Abstract
Background
The majority of infectious diseases of cultured fish is caused by bacteria. Rapid identification of bacterial pathogens is necessary for immediate management. The present study developed a custom Main Spectra Profile (MSP) database and validate the method using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) for rapid identification of fish bacterial pathogens. Streptococcus agalactiae, Streptococcus iniae, Aeromonas hydrophila, Aeromonas veronii, and Edwardsiella tarda obtained from diseased fish were used as representative bacterial pathogens in this study. Bacterial peptides were extracted to create a Main Spectra Profile (MSP), and the MSPs of each bacterial species was added into the MALDI Biotyper database. Fifteen additional isolates of each bacterial species were tested to validate the utilized technique.
Results
The MSPs of all field isolates were clearly distinguishable, and the MSPs of the same species were clustered together. The identification methodology was validated with 75 bacterial isolates. The reliability and specificity of the method were determined with MALDI Biotyper log score values and matching results with 16 s rDNA sequencing. The species identification using the public MALDI Biotyper library (Bruker MALDI Biotyper) showed unreliable results (log score < 2.000) with 42.67% matching result with the reference method. In contrast, accurate identification was obtained when using the custom-made database, giving log score > 2.115, and a 100% matching result.
Conclusion
This study demonstrates an effective identification of fish bacterial pathogens when a complete custom-made MSP database is applied. Further applications require a broad, well-established database to accommodate prudent identification of many fish bacterial pathogens by MALDI-TOF MS.
Keywords: Mass spectrometry, Proteomic, Fish disease, Biotyper
Background
Bacterial pathogens are a major etiology of infectious diseases of cultured fish [1]. Among those bacteria, Streptococcus spp., Aeromonas spp., and Edwardsiella spp., are commonly found in several important aquaculture species, such as the Asian catfish Clarias batrachus [2], barramundi Lates calcarifer [3], and Nile tilapia Oreochromis niloticus [4]. In many cases of bacterial infection, clinical signs and lesions are not obviously apparent and may mislead the diagnosis. Therefore, identification of disease-causing bacterial species is necessary in order to carry out proper disease management.
Conventional microbiology techniques, including morphological, physiological and biochemical tests, and molecular techniques based on 16S rDNA sequencing, are the gold standard for bacterial species identification [5]. However, these techniques require a substantial amount of time and expensive reagents [6]. In recent developments of mass spectrometry (MS), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) has been implemented in human and veterinary medicine as an alternative diagnostic tool with increasing popularity due to its quickness, simplicity, cost-effectiveness, and strong discriminating power [7, 8]. The MALDI-TOF MS detects mass signals from bacterial proteins or peptides and determines their unique mass spectra or peptide mass fingerprints (PMFs). The obtained PMFs are then compared with reference bacterial strains in the public proteomics/genomics databases, or in a dedicated mass spectra library (library based approach) [9]. These mass spectra libraries are able to differentiate the bacteria to their genus, species or sub-group levels subject to sufficient pre-existing reference strains in the database [10].
The MALDI-TOF MS approach has been adopted as a routine diagnostic tool for human medicine [11] and has also been widely evaluated for its ability to differentiate bacterial species of veterinary and public health importance. For example, S. equi at the subspecies level [8], Streptococcus species isolated from diseased pigs [12], pathogenic Gram-negative bacteria in seafood [13] and Aeromonas species found in a drinking water system [14] have been assessed. In fish, MALDI-TOF was evaluated for the rapid identification of Gram-positive bacterial pathogens, including S. agalactiae, Lactococcus garvieae, S. iniae, and S. dysgalactiae isolated from Nile tilapia [15] and S. iniae isolated from the olive flounder Paralichthys olivaceus [16]. These studies found that the public database, the Bruker MALDI Biotyper library, was insufficient for identifying bacterial species isolated from fish with MALDI-TOF MS.
Therefore, the present study aims to develop a custom Main Spectra Profile (MSP) database and validate the method using MALDI-TOF MS for a rapid and accurate identification of S. agalactiae, S. iniae, A. hydrophila, A. veronii and E. tarda isolated from economically important fish species.
