Abstract
Background
The current carriage study was set up to reinforce surveillance during/after the PCV13-to-PCVC10 switch in Belgium.
Aim
This observational study monitored carriage of Streptococcus pneumoniae (Sp) serotypes, particularly those no longer covered (3, 6A, 19A), as well as Haemophilus influenzae (Hi), because PCV10 contains the non-typeable Hi protein D.
Methods
A total of 2,615 nasopharyngeal swabs from children (6–30 months old) attending day care were collected in three periods over 2016–2018. Children’s demographic and clinical characteristics and vaccination status were obtained through a questionnaire. Sp and Hi were identified by culture and PCR. Pneumococcal strains were tested for antimicrobial (non-)susceptibility by disc diffusion and serotyped by Quellung-reaction (Quellung-reaction and PCR for serotypes 3, 6A, 19A).
Results
The carriage prevalence of Sp (> 75%) remained stable over the successive periods but that of Hi increased (87.4%, 664 Hi-carriers/760 in 2016 vs 93.9%, 895/953 in 2017–2018). The proportion of non-PCV13 vaccine serotypes decreased (94.6%, 438 isolates/463 in 2016 vs 89.7%, 599/668 in 2017–2018) while that of PCV13-non-PCV10 vaccine serotypes (3 + 6A + 19A) increased (0.9%, 4 isolates/463 in 2016 vs 7.8%, 52/668 in 2017–2018), with serotype 19A most frequently identified (87.9%, 58/66 isolates). Non-susceptibility of pneumococci against any of the tested antibiotics was stable over the study period (> 44%).
Conclusions
During and after the PCV13-to-PCV10 vaccine switch, the proportion of non-PCV13 serotypes decreased, mainly due to a serotype 19A carriage prevalence increase. These results complement invasive pneumococcal disease surveillance data, providing further basis for pneumococcal vaccination programme policy making.
Keywords: Nasopharyngeal carriage, Streptococcus pneumoniae, Haemophilus influenzae, Pneumococcal conjugate vaccines, Children, Day-care centres
Introduction
Nasopharyngeal carriage of Streptococcus pneumoniae (Sp) frequently occurs asymptomatically [1-5]. Nevertheless, it may evolve to respiratory infections such as otitis media and pneumonia or even invasive diseases including bacteraemia and meningitis [2,3,5]. Besides the elderly, young children are prone to (invasive) pneumococcal diseases ((I)PD) [6-10]. Before pneumococcal conjugate vaccines (PCVs) were introduced, the global annual number of serious pneumococcal disease cases (pneumonia, meningitis, and bacteraemia) in children under 5 years of age was estimated to be 14.5 million [11].
The primary virulence factor of Sp is its polysaccharide capsule, which also determines the serotype. More than 95 serotypes exist and they vary in their capacity to activate the host immune system and to invade [12-15]. PCVs provide direct protection to the vaccinated individuals against a number of clinically relevant serotypes [12]. In addition, the wider population experiences indirect protection against pneumococcal disease through reduced nasopharyngeal carriage of pneumococcal vaccine serotypes (VTs). However, the observed magnitude of this indirect effect varies in different contexts, and it is eroded by the rising incidence of non-VT-(NVT-)related diseases [16]. Several studies on carriage or IPD in the pre- and post-PCV era reported on serotype replacement, i.e. VTs being largely replaced by NVTs [17,18]. Furthermore, co-colonisation with other pathogens such as Haemophilus influenzae (Hi), Moraxella catarrhalis (Mc), Staphylococcus aureus (Sa), and Streptococcus pyogenes (GAS) may be changed after PCV-introduction because of mutual interactions [19-21].
Belgium initiated a universal childhood PCV-programme according to a two plus one schedule in 2007 (at 8 weeks, 16 weeks, and 12 months of age). The seven-valent vaccine (PCV7, including serotypes 4, 6B, 9V, 14, 18C, 19F, 23F) was superseded by the 13-valent vaccine (PCV13, including PCV7 serotypes plus 1, 5, 7F, 3, 6A, 19A, same 2 + 1 schedule) in 2011, which was in turn replaced by the 10-valent vaccine (PCV10, including PCV7 serotypes plus 1, 5, 7F, same 2 + 1 schedule) in 2015–2016. The implementation of immunisation programmes constitutes a regional responsibility in Belgium. PCV10 was introduced in the Flemish (Northern) region in July 2015 and in the Walloon (Southern) region in May 2016 [22]. In the Brussels (Capital) region either the Flemish or the Walloon programme was followed, depending on the consulting physician. The pneumococcal vaccination programme rapidly achieved high three-dose coverage in children (coverage in Belgium; > 80% in all regions in 2008–2009 vs > 94% in all regions in 2015–2016 [23-26]) and the overall incidence of IPD in Belgium significantly decreased after implementation of the vaccination programme; post-PCV7 period (2007–2010) vs pre-PCV7 period (pre 2007): decrease of 35%; post-PCV13 (2015) vs PCV7-era (2007–2010): decrease of 42% [22].
The current carriage study was set up to reinforce surveillance after the PCV13-to-PCV10 vaccination programme switch, in order to monitor the three pneumococcal serotypes that were no longer covered (3, 6A, 19A), as well as Hi, because PCV10 contains the non-typeable Hi (NTHi) protein D. To this end, we studied nasopharyngeal carriage of Sp and Hi in children between 6 and 30 months of age attending day care centres (DCCs) during three consecutive periods between 2016 and 2018. High pneumococcal carriage rates (range: 21–89%) have been reported in young children attending day care [17,27-29]. As such, the impact of the PCV-programme change was monitored in a random sample of this target population, to complement sentinel laboratory-reported IPD-surveillance. In this paper, we focus on pneumococcal serotype distribution and antimicrobial (non-)susceptibility during and after the PCV13-to-PCV10 vaccination programme switch.
Methods
Ethical statement
The current study was in line with the Declaration of Helsinki, as revised in 2013. Approval to conduct the current study with ID 15/45/471 was obtained from the University of Antwerp and University Hospital of Antwerp ethics committee (Commissie voor Medische Ethiek van UZA/UA) on 30 November 2015.
Study design
The design of this observational study was previously described in detail and is summarised here for the complete study period (from Period 1 in 2016 up to Period 3 in 2017–2018) [30,31].
Nasopharyngeal sampling was performed between March and July in Period 1 (2016) and between November and March in the consecutive periods (Period 2: 2016–2017 and Period 3: 2017–2018). Healthy children were recruited in DCCs randomly selected over the three Belgian regions (Wallonia, Flanders, Brussels), according to a population-proportionate distribution at regional level based on Belgian Federal Government Statistics for the 0–4 year population. In the consecutive periods, 85, 112, and 102 DCCs participated in the study, of which 66 DCCs participated in all three periods, 44 in two periods, and 24 in one period. A population-proportionate sample at the regional level was achieved from 2016 to 2017 onwards, after deliberate over-recruitment in Wallonia in 2016, in order to include a maximum of children who received PCV13 for both primary vaccine doses and their booster, since at that time, PCV10 was not yet introduced in Wallonia. The inclusion criteria were: no treatment with oral antibiotics (ABs) in the 7 days before sampling and age between 6 and 30 months included. An additional age criterion (date of birth before 1 January 2015) was applied in Flanders and Brussels for 2016, in order to exclusively include children who received PCV13 for their primary vaccine doses [30]. In this way, children recruited in the first period were on average older compared with children recruited in the subsequent periods.
