was withdrawn after dosing with intervention NALC (abnormal ECG). No participants were withdrawn after intervention ALC.
Adverse events
An overview of adverse events is in Table 4 and described in Table 5. The incidence of AEs was low; five of the 12 participants reported a total of nine AEs, which were mild (8) or moderate (1). There was no notable dose-related trend in the incidence of AEs and there was no notable difference between dosing in the presence or absence of alcohol. There were no serious or severe AEs.
Table 4.
Human Study: Overall incidence adverse events: participants (%)
| 300-mg GET 73 | Experiment 3 (once) | Experiment 4 (trice) | overall | ||
|---|---|---|---|---|---|
| Intervention | NALC a | ALC a | NALC a | ALC a | |
| N | 13 | 12 | 11 | 10 | 14 |
| AEs | 3 (23.1) | 1 (8.3) | 2 (18.2) | 1 (10) | 5 (35.7) |
| Medications-related AEs | 1 (7.7) | 1 (8.3) | 1 (9.1) | 0 | 3 (21.4) |
| Severe AEs | 0 | 0 | 0 | 0 | 0 |
| AEs leading to withdrawal | 0 | 1 (8.3) | 1 (9.1) | 0 | 2 (14.3) |
| Number of AEs | 4 | 2 | 2 | 1 | 9 |
| Number of medication-related AEs | 2 | 2 | 1 | 0 | 5 |
NALC: soda with No Alcohol, ALC: soda with Alcohol
Table 5.
Human Study: Display of adverse events: participants (%)
| 300-mg GET 73 | Experiment 3 (once) | Experiment 4 (trice) | overall | ||
|---|---|---|---|---|---|
| System organ class | NALC a | ALC a | NALC a | ALC a | |
|
Nervous System Headache Sciatica |
2 (15.4) 1 (7.7) 1 (7.7) |
0 0 0 |
1 (9.1) 0 1 (9.1) |
0 0 0 |
2 (14.3) 1 (7.1) 1 (7.1) |
|
Cardiac Disorders Atrioventricular block second degree |
0 0 |
0 0 |
1 (9.1) 1 (9.1) |
0 0 |
1 (7.1) 1 (7.1) |
|
Gastrointestinal Disorders Nausea |
1 (7.7) 1 (7.7) |
0 0 |
0 0 |
0 0 |
1 (7.1) 1 (7.1) |
|
Infections and Infestations Upper respiratory tract infection |
0 0 |
0 0 |
0 0 |
1 (10.0) 1 (10.0) |
1 (7.1) 1 (7.1) |
|
Injury, Poisoning and Procedural Complications Splinter |
1 (7.7) 1 (7.7) |
0 0 |
0 0 |
0 0 |
1 (7.1) 1 (7.1) |
|
Skin and Subcutaneous Tissue Disorders Urticaria |
0 0 |
1 (7.7) 1 (7.7) |
0 0 |
0 0 |
1 (7.1) 1 (7.1) |
NALC: soda with No Alcohol, ALC: soda with Alcohol
No AE was reported by more than one individual throughout the study. In Experiment 3, one participant had sciatica after dosing with intervention NALC. All AEs were mild in severity, except for moderate urticaria reported by one participant after dosing with intervention NALC (withdrawn from the study). Three participants had the following AEs: one reported headache and nausea after dosing with intervention NALC; one had two AEs urticaria after dosing with intervention ALC. In Experiment 4, one participant had second degree atrioventricular block after dosing with intervention NALC (withdrawn from the study). Two participants had AEs that had not resolved when they completed the study (upper respiratory tract infection and a splinter). Both these events were mild and unrelated to study drug. Other clinical laboratory evaluations including vital signs and electrocardiograms are in Text S5.
PK analysis for GET 73
The geometric mean and median of key PK parameters for GET 73 are in Table 6 and additional PK considerations are in Text S6.
Table 6.