Results
Maldi-Tof Ms. for bacterial species differentiation
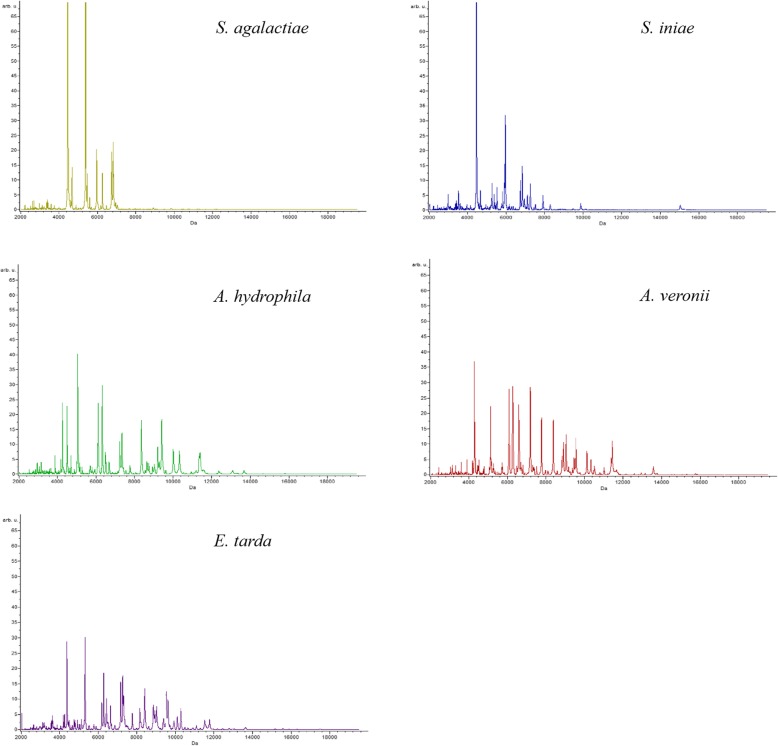
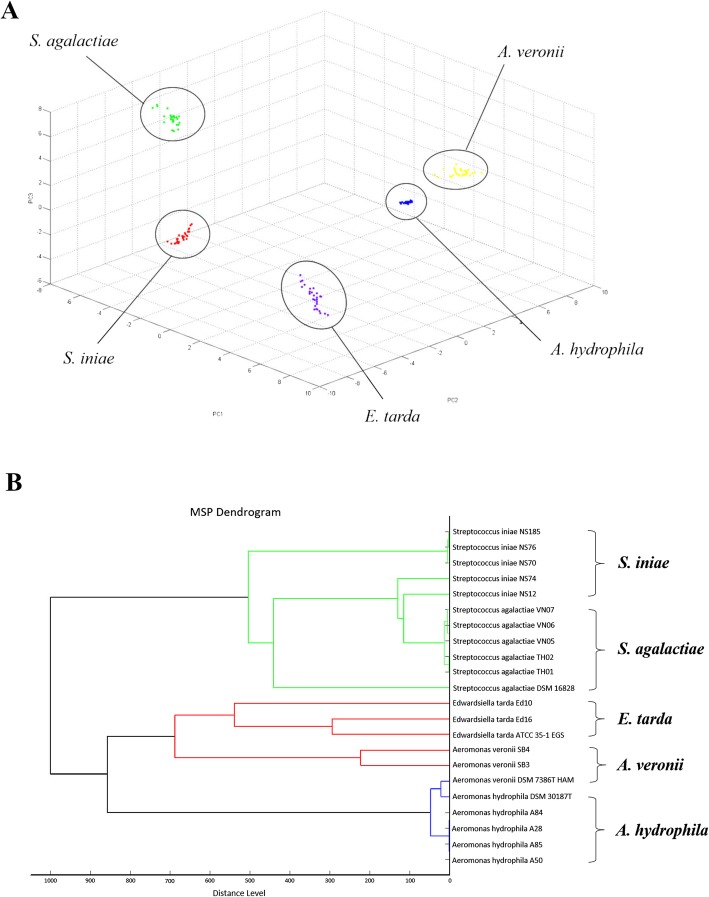
The high reliability of the MALDI-TOF MS was indicated by the obtained 100% recognition capabilities and by the cross-validation values of 87.8, 97.1, 100, 100, and 100% for S. agalactiae, S. iniae. A. hydrophila, A. veronii, and E. tarda, respectively. The five bacterial species showed distinguishing spectral peaks ranging between 2000 and 15,000 Da (Fig. 1). The three-dimensional principal component analysis (3D-PCA) scatterplot presented clearly distinguishable clusters, each cluster presented in the 3D-PCA scatterplot (Fig. 2a) indicates MSPs or distinctive peptide fingerprint of the bacterial species. Bacterial isolates of the same species were grouped within the same clade of MSP dendrogram (Fig. 2b).
Fig. 1.
Mass peptide fingerprints of five bacterial pathogens (S. agalactiae, S. iniae, A. hydrophila, A. veronii and E. tarda) isolated from cultured fish
Fig. 2.
Cluster analysis of the five bacterial pathogens (S. agalactiae, S. iniae, A. hydrophila, A. veronii and E. tarda) isolated from cultured fish. a The 3D-PCA scatterplot representing clusters of each species (dashed circles) and the (b) MSP dendrogram of the representative bacterial isolates analyzed in the present study with the reference ATCC strains
Bacterial identification with MALDI Biotyper
The 75 tested isolates, when blasted with the reference strains available in the Bruker database, gave no-reliable identification for 23 isolates (log score 1.432–1.669), probable genus-level identification for 42 isolates (log score 1.707–1.998), and secure genus-level identification for 10 isolates (log score 2.018–2.254). No species-level identification was obtained, particularly for S. iniae since it is not available in the Bruker database (Table 1). Differently, all 75 tested bacterial isolates yielded accurate genus-level identification with a custom MSP database, 66 isolates were identified highly probable species-level identification (log score 2.301–3.001), and 9 isolates were identified a probable species-level identification (log score 2.115–2.264). Repeatability of the method was considered with ≤10% deviations of log scores (Table 2). For specificity, a custom MSP database provided 100% match with 16 s rDNA sequencing, while the Bruker database yielded matching result of 42.67% (32 out of 75 isolates).
Table 1.
