Abstract
A series of 2-thio- and 2-seleno-acetamides bearing the benzenesulfonamide moiety were evaluated as Carbonic Anhydrase (CA, EC 4.2.1.1) inhibitors against different pathogenic bacteria such as the Vibrio cholerae (VchCA-α and VchCA-β), Burkholderia pseudomallei (BpsCA-β and BpsCA-γ), Mycobacterium tuberculosis (Rv3723-β) and the Salmonella enterica serovar Typhimurium (StCA2-β). The molecules represent interesting leads worth developing as innovative antibacterial agents since they possess new mechanism of action and isoform selectivity preferentially against the bacterial expressed CAs. The identification of potent and selective inhibitors of bacterial CAs may lead to tools also useful for deciphering the physiological role(s) of such proteins.
Keywords: carbonic anhydrase, inhibitors, metalloenzymes, selenium, Vibrio cholera, Burkholderia pseudomallei, Mycobacterium tuberculosis, salmonella
1. Introduction
Infectious diseases caused by human pathogens namely bacteria, fungi and virus are among the major challenges for humankind. A large number of bacteria, in recent years, showed increasing resistance to the more common antibiotics clinically used [1,2,3]. Despite the progresses in fundamental knowledge on various pathogens, the current chemotherapy still is unsatisfactory due to limited efficacy, long-term treatment, drug resistance and undesired side effects. Thus, there is an urgent need for the development of new and efficient drugs against human affecting pathogens. Recently, the inhibition of bacterial Carbonic Anhydrases (CAs EC 4.2.1.1) was demonstrated to influence both growth and pathogenicity of microorganisms [4,5,6], and this allows to properly define a new approach for obtaining anti-infective agents with a new mechanism of action when compared to classical antibiotics [7]. These essential enzymes are a superfamily of metalloenzymes which catalyze the reversible hydration of CO2 in H2CO3 [8]. To date, seven genetically distinct families were reported and were named as α- (present in vertebrates, protozoa, algae, cytoplasm of green plants, and in many gram-negative Bacteria), β- (in both gram-negative and -positive Bacteria, mono and dicotyledons plants, fungi and Archaea), γ- (Bacteria, cyanobacteria and Archaea), δ-, ζ-, θ- (in marine diatoms) and ɳ- CAs (protozoa belonging to the Plasmodium spp) [9,10,11,12].
Since our proof-of-concept on organochalcogen derivatives having interesting inhibition activity against some bacterial expressed CAs [13] further successful investigations were carried-out [10,14]. The study herein proposed is placed on this spot with the intent to further investigate such a class of compounds for their inhibition properties against selected bacterial expressed CAs.
2. Results and Discussion
2.1. Chemistry
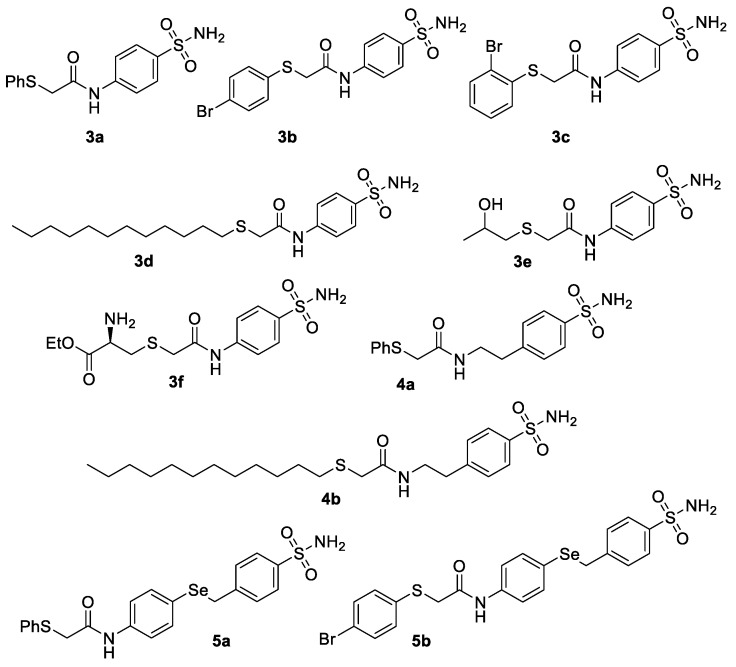
We focused our attention onto the study of substituted 2-thio- and 2-seleno-acetamides bearing the benzenesulfonamide moiety. We sought to employ 2-chloroacetamides 2a–c, obtained according to previously reported procedures [15], as precursors of the synthesis of the polyfunctionalised target compounds through the reaction with suitable sulfur- and selenium-containing nucleophiles. As reported previously by our group [16], this methodology enables the synthesis of differently substituted sulfur-containing N-(4-sulfamoylphenyl)acetamides as outlined in Figure 1.
