Abstract
Glioblastomas are among the most lethal cancers, with a 5 year survival rate below 25%. Temozolomide is typically used in glioblastoma treatment; however, the enzymes alkylpurine-DNA-N-glycosylase (APNG) and methylguanine-DNA-methyltransferase (MGMT) efficiently mediate the repair of DNA damage caused by temozolomide, reducing treatment efficacy. Consequently, APNG and MGMT inhibition has been proposed as a way of overcoming chemotherapy resistance. Here, we develop a mechanistic mathematical model that explicitly incorporates the effects of chemotherapy on tumour cells, including the processes of DNA damage induction, cell arrest and DNA repair. Our model is carefully parametrized and validated, and then used to virtually recreate the response of heteroclonal glioblastomas to dual treatment with temozolomide and inhibitors of APNG/MGMT. Using our mechanistic model, we identify four combination treatment strategies optimized by tumour cell phenotype, and isolate the strategy most likely to succeed in a pre-clinical and clinical setting. If confirmed in clinical trials, these strategies have the potential to offset chemotherapy resistance in patients with glioblastoma and improve overall survival.
Keywords: alkylpurine-DNA-N-glycosylase, glioblastoma, methylguanine-DNA-methyltransferase, mathematical model, temozolomide
1. Introduction
Glioblastoma, or glioblastoma multiforme (GBM), is the most common and malignant of glial tumours, accounting for 60–75% of all astrocytomas. More than 90% of GBM are estimated to develop over a period of only a few days to weeks [1,2]. The malignancy and lethality of GBM is driven by a rapid rate of cancer cell proliferation, coupled with a high degree of vascularity. Indeed, with improvements in leukaemia survival since the 1980s, brain cancers have taken over as the leading cause of childhood cancer death [3]. The first step in treating GBM is maximal resection or surgery, if possible. Radiation and chemotherapy with temozolomide (TMZ) may be administered subsequently [4,5]. With standard treatment, the median survival of adults diagnosed with high-grade brain cancer (grades III and IV) is less than 15 months, and fewer than 10% survive beyond 5 years; in children, GBM 5 year survival is below 25% [6]. Without treatment, patients with GBM have a life expectancy of less than three to four months [4]. GBM-associated death rates remain high, in part because the last few decades have produced only modest advances in treatment. Consequently, the standard therapy for GBM remains palliative, rather than curative, and patients ultimately die from this disease [1].
Chemotherapy has proven to be effective against cancers in general; however, in the case of brain tumours, it has failed to produce sustained remission. GBM typically responds well in the first few cycles of TMZ administration. However, the emergence of resistance diminishes the cytotoxic effects of TMZ in subsequent cycles until there is little or no response to treatment [4]. Several factors may contribute to TMZ resistance (for a comprehensive review, see [7]). Here, we are concerned with resistance mediated by the efficient repair of treatment-induced DNA damage in cancer cells.
The chemical mechanism by which TMZ induces cell death is DNA methylation, leading to double-stranded breaks (DSBs) and thus to apoptosis. A range of mechanisms by which methylation causes DSBs are ably reviewed in [8]. The cell-killing potential of this methylation depends on the position within the purine bicyclic ring where the methyl-adduct is formed. The majority of TMZ-induced methylation sites are N7-meG (greater than 70%) and N3-meA (9.2%). Less frequently (less than 6%) TMZ creates O6-meG adducts [9]. N7-meG and N3-meA contribute minimally to the cytotoxicity of TMZ as they are efficiently repaired via the base excision repair (BER) pathway, mediated by the alkylpurine-DNA-N-glycosylase (APNG) enzyme. O6-meG is more lethal for the cell and is repaired via the methylguanine-DNA-methyltransferase (MGMT) pathway, mediated by the MGMT enzyme [9,10]. Additionally, the mismatch repair (MMR) pathway may aid in DNA demethylation. However, the expression of MMR genes is linked to TMZ sensitivity [7]; therefore, this pathway is not considered here. The repair of TMZ-induced DNA damage reduces its therapeutic efficacy. There is a consensus in the literature that over-expression of MGMT is a primary factor driving TMZ resistance [7,11–13], and a landmark study [14] demonstrated that MGMT promoter methylation was associated with almost a doubling of both progression-free survival and overall survival, in comparison with unmethylated glioblastoma. Finally, in a multivariate model including both MGMT methylation and APNG expression, high APNG expression was associated with improved survival cumulative with the benefit of MGMT methylation [15]. Consequently, it has been hypothesized that MGMT and APNG expression or methylation may be used as biomarkers for glioma response to treatment with alkylating agents such as TMZ [15–17].
Preventing the onset of chemotherapy resistance by inhibiting MGMT or APNG in combination with TMZ administration represents an exciting new avenue of research with the potential for high impact in GBM treatment. Small molecule inhibitors of MGMT, such as bortezomib, and APNG are in various stages of pre-clinical or clinical development, with a majority of this effort being directed at MGMT inhibition [8,18]. With all of these new possibilities, a critical challenge in brain cancer therapeutics is the optimization of dosing and scheduling when alkylating agents such as TMZ are combined with DNA repair enzyme inhibitors. At the time of writing, no experimental studies could be found that consider the simultaneous inhibition of the two repair enzymes. Here, we propose a quantitative modelling framework that is used to conduct in silico pre-clinical and clinical trials with a view to predicting the potential of—and optimizing—such a combination.
Specifically, we develop a mathematical model of the response of GBM cells to TMZ administration, which incorporates TMZ-induced DNA damage, and its subsequent repair, at the level of intracellular molecular mechanisms. This allows us to simulate the effect of APNG and MGMT inhibition in a mechanistic, rather than phenomenological, manner. The model is extensively calibrated and validated versus available experimental data. We then optimize dosing and scheduling for the triple combination of TMZ and small molecule inhibitors of APNG and MGMT by simulating the treatment of phenotypically diverse GBM xenografts grown in virtual mice. Finally, the effect on patient survival of the combination with maximum anti-tumour potential is investigated by enrolling virtual human patients in an in silico clinical trial. Analysis of our results reveals critical features driving resistance to TMZ and evolution of tumour cell phenotypes under various treatment strategies. Our approach offers the key advantage of consistent comparison across strategies since a virtual patient may be treated with as many strategies as needed, allowing us to compare results without the confounding factor of inter-patient variability. Mathematical models have been employed extensively to describe the growth and treatment of gliomas (for instance, see [19–22]; see [23] for a review). However, to the best of our knowledge, ours is the first to explicitly incorporate DNA methylation and repair pathways, and to consider DNA repair inhibition in combination with chemotherapy.