Method validation of the MALDI Biotyper of five bacterial pathogens showing the matching results with the 16 s rDNA sequencing, custom MSP and Bruker MSP database. The identity was calculated against NCBI database
| Isolate number | Source | 16S rDNA sequencing | MALDI-TOF | ||||
|---|---|---|---|---|---|---|---|
| Custom MSP database | Bruker MSP database | ||||||
| Organism best match | % identity | Organism best match | Log score | Organism best match | Log score | ||
| Streptococcus spp | |||||||
| S147-Ja | Nile tilapia | S. agalactiae | 100 | S. agalactiae | 2.999 | S. parauberis | 1.877 |
| S183-Ja | Red tilapia | S. agalactiae | 100 | S. agalactiae | 2.897 | S. parauberis | 1.665 |
| S187-Ja | Nile tilapia | S. agalactiae | 99 | S. agalactiae | 2.988 | S. agalactiae | 1.545 |
| S190-Ja | Red tilapia | S. agalactiae | 99 | S. agalactiae | 2.856 | S. agalactiae | 1.998 |
| SV1/1-Ja | Nile tilapia | S. agalactiae | 100 | S. agalactiae | 2.945 | S. agalactiae | 2.019 |
| S71 | Red tilapia | S. agalactiae | 99 | S. agalactiae | 2.244 | S. urinalis | 1.666 |
| S96 | Red tilapia | S. agalactiae | 100 | S. agalactiae | 2.377 | S. agalactiae | 1.756 |
| S100 | Nile tilapia | S. agalactiae | 99 | S. agalactiae | 2.442 | S. agalactiae | 1.582 |
| S101 | Nile tilapia | S. agalactiae | 99 | S. agalactiae | 2.375 | S. parauberis | 1.829 |
| S102 | Nile tilapia | S. agalactiae | 98 | S. agalactiae | 2.544 | S. parauberis | 1.876 |
| S183 | Red tilapia | S. agalactiae | 100 | S. agalactiae | 2.388 | S. agalactiae | 1.716 |
| S184 | Red tilapia | S. agalactiae | 100 | S. agalactiae | 2.591 | S. agalactiae | 1.749 |
| S191 | Red tilapia | S. agalactiae | 99 | S. agalactiae | 2.347 | S. urinalis | 1.564 |
| S195 | Nile tilapia | S. agalactiae | 97 | S. agalactiae | 2.455 | S. agalactiae | 1.632 |
| S198 | Nile tilapia | S. agalactiae | 99 | S. agalactiae | 2.544 | S. agalactiae | 1.908 |
| NS12-Ja | Red tilapia | S. iniae | 100 | S. iniae | 2.898 | S. agalactiae | 1.432 |
| NS70-Ja | Nile tilapia | S. iniae | 100 | S. iniae | 2.988 | S. agalactiae | 1.688 |
| NS74-Ja | Barramundi | S. iniae | 100 | S. iniae | 2.878 | S. parauberis | 1.987 |
| NS76-Ja | Barramundi | S. iniae | 99 | S. iniae | 2.999 | S. pyogenes | 1.555 |
| NS185-Ja | Barramundi | S. iniae | 99 | S. iniae | 2.778 | S. parauberis | 1.654 |
| NS11 | Nile tilapia | S. iniae | 98 | S. iniae | 2.376 | S. agalactiae | 1.997 |
| NS18 | Nile tilapia | S. iniae | 100 | S. iniae | 2.419 | S. agalactiae | 1.707 |
| NS26 | Red tilapia | S. iniae | 99 | S. iniae | 2.534 | S. pyogenes | 1.728 |
| NS34 | Barramundi | S. iniae | 96 | S. iniae | 2.544 | S. agalactiae | 1.886 |
| NS50 | Barramundi | S. iniae | 97 | S. iniae | 2.308 | S. pyogenes | 1.679 |
| NS84 | Barramundi | S. iniae | 98 | S. iniae | 2.118 | S. pyogenes | 1.679 |
| NS85 | Barramundi | S. iniae | 98 | S. iniae | 2.445 | S. agalactiae | 1.864 |
| NS89 | Red tilapia | S. iniae | 99 | S. iniae | 2.342 | S. agalactiae | 1.975 |
| NS90 | Red tilapia | S. iniae | 100 | S. iniae | 2.221 | S. agalactiae | 1.873 |
| NS91 | Nile tilapia | S. iniae | 100 | S. iniae | 2.464 | S. parauberis | 1.593 |
| Aeromonas spp | |||||||
| A28-Ja | Nile tilapia | A. hydrophila | 100 | A. hydrophila | 2.978 | A. veronii | 1.495 |
| A29-Ja | Hybrid catfish | A. hydrophila | 100 | A. hydrophila | 2.991 | A. veronii | 1.767 |
| A49-Ja | Red tilapia | A. hydrophila | 100 | A. hydrophila | 2.965 | A. hydrophila | 1.869 |
| A50-Ja | Nile tilapia | A. hydrophila | 100 | A. hydrophila | 2.889 | A. ichthiosmia | 2.094 |
| A84-Ja | Snakehead fish | A. hydrophila | 100 | A. hydrophila | 2.945 | A. hydrophila | 1.755 |
| A90 | Nile tilapia | A. hydrophila | 99 | A. hydrophila | 2.312 | A. veronii | 2.018 |