Trained nurses and physicians conducted a questionnaire collecting demographic and clinical characteristics of the study participants. The vaccination status of the participating child was based on vaccination documentation or parental reporting. A single nasopharyngeal swab was taken with a flocked nylon fibre swab, transported in 1 mL skim milk-tryptone-glucose-glycerol (STGG) and cultured or stored at − 80 °C within 24 hours.
Culture analyses
At the National Reference Centre (NRC) for pneumococci, nasopharyngeal samples were plated on blood agar plates for identification of Sp, Mc, Sa, GAS and a selective plate for identification of Hi, following overnight enrichment in brain-heart infusion (BHI) broth (entire study period for blood agar plates, 2016 only for selective plate) and directly (from 2016–2017 onwards for blood agar plates and selective plate). Sp-strains were serotyped via Quellung-reaction. Antimicrobial (non-)susceptibility of the Sp-strains for erythromycin, levofloxacin, penicillin, tetracycline and trimethoprim/sulfamethoxazole was determined by disc diffusion according to the guidelines of Clinical and Laboratory Standards Institute (CLSI; 2016 and 2016–2017) [32] and European Committee on Antimicrobial Susceptibility Testing (EUCAST; 2017–2018) [33]. If non-susceptibility for penicillin or levofloxacin was identified by disc diffusion, the minimum inhibitory concentration (MIC) was determined by Etest (Biomérieux, Craponne, France). A MIC of > 0.06 mg/L for penicillin or > 2 mg/L for levofloxacin was interpreted as non-susceptible.
Molecular analyses
DNA was extracted from 200 µL of nasopharyngeal sample and tested in real-time PCR targeting lytA (for Sp) or P6 (for Hi) [30,34,35]. Real-time PCR was performed for Sp on all samples and for Hi on culture-negative samples. Samples were classified as positive for Sp or Hi when cycle threshold (CT) values were ≤ 40 or ≤ 35, respectively. LytA-positive samples were pooled and screened for presence of the three pneumococcal serotypes included in PCV13, but not in PCV10 (PCV13-non-PCV10-VTs: 3, 6A, 19A). If found positive for serotype 3, 6A, or 19A, pooled samples were unpooled and positivity of the individual sample was determined. Serotype-specific PCRs were performed in a 20 µL reaction volume containing 2x Taqman Universal PCR Master Mix (Applied Biosystems), 200 nM concentrations of serotype 3 [36], 6A [37], or 19A [36] primers and probe and 2 µL of DNA template (pooled PCR-reaction contains four times 2 µL of DNA). Samples positive for 6A real-time PCR were further subjected to 6C real-time PCR targeting wciN β to discriminate between serotypes 6A and 6C [38]. The serotype-specific 6C assay was performed as described above, with the exception that higher primer concentrations of 500 nM were used. Amplification was carried out on a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, California, United States) using the following cycling parameters: 10 min at 95 °C and 40 cycles of 15 s at 95 °C and 1 min at 59.5 °C (3, 19A) or 60 °C (6A, 6C). Serotype-specific PCRs were classified as positive when CT values were ≤ 35.
Samples were considered positive for any of the respective pathogens if either culture or PCR was positive. The presented carriage prevalences of Sp and Hi were based on culture and PCR-results, whereas overall serotype distribution and antimicrobial (non-)susceptibility were based on Quellung-reaction and culture results respectively. The reported carriage prevalence of serotypes 3, 6A, and 19A was based on Quellung-reaction and PCR-results.
Statistics
Sample size and power were calculated using the R-package ‘power’ [30]. A sample size of 700 children in 2016 and 900 children from the second period onwards allows the detection of 4% changes in carriage prevalence of Sp-serotypes 19A or 6A over the observation period of the study period with 80% power and assuming a starting carriage prevalence below 2%.
In IBM SPSS Statistics 25, the chi-squared (Chi2) or Fisher’s Exact Test (FET) and the Mann–Whitney U Test (MWU) were used to test for significance at a level of 5%. To identify predictors of carrying Sp, PCV13-non-PCV10-VTs, and Hi in DCC-children (three periods pooled), univariate and multiple binary logistic regressions were performed and adjusted using generalised estimating equations (GEEs) with an exchangeable correlation structure since 148 children (303 isolates) contributed more than one sample over the 3-year study period. The GEE model analyses were performed using the statistical software R (version 3.6.1) with the geepack package (version 1.2–1). Variables with a p value < 0.1 in the univariate analysis were included in the multiple regression analysis. Since no children were sampled in the youngest age category in Flanders and in Brussels in 2016, no adjustments can be made for the different sampling probabilities in the different study years. A continuity correction was applied for 95% confidence intervals (95% CI) on proportions. Missing values were not replaced.
Results
Study population
Over the three successive periods, nasopharyngeal samples from 2,883 children attending DCCs were collected. In total, 2,615 samples (760 in 2016, 902 in 2016–2017, 953 in 2017–2018) – corresponding to 2,621 pneumococcal isolates, as more than one serotype could be found per child (761 in 2016, 904 in 2016–2017, 956 in 2017–2018), were included in the final analyses, i.e. after exclusion of a random selection of 194 samples collected in 2016–2017 (not analysed by PCR as a consequence of over-recruitment), and after exclusion of 74 samples not fulfilling the inclusion criteria regarding age or use of ABs. Of the 2,615 nasopharyngeal samples, 148 (5.7%) originated from children who contributed more than one sample, but never in the same period. The univariate and multiple binary logistic regression models were adjusted for this through GEEs with an exchangeable correlation structure.
The main demographic and clinical characteristics of the child population over the study period are shown in Table 1, with the majority of these characteristics remaining similar over the study period. Nevertheless, a decreasing trend (p < 0.001) was observed for history of acute otitis media (AOM-history) and AB-use in the 3 months before sampling. The proportions of children being breastfed for more than 6 months (p = 0.026) and with symptoms (runny nose and/or cough) of common cold (p < 0.001) increased over the study period.
Table 1. Demographic and clinical characteristics of the healthy child population in day care per period, Belgium, 2016–2018 (n = 760 children in 2016, 902 in 2016–2017, 953 in 2017–2018).
| Characteristics | Healthy children attending day care | |||||||
|---|---|---|---|---|---|---|---|---|
| Period 1 2016 (N = 760) |
Period 2 2016–2017 (N = 902) |
Period 3 2017–2018 (N = 953) |
p value chi2 for trend |
|||||
| n | %a | n | %a | n | %a | |||
| Region | Wallonia | 353 | 46.4 | 287 | 31.8 | 282 | 29.6 | < 0.001 |
| Flanders | 332 | 43.7 | 488 | 54.1 | 552 | 57.9 | ||
| Brussels | 75 | 9.9 | 127 | 14.1 | 119 | 12.5 | ||
| Age in months | 6–12 | 98 | 12.9 | 217 | 24.1 | 209 | 21.9 | < 0.001 |
| 13–24 | 415 | 54.6 | 457 | 50.7 | 528 | 55.4 | ||
| 25–30 | 247 | 32.5 | 228 | 25.3 | 216 | 22.7 | ||
| Sex | Male | 387 | 50.9 | 455 | 50.4 | 469 | 49.2 | 0.474 |
| Preterm delivery | Yes | 60 | 8.0 | 71 | 7.9 | 78 | 8.2 | 0.872 |
| Breastfeedingb | Yes | 230 | 30.4 | 289 | 32.1 | 336 | 35.4 | 0.026 |
| Parental smokingc | Yes | 170 | 22.4 | 183 | 20.4 | 190 | 20.0 | 0.231 |
| Siblings | Yes | 459 | 62.9 | 548 | 61.0 | 599 | 63.3 | 0.813 |
| Common cold symptoms | Yes | 169 | 22.4 | 344 | 38.2 | 429 | 45.0 | < 0.001 |
| AOM-historyd | Yes | 258 | 34.8 | 225 | 25.5 | 199 | 21.8 | < 0.001 |
| AB < 3 monthse | Yes | 248 | 35.4 | 254 | 30.5 | 217 | 23.5 | < 0.001 |
AB: antibiotic; AOM: acute otitis media.
a Due to missing information on some characteristics for some children, the denominator can at times differ from ‘N’ in the column heading.
b Child was breastfed for more than 6 months.
c At least one parent smokes.
d Child with a history of AOM based on parental recall.
e Use of antibiotics in the 3 months before sampling.