2. Methods
2.1. Mathematical framework
Our modelling framework connects subcellular molecular interactions that govern outcomes at a cellular level with the resultant population-level behaviour of the tumour. A model schematic is shown in figure 1, and a description of our model derivation follows. Within the nucleus, TMZ induces methylation of nucleic acids, a process wherein a hydrogen atom on a DNA base is replaced by a methyl group (R − CH3). Methylation of DNA, in turn, leads to the formation of DNA adducts [9,10]. Briefly, the active metabolite of TMZ binds to DNA reversibly, forming drug–DNA complexes. Subsequently, the methyl group on the drug is transferred irreversibly to DNA, resulting in adduct formation, and TMZ is rendered inactive. In our formulation, this process is approximated by the following reactions:
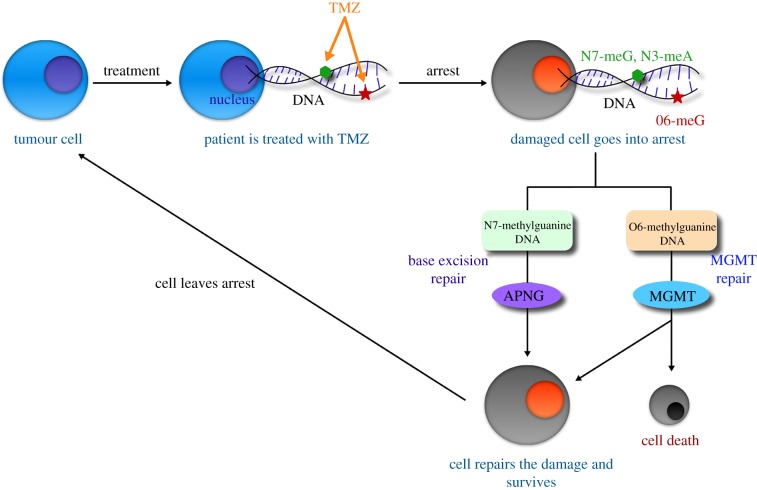
Figure 1.
Model schematic. Treatment with TMZ causes DNA methylation, leading to cell arrest. TMZ-induced methylation sites are N7-meG, N3-meA (repaired by the APNG-mediated BER pathway) and O6-meG (repaired by the MGMT pathway). If DNA damage repair is successful, the cell recovers to the proliferating pool, or undergoes apoptosis otherwise. (Online version in colour.)
Here, capital letters denote concentrations of intracellular species: Tin, intracellular TMZ; Tout, extracellular TMZ; N7, unmethylated DNA at N7-G or N3-A positions; O6, unmethylated DNA at O6-G positions; D7 and D6, position-specific drug–DNA complexes; A7 and A6, DNA adducts N7-meG/N3-meA and O6-meG, respectively; and uTin, inactive intracellular TMZ. Lowercase ks with sub-/superscripts denote the respective rates of reaction. The above chemical reactions are translated into mathematical equations assuming mass action kinetics:
| 2.1 |
In the above equations, we distinguish between intracellular (Tin) and extracellular (Tout) TMZ. The rate constants γj, j = {oi, io} represent the transport of TMZ in and out of the cell, while Kd represents the rate of clearance of TMZ from the extracellular compartment. The constants Vj, j = {in, out} represent the apparent volumes of distribution of TMZ in the intra- and extracellular compartments. Once DNA damage exceeds a critical threshold, the cell enters a state of arrest and different DNA repair mechanisms are triggered depending on the site of damage. For clarity, subcellular species in arrested cells are represented with the superscript *. For instance, if the damage is O6-meG, the MGMT pathway is triggered. When MGMT encounters O6-meG adducts, it transfers the methyl group to an internal cytosine residue. After successfully receiving the methyl group, MGMT inactivates itself, becoming a suicidal protein [24]. In our formulation, this process is approximated by the following reaction:
Briefly, MGMT binds reversibly to the methyl group on O6-meG, forming a repair enzyme–DNA adduct complex. Subsequently, the methyl group from the O6 position of guanine is removed, and O6-meG is restored irreversibly to its standard form, O6-G. A similar mechanism is assumed for APNG-mediated demethylation of N7-meG and N3-meA, that is,
Here, MGMT*, , uMGMT*, APNG*, and uAPNG* denote the intracellular concentration of MGMT, MGMT-O6-meG complex, inactive MGMT, APNG, APNG-N7-meG complex and inactive APNG, respectively. Assuming mass action kinetics, and including production and degradation of species when appropriate, the above reactions yield the following equations governing DNA repair in arrested cells:
| 2.2 |
We remark that, here, a is a second time variable that represents how long a cell has spent in the arrested compartment. The constants Si and λi, i = {APNG, MGMT} represent the rates of repair enzyme production and degradation, respectively. At the population level, the tumour is compartmentalized into proliferating and arrested cells. Following experimentally observed growth patterns, proliferating cells are assumed to grow either logistically, when simulating in vitro cell growth inhibition assays and in vivo xenograft assays, or exponentially, when simulating GBM growing in situ. TMZ application induces DNA damage, leading to cell cycle arrest and triggering of the BER and MGMT pathways [7]. An age structure is imposed on the arrested compartment to account for the time taken from induction of DNA damage to cell death, or recovery following successful completion of damage repair. We remark that TMZ targets actively proliferating cells, inducing arrest in G2/M phase [10,25]. Consequently, cells in the arrested compartment are assumed to be unaffected by its further application. The following equations are taken to represent these population-level processes:
| 2.3 |
Here, P(t) represents the number of proliferating tumour cells at time t, and A(t, a) the number of arrested cells at time t that have been arrested for a units of time. Furthermore, α is the proliferation rate of tumour cells and K is the carrying capacity (when needed).