| A108 | Hybrid catfish | A. hydrophila | 100 | A. hydrophila | 2.464 | A. hydrophila | 1.956 |
| A109 | Red tilapia | A. hydrophila | 100 | A. hydrophila | 2.115 | A. hydrophila | 1.848 |
| A110 | Snakehead fish | A. hydrophila | 98 | A. hydrophila | 2.394 | A. hydrophila | 2.181 |
| A112 | Snakehead fish | A. hydrophila | 98 | A. hydrophila | 2.601 | A. hydrophila | 2.011 |
| A114 | Nile tilapia | A. hydrophila | 97 | A. hydrophila | 2.451 | A. veronii | 1.995 |
| A115 | Nile tilapia | A. hydrophila | 97 | A. hydrophila | 2.551 | A. ichthiosmia | 2.045 |
| A120 | Hybrid catfish | A. hydrophila | 99 | A. hydrophila | 2.009 | A. veronii | 1.454 |
| A126 | Snakehead fish | A. hydrophila | 100 | A. hydrophila | 2.567 | A. veronii | 1.777 |
| A127 | Snakehead fish | A. hydrophila | 99 | A. hydrophila | 2.301 | A. shigelloides | 1.985 |
| SB1-Ja | Nile tilapia | A. veronii | 100 | A. veronii | 2.969 | A. hydrophila | 1.559 |
| SB2-Ja | Nile tilapia | A. veronii | 99 | A. veronii | 3.000 | A. shigelloides | 1.997 |
| SB3-Ja | Barramundi | A. veronii | 99 | A. veronii | 2.897 | A. veronii | 1.787 |
| SB4-Ja | Barramundi | A. veronii | 100 | A. veronii | 2.888 | A. veronii | 1.945 |
| SB7-Ja | Red tilapia | A. veronii | 98 | A. veronii | 2.899 | A. shigelloides | 1.658 |
| SB5 | Nile tilapia | A. veronii | 98 | A. veronii | 2.327 | A. ichthiosmia | 2.027 |
| SB6 | Nile tilapia | A. veronii | 100 | A. veronii | 2.454 | A. hydrophila | 1.787 |
| SB8 | Barramundi | A. veronii | 98 | A. veronii | 2.382 | A. hydrophila | 1.844 |
| SB9 | Barramundi | A. veronii | 98 | A. veronii | 2.511 | A. veronii | 1.906 |
| SB10 | Barramundi | A. veronii | 97 | A. veronii | 2.377 | A. veronii | 1.733 |
| SB12 | Barramundi | A. veronii | 100 | A. veronii | 2.401 | A. veronii | 1.667 |
| SB13 | Barramundi | A. veronii | 99 | A. veronii | 2.359 | A. hydrophila | 1.872 |
| SB14 | Barramundi | A. veronii | 97 | A. veronii | 2.228 | A. ichthiosmia | 1.560 |
| SB15 | Barramundi | A. veronii | 100 | A. veronii | 2.553 | A. veronii | 1.924 |
| SB17 | Barramundi | A. veronii | 100 | A. veronii | 2.198 | A. veronii | 1.855 |
| Edwardsiella tarda | |||||||
| Ed10-Ja | Hybrid catfish | E. tarda | 100 | E. tarda | 2.899 | E. tarda | 2.000 |
| Ed12-Ja | Hybrid catfish | E. tarda | 100 | E. tarda | 2.932 | E. tarda | 1.887 |
| Ed14-Ja | Nile tilapia | E. tarda | 99 | E. tarda | 2.984 | E. tarda | 1.666 |
| Ed16-Ja | Nile tilapia | E. tarda | 98 | E. tarda | 3.000 | E. hoshinae | 1.669 |
| Ed18-Ja | Nile tilapia | E. tarda | 100 | E. tarda | 2.999 | E. hoshinae | 1.842 |
| Ed8 | Hybrid catfish | E. tarda | 99 | E. tarda | 2.382 | E. hoshinae | 1.584 |
| Ed9 | Hybrid catfish | E. tarda | 100 | E. tarda | 2.367 | E. hoshinae | 1.624 |
| Ed11 | Hybrid catfish | E. tarda | 98 | E. tarda | 2.203 | E. hoshinae | 1.782 |
| Ed17 | Nile tilapia | E. tarda | 99 | E. tarda | 2.471 | E. tarda | 1.977 |
| Ed20 | Nile tilapia | E. tarda | 100 | E. tarda | 2.457 | E. tarda | 1.945 |
| Ed23 | Hybrid catfish | E. tarda | 98 | E. tarda | 2.457 | E. tarda | 2.254 |
| Ed25 | Hybrid catfish | E. tarda | 100 | E. tarda | 2.342 | E. tarda | 1.963 |
| Ed27 | Nile tilapia | E. tarda | 100 | E. tarda | 2.264 | E. tarda | 2.014 |
| Ed30 | Nile tilapia | E. tarda | 100 | E. tarda | 2.445 | E. tarda | 2.002 |
| Ed31 | Nile tilapia | E. tarda | 100 | E. tarda | 2.116 | E. tarda | 1.956 |
aIsolate used for MSP database construction
log score values: < 1.700 = no reliable identification, ≥ 1.700–1.999 = probable genus-level identification, 2.000–2.229 = a secure genus-level identification and a probable species-level identification, and 2.300–3.000 = a secure species-level identification and highly probable species-level identification
Table 2.