Significant p values (<0.05) are indicated in bold.
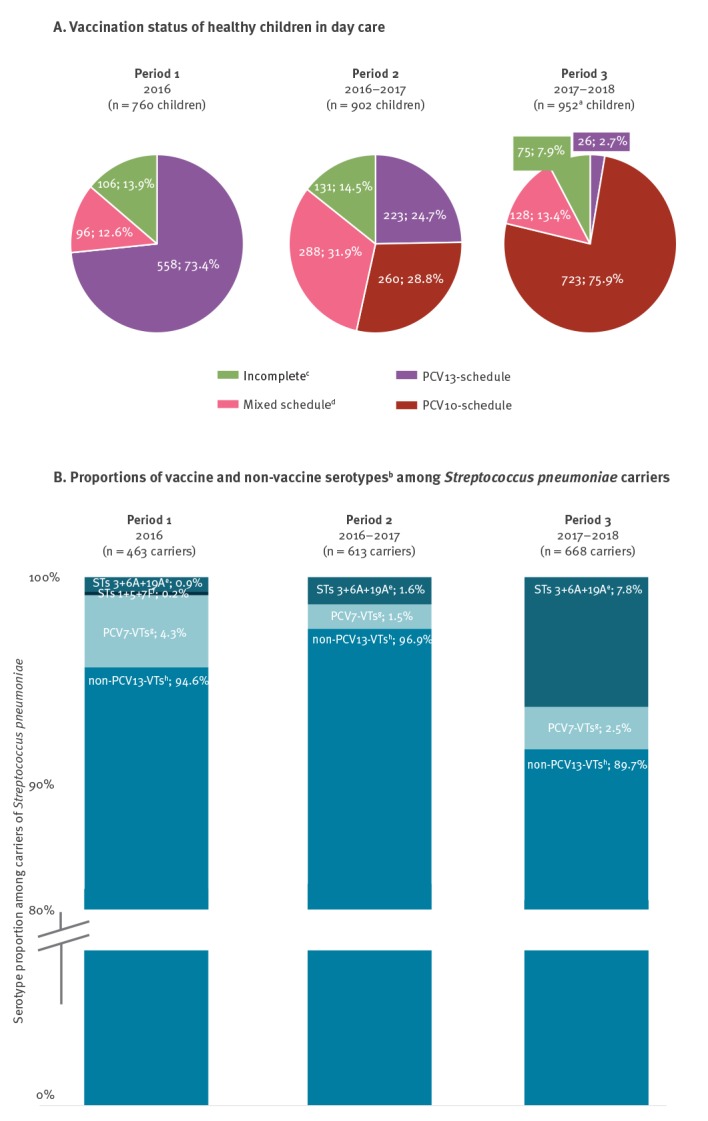
As a result of the recruitment strategy to include older children in 2016 (mean age: 21.0 months in 2016 vs 18.4 months in 2016–2017 vs 18.4 months in 2017–2018), the majority of the Period 1 population was vaccinated with PCV13 only (Figure 1: 73.4%; 558/760 children). The proportion of PCV13-vaccinated children decreased over the study period to 2.7% (26/952 children; vaccination status was missing for one of the 953 children in 2017–2018), whereas the proportion of PCV10-vaccinated children increased from 0.0% to 75.9% (723/952 children).
Figure 1.
(A) Vaccination status of healthy children in day care (n = 760 in 2016, 902 in 2016–2017, 952a in 2017–2018) and (B) proportions of vaccine and non-vaccine serotypesb among Streptococcus pneumoniae carriers (n = 463 carriers in 2016, 613 in 2016–2017, 668 in 2017–2018), Belgium, 2016–2018
PCV7/10/13: 7/10/13-valent pneumococcal conjugate vaccine; STs: serotypes; VTs: vaccine serotypes.
a A total of 952 is used because vaccination status was missing for one of the 953 children in 2017–2018.
b Serotypes are determined by Quellung reaction.
c Incomplete schedule: children who were not or incompletely vaccinated.
d Mixed schedule: children vaccinated with a combination of PCV13 and PCV10.
e STs 3 + 6A + 19A: serotypes included in PCV13, but not in PCV10.
f STs 1 + 5 + 7F: serotypes included in PCV10 but not in PCV7.
g PCV7-VTs: vaccine serotypes included in PCV7 (4, 6B, 9V, 14, 18C, 19F, 23F).
h Non-PCV13-VTs: vaccine serotypes not included in PCV13.
Children in the study were 6–30 months old. Vaccination status was based on vaccination documentation or parental reporting.
Carriage prevalence of Streptococcus pneumoniae and Haemophilus influenzae
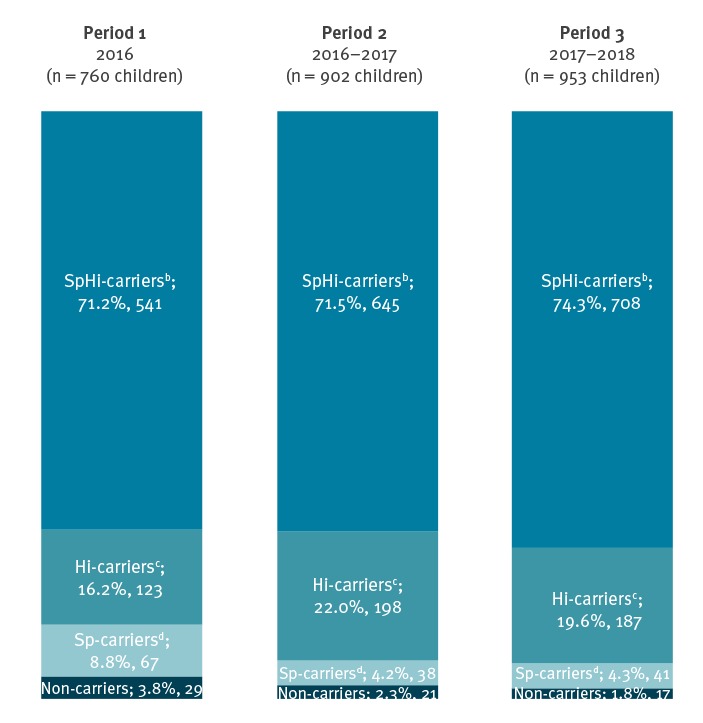
As determined by PCR, very few children carried neither Sp, nor Hi, namely 3.8% (29/760 children), 2.3% (21/902 children), 1.8% (17/953 children) in the consecutive periods (Figure 2). The carriage prevalence of Sp was stable and high, ranging from 75.7% Sp-carriers (683/902 children) in 2016–2017 to 80.0% Sp-carriers (608/760 children) in 2016. The carriage prevalence of Hi increased significantly (p < 0.001) over the study period (87.4% Hi-carriers, 664/760 in 2016 vs 93.5% Hi-carriers, 843/902 in 2016–2017 vs 93.9% Hi-carriers, 895/953, in 2017–2018). Co-colonisation with Sp and Hi was frequent and did not change over the study period; it ranged from 71.2% (541/760 Sp and Hi-carriers) in 2016 to 74.3% (708/953 Sp and Hi-carriers) in 2017–2018.