The subcellular and population scales are connected by decisions made at the cellular level, represented in the above equations with upper case Greek letters. For instance, cells enter a state of arrest (with age 0) at a rate Γ, which is assumed to depend on the level of DNA damage. From an arrested state, cells may recover to the proliferating pool at a rate Ψ, if DNA damage repair has been successful, which is assumed to be proportional to the fraction of DNA that has been repaired. Arrested cells may also undergo apoptosis at rates Φ6 and Φ7, taken to be functions of the levels of damaged DNA and repair enzymes and the amount of time spent in an arrested state. Specifically, these rates are taken to be:
| 2.4 |
The rates of cell arrest and recovery are taken to be proportional to the fraction of damaged and healthy DNA, respectively, with constants of proportionality μ and ρ, respectively. The functional forms of the rates of arrested cell death are chosen to reflect the following empirical observations. The cytotoxicity of TMZ has been shown to correlate positively with time spent in an arrested state [26–28]. Thus, the rates of arrested cell death are assumed to be increasing functions of time spent in an arrested state, with a0 denoting a characteristic waiting time before apoptosis is initiated and c1 denoting the sensitivity of arrested cells to this waiting time. bi, i = {1, 2} are the maximum rates of arrested cell death due to O6-meG or N7-meG. Furthermore, it has been shown that MGMT expression protects cells from TMZ-induced apoptosis [29]; thus the rate of death due to O6-meG (Φ6) is assumed to decrease exponentially as MGMT expression increases, with c2 representing sensitivity to intracellular MGMT. On the other hand, high levels of N3-meA and N7-meG trigger apoptosis [29]; thus the rate of death due to this type of damage (Φ7) is assumed to be a decreasing function of N7-meG, with c3 representing sensitivity to .
The various model variables and parameters, together with their values, meaning and units, are listed in the electronic supplementary material, tables S1 and S2.
2.2. Structural identifiability and estimation of model parameters
Our model has 31 parameters that need to be estimated from available experimental data (see the electronic supplementary material, section S2 for a description of the experimental data used for model parametrization). With such a large number of unknowns, it is crucial to check whether the estimation problem is well posed [30]. This question is addressed by conducting a structural identifiability analysis, a process which determines whether indistinguishable outputs of the model imply uniqueness of parameters, disregarding issues related to data quantity or quality [31]. This analysis reveals the maximum possible information about parameters a given type of data contains and is therefore essential if we are to have any degree of confidence in parameter estimates and, by extension, model predictions.
We use the Matlab toolbox GenSSI 2.0, which uses a series approach to determine structural identifiability and presents the results using identifiability tableaus (for details, see [32]). Briefly, Lie derivatives are employed to arrive at a system of equations for model parameters in terms of model variables. The analysis has three possible outcomes for each parameter. A given parameter is deemed globally identifiable if the resulting system can be solved uniquely for it, that is, the parameter can be uniquely determined from the system output. A parameter is locally identifiable if the above result holds in a neighbourhood of it. Finally, a parameter is unidentifiable if infinitely many values of it yield the same model output [31]. All model parameters were found to be globally or locally structurally identifiable, thus validating the structure of our proposed model.
We next extensively calibrated parameters using available clinical data from a wide array of experiments. Moreover, model output was found to match experimental data that were excluded from the fitting process, thus validating our model formulation. A detailed description of the structural identifiability analysis, clinical data, parameter estimates and model validation is provided in the electronic supplementary material. Identifiability tableaus for this analysis are presented in electronic supplementary material, figure S1 F–H; best fits are shown in electronic supplementary material, figure S2; model validation in electronic supplementary material, figure S3; with the parameter value estimates recorded in electronic supplementary material, tables S3 and S4.
2.3. Sensitivity analysis and energy constraints yield phenotypically diverse virtual GBM cell lines
Predicting optimal treatment protocols requires not only a validated mechanistic model but also a phenotypically diverse population of cells that is representative of different responses to treatment. We generate such a cohort of virtual cell lines by first identifying, by means of local and global sensitivity analyses, which features—or model parameters—are critical drivers of resistance to TMZ. Next, diverse cell phenotypes are generated by allowing these features to vary randomly, keeping in mind that all cellular processes require energy, only a limited amount of which is available to each cell.
Significant variance has been observed across cell populations in proliferation rates (or doubling times) [33] and repair enzyme expression levels [34]. Furthermore, repair enzyme turnover time scales may affect how fast DNA damage repair is completed. Therefore, in our sensitivity analysis, we focus on the rates of: cell proliferation (α); repair enzyme expression (SMGMT, SAPNG); and repair enzyme degradation (λAPNG, λMGMT).