Human Study: Geometric Mean (CV%) Values of key pharmacokinetic parameters for GET 73
| 300-mg GET 73 | Experiment 3 (300-mg GET 73 once) | Experiment 4 (300-mg GET 73 trice) | ||
|---|---|---|---|---|
| intervention | NALC a | ALC a | NALC a | ALC a |
| n | 13 | 12 | 11 | 10 |
| Cmax(ng/mL) | 1480 (61.5) | 2920 (59.7) | 1660 (78.7) | 1760 (64.8) |
| tmax (h)b | 0.50 (0.25–3.00) | 0.75 (0.25–1.50) | 2.00 (0.50–14.00) | 6.50 (0.50–14.00) |
| tlag (h)b | 0.00 (0.00–0.25) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| AUClast(h.ng/mL) | 3260 (63.8) | 4817 (57.7) | 8885 (71.9) | 9737 (67.9) |
| AUCinf(h.ng/mL) | 3282 (63.5) | 4851 (57.0) | 10180 (52.9)c | 9764 (67.8) |
| t1/2 (h) | 1.06 (29.6) | 1.06 (22.9) | 1.44 (13.9)c | 1.34 (18.9) |
NALC: soda with No Alcohol, ALC: soda with Alcohol
Results reported as median range
n = 10
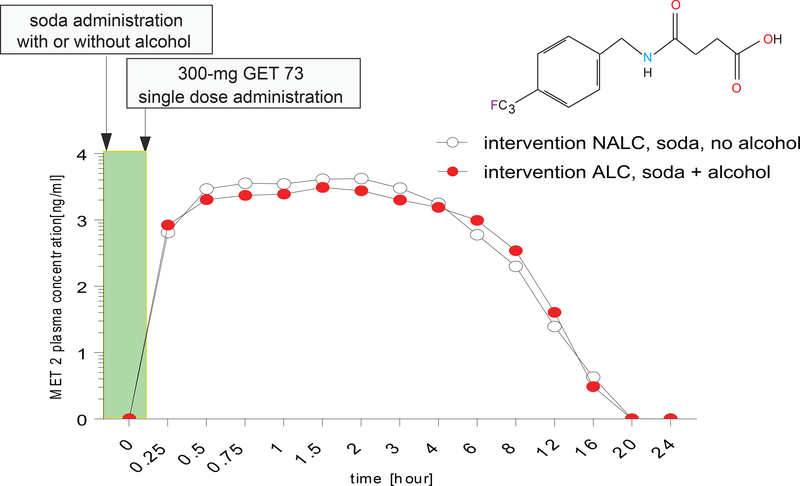
Experiment 3: 300-mg GET 73 once
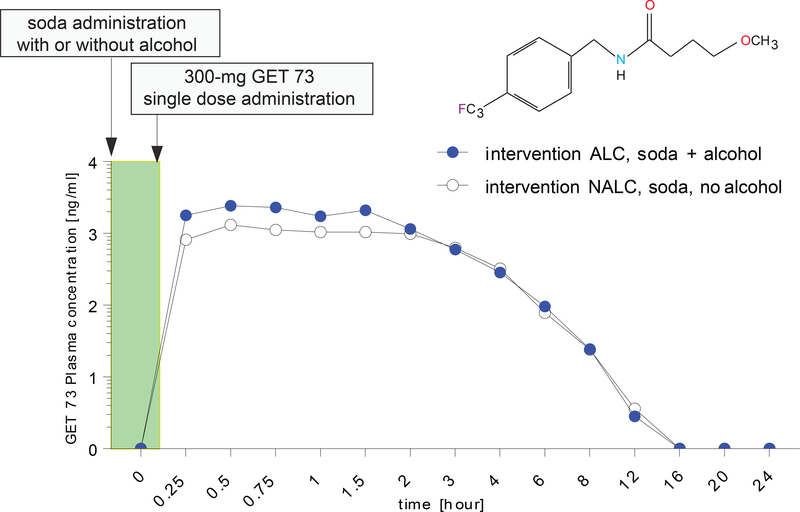
The GET 73 mean plasma concentrations after the administration of 300-mg GET 73 once, in the absence/presence of alcohol, are on Figure 4. Following dosing with intervention NALC, no lag phase was apparent before the post-dose onset of quantifiable plasma concentrations of GET 73. Following an absorption phase, GET 73 concentrations were highest between 0.25–3.00 h post-dose, with evidence of multiple concentration maxima for some participants. Terminal half-life estimates ranged between 0.63–1.57 hour for GET 73 following intervention NALC. Inter-subject variability in systemic exposure was high after dosing with intervention NALC; geometric CV% was 61.5% and 63.50% for Cmax and AUC∞.