Figure 1.
Sulfur-containing acetamides bearing the benzenesulfonamide moiety.
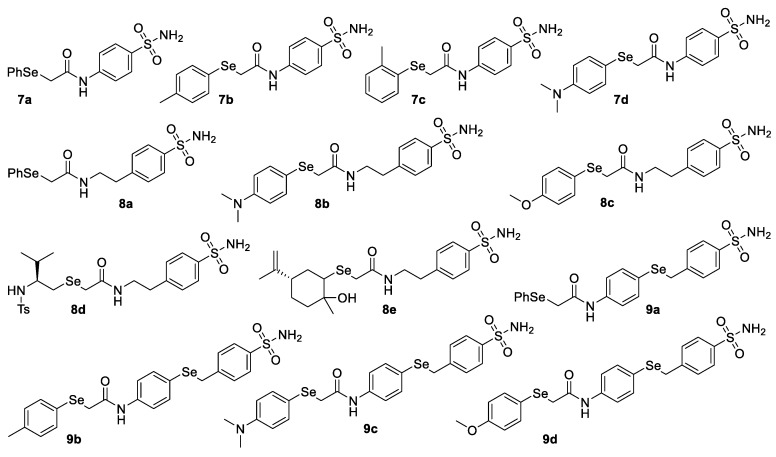
In order to enlarge the library of these novel chalcogen-containing molecules as potential CA inhibitors, we also synthetized the selenium-containing analogues of compounds 3–5. Diselenides were employed as precursors of selenolate anions and in situ treated with 2-chloroacetamides 2a–c. Diselenides 6b–d, bearing p-methyl-, o-methyl-, and p-(N,N-dimethylamino)-substituted aromatic rings, were efficiently converted into the corresponding 2-(arylseleno)acetamides 7b–d in good yields under the same reaction conditions (Figure 2).
Figure 2.
Selenium-containing acetamides bearing the benzenesulfonamide moiety.