Figure 2.
Carriage prevalencea of Streptococcus pneumoniae and Haemophilus influenzae among healthy children in day care, Belgium, 2016–2018 (n = 760 children in 2016, 902 in 2016–2017, 953 in 2017–2018)
Hi: Haemophilus influenza; Sp: Streptococcus pneumoniae.
a Prevalence was inferred from results of culture and PCR combined.
b SpHi-carriers: carriers of Sp and Hi.
c Hi-carriers: carriers of Hi.
d Sp-carriers: carriers of Sp.
In a multiple regression analysis using pooled data of the three periods, positive predictors for Sp-carriage among DCC-children (Table 2) were being sampled in 2016, being female, having symptoms of common cold, carrying Hi or Mc, and having siblings, whereas AB-use in the 3 months before sampling and carrying Sa were associated with a lower likelihood of Sp-carriage. Predictors of carrying PCV13-non-PCV10-VTs (based on Quellung-reaction) were similarly evaluated using binary logistic regression. The only variable that remained significant in the multiple regression model (Table 2) was study period, indicating higher PCV13-non-PCV10-VT-carriage in 2017–2018 compared with the previous periods. The predictors for Hi-carriage that were significant in the multiple regression model were having symptoms of common cold (odds ratio (OR): 1.63; 95% CI: 1.15–2.31), having siblings (OR: 1.45; 95% CI: 1.08–1.94), and carrying Sp (OR: 1.54; 95% CI: 1.12–2.12), Mc (OR: 1.70; 95% CI: 1.11–2.59), or GAS (OR: 8.52; 95% CI: 1.17–62.00) and all of these were positive predictors. Being in the age category of 13–24 months (compared to the two other age categories: 6–12 months (OR: 0.42; 95% CI: 0.29–0.60) and 25–30 months (OR: 0.59; 95% CI: 0.42–0.83)), or being sampled in 2016–2017 (OR: 2.28; 95% CI: 1.60–3.26) or 2017–2018 (OR: 2.26; 95% CI: 1.58–3.24, compared with 2016) was associated with a higher likelihood of Hi-carriage. Study period and vaccination schedule were both included in all regression analyses since no collinearity between these variables was observed based on a linear regression analysis.
Table 2. Predictors through binary logistic regression of Streptococcus pneumoniae carriagea (n = 2,615 nasopharyngeal samples) and of PCV13-non-PCV10 vaccine serotype carriageb (n = 1,744) among children attending day care (pooled over study periods), Belgium, 2016–2018.
| Number of samples/isolatesc | Univariate regression | Multiple regression | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | n | % | OR | 95% CId | OR | 95% CId | ||
| Predictors of Sp-carriagea | ||||||||
| Study period | ||||||||
| 2016 | 760 | 29.1 | REF | REF | REF | REF | ||
| 2016–2017 | 902 | 34.5 | 0.78 | 0.62–0.99 | 0.64 | 0.47–0.87 | ||
| 2017–2018 | 953 | 36.4 | 0.92 | 0.73–1.16 | 0.71 | 0.52–0.96 | ||
| Region | ||||||||
| Wallonia | 922 | 35.3 | REF | REF | REF | REF | ||
| Flanders | 1,372 | 52.5 | 1.29 | 1.06–1.57 | 1.09 | 0.83–1.42 | ||
| Brussels | 321 | 12.3 | 1.42 | 1.04–1.95 | 1.16 | 0.81–1.66 | ||
| Sex | ||||||||
| Female | 1,304 | 49.9 | REF | REF | REF | REF | ||
| Male | 1,311 | 50.1 | 0.75 | 0.63–0.91 | 0.76 | 0.62–0.93 | ||
| Common cold symptomse | ||||||||
| Yes | 942 | 36.1 | REF | REF | REF | REF | ||
| No | 1,668 | 63.9 | 0.65 | 0.53–0.80 | 0.64 | 0.51–0.80 | ||
| Sa-carriagef | ||||||||
| Yes | 116 | 4.4 | REF | REF | REF | REF | ||
| No | 2,499 | 95.6 | 1.71 | 1.14–2.55 | 1.79 | 1.13–2.85 | ||
| Hi-carriageg | ||||||||
| Yes | 2,402 | 91.9 | REF | REF | REF | REF | ||
| No | 213 | 8.1 | 0.58 | 0.43–0.79 | 0.64 | 0.45–0.90 | ||
| Mc-carriageg | ||||||||
| Yes | 2,382 | 91.1 | REF | REF | REF | REF | ||
| No | 233 | 8.9 | 0.26 | 0.19–0.34 | 0.31 | 0.23–0.42 | ||
| Siblingsh | ||||||||
| Yes | 1,606 | 62.4 | REF | REF | REF | REF | ||
| No | 969 | 37.6 | 0.72 | 0.59–0.87 | 0.73 | 0.61–0.89 | ||
| AOM-historyI,j | ||||||||
| Yes | 682 | 26.9 | REF | REF | REF | REF | ||
| No | 1,855 | 73.1 | 1.36 | 1.11–1.67 | 1.14 | 0.91–1.44 | ||
| AB-use < 3 monthsk,l | ||||||||
| Yes | 719 | 29.3 | REF | REF | REF | REF | ||
| No | 1,739 | 70.7 | 1.79 | 1.47–2.18 | 1.63 | 1.30–2.05 | ||
| Age (months) | ||||||||
| 6–12 | 524 | 20.0 | 0.81 | 0.64–1.03 | 0.94 | 0.73–1.22 | ||
| 13–24 | 1,400 | 53.5 | REF | REF | REF | REF | ||
| 25–30 | 691 | 26.4 | 0.79 | 0.64–0.99 | 0.84 | 0.65–1.07 | ||
| Sampled during influenza-peak | ||||||||
| Yes | 1,072 | 41.0 | REF | REF | REF | REF | ||
| No | 1,543 | 59.0 | 1.23 | 1.02–1.48 | 1.01 | 0.77–1.33 | ||
| Predictors of PCV13-non-PCV10-VT-carriageb | ||||||||
| Study period | ||||||||
| 2016 | 463 | 26.5 | REF | REF | REF | REF | ||
| 2016–2017 | 613 | 35.1 | 1.90 | 0.59–6.11 | 1.36 | 0.36–5.07 | ||
| 2017–2018 | 668 | 38.3 | 9.69 | 3.48–27.00 | 5.88 | 1.56–22.19 | ||
| Vaccination schedulem | ||||||||
| PCV13 | 486 | 27.9 | REF | REF | REF | REF | ||
| PCV10 | 707 | 40.6 | 6.85 | 2.70–17.35 | 1.79 | 0.53–6.01 | ||
| Incomplete | 222 | 12.7 | 1.77 | 0.47–6.64 | 0.92 | 0.22–3.87 | ||
| Mix | 328 | 18.8 | 3.03 | 1.02–8.93 | 1.71 | 0.50–5.82 | ||
| Sampled during RSV-peak | ||||||||
| Yes | 601 | 34.5 | REF | REF | REF | REF | ||
| No | 1143 | 65.5 | 0.512 | 0.313-0.838 | 0.88 | 0.52–1.50 | ||
AB: antibiotic; AOM: acute otitis media; CI: confidence interval; Hi: Haemophilus influenza; Mc: Moraxella catarrhalis; OR: odds ratio; PCV: pneumococcal conjugate vaccine; PCV13-non-PCV10-VT: vaccine serotypes included in PCV13, but not in PCV10 (serotypes: 3, 6A, 19A); REF: reference group for the regression analysis; RSV: respiratory syncytial virus; Sa: Staphylococcus aureus; Sp: Streptococcus pneumoniae.