FIGURE 4– Humans: Experiment 3: GET 73 plasma concentration after 300-mg GET 73 once in the absence/presence of alcohol.
GET 73 plasma concentration of intervention NALC (n =12) 300-mg single oral dose of GET 73 within 15 minutes of receiving 240 mL of soda and intervention ALC (n = 12), 300-mg single oral dose of GET 73 within 15 minutes of receiving 4 units of alcohol with 240 mL of soda. Results are reported as the Mean ± S.E.M.
Following dosing with intervention ALC, concentrations of GET 73 peaked between 0.25–1.50 h post-dose. Median tmax values were similar to those observed for intervention NALC.A lag phase was not seen for any of the participants. Individual terminal half-life estimates were comparable to those from intervention NALC, ranging between 0.71–1.49 h post-dose. Geometric mean estimates of Cmax and AUC∞ were higher than the corresponding values seen for intervention NALC and were reflected in the apparent bioavailability of intervention NALC relative to intervention ALC. Inter-subject variability was comparable to that seen for intervention ALC, with geometric CV% of 59.7% and 57.0% for Cmax and AUC∞.
For the comparison of intervention NALC versus ALC, the CIs for GET 73 AUClast, Cmax and AUC∞, were all below 1. This suggests that, at the 10% level, the adjusted geometric means for these parameters were lower when a single 300-mg dose GET 73 was administered in the absence of alcohol than when administered in the presence of alcohol. Finally, there was not statistical difference in the tmax between the NALC versus ALC intervention (P>0.05).
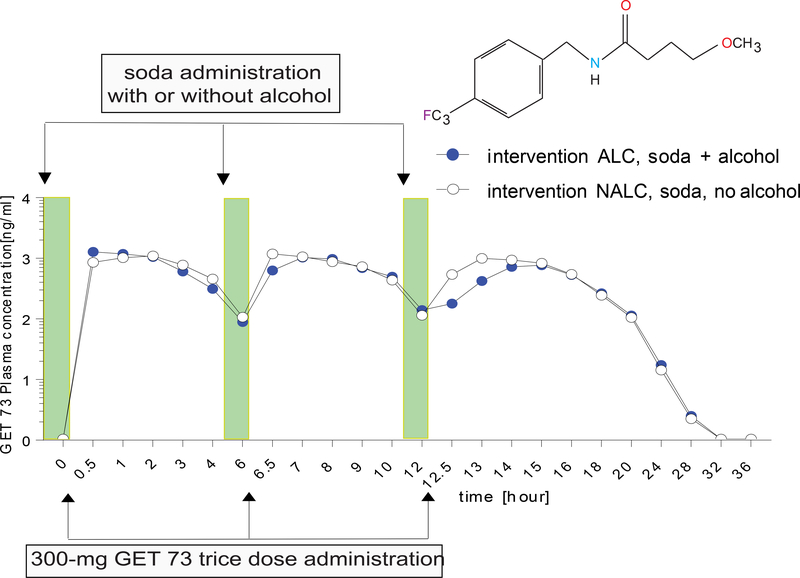
Experiment 4: 300-mg GET 73 trice
The GET 73 mean plasma concentration after the administration of 300-mg GET 73 trice, in the absence/presence of alcohol, is in Figure 5. Following dosing with intervention NALC, plasma concentration versus time profiles of GET 73 were consistent with three doses administered 6 hour apart. A lag phase was not observed for any participant, and concentration maxima occurred after each of the 3 doses during the dosing cycle. The inter-subject variability was high with a geometric CV% of 78.7% and 52.9% for Cmax and AUC∞. The reported terminal half-life estimates ranged between 1.24–1.79 hour post-dose.
FIGURE 5– Humans: Experiment 4: GET 73 plasma concentration after 300-mg GET 73 trice in the absence/presence of alcohol.