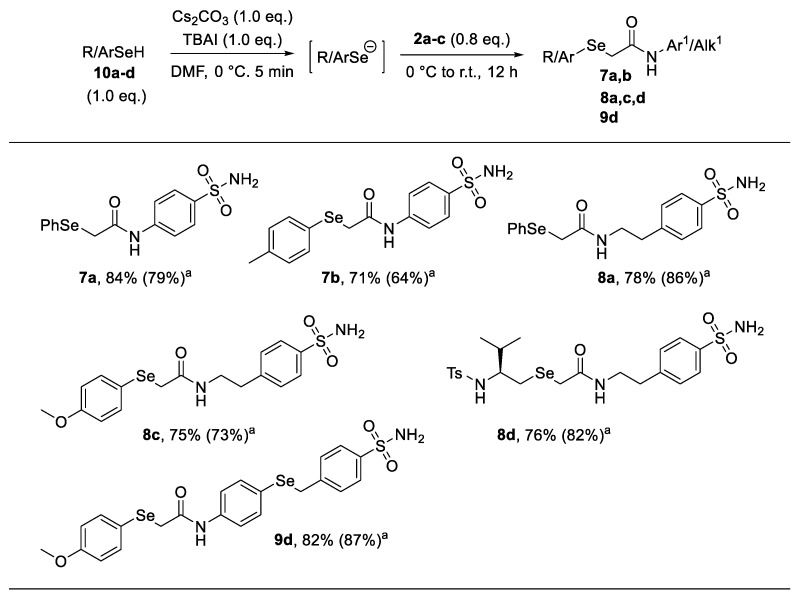
Finally, we further enlarge the scope of this procedure with variously substituted diselenides allowing the synthesis of organoselenides 8a–c from diselenides 6a–c and diselenides 6a,b,d,e gave N-(4-(benzylselanyl)phenyl)acetamides 9a–d, bearing two selenated moieties onto the same molecular skeleton (Figure 2). Additionally, on the basis of our recent findings on the reactivity of selenols with elecrophiles [17,18,19,20], we also evaluated an alternative and milder approach to 2-seleno-acetamides 7–9. Thus, aryl- and alkyl-selenols 10a–d were efficiently deprotonated by using a Cs2CO3/TBAI system [21,22] and then treated with 2-chloroacetamides 2a–c to afford the corresponding selenium-containing acetamides 7–9 in good yield (Scheme 1). Notably, owing to the mildness of the reaction conditions, this methodology could represent a valuable alternative route for the synthesis of 2-seleno-acetamides bearing labile moieties, poorly stable under strong reducing conditions. All obtained compounds were characterized by means of 1H-NMR, 13C-NMR, 77Se-NMR and mass spectra analysis and were in agreement with the previously reported data [16].
Scheme 1.
Alternative route for the synthesis of selenium-containing acetamides bearing the benzenesulfonamide moiety exploiting the reactivity of selenols 10a–d. Yields refer to isolated products. a Yields refer to ref. [16].
2.2. Carbonic Anhydrase Inhibition
Our main interest was to investigate structure-activity relationship (SAR) features of our 3a-f, 4a-b, 5a-b, 7a-c, 8a-c, 9a-d compounds underpinning the inhibition against different bacterial CA isoforms such as Vibrio cholerae (VchCA-α and VchCA-β), Burkholderia pseudomallei (BpsCA-β and BpsCA-γ), Mycobacterium tuberculosis (Rv3723-β) and Salmonella enterica serovar Typhimurium (StCA2-β). The inhibition studies were performed by means of the stopped-flow carbon dioxide hydration assay [23], compared to the standard and clinically used CAI acetazolamide (AAZ) (Table 1).
Table 1.
Inhibition data against bacterial enzymes VchCAα-β, BpsCAβ-γ, StCA2-β and Rv3273-β of compounds 3a-f, 4a-b, 5a-b, 7a-c, 8a-c, 9a-d and acetazolamide (AAZ) by a stopped flow CO2 hydrase assay [23].