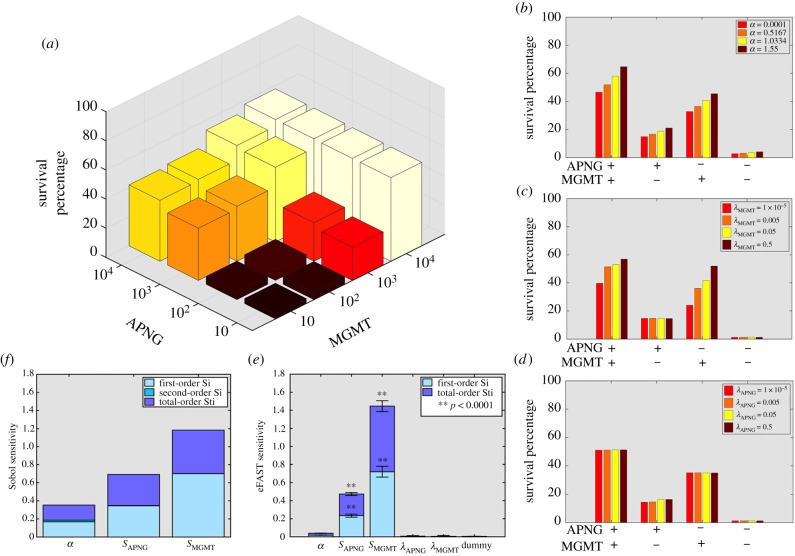
We first conducted a local sensitivity analysis, the results of which are presented in figure 2 (and in electronic supplementary material, figure S4). Following the experimental protocol in [35], cell growth inhibition assays were simulated, with 105 cells cultured in the presence of 500 μM TMZ for one week, and surviving cells counted at the end of the experiment. Figure 2a reveals that cell survival increases as repair enzyme expression rates are increased, this effect being more pronounced as MGMT expression is varied for a fixed level of APNG expression. Interestingly, cells expressing high levels of MGMT show similarly high survival rates even if APNG expression is suppressed. We next investigate the effect of varying the rates of cell proliferation and repair enzyme turnover on cell survival. For each parameter, four distinct cell phenotypes are considered, based on whether or not MGMT and APNG are expressed. From figure 2b, we observe that, in cells expressing at least one repair enzyme, increasing the rate of cell proliferation—or, equivalently, decreasing cell doubling time—is associated with improved cell survival, and hence greater resistance to TMZ. Further, as can be seen in figure 2c, whenever cells express MGMT, cell survival increases as the rate of MGMT turnover increases. On the other hand, figure 2d reveals that the rate of APNG turnover has little impact on cell survival. This is because we account for the experimentally observed [29] pro-survival effect of MGMT on TMZ-treated cells. In all cases, cells not expressing either repair enzyme are most sensitive to TMZ and their survival is minimally affected by varying any of the considered parameters.
Figure 2.
Local and global sensitivity analyses. (a) Three-dimensional histogram of survival percentage of cells following TMZ treatment in a cell growth inhibition assay as the expression levels of APNG (y-axis) and MGMT (x-axis) are varied. (b) Survival percentage of cells as proliferation rate is varied in cell lines with MGMT and/or APNG expression turned on/off. (c,d) Survival percentage of cells as MGMT turnover rate (c) or APNG turnover rate (d) is varied in cell lines with MGMT and/or APNG expression turned on/off. (e) eFAST sensitivity indices of various model parameters (α, proliferation rate; SAPNG and SMGMT, rates of APNG and MGMT expression, respectively; and λAPNG and λMGMT, rates of APNG and MGMT degradation, respectively). A dummy variable is used to perform a t-test. (f) Sobol sensitivity indices of the parameters α, SAPNG and SMGMT. See also electronic supplementary material, figure S4. (Online version in colour.)
Local sensitivity analysis ignores the effect of any correlation between various parameters, and, hence, the biological processes they represent. Therefore, we also performed a global sensitivity analysis with all parameters being varied simultaneously, using the eFAST and Sobol methods [36]. Figure 2e,f plots eFAST first- and total-order indices and Sobol’s first-order and total effect indices, respectively, for the various parameters considered. We see that resistance to TMZ is maximally affected by MGMT expression, followed by APNG expression and, to a lesser extent, cell proliferation rate. The rates of turnover of both enzymes have a minimal impact on cell survival, with their sensitivity indices indistinguishable from that of a dummy variable (figure 2e).
From the above analyses, we conclude that the rates of MGMT and APNG expression and cell proliferation may be significant determinants of tumour cell sensitivity to TMZ. Therefore, virtual cell lines with phenotypes capable of capturing diverse responses to TMZ are created by allowing SMGMT, SAPNG and α to vary randomly, keeping all other model parameters fixed. However, cellular activities such as proliferation and repairing DNA damage require energy, and tumour cells have to balance the allocation of limited resources towards such activities. Motivated by the approach of Nagy & Armbruster [37], we derive an energy constraint inequality to which all cells must adhere. This places bounds on how SMGMT, SAPNG and α may vary relative to each other.
Specifically, tumour cells perform the following activities: cell division; APNG and/or MGMT expression; and maintenance of normal physiological function (inclusive of all other cellular processes). Each activity requires the production of relevant cellular material, for which energy—provided by ATP—is consumed. Based on data relating to ATP reserves maintained in a cell, and typical rates of glycolysis and ATP hydrolysis, an upper bound for the energy available to a cell () is derived, resulting in the following energy constraint inequality. We define energy as the effort required to create 1 μM material in 1 min, where effort relates to the rate of ATP consumption (see electronic supplementary material for details):
| 2.5 |
Here ηP, ηA , ηM and ηβ are efforts related to proliferation, APNG production, and MGMT production and maintenance, respectively, and are assumed to be constant across all cell lines. SP (taken to be proportional to α) and Sβ are the production rates of cellular material needed for division and maintenance, respectively. Virtual cell lines are generated by allowing SP , SAPNG and SMGMT to vary, while ensuring the above inequality is satisfied. Thus, for instance, if a particular cell line over-expresses APNG, SAPNG would have a high value. This would result in a correspondingly high energy investment towards producing APNG, given by the product ηA SAPNG, and the above inequality would limit how fast the cell divides or how much MGMT it can produce by constraining the possible choices for SP and SMGMT. We remark that, in the absence of such a constraint, cells with the least doubling time and maximum repair enzyme expression would dominate. However, such cells would consume biologically unrealistic amounts of energy.
3. Results
3.1. MGMT and APNG expressions levels, rather than cell doubling time, are indicators of resistance to TMZ
Next, we simulate xenograft treatment assays to determine the relative contribution to TMZ resistance of the key features identified above. Briefly, 500 ‘virtual’ mice received intracranial implantations of 105 GBM cells. This initial tumour size reflects the experimental protocol followed in [38–42]. Each such implantation comprised equal numbers of 10 randomly chosen cell lines, thereby ensuring phenotypic heterogeneity in the resultant xenografts. At day 0, each animal was treated with a single dose of 500 μM TMZ, and tumour volume and composition were recorded one week later. Cell phenotypes were classified by the percentage of the tumour they occupied at the end of the experiment, with those cell lines assigned to quartile 1 that occupy between 0% and 25% of the tumour, those to quartile 2 that occupy between 25% and 50% of the tumour, and so on. Thus, cell lines belonging to quartile 4, for instance, dominate the tumour and represent phenotypes most resistant to TMZ.