GET 73 plasma concentration in intervention NALC (n =12) 300-mg trice oral dose of GET 73 within 15 minutes of receiving 240 mL of soda versus intervention ALC (n = 12), 300-mg trice oral dose of GET 73 within 15 minutes of receiving 4 units of alcohol with 240 mL of soda. Results are reported as the Mean ± S.E.M.
Following administration of intervention ALC, peak concentrations of GET 73 occurred following every dose. As with intervention NALC, no lag phase was observed. Concentrations of GET 73 were similar to those following intervention NALC. The Cmax and AUC∞, values were also similar. The median tmax values were different for intervention NALC and ALC (2.0 hour and 6.5 hour). The mean t1/2 values were similar to those seen for intervention NALC as was the inter-subject variability with geometric CV% of 64.8% and 67.8% for Cmax and AUC∞. Terminal half-life estimates ranged betwen1.07–1.72 hour post-dose. For the comparison of intervention NALC versus ALC, the CIs for GET 73AUClast, Cmax and AUC∞, included 1. This suggests, that at the 10% level, the adjusted geometric means were similar when a 300-mg trice dose of GET 73 was administered in the presence/absence of alcohol.
Results for the PK parameters Cmax, AUClast and AUC∞ are in Table 7. Finally, there was not statistical difference in the tmax between the NALC versus ALC intervention (p>0.05).
Table 7.
Human Study: Geometric Mean (CV%) Values of key pharmacokinetic parameters for MET 2
| 300-mg GET 73 | Experiment 3 (300-mg GET 73 once) | Experiment 4 (300-mg GET 73 trice) | ||
|---|---|---|---|---|
| Intervention | NALC a | ALC a | NALC a | ALC a |
| n | 13 | 12 | 11 | 10 |
| Cmax(ng/mL) | 5120 (22.2) | 3460 (22.8) | 5890 (21.8) | 4440 (27.6) |
| tmax (h)b | 2.00 (0.50–4.00) | 1.50 (0.50–3.00) | 8.00 (0.50–16.00) | 9.00 (3.00–16.00) |
| tlag (h)b | 0.00 (0.00–0.25) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| AUClast(h.ng/mL) | 15660 (16.1) | 13090 (17.4) | 51230 (21.9) | 49070 (22.) |
| AUCinf(h.ng/mL) | 15750 (16.1) | 13180 (17.2) | 51370 (21.7) | 49210 (22.7) |
| t1/2 (h) | 1.29 (24.2) | 1.29 (13.2) | 1.47 (20.2) | 1.61 (26.5) |
NALC: soda with No Alcohol, ALC: soda with Alcohol
Result s reported
PK analysis of Metabolite MET 2
The key PK parameters of MET 2 are in Table 7.
Experiment 3: 300-mg GET 73 once
The MET 2 plasma concentration after the administration of 300-mg GET 73 once, in the absence/presence of alcohol, is on Figure 6. Following dosing with intervention NALC, MET 2 plasma concentrations were highest between 0.5–4.00 h post-dose. A lag phase of 0.25 h was identified in two participants only. Inter-subject variability was low, with a geometric CV% of 22.2% and 16.1% for Cmax and AUC∞. Terminal half-life estimates rangebetween0.96–1.89 h post-dose.
FIGURE 6– Humans: Experiment 3: MET 2 plasma concentration after 300-mg GET 73 in the absence/presence of alcohol.
MET 2 plasma concentration of intervention NALC (n =12) 300-mg single oral dose of GET 73 within 15 minutes of receiving 240 mL of soda versus intervention ALC (n = 12), 300-mg single oral dose of GET 73 within 15 minutes of receiving 4 units of alcohol with 240 mL of soda. Results are reported as the Mean ± S.E.M.
Following dosing with intervention ALC, concentrations of MET 2 peaked between 0.5–3.0 h post-dose and were similar to intervention NALC. A lag phase was not apparent in any subject. The Cmax and AUC∞ estimates for MET 2 were lower than the corresponding values seen for intervention NALC and the geometric mean value for the AUC∞ ratio (Intervention ALC/NALC) was 0.84. The t1/2 and tmax values were similar to those seen for intervention NALC, as was the inter-subject variability with a geometric CV% of 22.8% and 17.2% for Cmax and AUC∞, respectively. Terminal half-life estimates were similar to those reported for MET 2 following intervention NALC, ranging between 1.07–1.69 h. For MET 2tmaxvalues were not significantly different in NALC versus ALC interventions (p>0.05).