| KI (nM) * | ||||||
|---|---|---|---|---|---|---|
| Cmp | VchCA-α | VchCA-β | StCA2-β | BpsCA-β | BpsCA-γ | Rv3273-β |
| 3a | 49.9 | 4648 | 797.9 | >10,000 | 4168 | 2779 |
| 3b | 46.7 | 700.0 | 85.6 | >10,000 | 2750 | 1833 |
| 3c | 33.5 | 865.0 | 84.1 | >10,000 | 404.7 | 269.8 |
| 3d | >10,000 | 581.4 | 752.7 | >10,000 | 3819 | 2546 |
| 3e | 44.7 | 6651 | 77.7 | >10,000 | 321.5 | 214.3 |
| 3f | 84.9 | >10,000 | 482.8 | >10,000 | >10,000 | >10,000 |
| 4a | 51.1 | 700.0 | 86.7 | >10,000 | 3515 | 2343 |
| 4b | >10,000 | 7187 | 881.1 | >10,000 | >10,000 | >10,000 |
| 5a | 829.1 | 6225 | 865.0 | >10,000 | 1517 | 1011 |
| 5b | 45.8 | 580.7 | 8.2 | >10,000 | >10,000 | >10,000 |
| 7a | 2.2 | 96.3 | 814.7 | 4094 | 303.8 | 202.5 |
| 7b | 7.7 | 762.2 | >10,000 | >10,000 | 424.2 | 282.8 |
| 7c | 41.7 | 6210 | 451.3 | >10,000 | >10,000 | >10,000 |
| 8a | 44.4 | 6590 | 86.9 | >10,000 | 2250 | 1500 |
| 8b | 85.6 | 607.8 | 550.0 | >10,000 | 3634 | 2422 |
| 8c | 3.2 | 85.0 | 9.3 | >10,000 | >10,000 | >10,000 |
| 8d | 62.3 | 828.1 | 9.1 | >10,000 | >10,000 | >10,000 |
| 8e | 6.1 | 308.9 | 8.9 | >10,000 | >10,000 | >10,000 |
| 9a | 902.2 | 7468 | >10,000 | >10,000 | 449.7 | 299.8 |
| 9b | 88.6 | 729.5 | 27.3 | >10,000 | 4172 | 2781 |
| 9c | 730.3 | 7985 | 820.0 | >10,000 | 3656 | 2437 |
| 9d | 8.9 | 82.9 | 7.5 | >10,000 | >10,000 | >10,000 |
| AAZ | 6.8 | 451 | 89.0 | 745 | 149 | 104 |
* Mean from 3 different assays, by a stopped flow technique (errors were in the range of 5–10% of the reported values).
The following SARs can be drawn out of the data reported in Table 1:
(i) The inhibition profile of the only α-CA isoform reported in this study (i.e., the VchCA-α) was deeply influenced by compounds herein tested. The range of KI values spanned from low to high nanomolar range (KI 2.2–902.2 nM) and the compounds 3d and 4b bearing the nonanethiol tail were devoid of any activity (Table 1). The insertion of the bromine atom within the thiophenol moiety as in compounds 5b increased up to 18 folds the inhibition activity when compared to 5a which does not have any halogen (KI 829.1 to 45.8 nM). An interesting SAR case was the replacement of the sulfur atom in 3a-b with selenium one to afford compounds 7a-b. Such an isosteric substitution increased the inhibition potency up to low nanomolar values (KIs 49.9, 46.7 and 2.2, 7.7 nM respectively).
(ii) The second isoform here tested from the Gram-negative bacterium Vibrio Cholerae, VchCA-β, was less inhibited when compared to the α- as the associated KI values were comprised between high nanomolar to micromolar range (KI 82.9–7985 nM). On the other hand, compound 3f did not show any inhibitory activity against this isoform. The replacement of sulfur with selenium atom by compounds 3a and 7a, also for this isoform, lead to increase the activity over 48 times (KI 4648 nM for 3a and 96.3 nM for 7a). Conversely, such a replacement was not effective for compounds 5a and 9a as the inhibition constants were perfectly superimposable (KIs 829.1 and 902.2 nM, respectively) or were decreased as for compounds 4a and 8a (700 and 6590 nM respectively).
(iii) All compounds herein considered had not effects on the BpsCA-β except for derivative 7a, which was a weak inhibitor (KI 4094 nM).
(iv) As for the BpsCA-γ the replacement of sulfur atom with selenium one (compounds 3a and 7a) lead to increase activity over 100 folds (KI 4168 and 303.8 nM, respectively). Moreover, for the series 9a-d, substituents on the aromatic ring proved to be detrimental for the inhibition potency (KI 449.7–10000 nM). Substitutions on the aromatic rings within the series 7a-d proved to be crucial for the activity as reported for the ortho CH3 in 7c which resulted in loss of the inhibition potency. The BpsCA-β was inhibited only from compound 7a (KI 4094 nM).