Comparison of the test results for bacterial species identification using a custom MSP database and Bruker database
| Pathogen | MSP database | No. of isolate matched with 16 s rDNA sequencing (%) | Mean log score ± SD (Min - Max) |
|---|---|---|---|
| S. agalactiae | Custom | 15/15 (100%) | 2.599 ± 0.26 (2.244–2.999) |
| Bruker | 9/15 (60%) | 1.758 ± 0.15 (2.545–2.019) | |
| S. iniae | Custom | 15/15 (100%) | 2.554 ± 0.25 (2.118–2.999) |
| Bruker | 0/15 (0%) | 1.753 ± 0.17 (1.432–1.997) | |
| A. hydrophila | Custom | 15/15 (100%) | 2.568 ± 0.22 (2.009–2.991) |
| Bruker | 6/15 (40%) | 1.883 ± 0.20 (1.454–2.181) | |
| A. veronii | Custom | 15/15 (100%) | 2.562 ± 0.25 (2.198–3.000) |
| Bruker | 7/15 (46.67%) | 1.808 ± 0.14 (1.559–2.027) | |
| E. tarda | Custom | 15/15 (100%) | 2.554 ± 0.24 (2.116–3.000) |
| Bruker | 10/15 (66.67%) | 1.877 ± 0.18 (1.584–2.254) | |
| Total | Custom | 75/75 (100%) | 2.568 ± 0.25 (2.009–3.000) |
| Bruker | 32/75 (42.67%) | 1.816 ± 0.17 (1.432–2.254) |
Discussion
An accurate and repeatable method for identification of the important bacterial pathogens of aquaculture species was established in this study. This method can be performed in a relatively short time compared to conventional microbiological methods. However, the present study found that reliable identification of bacterial species was only obtained when a custom MSP database was constructed since the reference database does not always accommodate the tested pathogens. Our study shows that identification was significantly improved when a custom MSP database was applied. All 75 isolates were secure at a genus-level identification and up to 88% (66 out of 75 isolates) were identified a highly probable species level when the identification was made on a custom MSP database. The public database may predominantly contain bacterial species that are only significant to humans but not species of veterinary importance, particularly from aquatic species [17]. The failure in species identification from the Bruker database may also result from inconsistent peptide profiles due to the use of different sample preparation protocols. The extraction method usually involves the use of organic acid to extract small-sized protein molecules, such as ribosomal proteins, cold shock proteins, and nucleic-acid binding proteins [18]. The different percentage of acid used in other studies [50% ACN and 2.5% TFA] [16, 19, 20] may alter the pattern of those extracted proteins. Nevertheless, the ability to tailor a database expands the application of MALDI Biotyper as an identification tool for bacterial species specific to a host or location, and at below species-levels, such as sub-species, strain, or serotype [21, 22].
The 3D-PCA scatterplot and MSP dendrogram generated from the MSPs can also be used for grouping or discriminating the type of organisms. The analyzed peptides are mainly ribosomal peptide molecules which uniquely present in the organisms [23]. In the present study, we provide an example of a MSP dendrogram created by the Biotyper software, by grouping the bacteria based on their phenotypic traits instead of their genetic traits (Fig. 2b). The software allows us to insert additional MSPs of other bacterial strains available in the reference database. Interestingly, the ATCC strains from the reference database are located in a different clade from our field strains. This may explain the failure of species identification described previously. Genotyping is usually based on phylogenetic analysis of a highly conserved region of the ribosomal RNA of the bacteria and this conservative feature may limit classification of the bacteria. Several studies have used MALDI-TOF MS as a discriminatory tool for typing bacterial pathogens [17, 24, 25] and have found that genotypic and phenotypic traits do not always concur [26]. Thus, MALDI-TOF MS can be used as an additional method for bacterial taxonomic classification when a complete MSP database is used, which may benefit further research, such as epidemiology, identification of protein biomarkers, and virulence studies. For example, MALDI-TOF MS has been used to distinguish antimicrobial resistant Enterobacteriaceae [27], identify Burkholderia pseudomallei mutants [18], and Carbapenem-resistant Klebsiella pneumoniae [28].
Conclusions
To our knowledge, the present study is the first to describe a MSP database for both Gram-positive (Streptococcus) and Gram-negative (Aeromonas and Edwardsiella) bacterial pathogens of cultured fish. This analysis could establish species-level identifications, even when the sources of those bacteria are from different geographical locations or host species. However, this specificity can be obtained only when a custom MALDI Biotyper database is constructed with a standard sample preparation protocol. For the most reliable results, we suggest that the database of each user should contain a custom MSP of the active local pathogenic strains.
Methods
Bacterial samples
All bacterial isolates were obtained from clinical cases that were submitted for disease diagnosis at the Faculty of Veterinary Science, Chulalongkorn University, Thailand. Bacteriology was conducted by the methods described previously [4]. Diseased fish were dissected dorsoventrally with a sterile blade to expose the kidney. Bacterial isolation was then performed using a kidney swab onto Columbia blood agar supplemented with 5% sheep blood (Oxiod, Basingstoke, UK) and incubated at 28 °C for 24 h. A single colony of the pure (homogeneous colony appearance) bacterial culture on an agar plate was selected for species confirmation by conventional microbiology methods, including Gram staining, catalase and oxidase production tests, and API identification (BioMérieus®, France). Bacterial species were confirmed using PCR amplification and sequencing of the 16S rDNA [29]. All bacterial isolates were stored in a nutrient broth (NB; Oxiod) containing 10% fetal calf serum and 20% glycerol at -80 °C for further analysis.
Sample preparation for MALDI-TOF MS
Each bacterial isolate was revived from the stock onto Columbia blood agar and incubated at 28 °C for 18 h. Extraction of bacterial proteins was performed as previously described [30]. A loopful of bacterial colonies was suspended in 70% ethanol and the suspension was centrifuged at 11,000 g for 2 min. The supernatant was removed, and the bacterial pellet was resuspended and mixed thoroughly with 100% acetonitrile (ACN) containing 5% (w/v) trifluoroacetic acid (TFA). The suspension was centrifuged and the supernatant was collected for peptide measurement using Lowry’s assay at 690 nm absorbance [31]. The concentration of peptide was adjusted to 0.1 μg μL− 1 for the MALDI-TOF MS analysis.