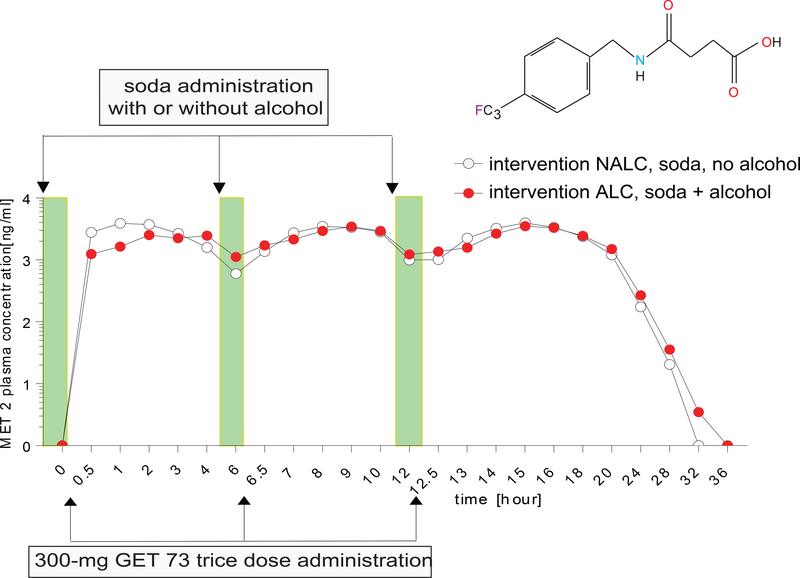
Experiment 4: 300-mg MET 2 trice
The MET 2 plasma concentration after the administration of 300-mg GET 73 trice, in the absence/presence of alcohol, is on Figure 7. Following dosing with intervention NALC, plasma concentrations of MET 2 peaked following each dose of GET 73 within the dose cycle. A lag phase was not seen for any of the subjects. The inter-subject variability was low with a geometric CV% of 21.8% and 21.7% for Cmax and AUC∞, respectively. Terminal half-life estimates ranged between1.22–2.54 h.
FIGURE 7– Humans: Experiment 4: MET 2 plasma concentration after 300-mg GET 73 trice in the absence/presence of alcohol.
MET 2 plasma concentration of intervention NALC (n =12) 300-mg trice a day oral dose of GET 73 within 15 min of receiving 240 mL of soda versus intervention ALC (n = 12), 300-mg trice oral dose of GET 73 within 15 minutes of receiving 4 units of alcohol with 240 mL of soda. Results are reported as the Mean ± S.E.M.
Following dosing with intervention ALC, MET 2 concentration maxima were apparent following each dose of GET 73, with no lag phase seen for any participants. While mean estimates of Cmax intervention ALC were similar to those seen for intervention NALC; the geometric mean value for the AUC∞ ratio (Intervention ALC/ NALC) was 0.96, which was closer to unity than that reported for intervention NALC. The t1/2and tmax values were similar to those seen for intervention NALC and the inter-subject variability was low with a geometric CV% of 27.6% and 22.7% for Cmax and AUC∞. Individual terminal half-life estimates range between 1.18–2.68 h. The results of the statistical analyses for MET 2 for the PK parameters Cmax, AUClast and AUC∞, are in Table 7 and for the bioavailability analysis of MET 2 in Table 8.