a Knowledge of Sp carriage was through results of culture and PCR combined.
b Knowledge of PCV13-non-PCV10 vaccine serotype carriage was based on culture or Quellung-reaction results.
c The number of samples was used for the analyses regarding Sp-carriage predictors; the number of isolates was used for the analyses regarding PCV13-non-PCV10-VT-carriage predictors.
d Confidence intervals that do not overlap the null value of OR = 1 are indicated in bold.
e Information on common cold symptoms was not available for five children.
f Based on culture-results or Quellung-reaction.
g Based on the combination of culture and PCR-results.
h Information on siblings was not available for 40 children.
i Child with a history of AOM.
j Information on history of AOM was not available for 78 children.
k Use of antibiotics in the 3 months before sampling.
l Information on use of antibiotics in the 3 months before sampling was not available for 157 children.
m Information on vaccination status was missing for one child.
Besides the shown confounders, other variables were assessed, but not significant in univariate analysis: preterm delivery, previous hospitalisation, age-appropriate vaccination, carriage of Streptococcus pyogenes (based on culture-results or Quellung-reaction), parental smoking, breastfeeding.
Trends over time in carriage prevalence of Streptococcus pneumoniae serotypes
Among the Sp-carriers based on Quellung-results, a decreasing trend (p < 0.001) in the proportion of non-PCV13-VTs (94.6%, 438/463 isolates in 2016 vs 96.9%, 594/613 isolates in 2016–2017 vs 89.7%, 599/668 isolates in 2017–2018) was accompanied by an increasing trend (p < 0.001) in the proportion of PCV13-non-PCV10-VTs (3 + 6A + 19A: 0.9%, 4/463 isolates in 2016 vs 1.6%, 10/613 isolates in 2016–2017 vs 7.8%, 52/668 isolates in 2017–2018; Figure 1).
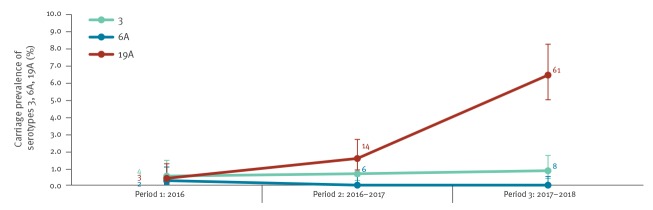
Among the latter, serotype 19A was most frequently identified (87.9%, 58/66 isolates), followed by serotype 3 (10.6%, 7/66 isolates), and serotype 6A, which was identified once (1.5%, 1/66 isolates). The Quellung-reaction and PCR combined results of these serotypes among DCC-children’s isolates (Figure 3) showed a stable carriage prevalence for serotypes 3 (0.5%, 4/761 isolates in 2016 vs 0.7%, 6/904 isolates in 2016–2017 vs 0.8%, 8/956 isolates in 2017–2018) and 6A (0.3%, 2/761 isolates; 0.0%; 0.0%), but a significant increase (p < 0.001) was observed for serotype 19A (0.4%, 3/761 isolates in 2016 vs 1.5%, 14/904 isolates in 2016–2017 vs 6.4%, 61/956 isolates in 2017–2018).
Figure 3.
Carriage prevalence of PCV13-non-PCV10 vaccine serotypes 3, 6A, 19Aa, Belgium, 2016–2018 (n = 761 in 2016, 904 in 2016–2017, 956 in 2017–2018)
a Carriage prevalence was determined from the results of Quellung-reaction and PCR combined.
Error bars indicate 95% confidence intervals, numbers added to the respective line charts indicate the total number of isolates for the respective serotype during the indicated study period.
Based on Quellung-results, non-PCV13-VTs dominated Sp-carriage over the entire study period. The serotypes 23B, 23A, 11A, 15B, 15A, and 10A constituted nearly 50% of the total non-PCV13-VTs among Sp-carriers in all three periods. The separate proportions of the different serotypes identified among Sp-carriers fluctuated over the study period except for three serotypes. The proportions of serotypes 19A and 6C consistently increased (p < 0.001); from 0.4% (2/463 isolates) in 2016, to 1.5% (9/613 isolates) in 2016–2017, to 7.0% (47/668 isolates) in 2017–2018 for serotype 19A and from 0.9% (4/463 isolates) in 2016, to 1.5% (9/613 isolates) in 2016–2017, to 5.8% (39/668 isolates) in 2017–2018 for serotype 6C. The proportion of serotype 15A consistently decreased (p = 0.042); from 6.7% (31/463 isolates) in 2016 to 5.2% (32/613 isolates) in 2016–2017 to 3.4% (23/668 isolates) in 2017–2018.
Streptococcus pneumoniae and its antimicrobial non-susceptibility
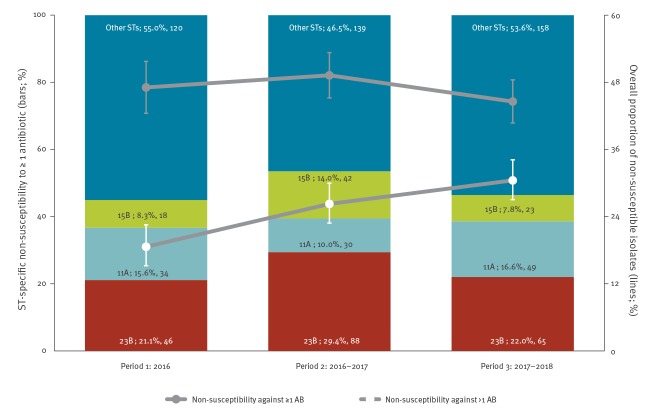
The proportion of Sp-strains that were non-susceptible against any of the five tested antibiotics remained stable over the study period (47.1%, 218/463 isolates in 2016 vs 49.3%, 299/607 isolates in 2016–2017 vs 44.6%, 295/662 isolates in 2017–2018), whereas non-susceptibility against more than one antibiotic increased (18.6%, 86/462 isolates in 2016 vs 26.3%, 160/609 isolates in 2016–2017, vs 30.5%, 203/666 isolates in 2017–2018; p < 0.001). Non-susceptibility against levofloxacin (cut-off MIC > 2.00 mg/L) was inexistent. Non-susceptibility against penicillin (cut-off MIC > 0.06 mg/L; 13.4%, 62/463 isolates in 2016 vs 19.2%, 117/609 isolates in 2016–2017 vs 18.5%, 123/666 isolates in 2017–2018; p = 0.041), erythromycin (17.3%, 80/463 isolates in 2016 vs 16.1%, 98/608 isolates in 2016–2017 vs 22.0%, 146/664 isolates in 2017–2018; p = 0.028), and tetracycline (11.7%, 54/463 isolates in 2016 vs 12.6%, 77/610 isolates in 2016–2017 vs 20.0%, 133/666 isolates in 2017–2018; p < 0.001) increased over the study period, whereas non-susceptibility against trimethoprim/sulfamethoxazole fluctuated over the study period (35.2%, 163/463 isolates in 2016 vs 40.3%, 246/610 isolates in 2016–2017 vs 30.3%, 202/666 isolates in 2017–2018; p = 0.042). Nevertheless, trimethoprim/sulfamethoxazole was the antibiotic against which most strains (35.1%; 611/1,739) were non-susceptible over the entire study period. The serotypes (based on Quellung-reaction) that were most often found to be non-susceptible against at least one of the tested antibiotics among Sp-carriers are shown in Figure 4.