MALDI-TOF MS for database generation
Five bacterial isolates of S. agalactiae, S. iniae, A. hydrophila, A. veronii and E. tarda were used as a representative for the MSP database preparation (Table 3). The peptide extraction was performed once for each bacterial isolate as described above. The protocol for database construction was referred to the previous study [32]. Peptide patterns of all isolates were identified by MALDI-TOF MS to generate a database specific to each bacterial species. The MALDI matrix solution [10 mg mL− 1 sinapinic acid in 100% ACN containing 5% (w/v) TFA] was added to each sample (0.1 μg μL− 1 peptide) at a 1:1 (v/v) ratio. The mixed samples were spotted and air dried onto a MTP 384 ground steel target plate (Bruker Daltonics, Billerica, MA, USA) as 29 individual replicates. Mass spectra were obtained using an Ultraflex III MALDI-TOF/TOF (Bruker Daltonik, GmbH, Germany) in a linear positive mode with a mass range between 2 and 20 kDa, a laser frequency of 50 Hz and 500 laser shots. A ProteoMass Peptide & Protein MALDI-MS Calibration Kit (Sigma Aldrich, MO, USA) was applied for external calibrations which consisted of human angiotensin II (m/z 1046), P14R (m/z 1533), human adrenocorticotropic hormone fragment 18–39 (m/z 2465), bovine insulin oxidized B chain (m/z 3465), bovine insulin (m/z 5731), and cytochrome C (m/z 12,362).
Table 3.
Bacterial pathogens used for the development the custom MSP database. The isolates were obtained from cultured Nile tilapia Oreochromis niloticus, red tilapia Oreochromis spp., barramundi Lates calcarifer, hybrid catfish Clarias macrocephalus × C. gariepinus, and Snakehead fish Channa striata
| Bacterial species | Isolate number | Year | Source | Region |
|---|---|---|---|---|
| S. agalactiae | S147-J | 2013 | Nile tilapia | Western Thailand |
| S183-J | 2015 | Red tilapia | Central Thailand | |
| S187-J | 2017 | Nile tilapia | Eastern Thailand | |
| S190-J | 2018 | Red tilapia | Southern Thailand | |
| SV1/1-J | 2018 | Nile tilapia | Northern Vietnam | |
| S. iniae | NS12-J | 2007 | Red tilapia | Northeastern Thailand |
| NS70-J | 2012 | Nile tilapia | Northeastern Thailand | |
| NS74-J | 2014 | Barramundi | Eastern Thailand | |
| NS76-J | 2014 | Barramundi | Eastern Thailand | |
| NS185-J | 2018 | Barramundi | Eastern Thailand | |
| A. hydrophila | A28-J | 2011 | Nile tilapia | Eastern Thailand |
| A29-J | 2011 | Hybrid catfish | Eastern Thailand | |
| A49-J | 2013 | Red tilapia | Central Thailand | |
| A50-J | 2015 | Nile tilapia | Eastern Thailand | |
| A84-J | 2017 | Snakehead fish | Central Thailand | |
| A. veronii | SB1-J | 2017 | Nile tilapia | Eastern Thailand |
| SB2-J | 2017 | Nile tilapia | Eastern Thailand | |
| SB3-J | 2018 | Barramundi | Eastern Thailand | |
| SB4-J | 2019 | Barramundi | Eastern Thailand | |
| SB7-J | 2019 | Red tilapia | Eastern Thailand | |
| E. tarda | Ed10-J | 2012 | Hybrid catfish | Central Thailand |
| Ed12-J | 2013 | Hybrid catfish | Central Thailand | |
| Ed14-J | 2015 | Nile tilapia | Eastern Thailand | |
| Ed16-J | 2016 | Nile tilapia | Eastern Thailand | |
| Ed18-J | 2017 | Nile tilapia | Central Thailand |
Fingerprint spectra were calibrated and analyzed by the flexAnalysis software version 3.4 to assess high levels of reproducibility. The uniformity and homogeneity of the sample group as PMF and 3D-PCA were determined by t-test/ANOVA incorporated in the ClinProTools software version 3.0 [28]. A construction of a 3D-PCA scatterplot was performed using ClinProTools software.
The custom MSP database construction was performed according to Bruker’s recommendation. Twenty apparent spectra were chosen from MALDI-TOF analysis of each bacterial isolate, then a total of 100 spectra from 5 isolates of one bacterial pathogen were uploaded into MALDI Biotyper software (version 4.0) and assembled to generate a MSP database for the species using the standard method of BioTyper MSP creation. The MSP dendrogram was then created to determine the relatedness of each bacterial species based on their peptide fingerprint.
Method validation
Reliability, repeatability and specificity of the method were evaluated by testing 15 bacterial isolates per bacterial species (Table 1). The bacteria were retrieved from -80 °C stock and processed through bacterial protein extraction, MAILDI-TOF MS, and species identification via BioTyper software using a similar protocol described above, each extracted sample was spotted as four replicates on the MALDI plate. The MSPs of these isolates were then blasted against the Bruker database and a custom MSP database. The reliability of the method was determined based on log score values computed by Biotyper software [33]; < 1.700 = no reliable identification (indicating inaccurate identification), ≥ 1.700–1.999 = probable genus-level identification, 2.000–2.229 = a secure genus-level identification and a probable species-level identification, and 2.300–3.000 = a secure genus-level identification and highly probable species-level identification. The ≤10% variation of log scores justified repeatability. Specificity was evaluated against the reference method, 16S rDNA sequencing. Numbers of the bacterial isolate that provided similar identification as detected by the reference method indicated a degree of specificity of the MALDI-TOF application.
Acknowledgments
We thank for the technical advice from the Agricultural Research Development Agency (Public organization), Bangkok, Thailand.