TABLE 8 –
Human Study: Bioavailability Analysis of MET 2
| Pharmacokinetic Parameter | Study Experiment | N | NALC a,b | ALC a,b | NALC/ALC c | NALC/ALC90% CI d |
|---|---|---|---|---|---|---|
| AUClast (h.ng/mL) | 1 | 12 | 15613 | 13178 | 1.18 | (1.10, 1.27) |
| 2 | 10 | 51598 | 49421 | 1.04 | (0.97, 1.13) | |
| AUC∞ (h.ng/mL) | 1 | 12 | 15519 | 13093 | 1.19 | (1.10, 1.27) |
| 2 | 10 | 51459 | 49282 | 1.04 | (0.96, 1.13) | |
| Cmax (ng/mL) | 1 | 12 | 5136 | 3462 | 1.48 | (1.32, 1.67) |
| 2 | 10 | 5722 | 4401 | 1.30 | (1.14, 1.48) | |
Results reported as mean and lower to upper 95% (CI)
NALC: soda with No Alcohol, ALC: soda with Alcohol
Adjusted geometrics mean from ANOVA model
Ratio of adjusted geometric mean
Confidence interval for the ratio adjusted geometric means
In Experiment 3, for the comparison of intervention NALC versus intervention ALC, the CIs for MET 2 Cmax, AUClast and AUC∞ were all above 1. This suggests, that at the 10% level, the adjusted geometric means for these parameters were higher when a single 300-mg dose GET 73 was administered in the absence of alcohol than when administered in the presence of alcohol.
In Experiment 4, for the comparison of intervention NALC versus ALC, the CIs for MET 2AUClast and AUC∞ included 1. This suggests that, at the 10% level, the adjusted geometric means for these parameters were similar when a 300-mg trice a day dose of GET 73 was administered in the presence or absence of alcohol. For MET 2 Cmax, the CI was entirely above 1, suggesting that, at the 10% level, the adjusted geometric mean Cmax was higher when a 300-mg trice a day dose of GET 73 was administered in the absence of alcohol than when administered in the presence of alcohol.
For MET 2 tmax values were not significantly different in NALC versus ALC intervention (P >0.05). For the statistical analysis of relative bioavailability, the residual plots, the normal probability plots and the plots of the residuals against the predicted values (i.e. the distributional assumptions underlying the statistical analysis, the assumptions of normality and homogeneity of variance) were satisfied.
Discussion
This study was the first to investigate and provide evidence of the safety of the administration of GET 73 with alcohol both in rats and humans. In humans, we also measured the bioavailability of GET 73 and of its major metabolite MET 2 after GET 73 was administered with alcohol. Based on the PK of GET 73 and MET 2, there were no significant differences in bioavailability between administering 300-mg GET 73 either once or trice in the presence or absence of alcohol.
In rats, when given a dose equivalent to the human dose alone, GET 73 did not alter spontaneous locomotor activity (parameter highly sensitive to the rat well-being), indicating that this dose of GET 73 was per se devoid of any motor-incoordinating, ataxic, and sedative effects. Second, neither 30 mg/kg nor 100 mg/kg GET 73 altered the intoxicating effects of alcohol. The doses of alcohol used in the rat experiments were selected to produce different degrees of alcohol intoxication, varying from hypolocomotion to sedation/hypnosis. The results of these two rat experiments suggest that GET 73 did not potentiate alcohol-induced intoxication. These results are relevant from a translational standpoint given that we tested doses of GET 73 that are equivalent to the doses employed in humans and in the range of those found to be pharmacologically effective in reducing alcohol drinking and anxiety-related behaviors in a rat model of AUD (Loche et al., 2012). The lack of any GET 73-induced potentiation of alcohol intoxication observed in these two rat experiments complements the lack of notable differences in AEs in humans receiving GET 73 in the presence/absence of alcohol.
Our results in humans hold clinical importance because GET 73 was well-tolerated when administered with alcohol with no severe or serious AEs. The overall incidence of AEs was low, and there was no notable difference between dose levels. Furthermore, for the few AEs observed, it is not possible to fully determine whether these AEs were attributed to the medication since the study did not included a placebo-controlled arm.