Figure 4.
Non-susceptibility against at least one (n = 463 isolates in 2016, 607 in 2016–2017, 662 in 2017–2018) or more than one (n = 462 isolates in 2016, 609 in 2016–2017, 666 in 2017–2018) of the tested antibioticsa and the dominating serotypes among strains of Streptococcus pneumoniae non-susceptible to at least one antibioticb, Belgium, 2016–2018
AB: antibiotic; ST(s): serotype(s).
a Non susceptibility to antibiotics was assessed using cultures and the overall proportions of non-susceptible isolates (against ≥ 1 and > 1 antibiotic) are represented by lines.
b Dominating serotypes among strains of Streptococcus pneumoniae non-susceptible to ≥ 1 antibiotic as well as all the other remaining serotypes are shown within the chart bars. For each particular serotype presented, the serotype number, followed by the proportion and number of isolates for this serotype is indicated. Serotypes were determined by Quellung-reaction.
Error bars indicate 95% confidence intervals. A minimum inhibitory concentration (MIC) of > 0.06 mg/L (penicillin) and > 2.00 mg/L (levofloxacin) was interpreted as non-susceptible.
Over the 3-year study period, 23.2% (13/56 19A-strains) of the 19A-strains were non-susceptible to more than one of the tested antibiotics, whereas two 19A-strains (3.6%; 2/56 19A-strains) were non-susceptible to only one of the tested antibiotics; erythromycin and trimethoprim/sulfamethoxazole for the respective strains. The isolated 19A-strains were most frequently non-susceptible to erythromycin (93.3%; 14/15 19A-strains), followed by tetracycline (86.7%; 13/15 19A-strains), trimethoprim/sulfamethoxazole (46.7%; 7/15 19A-strains), and penicillin (20.0%; 3/15 19A-strains).
Discussion
In this pneumococcal carriage study, we evaluated during 3 years any changes in the nasopharyngeal carriage prevalence, serotype distribution and antimicrobial (non-)susceptibility of Sp in healthy children (aged 6–30 months) attending DCCs in Belgium, from 2016 onwards, i.e. during and immediately after a PCV13-to-PCV10 vaccination programme switch. Common co-colonising bacteria were followed as well, with a special focus on Hi.
Demographics and clinical characteristics of the study population showed higher percentages of common cold symptoms in 2016–2017 and 2017–2018 compared with 2016, which might be due to differences in sampling period. In 2016, nasopharyngeal samples were taken during spring (March–July), whereas the subsequent periods encompassed autumn and winter (November–March), during which common cold frequently occurs. The higher percentages of AOM-history in 2016 compared with the other periods might be due to recruitment of (on average) older children in this year compared with the subsequent periods and in 2016 more AB-treatments in the 3 months before sampling were observed. With regard to breastfeeding, it is unclear why it is more frequent in 2017–2018 compared with the previous periods. It is likely that several factors contributed to fluctuations in breastfeeding practices, one of which may be the coinciding extensive campaigns (personal communication: Marc Hainaut, 24 Oct 2018) on the importance of breastfeeding in Brussels’ maternity hospitals (in 2017–2018, > 54% of the included Brussels children were breastfed for more than 6 months).
During the study period, the vaccination status of the child populations gradually changed; from mainly PCV13-vaccinated children in 2016 to mainly PCV10-vaccinated children in 2017–2018. Sp-carriage prevalence was consistently high (> 75%) over the study period. Real-life carriage in DCC might be slightly lower since children who were treated with oral ABs in the 7 days before sampling could not take part in the study.
In contrast, the carriage prevalence of Hi increased significantly over the study period (especially in the two oldest age categories), despite the increasing number of children vaccinated with PCV10, containing the NTHi protein D. Other reports also indicated the absence of PCV10-induced protection against NTHi [39-41].
The predictors of Sp-carriage identified in our study include study period, sex, siblings, common cold symptoms, use of antibiotics, Hi-carriage, Mc-carriage, Sa-carriage and confirm the findings of other reports (besides study period and sex) [5,19,20,42,43].
Based on Quellung-reaction, the proportion of non-PCV13-VTs decreased significantly over the study period, associated with an increase in the proportion of the three PCV13-non-PCV10-VTs 3, 6A, 19A (mainly 19A). We verified for confounders (preterm delivery, previous hospitalisation, age-appropriate vaccination, GAS-carriage, parental smoking, breastfeeding, and the variables shown in Table 2) that could have caused increased PCV13-non-PCV10-VT-carriage, but could not identify any besides study period, which strengthens the hypothesis that the increase was caused by the vaccine switch.
Serotype 19A became the most frequent vaccine serotype in 2017–2018 and its PCR-based prevalence rose from 0.4% in 2016 to 6.4% in 2017–2018. According to surveillance data on IPD from the NRC in 2017, serotype 19A was the second most frequent serotype (after serotype 12F) among IPD-isolates of children younger than 2 years of age. While no increase in serotype 19A frequency had been noted since the introduction of PCV13 in 2011, an increase was observed for the first time in 2016, when the frequency changed from 2.1% in 2016 to 14.2% in 2017 [44]. These findings were confirmed in 2018, 2 to 3 years after the switch from PCV13 to PCV10 [45]. In addition to being reported as an invasive serotype, 19A has also been reported as a serotype that is frequently non-susceptible to antimicrobials [46]. Nevertheless, in our study other serotypes dominated among the non-susceptible strains (23B, 11A, 15B) as they were more prevalent than 19A. Since we excluded children who were treated with oral ABs in the 7 days before sampling, we possibly missed some non-susceptible strains (including 19A). Besides for serotype 19A, a consistently increasing proportion was also observed for the carriage of serotype 6C, which increased from 0.9% in 2016 to 5.8% in 2017–2018. Sweden, where PCV13 is used in some regions, while PCV10 is used in others, also reported an increase in serotype 6C in PCV10-regions [47]. This could have implications for the non-vaccinated elderly, as reported in Finland, where serotypes 19A and 6C are frequently isolated from IPD in adults older than 65 years [48].
Our results should be interpreted in the context of several limitations. First, a decreasing age trend was introduced by recruiting older children in 2016 in order to include a maximum of children vaccinated with PCV13 and for the same reason, Wallonia was over-recruited in 2016. Since this is intrinsic to our study design, we cannot adjust for this: the sampling probabilities did not allow re-weighting for any of the analyses because no children were sampled in the youngest age category in Flanders and in Brussels in 2016.
Furthermore, the Sp-carriage prevalence was stable within the different age categories (6–12, 13–24, 25–30 months) over the study period, but the Hi-carriage prevalence in the two oldest age categories increased over the study period. Second, we over-recruited in Wallonia in 2016 to enlarge the PCV13-vaccinated population, but in the first season no regional differences in overall Hi-carriage or pneumococcal carriage, and vaccine type carriage were found. Third, a comparative analysis based on the children’s vaccination schedule was not performed due to the small size of these subpopulations in either 2016 (few PCV10-vaccinated children) or 2017–2018 (few PCV13-vaccinated children). Finally, a 3-year study period is short to completely exclude natural fluctuations in the carriage prevalence of specific serotypes; a follow-up study should confirm whether or not a new equilibrium is reached.
Despite these limitations, our study allowed to monitor the impact of the PCV13-to-PCV10 vaccine switch on nasopharyngeal carriage, serotype distribution, and antimicrobial (non-)susceptibility of Sp. This is a complementary activity to the analysis of IPD-surveillance data, thus providing further basis for policy making on pneumococcal vaccination programme options.