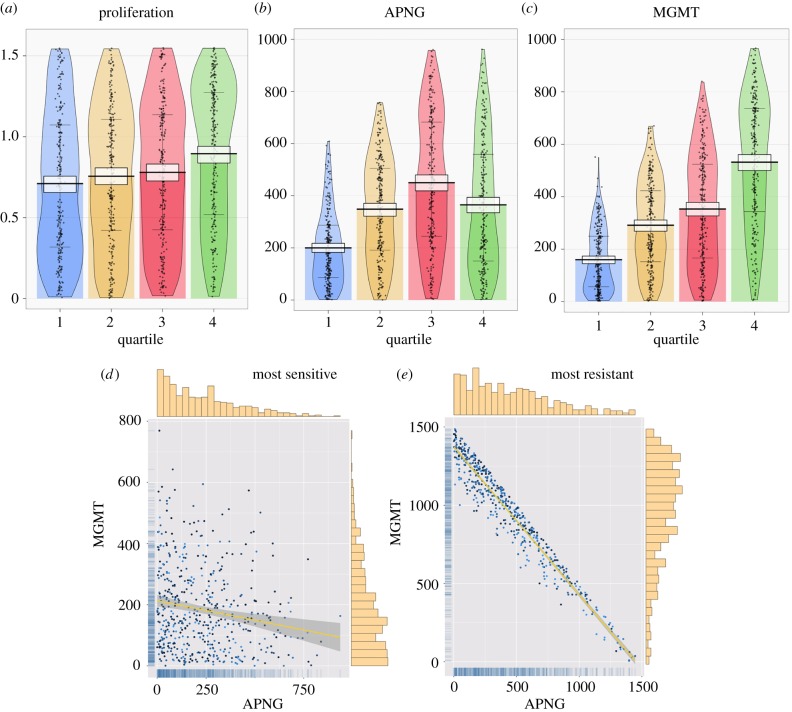
The resultant tumour composition profiles are shown in figure 3a–c as RDI (raw data, descriptive statistics and inferential statistics) plots. Figure 3a reveals that faster proliferating cells are more resistant to TMZ, with the smoothed density of cells in quartile 4 showing a top-heavy distribution. However, the differences in the central tendencies of cell proliferation rates across the various quartiles are minimal. Indeed, for cell lines occupying quartiles 1–3, these differences are not statistically significant. Therefore, we conclude that cell doubling time alone cannot guarantee resistance to TMZ.
Figure 3.
Heteroclonal tumour xenograft response to a single dose of TMZ. (a–c), RDI (raw data, descriptive statistics and inferential statistics) plots showing raw data points (black dots), central tendencies (vertical bar), smoothed densities (irregular coloured regions) and Bayesian highest density intervals (HDIs; white rectangles). Tumour cell lines are divided into quartiles according to the percentage of the tumour they occupy at the end of the experiment: quartile 1, 0–25%; quartile 2, 25–50%; quartile 3, 50–75%; and quartile 4, 75–100%. Cell lines in quartile 1 are most sensitive to TMZ, while those in quartile 4 are most resistant. Three cellular phenotypes are considered: (a) rate of cell proliferation, (b) APNG expression and (c) MGMT expression. (d,e) Scatter plots of APNG expression versus MGMT expression in the most TMZ-sensitive cells (d) and most TMZ-resistant cells (e). The colour of the data dots is a gradient based on proliferation rate with darker dots representing cells with smaller doubling time. Linear correlation is shown as yellow lines, and corresponding 95% confidence intervals are shown as shaded areas. Distributions of enzyme expression are shown as marginal histograms (top, APNG; right, MGMT) and marginal rugs (left, MGMT; bottom, APNG). (Online version in colour.)
The expression levels of repair enzymes reveal more differentiating attributes (figure 3b,c). As expected, cell lines most sensitive to TMZ (quartile 1) express the least amounts of both APNG and MGMT, with clear bottom-heavy distributions and narrow 95% highest density intervals (HDIs) around the central tendency. Interestingly, figure 3b reveals that, although the central tendency of APNG expression increases from quartile 1 to 3, the most resistant cell lines (quartile 4) show a significant decrease in the most credible values of APNG expression. The reason for such an unexpected decline becomes clear when we look at the expression of MGMT in figure 3c. We observe that resistance to TMZ increases with MGMT expression monotonically. Furthermore, the smoothed density of cells in quartile 4 is top heavy, indicating that the most resistant cell lines invest maximally in MGMT expression. This comes at the cost of APNG expression because of the imposed energy constraint. Thus, we conclude that MGMT expression and, perhaps to a lesser extent, APNG expression are reliable predictors of TMZ resistance.
Further verification of our conclusion comes from looking at scatter plots of APNG versus MGMT expression in the most sensitive (quartile 1) and most resistant (quartile 4) cell lines, shown in figure 3d,e, respectively. Linear regressions, shown in yellow, reveal that rates of APNG and MGMT expression correlate poorly in TMZ-sensitive cell lines (adjusted R2: 0.03), while resistant cell lines maximize their efforts towards repairing cell damage. In these cells, a clear linear trend in APNG versus MGMT expression is observed (adjusted R2: 0.94), with high values of MGMT expression favoured. Dots represent individual cell lines, with darker colours corresponding to cells with higher proliferation rates, and vice versa. Neither plot shows a discernible pattern in the colour distribution of dots, indicating a lack of correlation between resistance to TMZ and cell proliferation rate.