After a single oral dose of 300-mg GET 73 (absence of alcohol), the geometric mean Cmax and AUClast and median tmax values were consistent to those reported in the previous first-in-man study at the 300-mg dose level which confirm the reproducibly of the GET 73 PK profile in healthy males (Haass-Koffler et al., 2017a; Haass-Koffler et al., 2017b). When GET 73 was administered with alcohol the geometric means of Cmax and AUC∞ were higher, with a 2-fold and 1.5-fold increase, compared to GET 73 administration without alcohol. In contrast, both tmax and t1/2 appeared to be largely unchanged by the presence of alcohol. For MET 2, there appeared to be a decrease in Cmax (32%) and AUC∞ (16%) in the presence of alcohol. However, tmax and t1/2 of MET 2 were similar in the absence and presence of alcohol. These data indicate that following a single 300-mg oral dose in the presence of alcohol, systemic exposure to GET 73 increased; this increase might be due, in part, to a reduction in the extent of metabolic conversion to MET 2.
The trend towards greater systemic exposure in the presence of alcohol was not observed following repeat (300-mg trice) doses of GET 73. The mean Cmax and AUC∞ values of GET 73 were similar in the absence and presence of alcohol. The median tmax values were different for intervention NALC and intervention ALC (2.0 hour and 6.5 hour); however, the range of individual tmax estimates was similar for both treatments.
We observed a decrease in Cmax for MET 2 (25%) in the presence of alcohol, although the extent of total systemic exposure was similar. The occurrence of tmax was variable following each dose. The terminal half-life of MET 2 was similar following multiple doses of GET 73 in the presence/absence of alcohol. Total systemic exposure to GET 73 following the trice a day dosing of 300-mg GET 73 was approximately 3-fold greater than that observed following a single 300-mg dose (intervention NALC) and was therefore broadly proportional to the total dose administered. Observed concentration maxima were, however, comparable between intervention NALC in the 300-mg once dose and 300-mg trice dose, suggesting little or no accumulation of GET 73 over the trice dosing cycle. Corresponding treatment comparisons in the presence of alcohol, intervention ALC also suggested proportionality of total systemic exposure to total dose of GET 73 administered. Metabolite MET 2 also exhibited a proportional relationship between GET73 dose and systemic exposure (regardless of alcohol status) once again with little evidence of accumulation following trice a day dosing. This study provides preliminary evidence of no accumulation of GET 73 or its metabolite with the trice 300-mg dosing both in absence/presence of alcohol.
This study should be seen in light of both its strengths and limitations. Strengths include: (a) this study was a direct rat-to-human translation work on the drug-alcohol interaction of a putative compound for AUD; (b) it collected human PK information not only on the parent drug, but also on its main metabolite MET 2; and (c) GET 73 was tested both as single and as multiple dosing administrations. Limitations include that: (a) the study was not a fully factorial design (open-label, no GET 73-matched placebo); (b) only male rats and male humans were included; and (c) the amount of alcohol co-administered was relatively low in humans. Nonetheless, given this was the first study of its kind, it was prudent to start with low alcohol doses to assess safety as it relates to drug-alcohol interactions. Also, there is a general consensus on the need for women inclusion in research trials, in order to ensure a correct evaluation of gender differences during drug development. However, no sufficient genotoxicity data were available at the time of the study; in addition the small sample size did not allow a reliable gender difference evaluation. Future studies will need to look as the safety and PK profile of GET 73 when administered with higher doses of alcohol. Finally, a recent study showed a decrease in mGluR5 receptor binding in smokers and ex-smokers (Akkus et al., 2013); given the high incidence of alcohol and smoking comorbidity, future studies could investigate the role of GET 73 in tobacco use.
In conclusion, this study provides evidence on the safety and tolerability of GET 73 during an acute alcohol challenge experimental procedure and further supports future research towards investigating GET 73 as a novel pharmaceutical to treat patients with AUD.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Phillip Evans and the research staff at Quotient Clinical, Fordham, UK (human study), for the logistic support of the studies.
Funding and conflict of interest
These studies were funded by CT Laboratories, San Remo, Italy. RC and AL are employees of CT Laboratories. RMS has received consultant fees from CT Laboratories and travel and honoraria from D&A Pharma and Lundbeck. The other authors report no biomedical financial interests or potential conflicts of interest.