(v) The Mycobacterium tuberculosis β-CA Rv3273 was weakly or not inhibited at all by compounds here reported. In general, the series 9a-d and 7a-d showed the same inhibition features of BpsCA-γ.
(vi) Finally, the StCA2-β from the bacterial pathogen Salmonella enterica serovar Typhimurium was deeply influenced by these compounds. As VchCA-α the range of inhibition spanned from low to high nanomolar range (KI 7.5–88.1 nM) and for compounds 7b, 9a no activity was recorded. Within the 3a-f series the bromine atom proved to be essential for the potency (3b-c), indeed, the activity increased 9 times than the analog without substituent (3a) or different derivatives as in 3d-f. In analogy, as mentioned above, compound 5b with bromine atom showed an inhibition constant 100 folds better than 5a (KI 8.2–865 nM, respectively). StCA2-β was the only isoform here reported where the replacement of sulfur atom of compound 3a with selenium one (7a) did not show improvement in the inhibition activity. An interesting case was for the series 9a-d where the substituents on aromatic ring were fundamental for the inhibition of this isoform showing no activity for compound 9a without them.
Overall, the substituted 2-thio- and 2-seleno-acetamides bearing the benzenesulfonamide revealed interesting inhibition features and selectivity profiles. Compounds 3f did not show inhibition against the β CA isoforms except for the StCA2-β, which was weakly inhibited and thus proving this molecule to possess a good selectivity for the α isoforms such as the VchCA-α. Derivatives 4b and 5b did not show any activity against the bacterial isoforms of Burkholderia pseudomallei and Mycobacterium tuberculosis proving good inhibitors of the β isoform of Salmonella. In general, series 7a-c had selectivity for α isoform of Vibrio cholera. Finally, series 8a-e and 9a-d did not show particular potency of inhibition for BpsCA-β and γ but activity against the VchCA isoforms.
3. Materials and Methods
3.1. General
Anhydrous solvents and all reagents were purchased from Sigma-Aldrich, Alfa Aesar and TCI. The solvents used in MS measures were acetone, DMSO, acetonitrile (Chromasolv grade), purchased from Sigma-Aldrich (Milan, Italy), and mQ water 18 MΩ, obtained from Millipore’s Simplicity system (Milan, Italy).
3.2. Chemistry
Characterization of compounds 3a-f, 4a-b, 5a-b, 7a-c, 8-e and 9-a-d was reported earlier by our group [16]. β-mercapto alcohol 1e [24], alkyl selenols [22] and aryl selenols [25] were prepared according previously reported procedures.
Typical procedure for the synthesis of 2-seleno-acetamides through alkylation of selenols:
A solution of selenol (1.0 mmol) in dry DMF (5 mL) was cooled under inert atmosphere at 0 °C and treated with Cs2CO3 (326 mg, 1.0 mmol) and TBAI (369 mg, 1.0 mmol). Then, a DMF solution (1 mL) of the suitable 2-chloroacetamide 2a-c (0.8 mmol) was added and the mixture was allowed to warm to r.t. and stirred for 12 h. Afterwards, the reaction was treated with saturated NH4Cl solution (2 mL), extracted with EtOAc (10 mL) and washed with water (2 × 10 mL) and brine (2 × 10 mL). The organic phase was dried over Na2SO4, the solvent was evaporated under vacuum and the crude product was purified by flash column chromatography or precipitated from EtOAc/petroleum ether to yield substituted 2-selenoacetamides 7,8,9.