Conclusions
As the proportion of children vaccinated exclusively with PCV10 increased, the proportion of the serotypes not included in PCV13 decreased over the 3-year study period, mainly due to an increase in the carriage prevalence of serotype 19A.
Acknowledgements
Funding statement
Research grant from Research Foundation Flanders (1150017N); investigator-initiated research grant from Pfizer.
NPcarriage Study Group
David Tuerlinckx, CHU Dinant-Godinne, Université Catholique de Louvain, Yvoir, Belgium; Adam Finn, University of Bristol, School of Cellular and Molecular Medicine, Bristol, UK; Koen Van Herck, Department of Public Health and Primary Care, Ghent University, Gent, Belgium, Centre for the Evaluation of Vaccination, Vaccine and Infectious Disease Institute, University of Antwerp, Wilrijk, Belgium; Robert Cohen, Université Paris Est, IMRB-GRC GEMINI, 94000 Créteil, France; Christine Lammens, Laboratory of Medical Microbiology, Vaccine and Infectious Disease Institute, University of Antwerp, Wilrijk, Belgium.
Conflict of interest: Investigator-initiated research grant from Pfizer.
Authors’ contributions: HT designed and supervised the study including contribution to collection, analysis and interpretation of the data and writing; IW, LVH and SD contributed to the collection, analysis and interpretation of data and writing; SH contributed to the analysis and interpretation of the data; IW initiated and handled the manuscript; PB, JV, HG, PVD and SMK contributed to writing; NPcarriage Study Group contributed to study design and data interpretation.
References
- 1. Satzke C, Dunne EM, Porter BD, Klugman KP, Mulholland EK, PneuCarriage project group The PneuCarriage Project: A Multi-Centre Comparative Study to Identify the Best Serotyping Methods for Examining Pneumococcal Carriage in Vaccine Evaluation Studies. PLoS Med. 2015;12(11):e1001903 , discussion e1001903. 10.1371/journal.pmed.1001903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simell B, Auranen K, Käyhty H, Goldblatt D, Dagan R, O’Brien KL, Pneumococcal Carriage Group The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines. 2012;11(7):841-55. 10.1586/erv.12.53 [DOI] [PubMed] [Google Scholar]
- 3. Syrjänen RK, Kilpi TM, Kaijalainen TH, Herva EE, Takala AK. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. J Infect Dis. 2001;184(4):451-9. 10.1086/322048 [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization (WHO) Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper - February 2019. WER. 2019;94(8):85-104. [Google Scholar]
- 5. Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144-54. 10.1016/S1473-3099(04)00938-7 [DOI] [PubMed] [Google Scholar]
- 6. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;46(RR-8):1-24. [PubMed] [Google Scholar]
- 7. Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. J Infect Dis. 1997;175(6):1440-5. 10.1086/516477 [DOI] [PubMed] [Google Scholar]
- 8. Gray BM, Converse GM, 3rd, Dillon HC., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142(6):923-33. 10.1093/infdis/142.6.923 [DOI] [PubMed] [Google Scholar]
- 9. Principi N, Marchisio P, Schito GC, Mannelli S, Ascanius Project Collaborative Group Risk factors for carriage of respiratory pathogens in the nasopharynx of healthy children. Pediatr Infect Dis J. 1999;18(6):517-23. 10.1097/00006454-199906000-00008 [DOI] [PubMed] [Google Scholar]
- 10. Vanderkooi OG, Church DL, MacDonald J, Zucol F, Kellner JD. Community-based outbreaks in vulnerable populations of invasive infections caused by Streptococcus pneumoniae serotypes 5 and 8 in Calgary, Canada. PLoS One. 2011;6(12):e28547. 10.1371/journal.pone.0028547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Hib and Pneumococcal Global Burden of Disease Study Team Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893-902. 10.1016/S0140-6736(09)61204-6 [DOI] [PubMed] [Google Scholar]
- 12. Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, et al. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin Microbiol Rev. 2015;28(3):871-99. 10.1128/CMR.00024-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jauneikaite E, Tocheva AS, Jefferies JM, Gladstone RA, Faust SN, Christodoulides M, et al. Current methods for capsular typing of Streptococcus pneumoniae. J Microbiol Methods. 2015;113:41-9. 10.1016/j.mimet.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 14. Pichichero ME, Khan MN, Xu Q. Next generation protein based Streptococcus pneumoniae vaccines. Hum Vaccin Immunother. 2016;12(1):194-205. 10.1080/21645515.2015.1052198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Varghese R, Jayaraman R, Veeraraghavan B. Current challenges in the accurate identification of Streptococcus pneumoniae and its serogroups/serotypes in the vaccine era. J Microbiol Methods. 2017;141:48-54. 10.1016/j.mimet.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 16. Tsaban G, Ben-Shimol S. Indirect (herd) protection, following pneumococcal conjugated vaccines introduction: A systematic review of the literature. Vaccine. 2017;35(22):2882-91. 10.1016/j.vaccine.2017.04.032 [DOI] [PubMed] [Google Scholar]
- 17. Steens A, Caugant DA, Aaberge IS, Vestrheim DF. Decreased Carriage and Genetic Shifts in the Streptococcus pneumoniae Population After Changing the Seven-valent to the Thirteen-valent Pneumococcal Vaccine in Norway. Pediatr Infect Dis J. 2015;34(8):875-83. 10.1097/INF.0000000000000751 [DOI] [PubMed] [Google Scholar]
- 18. Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11(10):760-8. 10.1016/S1473-3099(11)70090-1 [DOI] [PubMed] [Google Scholar]
- 19. Navne JE, Børresen ML, Slotved HC, Andersson M, Melbye M, Ladefoged K, et al. Nasopharyngeal bacterial carriage in young children in Greenland: a population at high risk of respiratory infections. Epidemiol Infect. 2016;144(15):3226-36. 10.1017/S0950268816001461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pan H, Cui B, Huang Y, Yang J, Ba-Thein W. Nasal carriage of common bacterial pathogens among healthy kindergarten children in Chaoshan region, southern China: a cross-sectional study. BMC Pediatr. 2016;16(1):161. 10.1186/s12887-016-0703-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spijkerman J, Prevaes SM, van Gils EJ, Veenhoven RH, Bruin JP, Bogaert D, et al. Long-term effects of pneumococcal conjugate vaccine on nasopharyngeal carriage of S. pneumoniae, S. aureus, H. influenzae and M. catarrhalis. PLoS One. 2012;7(6):e39730. 10.1371/journal.pone.0039730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendes da Costa E, et al. Infectieziekten bij kinderen, die voorkomen kunnen worden door vaccinatie, jaarrapport 2015. Brussel: Wetenschappelijk Instituut Volksgezondheid; 2016.
- 23.Robert E, Swennen B. Enquête de couverture vaccinale des enfants de 18 à 24 mois en Fédération Wallonie-Bruxelles (Bruxelles exceptée). Bruxelles: ULB; 2015. [Google Scholar]
- 24.Vandermeulen C, Hoppenbrouwers K, Roelants M, Theeten H, Braeckman T, Maertens K, Blaizot S, Van Damme P. Studie van de vaccinatiegraad in Vlaanderen, 2016. Vlaamse Overheid: Vlaams Agentschap Zorg en Gezondheid; 2017.
- 25.Hoppenbrouwers K, et al. Studie van de vaccinatiegraad bij jonge kinderen en adolescenten in Vlaanderen in 2008. Vlaamse Overheid: Vlaams Agentschap Zorg en Gezondheid; 2009.