Abbreviations
- 3D-PCA
Three-Dimensional Principal Component Analysis
- MALDI-TOF MS
Matrix-Assisted Laser Desorption/ionization Time-Of-Flight Mass Spectrometry
- MSP
Main Spectra Profile
- PMF
Peptide Mass Fingerprint
Authors’ contributions
PP and JW committed study design. PP, SR and JW organized the project and agreed the objectives of the study. PP and TQH executed the sample collection. PP and JJ performed the sample treatment, method validation, and instrumental analysis. PP and JW prepared initial draft of the manuscript. SR and JW made revision and refinement of the manuscript. SR and JW applied for project’s funding. All authors read and approved the final manuscript.
Funding
This study was supported by Grants for Development of New Faculty Staff, Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University.
Availability of data and materials
The datasets used and analyzed during the study are available from the corresponding author upon request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lafferty KD, Harvell CD, Conrad JM, Friedman CS, Kent ML, Kuris AM, Powell EN, Rondeau D, Saksida SM. Infectious diseases affect marine fisheries and aquaculture economics. Annu Rev Mar Sci. 2015;7:471–496. doi: 10.1146/annurev-marine-010814-015646. [DOI] [PubMed] [Google Scholar]
- 2.Sahoo P, Mukherjee S, Sahoo S. Aeromonas hydrophila versus Edwardsiella tarda: a pathoanatomical study in Clarias batrachus. J Aquacult. 1998;6:57–66. [Google Scholar]
- 3.Bromage ES, Owens L. Infection of barramundi Lates calcarifer with Streptococcus iniae: effects of different routes of exposure. Dis Aquat Org. 2002;52(3):199–205. doi: 10.3354/dao052199. [DOI] [PubMed] [Google Scholar]
- 4.Jantrakajorn S, Maisak H, Wongtavatchai J. Comprehensive investigation of streptococcosis outbreaks in cultured Nile tilapia, Oreochromis niloticus, and red tilapia, Oreochromis sp., of Thailand. J World Aquacult Soc. 2014;45(4):392–402. doi: 10.1111/jwas.12131. [DOI] [Google Scholar]
- 5.Laupland KB, Valiquette L. The changing culture of the microbiology laboratory. Can J Infect Dis Med Microbiol. 2013;24(3):125–128. doi: 10.1155/2013/101630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buller NB. Bacteria from fish and other aquatic animals: a practical identification manual. Oxfordshire: Cabi Publishing; 2004.
- 7.Angeletti S. Matrix assisted laser desorption time of flight mass spectrometry (MALDI-TOF MS) in clinical microbiology. J Microbiol Methods. 2017;138:20–29. doi: 10.1016/j.mimet.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Kudirkiene E, Welker M, Knudsen NR, Bojesen AM. Rapid and accurate identification of Streptococcus equi subspecies by MALDI-TOF MS. Syst Appl Microbiol. 2015;38(5):315–322. doi: 10.1016/j.syapm.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Popović NT, Kazazić SP, Strunjak-Perović I, Čož-Rakovac R. Differentiation of environmental aquatic bacterial isolates by MALDI-TOF MS. Environ Res. 2017;152:7–16. doi: 10.1016/j.envres.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Benagli C, Demarta A, Caminada A, Ziegler D, Petrini O, Tonolla M. A rapid MALDI-TOF MS identification database at genospecies level for clinical and environmental Aeromonas strains. PLoS One. 2012;7(10):e48441. doi: 10.1371/journal.pone.0048441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faron ML, Buchan BW, Hyke J, Madisen N, Lillie JL, Granato PA, Wilson DA, Procop GW, Novak-Weekley S, Marlowe E. Multicenter evaluation of the Bruker MALDI Biotyper CA system for the identification of clinical aerobic gram-negative bacterial isolates. PLoS One. 2015;10(11):e0141350. doi: 10.1371/journal.pone.0141350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pérez-Sancho M, Vela AI, García-Seco T, González S, Domínguez L, Fernández-Garayzábal JF. Usefulness of MALDI-TOF MS as a diagnostic tool for the identification of Streptococcus species recovered from clinical specimens of pigs. PLoS One. 2017;12(1):e0170784. doi: 10.1371/journal.pone.0170784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Böhme K, Fernández-No IC, Barros-Velázquez J, Gallardo JM, Calo-Mata P, Canas B. Species differentiation of seafood spoilage and pathogenic gram-negative bacteria by MALDI-TOF mass fingerprinting. J Proteome Res. 2010;9(6):3169–3183. doi: 10.1021/pr100047q. [DOI] [PubMed] [Google Scholar]
- 14.Donohue MJ, Best JM, Smallwood AW, Kostich M, Rodgers M, Shoemaker JA. Differentiation of Aeromonas isolated from drinking water distribution systems using matrix-assisted laser desorption/ionization-mass spectrometry. Anal Chem. 2007;79(5):1939–1946. doi: 10.1021/ac0611420. [DOI] [PubMed] [Google Scholar]
- 15.Assis GB, Pereira FL, Zegarra AU, Tavares GC, Leal CA, Figueiredo HC. Use of MALDI-TOF mass spectrometry for the fast identification of gram-positive fish pathogens. Front Microbiol. 2017;8:1492. doi: 10.3389/fmicb.2017.01492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SW, Nho SW, Im SP, Lee JS, Jung JW, Lazarte JMS, Kim J, Lee W-J, Lee J-H, Jung TS. Rapid MALDI biotyper-based identification and cluster analysis of Streptococcus iniae. J Microbiol. 2017;55(4):260–266. doi: 10.1007/s12275-017-6472-x. [DOI] [PubMed] [Google Scholar]