References
- Akkus F, Ametamey SM, Treyer V, et al. (2013) Marked global reduction in mGluR5 receptor binding in smokers and ex-smokers determined by [11C]ABP688 positron emission tomography. Proc Natl Acad Sci U S A 110: 737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggiato S, O’Connor WT, Tomasini MC, et al. (2013) GET73 increases rat extracellular hippocampal CA1 GABA levels through a possible involvement of local mGlu5 receptor. Synapse 67: 678–691. [DOI] [PubMed] [Google Scholar]
- Bell RL, Sable HJ, Colombo G, et al. (2012) Animal models for medications development targeting alcohol abuse using selectively bred rat lines: neurobiological and pharmacological validity. Pharmacol Biochem Behav 103: 119–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin JJ, et al. (2008) Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats. Alcohol Clin Exp Res 32: 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Lobina C, Carai MA, et al. (2006) Phenotypic characterization of genetically selected Sardinian alcohol-preferring (sP) and -non-preferring (sNP) rats. Addict Biol 11: 324–338. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A and Lindsley CW. (2009) Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov 8: 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza MS. (2015) Glutamatergic transmission in drug reward: implications for drug addiction. Front Neurosci 9: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR and Lawrence AJ. (2012) The role of metabotropic glutamate receptors in addiction: evidence from preclinical models. Pharmacol Biochem Behav 100: 811–824. [DOI] [PubMed] [Google Scholar]
- FDA FaDA. (2015) Alcoholism: Developing Drugs for Treatment. Center for Drug Evaluation and Research (CDER). [Google Scholar]
- Ferraro L, Beggiato S, Tomasini MC, et al. (2011) GET73 modulates rat hippocampal glutamate transmission: evidence for a functional interaction with mGluR5. Pharmacol Rep 63: 1359–1371. [DOI] [PubMed] [Google Scholar]
- Gilpin NW and Koob GF. (2008) Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Res Health 31: 185–195. [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Goodyear K, Long VM, et al. (2017a) Dataset for Phase I clinical trial for safety and tolerability of GET 73 in single and repeated ascending doses including preliminary pharmacokinetic parameters Data In Brief. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Goodyear K, Long VM, et al. (2017b) A Phase I randomized clinical trial testing the safety, tolerability and preliminary pharmacokinetics of the mGluR5 negative allosteric modulator GET 73 following single and repeated doses in healthy volunteers. Eur J Pharm Sci 109: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Leggio L and Kenna GA. (2014) Pharmacological approaches to reducing craving in patients with alcohol use disorders. CNS Drugs 28: 343–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loche A, Simonetti F, Lobina C, et al. (2012) Anti-Alcohol and Anxiolytic Properties of a New Chemical Entity, GET73. Front Psychiatry 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihov Y and Hasler G. (2016) Negative Allosteric Modulators of Metabotropic Glutamate Receptors Subtype 5 in Addiction: a Therapeutic Window. Int J Neuropsychopharmacol 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. (2009) Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev 2: 83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. (2010) Cognitive effects of Group I metabotropic glutamate receptor ligands in the context of drug addiction. Eur J Pharmacol 639: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottani A, Leone S, Vergara FB, et al. (2007) Preference for palatable food is reduced by the gamma-hydroxybutyrate analogue GET73, in rats. Pharmacol Res 55: 271–279. [DOI] [PubMed] [Google Scholar]
- Spooren W, Ballard T, Gasparini F, et al. (2003) Insight into the function of Group I and Group II metabotropic glutamate (mGlu) receptors: behavioural characterization and implications for the treatment of CNS disorders. Behav Pharmacol 14: 257–277. [DOI] [PubMed] [Google Scholar]
- Tacchi R, Ferrari A, Loche A, et al. (2008) Sucrose intake: increase in non-stressed rats and reduction in chronically stressed rats are both prevented by the gamma-hydroxybutyrate (GHB) analogue, GET73. Pharmacol Res 57: 464–468. [DOI] [PubMed] [Google Scholar]
- Tomasini MC, Borelli AC, Beggiato S, et al. (2016) GET73 Prevents Ethanol-Induced Neurotoxicity in Primary Cultures of Rat Hippocampal Neurons. Alcohol Alcohol 51: 128–135. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Marek GJ, Johnson BG, et al. (2007) Metabotropic glutamate receptors in the control of mood disorders. CNS Neurol Disord Drug Targets 6: 87–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.