3.2. Optimizing TMZ administration with APNG and MGMT inhibition
We now predict optimal drug dosing and scheduling protocols when small molecule inhibitors of APNG and MGMT are co-administered with TMZ. Briefly, 500 phenotypically diverse GBM cell lines were generated and cell growth inhibition assays initiated as described previously. A single cycle of ‘standard TMZ’ treatment was simulated, with drug administration possible on days 1–5, followed by a rest period from day 6 to day 28. This is consistent with typical clinical protocols for TMZ administration [43]. A genetic algorithm [44] was employed to arrive at optimal treatment strategies specific to individual cell lines, with variability allowed in the daily TMZ dosage, and a decision made whether or not to co-administer APNG and/or MGMT inhibitors. Genetic algorithms mimic the principles of genetic evolution to find the solution to an optimization problem—in our case, tumour response to treatment—with tumour burden taken as the fitness function we minimize. Our choice of fitness function follows current guidelines for treating patients with GBM. These seek to reduce tumour burden, which is directly related to increased intracranial pressure, responsible for most GBM symptoms and patient death [22,43,45]. The genetic algorithm was implemented as follows. An initial pool of treatment strategies was created randomly. These strategies were then ranked based on their reduction of tumour burden at the end of the treatment cycle. The top 20% of strategies were paired to create offspring. As in evolution, the offspring strategies inherit different properties from each of their parents. The resulting offspring were also allowed a small chance of mutation, thus creating a new generation of treatment protocols that were sorted once again based on fitness, and the process repeated until convergence. Further details of this algorithm are provided in the electronic supplementary material.
An analysis of the optimal protocols thus obtained reveals a natural grouping which is based on tumour cell phenotype. Specifically, we can classify the cell lines into four cohorts distinguished by the expression of each enzyme. For instance, cells expressing high levels of both repair enzymes are assigned to the ‘APNG+/MGMT+’ cohort, and so on. The ‘optimal’ protocol for each cohort is then obtained by averaging the TMZ dosage across cell lines in that cohort and finding the mode of the decision whether or not to inhibit APNG and/or MGMT. The resultant protocols are summarized in table 1.
Table 1.
Description of treatment strategies. Standard and optimal treatment strategies identified by GBM cell phenotype. Note that the specific daily dose of TMZ varies between strategies. Here, SMI stands for small molecule inhibitor. Standard TMZ refers to a TMZ protocol as it is typically administered in the clinic [43]. Optimal TMZ refers to an alternating high/low dosage schedule with no APNG/MGMT inhibitors administered.
| treatment strategy | TMZ | SMI APNG | SMI MGMT | ||
|---|---|---|---|---|---|
| standard TMZ | constant dose days 1–5 | none | none | ||
| rest days 6–28 | |||||
| optimal TMZ | high dose days 1, 3 and 5 | none | none | ||
| low dose days 2 and 4 | |||||
| rest days 6–28 | |||||
| cell phenotype | APNG−/MGMT− | A | high dose days 1, 3 and 5 | days 1–5 | days 1 and 5 |
| low dose days 2 and 4 | |||||
| rest days 6–28 | |||||
| APNG+/MGMT− | B | high dose days 1, 3 and 5 | days 1–5 | days 2, 3 and 4 | |
| low dose days 2 and 4 | |||||
| rest days 6–28 | |||||
| APNG−/MGMT+ | C | high dose days 1, 3 and 5 | days 1–5 | days 1, 2, 3 and 4 | |
| low dose days 2 and 4 | |||||
| rest days 6–28 | |||||
| APNG+/MGMT+ | D | high dose days 1, 3 and 5 | days 1–5 | days 1–5 | |
| low dose days 2 and 4 | |||||
| rest days 6–28 |
In all cases, alternating the daily dose of TMZ, with a high dose followed by a low dose, is predicted to be optimal (see insets in figure 4b,c). This is in contrast with typical clinical practice, where a fixed dose is administered daily, referred to here as standard TMZ. Indeed, the experiments of Wick et al. [46] provide indirect validation for this prediction, where it was observed that cells over-expressing MGMT were more susceptible to an alternating TMZ dose.
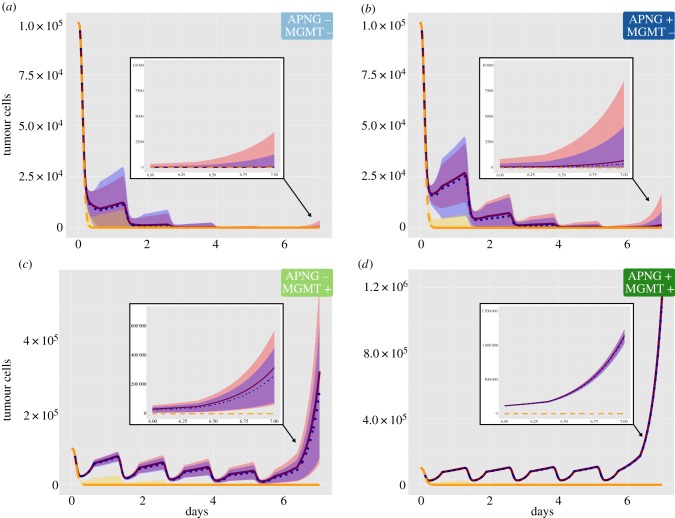
Figure 4.
Cell growth inhibition in response to various treatment strategies. Mean values and 95% confidence intervals of surviving cell numbers when cell growth inhibition assays are simulated with: standard TMZ (solid pink line, pink shaded area); optimal TMZ (dotted blue line, blue shaded area) and optimal combination treatment (dashed yellow line, yellow shaded area). (a) Cells expressing low levels of both repair enzymes (APNG−/MGMT−, 〈α〉 = 0.86, 〈SAPNG〉 = 51.96, 〈SMGMT〉 = 52.85). (b) Cells expressing high levels of APNG and low levels of MGMT (APNG+/MGMT−, 〈α〉 = 0.75, 〈SAPNG〉 = 977.42, 〈SMGMT〉 = 49.08). (c) Cells expressing low levels of APNG and high levels of MGMT (APNG−/MGMT+, 〈α〉 = 0.74, 〈SAPNG〉 = 48.93, 〈SMGMT〉 = 975.70). (d) Cells expressing high levels of both repair enzymes (APNG+/MGMT+, 〈α〉 = 0.66, 〈SAPNG〉 = 658.54, 〈SMGMT〉 = 655.32). Here, 〈 · 〉 denotes the average value of that parameter across all cell lines within the respective cohort. (Online version in colour.)