- 17.Kim SW, Jang HB, Lee JS, Im SP, Lazarte JMS, Seo JP, Lee WJ, Kim JS, Jung TS. Comparison of proteome typing and serotyping of Streptococcus parauberis isolates from olive flounder (Paralichthys olivaceus) J Microbiol Methods. 2015;118:168–172. doi: 10.1016/j.mimet.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Niyompanich S, Srisanga K, Jaresitthikunchai J, Roytrakul S, Tungpradabkul S. Utilization of whole-cell MALDI-TOF mass spectrometry to differentiate Burkholderia pseudomallei wild-type and constructed mutants. PLoS One. 2015;10(12):e0144128. doi: 10.1371/journal.pone.0144128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alatoom AA, Cunningham SA, Ihde SM, Mandrekar J, Patel R. Comparison of direct colony method versus extraction method for identification of gram-positive cocci by use of Bruker Biotyper matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol. 2011;49(8):2868–2873. doi: 10.1128/JCM.00506-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuda N, Matsuda M, Notake S, Yokokawa H, Kawamura Y, Hiramatsu K, Kikuchi K. Evaluation of a simple protein extraction method for species identification of clinically relevant staphylococci by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol. 2012;50(12):3862–3866. doi: 10.1128/JCM.01512-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mclean K, Palarea-Albaladejo J, Currie CG, Imrie LH, Manson ED, Fraser-Pitt D, Wright F, Alexander CJ, Pollock KG, Allison L. Rapid and robust analytical protocol for E. coli STEC bacteria subspecies differentiation using whole cell MALDI mass spectrometry. Talanta. 2018;182:164–170. doi: 10.1016/j.talanta.2018.01.055. [DOI] [PubMed] [Google Scholar]
- 22.Rothen J, Pothier JF, Foucault F, Blom J, Nanayakkara D, Li C, Ip M, Tanner M, Vogel G, Pflüger V. Subspecies typing of Streptococcus agalactiae based on ribosomal subunit protein mass variation by MALDI-TOF MS. Front Microbiol. 2019;10:471. doi: 10.3389/fmicb.2019.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santos IC, Hildenbrand ZL, Schug KA. Applications of MALDI-TOF MS in environmental microbiology. Analyst. 2016;141(10):2827–2837. doi: 10.1039/C6AN00131A. [DOI] [PubMed] [Google Scholar]
- 24.Carannante A, De Carolis E, Vacca P, Vella A, Vocale C, De Francesco MA, Cusini M, Del Re S, Dal Conte I, Cristaudo A. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) for identification and clustering of Neisseria gonorrhoeae. BMC Microbiol. 2015;15(1):142. doi: 10.1186/s12866-015-0480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batah R, Loucif L, Olaitan AO, Boutefnouchet N, Allag H, Rolain J-M. Outbreak of Serratia marcescens coproducing ArmA and CTX-M-15 mediated high levels of resistance to aminoglycoside and extended-spectrum beta-lactamases, Algeria. Microbial Drug Resistance. 2015;21(4):470–476. doi: 10.1089/mdr.2014.0240. [DOI] [PubMed] [Google Scholar]
- 26.Rim JH, Lee Y, Hong SK, Park Y, Kim M, D’Souza R, Park ES, Yong D, Lee K. Insufficient discriminatory power of matrix-assisted laser desorption ionization time-of-flight mass spectrometry dendrograms to determine the clonality of multi-drug-resistant Acinetobacter baumannii isolates from an intensive care unit. Biomed Res Int. 2015;2015:535027. doi: 10.1155/2015/535027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arpornsuwan Teerakul, Paveenkittiporn Wantana, Jaresitthikunchai Janthima, Roytrakul Sittiruk. BAMP-28 Antimicrobial Peptide Against Different MALDI Biotype of Carbapenam Resistant Enterobacteriaceae. International Journal of Peptide Research and Therapeutics. 2018;25(3):951–960. doi: 10.1007/s10989-018-9743-4. [DOI] [Google Scholar]
- 28.Meng X, Yang J, Duan J, Liu S, Huang X, Wen X, Huang X, Fu C, Li J, Dou Q. Assessing molecular epidemiology of Carbapenem-resistant Klebsiella pneumoniae (CR-KP) with MLST and MALDI-TOF in Central China. Sci Rep. 2019;9(1):2271. doi: 10.1038/s41598-018-38295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173(2):697–703. doi: 10.1128/JB.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaresitthikunchai J, Phanphiriya P. Proceeding of The 3rd International Conference on Biochemistry and Molecular Biology 2011. 2011. S. R: MALDI-TOF Mass Spectrometric Fingerprint for Direct Identification of Bacillus, Escherichia, Staphylococcus, Enterobacter, Pseudomonas from Colonies Grown on Agar Plates. [Google Scholar]
- 31.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 32.Sonthayanon P, Jaresitthikunchai J, Mangmee S, Thiangtrongjit T, Wuthiekanun V, Amornchai P, Newton P, Phetsouvanh R, Day NP, Roytrakul S. Whole cell matrix assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) for identification of Leptospira spp. in Thailand and Lao PDR. PLoS Negl Trop Dis. 2019;13(4):e0007232. doi: 10.1371/journal.pntd.0007232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cherkaoui A, Hibbs J, Emonet S, Tangomo M, Girard M, Francois P, Schrenzel J. Comparison of two matrix-assisted laser desorption ionization-time of flight mass spectrometry methods with conventional phenotypic identification for routine identification of bacteria to the species level. J Clin Microbiol. 2010;48(4):1169–1175. doi: 10.1128/JCM.01881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the study are available from the corresponding author upon request.