3.3. Carbonic Anhydrase Inhibition
An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity [23]. Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.5 for the α-CAs) or TRIS (pH 8.3 for the β-CAs) as buffers, and 20 mM Na2SO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalyzed CO2 hydration reaction for a period of 10–100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor, at least six traces of the initial 5% to 10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled-deionized water and dilutions up to 0.01 nM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the E-I complex. The inhibition constants were obtained by non-linear least-squares methods using PRISM 3 and the Cheng–Prusoff equation, as reported earlier [26,27,28,29,30,31], and represent the mean from at least three different determinations. All CA isoforms were recombinant ones obtained in-house as reported earlier [26,27,28,29,30,31].
4. Conclusions
In conclusion, we investigated a series of 2-thio- and 2-seleno-acetamides bearing the benzenesulfonamide for their inhibition against different carbonic anhydrase isoforms from several pathogenic bacteria such as Vibrio cholera, Burkholderia pseudomallei, Mycobacterium tuberculosis and Salmonella enterica serovar Typhimurium. Regarding their well-known zinc binding group, the primary sulfonamide, these compounds showed an interesting inhibition activity with different selectivity profile. Among them, compounds 3f and 7a-c had a good selectivity for VchCAα. Derivatives 4b and 5b proved good inhibitors of the β isoform of Salmonella and, finally, series 8a-e and 9a-d showed activity against both VchCA isoforms. Thus, this series of compounds might be considered promising lead compounds for obtaining more effective and selective CAIs targeting different bacterium isoforms and as useful pharmacological tools for understanding the physiological role(s) of these under-investigated enzymes.
Abbreviations
| CA | Carbonic Anhydrase |
| CAI | Carbonic Anhydrase Inhibitors |
Author Contributions
Data curation, A.A., D.T. and B.C.S.; funding acquisition, M.P.; investigation, A.M. and G.A.; methodology, A.C., C.C. and S.d.P.; supervision, C.T.S.; validation, S.S.M.; writing—original draft, F.C. All authors have read and agreed to the published version of the manuscript
Funding
This work was supported by a grant of the Romanian Ministry of Research and Innovation, CNCS–UEFISCDI, project number PN-III-P4-ID-PCCF-2016–0050, within PNCDI II.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.van Hecke O., Wang K., Lee J.J., Roberts N.W., Butler C.C. Implications of Antibiotic Resistance for Patients’ Recovery From Common Infections in the Community: A Systematic Review and Meta-analysis. Clin. Infect Dis. 2017;65:371–382. doi: 10.1093/cid/cix233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson D.A., Carter G.P., Howden B.P. Current and Emerging Topical Antibacterials and Antiseptics: Agents, Action, and Resistance Patterns. Clin. Microbiol. Rev. 2017;30:827–860. doi: 10.1128/CMR.00112-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ching C., Orubu E.S.F., Wirtz V.J., Zaman M.H. Bacterial antibiotic resistance development and mutagenesis following exposure to subminimal inhibitory concentrations of fluoroquinolones in vitro: A systematic literature review protocol. BMJ Open. 2019;9:e030747. doi: 10.1136/bmjopen-2019-030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Supuran C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin. Drug Discov. 2017;12:61–88. doi: 10.1080/17460441.2017.1253677. [DOI] [PubMed] [Google Scholar]