Interestingly, optimal protocols in every case require APNG inhibition to be applied on all treatment days. This is surprising since N7-meG and N3-meA typically contribute minimally to the cytotoxicity of TMZ [9]. However, our model accounts for the fact that high levels of N7-meG adducts trigger apoptosis [29]. Furthermore, APNG is predicted to be highly expressed in cell lines with a greater than average degree of TMZ resistance (figure 3b, quartile 3). Together, these features explain the predicted optimal schedule of APNG inhibitors. In fact, it is the scheduling of MGMT inhibitors that distinguishes the four cohorts, with cell lines in the APNG+/MGMT+ cohort—and, hence, most resistant to TMZ—requiring MGMT inhibition on all treatment days. It is worth noting that the optimal scheduling of MGMT inhibition is not dictated exclusively by its expression (compare rows 3 and 4 or 5 and 6 in table 1), but seems to also depend on APNG expression. This highlights a key advantage of our approach, namely that the mechanistic model of subcellular response to TMZ that underpins our simulations captures unexpected and potentially nonlinear interdependencies between the two DNA damage-repair pathways.
A comparison of how cell lines in each cohort respond to standard and optimal TMZ monotherapy, and optimal combination therapy, is shown in figure 4. For each cohort and for each treatment strategy, surviving tumour cell number together with 95% confidence intervals are plotted versus time. In each panel, the response under standard TMZ treatment is shown in red (solid line), under optimal TMZ without inhibitors in blue (dotted line), and strategies A, B, C, or D are shown in yellow in the corresponding panel (dashed line). In general, optimal TMZ performs marginally better than standard TMZ. Maximum cell growth inhibition, driven by a high degree of apoptosis early on, is achieved when TMZ is combined with APNG and MGMT inhibitors. Cell survival 7 days after treatment initiation is comparable across strategies when cell lines express low levels of MGMT (figure 4a,b). However, combination therapy is most effective when treating cells expressing high levels of MGMT (figure 4c,d).
3.3. Treatment strategy D outperforms all others in a virtual pre-clinical trial
When treating GBM in situ, the expression levels of MGMT and APNG may not be known a priori. Indeed, these may vary significantly even within a tumour. Therefore, we identify the protocol with maximum inhibitory potential when treating tumours comprising a heterogeneous population of cells. For this, a pre-clinical trial was conducted in silico as follows. Five hundred virtual mice, with heteroclonal tumour xenografts established as described previously, were randomly assigned to one of the optimal treatment strategies (A, B, C or D listed in table 1). Each mouse received a single cycle of treatment, and tumour volumes were recorded periodically. The resulting averages of the fold change in tumour volume relative to pre-treatment, together with 95% confidence intervals, are shown as a waterfall plot in figure 5.
Figure 5.
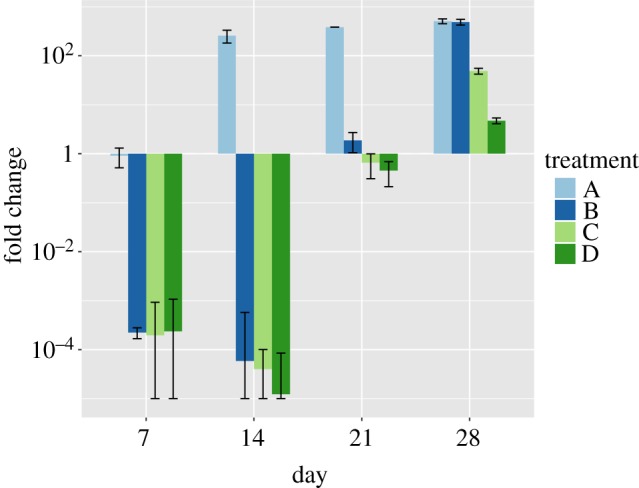
Heteroclonal xenograft response to optimal treatment protocols identified in table 1. Mice with heteroclonal tumour xenografts were randomly assigned to one of the strategies A, B, C or D. The mean values of fold change in tumour volume relative to pre-treatment are shown at days 7, 14, 21 and 28 after treatment initiation. Error bars represent 95% confidence intervals. (Online version in colour.)
Even at week 1, strategy A, optimal for APNG−/MGMT− cells, has failed to induce a significant reduction in tumour volume. By contrast, strategies B, C and D, optimal for APNG+/MGMT−, APNG−/MGMT+ and APNG+/MGMT+ cells, respectively, show comparable inhibition of tumour volumes at week 1. Remarkably, tumour volumes continue to shrink under these protocols even after treatment has ceased, with the maximum reduction in volume predicted for strategy D. This behaviour can be understood in terms of the long half-life of extracellular TMZ (48 h, estimated from [47]) and the time spent by DNA-damaged cells in an arrested state and consequent delay in their apoptosis. However, by the end of the treatment cycle, xenografts treated with strategy B have recovered to attain their maximum possible volumes. In the long term, only strategies C and D are predicted to have a significant impact on tumour growth, with strategy D outperforming all others by week 4. Thus, in the absence of any information regarding repair enzyme expression by tumour cells, we propose strategy D as the optimal protocol when treating heteroclonal tumours.