- 26.Robert E, Swennen B. Enquête de couverture vaccinale des enfants de 18 à 24 mois en Communauté Française (Bruxelles excepté) - Novembre 2009. Bruxelles: ULB; 2010. [Google Scholar]
- 27. Malfroot A, Verhaegen J, Dubru JM, Van Kerschaver E, Leyman S. A cross-sectional survey of the prevalence of Streptococcus pneumoniae nasopharyngeal carriage in Belgian infants attending day care centres. Clin Microbiol Infect. 2004;10(9):797-803. 10.1111/j.1198-743X.2004.00926.x [DOI] [PubMed] [Google Scholar]
- 28. Rodrigues F, Foster D, Caramelo F, Serranho P, Gonçalves G, Januário L, et al. Progressive changes in pneumococcal carriage in children attending daycare in Portugal after 6 years of gradual conjugate vaccine introduction show falls in most residual vaccine serotypes but no net replacement or trends in diversity. Vaccine. 2012;30(26):3951-6. 10.1016/j.vaccine.2012.03.058 [DOI] [PubMed] [Google Scholar]
- 29. Vestrheim DF, Høiby EA, Aaberge IS, Caugant DA. Phenotypic and genotypic characterization of Streptococcus pneumoniae strains colonizing children attending day-care centers in Norway. J Clin Microbiol. 2008;46(8):2508-18. 10.1128/JCM.02296-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wouters I, Van Heirstraeten L, Desmet S, Blaizot S, Verhaegen J, Goossens H, et al. NPcarriage Study Group Nasopharyngeal s. pneumoniae carriage and density in Belgian infants after 9 years of pneumococcal conjugate vaccine programme. Vaccine. 2018;36(1):15-22. 10.1016/j.vaccine.2017.11.052 [DOI] [PubMed] [Google Scholar]
- 31. Wouters I, Desmet S, Van Heirstraeten L, Blaizot S, Verhaegen J, Van Damme P, et al. NPcarriage Study Group Follow-up of serotype distribution and antimicrobial susceptibility of Streptococcus pneumoniae in child carriage after a PCV13-to-PCV10 vaccine switch in Belgium. Vaccine. 2019;37(8):1080-6. 10.1016/j.vaccine.2018.12.068 [DOI] [PubMed] [Google Scholar]
- 32.Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Supplement M100. Wayne, PA: CSLI; 2017.
- 33.European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. Version 7.1. Växjö: EUCAST ; 2017. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.pdf
- 34. Boelsen LK, Dunne EM, Lamb KE, Bright K, Cheung YB, Tikoduadua L, et al. Long-term impact of pneumococcal polysaccharide vaccination on nasopharyngeal carriage in children previously vaccinated with various pneumococcal conjugate vaccine regimes. Vaccine. 2015;33(42):5708-14. 10.1016/j.vaccine.2015.07.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakamura S, Yanagihara K, Morinaga Y, Izumikawa K, Seki M, Kakeya H, et al. Multiplex real-time polymerase chain reaction for rapid detection of beta-lactamase-negative, ampicillin-resistant Haemophilus influenzae. Diagn Microbiol Infect Dis. 2009;64(1):64-9. 10.1016/j.diagmicrobio.2009.01.023 [DOI] [PubMed] [Google Scholar]
- 36. Azzari C, Moriondo M, Indolfi G, Cortimiglia M, Canessa C, Becciolini L, et al. Realtime PCR is more sensitive than multiplex PCR for diagnosis and serotyping in children with culture negative pneumococcal invasive disease. PLoS One. 2010;5(2):e9282. 10.1371/journal.pone.0009282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Slinger R, Duval M, Langill J, Bromwich M, MacCormick J, Chan F, et al. Direct molecular detection of a broad range of bacterial and viral organisms and Streptococcus pneumoniae vaccine serotypes in children with otitis media with effusion. BMC Res Notes. 2016;9(1):247. 10.1186/s13104-016-2040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sakai F, Chochua S, Satzke C, Dunne EM, Mulholland K, Klugman KP, et al. Single-plex quantitative assays for the detection and quantification of most pneumococcal serotypes. PLoS One. 2015;10(3):e0121064. 10.1371/journal.pone.0121064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andrade DC, Borges IC, Bouzas ML, Oliveira JR, Käyhty H, Ruuskanen O, et al. Antibody responses against Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis in children with acute respiratory infection with or without nasopharyngeal bacterial carriage. Infect Dis (Lond). 2018;50(9):705-13. 10.1080/23744235.2018.1463451 [DOI] [PubMed] [Google Scholar]
- 40. Brandileone MC, Zanella RC, Almeida SCG, Brandao AP, Ribeiro AF, Carvalhanas TMP, et al. Pneumococcal Carriage Study Group Effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae among children in São Paulo, Brazil. Vaccine. 2016;34(46):5604-11. 10.1016/j.vaccine.2016.09.027 [DOI] [PubMed] [Google Scholar]
- 41. van den Bergh MR, Spijkerman J, Swinnen KM, François NA, Pascal TG, Borys D, et al. Effects of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D-conjugate vaccine on nasopharyngeal bacterial colonization in young children: a randomized controlled trial. Clin Infect Dis. 2013;56(3):e30-9. 10.1093/cid/cis922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Regev-Yochay G, Dagan R, Raz M, Carmeli Y, Shainberg B, Derazne E, et al. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in Children. JAMA. 2004;292(6):716-20. 10.1001/jama.292.6.716 [DOI] [PubMed] [Google Scholar]
- 43. Lewnard JA, Givon-Lavi N, Huppert A, Pettigrew MM, Regev-Yochay G, Dagan R, et al. Epidemiological Markers for Interactions Among Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus in Upper Respiratory Tract Carriage. J Infect Dis. 2016;213(10):1596-605. 10.1093/infdis/jiv761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verhaegen J. Surveillance van de pneumokokkeninfecties in België. Verslag voor 2017. UZ Leuven: Nationaal Referentiecentum voor invasieve pneumokokkeninfecties; 2018.
- 45.Desmet S, Peetermans WE, Patteet S, Top G, Verhaegen J, Lagrou K. Increase of ST19A invasive pneumococcal disease in young children after switch from PCV13 to PCV10. 39th Annual meeting of the European Society for Paediatric Infectious Dieseases (ESPID); 2019. Ljubljana (Slovenia). [Google Scholar]
- 46. Dagan R, Klugman KP. Impact of conjugate pneumococcal vaccines on antibiotic resistance. Lancet Infect Dis. 2008;8(12):785-95. 10.1016/S1473-3099(08)70281-0 [DOI] [PubMed] [Google Scholar]
- 47. Naucler P, Galanis I, Morfeldt E, Darenberg J, Örtqvist Å, Henriques-Normark B. Comparison of the Impact of Pneumococcal Conjugate Vaccine 10 or Pneumococcal Conjugate Vaccine 13 on Invasive Pneumococcal Disease in Equivalent Populations. Clin Infect Dis. 2017;65(11):1780-90. 10.1093/cid/cix685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finnish Institute for Health and Welfare (THL). Incidence of invasive pneumococcal disease in Finland. Helsinki: THL; 2017. [Accessed 11 Mar 2019]. Available from: https://thl.fi/en/web/thlfi-en/research-and-expertwork/projects-and-programmes/monitoring-the-population-effectiveness-of-pneumococcal-conjugate-vaccination-in-the-finnish-national-vaccination-programme/incidence-of-invasive-pneumococcal-disease-in-finland#figures