- 5.Capasso C., Supuran C.T. An overview of the selectivity and efficiency of the bacterial carbonic anhydrase inhibitors. Curr. Med. Chem. 2015;22:2130–2139. doi: 10.2174/0929867321666141012174921. [DOI] [PubMed] [Google Scholar]
- 6.Capasso C., Supuran C.T. Inhibition of Bacterial Carbonic Anhydrases as a Novel Approach to Escape Drug Resistance. Curr top Med Chem. 2017;17:1237–1248. doi: 10.2174/1568026617666170104101058. [DOI] [PubMed] [Google Scholar]
- 7.Supuran C.T., Capasso C. An Overview of the Bacterial Carbonic Anhydrases. Metabolites. 2017;7:E56. doi: 10.3390/metabo7040056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Supuran C.T. Structure and function of carbonic anhydrases. Biochem, J. 2016;473:2023–2032. doi: 10.1042/BCJ20160115. [DOI] [PubMed] [Google Scholar]
- 9.Aspatwar A., Kairys V., Rala S., Parikka M., Bozdag M., Carta F., Supuran C.T., Parkkila S. Mycobacterium tuberculosis β-Carbonic Anhydrases: Novel Targets for Developing Antituberculosis Drugs. Int. J. Mol. Sci. 2019;20:E5153. doi: 10.3390/ijms20205153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angeli A., Pinteala M., Maier S.S., Del Prete S., Capasso C., Simionescu B.C., Supuran C.T. Inhibition of bacterial α-, β- and γ-class carbonic anhydrases with selenazoles incorporating benzenesulfonamide moieties. J. Enzyme. Inhib. Med. Chem. 2019;34:244–249. doi: 10.1080/14756366.2018.1547287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capasso C., Supuran C.T. Anti-infective carbonic anhydrase inhibitors: A patent and literature review. Expert Opin. Ther. Pat. 2013;23:693–704. doi: 10.1517/13543776.2013.778245. [DOI] [PubMed] [Google Scholar]
- 12.Capasso C., Supuran C.T. An overview of the alpha-, beta- and gamma-carbonic anhydrases from Bacteria: Can bacterial carbonic anhydrases shed new light on evolution of bacteria? J. Enzyme. Inhib. Med. Chem. 2015;30:325–332. doi: 10.3109/14756366.2014.910202. [DOI] [PubMed] [Google Scholar]
- 13.Angeli A., Abbas G., Del Prete S., Carta F., Capasso C., Supuran C.T. Acyl selenoureido benzensulfonamides show potent inhibitory activity against carbonic anhydrases from the pathogenic bacterium Vibrio cholerae. Bioorg. Chem. 2017;75:170–172. doi: 10.1016/j.bioorg.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Angeli A., Abbas G., Del Prete S., Capasso C., Supuran C.T. Selenides bearing benzenesulfonamide show potent inhibition activity against carbonic anhydrases from pathogenic bacteria Vibrio cholerae and Burkholderia pseudomallei. Bioorg. Chem. 2018;79:319–322. doi: 10.1016/j.bioorg.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Alafeefy A.M., Ceruso M., Al-Tamimi A.-M.S., Del Prete S., Capasso C., Supuran C.T. Quinazoline-sulfonamides with potent inhibitory activity against the α-carbonic anhydrase from Vibrio cholerae. Bioorg. Med. Chem. 2014;22:5133. doi: 10.1016/j.bmc.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Tanini D., Capperucci A., Scopelliti M., Milaneschi A., Angeli A., Supuran C.T. Syntesis of thio- and seleno-acetamides bearing benzenesulfonamide as potent inhibitors of human carbonic anhydrase II and XII. Bioorg. Chem. 2019;89:102984. doi: 10.1016/j.bioorg.2019.102984. [DOI] [PubMed] [Google Scholar]
- 17.Tanini D., Scarpelli S., Ermini E., Capperucci A. Seleno-Michael reaction of stable functionalised alkyl selenols: A versatile tool for the synthesis of acyclic and cyclic unsymmetrical alkyl and vinyl selenides. Adv. Synth. Catal. 2019;361:2337–2346. doi: 10.1002/adsc.201900168. [DOI] [Google Scholar]
- 18.Tanini D., Lupori B., Malevolti G., Ambrosi M., Lo Nostro P., Capperucci A. Direct biocatalysed synthesis of first sulfur-, selenium- and tellurium containing L-ascorbyl hybrid derivatives with radical trapping and GPx-like properties. Chem. Commun. 2019;55:5705–5708. doi: 10.1039/C9CC02427A. [DOI] [PubMed] [Google Scholar]
- 19.Tanini D., Capperucci A. Ring opening reactions of heterocycles with selenium and tellurium nucleophiles. New. J. Chem. 2019;43:11451–11468. doi: 10.1039/C9NJ02320H. [DOI] [Google Scholar]
- 20.Mloston G., Capperucci A., Tanini D., Hamera-Faldyga R., Heimgartner H. Dialkyl Dicyanofumarates as Oxidizing Reagents for the Conversion of Thiols into Disulfides and Selenols into Diselenides. Eur. J. Org. Chem. 2017;46:6831–6839. doi: 10.1002/ejoc.201701066. [DOI] [Google Scholar]
- 21.Salvatore R.N., Smith R.A., Nischwitz A.K., Gavin T. A mild and highly convenient chemoselective alkylation of thiols using Cs2CO3-TBAI. Tetrahedron Lett. 2005;46:8931–8935. doi: 10.1016/j.tetlet.2005.10.062. [DOI] [Google Scholar]
- 22.Tanini D., Tiberi C., Gellini C., Salvi P.R., Capperucci A. A Straightforward access to stable β-functionalized alkyl selenols. Adv. Synth. Catal. 2018;360:3367–3375. doi: 10.1002/adsc.201800602. [DOI] [Google Scholar]
- 23.Khalifah R.G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971;246:2561. [PubMed] [Google Scholar]
- 24.Obijalska E., Pawelec M., Mlostoń G., Capperucci A., Tanini D., Heimgartner H. A Remarkable Influence of the Trifluoromethyl Group on the Reactions of β-Mercaptoalcohols with Fluorinated α-Bromoenones. Eur. J. Org. Chem. 2018:3716–3723. doi: 10.1002/ejoc.201701752. [DOI] [Google Scholar]
- 25.Angeli A., Tanini D., Nocentini A., Capperucci A., Ferraroni M., Gratteri P., Supuran C.T. Selenols: A new class of carbonic anhydrase inhibitors. Chem. Commun. 2019;55:648–651. doi: 10.1039/C8CC08562E. [DOI] [PubMed] [Google Scholar]
- 26.Da’dara A.A., Angeli A., Ferraroni M., Supuran C.T., Skelly P.J. Crystal structure and chemical inhibition of essential schistosome host-interactive virulence factor carbonic anhydrase SmCA. Commun. Biol. 2019;2:333. doi: 10.1038/s42003-019-0578-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angeli A., Tanini D., Capperucci A., Supuran C.T. First evaluation of organotellurium derivatives as carbonic anhydrase I, II, IV, VII and IX inhibitors. Bioorg. Chem. 2018;76:268–272. doi: 10.1016/j.bioorg.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Angeli A., Tanini D., Capperucci A., Supuran C.T. Synthesis of Novel Selenides Bearing Benzenesulfonamide Moieties as Carbonic Anhydrase I, II, IV, VII, and IX inhibitors. ACS Med. Chem. Lett. 2017;8:1213–1217. doi: 10.1021/acsmedchemlett.7b00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angeli A., Di Cesare Mannelli L., Trallori E., Peat T.S., Ghelardini C., Carta F., Supuran C.T. Design, Synthesis and X-ray of Selenides as New Class of Agents for Prevention of Diabetic Cerebrovascular Pathology. ACS Med. Chem. Lett. 2018;9:462–467. doi: 10.1021/acsmedchemlett.8b00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angeli A., Carta F., Bartolucci G., Supuran C.T. Synthesis of novel acyl selenoureido benzensulfonamides as carbonic anhydrase I, II, VII and IX inhibitors. Bioorg. Med. Chem. 2017;25:3567. doi: 10.1016/j.bmc.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Angeli A., Peat T.S., Bartolucci G., Nocentini A., Supuran C.T., Carta F. Intramolecular oxidative deselenization of acylselenoureas: A facile synthesis of benzoxazole amides and carbonic anhydrase inhibitors. Org. Biomol. Chem. 2016;14:11353–11356. doi: 10.1039/C6OB02299E. [DOI] [PubMed] [Google Scholar]