3.4. In a virtual human clinical trial, strategy D predicts a 30% improvement in patient survival
Finally, we predict the potential survival benefit of treating human GBM patients with the identified optimal combination of TMZ and APNG/MGMT inhibitors (strategy D) by conducting a clinical trial in silico. Briefly, 100 virtual patients with GBM were created, with each cancer comprising a heterogeneous population of cells. We remark that in this case the range over which proliferation rates vary was adjusted so that the mean doubling time was 49.6 days [48]. Each patient was treated with standard TMZ, optimal TMZ and combination strategy D. We remark that, were this an actual clinical trial, the patients would be randomized into one of the three treatment protocols and their progression monitored. However, the in silico experimentation proposed here has the advantage that a virtual patient may be treated with as many strategies as desired. Thus, response to treatment may be compared without having to account for the confounding factor of inter-patient variability. Treatment was initiated once tumours reached a size of 1 cm in diameter (corresponding to approx. ), the average size of GBM at diagnosis in a clinical setting [49], and continued for a maximum of seven 28-day cycles. This follows typical clinical protocol wherein TMZ is administered once (concomitant with focal radiotherapy, not considered here) followed by six cycles of TMZ alone [50]. Simulations were carried out until patient ‘death’, which was assumed to occur once tumours reached a critical size. This size was determined by the observation that, left untreated, patients with GBM die within three to four months [51]. Details of modifications to the model necessary to simulate GBMs growing in situ are presented in the electronic supplementary material.
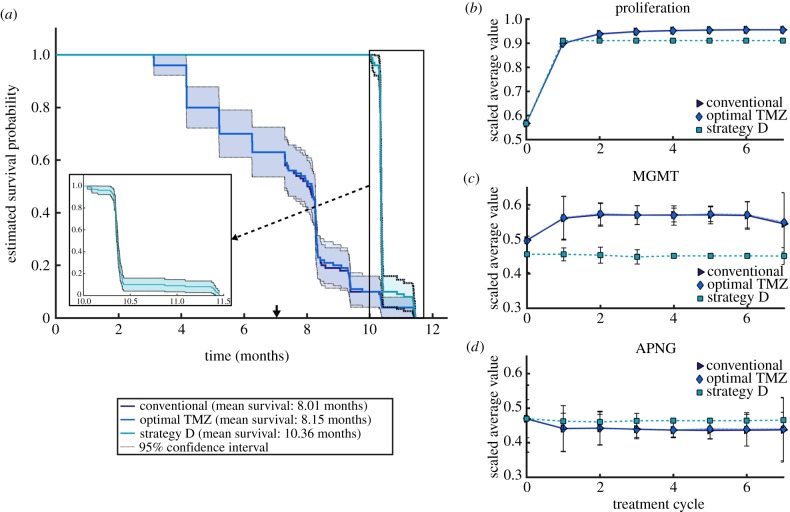
A Kaplan–Meier survival analysis was conducted on our simulations, the results of which are summarized in figure 6. Under standard and optimal TMZ monotherapy, patient survival is predicted to decrease similarly (figure 6a) with comparable mean survival times of about eight months. In particular, optimal TMZ improves mean survival by only one week as compared with standard TMZ. In contrast, under treatment with strategy D, all patients are expected to survive—barring adverse events not considered in our model—for up to three months after cessation of treatment. The mean survival under strategy D is over 10 months, an approximate 30% improvement over treatment with TMZ alone. A steep decline in survival is predicted thereafter (figure 6a, inset), owing to the rapid growth of tumour cells left unaffected by therapy administration.
Figure 6.
Results of an in silico human trial. (a) Kaplan–Meier survival probabilities when patients were treated with standard TMZ, optimal TMZ, and combination strategy D for seven 28-day treatment cycles. (b–d) Proliferation rates, and APNG and MGMT expression averaged over each tumour over the course of seven treatment cycles. Data points represent the mean across the 100 patients, with 95% confidence intervals. (Online version in colour.)
With the development of targeted therapeutics such as those considered here, an important question is: how do tumours evolve under the selection pressures created by the treatment? To answer this, we recorded the composition of each patient’s tumour periodically, under each treatment strategy. Given the diversity in cell lines across all patients, the data were first quantile-normalized to make the distributions identical in statistical properties [52], and the resulting parameter distributions scaled by their respective maxima. The results are shown in figure 6b–d, which plot time courses of the mean values and 95% confidence intervals of the scaled average tumour compositions.
Standard and optimal TMZ monotherapy exert similar selection pressures, resulting in tumours that have an overall bias towards cell lines with faster doubling times and high MGMT expression. Cellular investment in MGMT expression is compensated for by expressing lesser APNG, as observed in the case of heteroclonal xenograft response to TMZ (figure 3a–c). In particular, TMZ administration induces death in slow proliferating cells with low repair enzyme expression, leaving an abundance of space and resources for those cells over-expressing MGMT. Subsequent cycles of TMZ become less effective since they are targeting a population of cells capable of efficient DNA damage repair, underscoring the role played by MGMT in mediating TMZ resistance. The emergent tumour phenotype is markedly different under strategy D, which favours cell lines that invest less in proliferation, less in MGMT expression, and more in APNG expression. Furthermore, the emergent phenotype has very narrow 95% confidence intervals, indicating that tumours evolve to a more homogeneous phenotype under strategy D. This could explain the steep decline in patient survival after therapy cessation (figure 6a, inset).
4. Discussion
GBM lethality is driven, in part, by resistance to TMZ, the most commonly used chemotherapeutic drug for treating this disease [5]. TMZ-induced DNA damage is efficiently repaired by the APNG-mediated BER repair pathway or by the MGMT pathway. Therefore, it has been hypothesized that combining TMZ with novel small molecule inhibitors of APNG and/or MGMT may improve patient survival. Indeed, several such drugs are in various stages of clinical trials [8,18]. The mechanistic models studied here provide counterintuitive results, which could be crucial in the success or failure of TMZ-adjuvant clinical trials. First, in an emergent result, optimal scheduling across tumour phenotypes includes continuous inhibition of APNG-mediated DNA repair, even though MGMT repairs damage which is more lethal to the cancer cell. Second, only in MGMT over-expressing cancers is continuous MGMT inhibition optimal, whereas, even in tumours with low APNG expression, APNG inhibition is vital to cell-killing potential.
The mechanistic modelling approach employed here is central to the predictive power of the models. Crucially, this model includes TMZ-induced DNA methylation and its subsequent repair. Our model was extensively parametrized and validated with available experimental data, resulting in a computational framework ideally suited to testing the anti-cancer potential of various drug combinations. GBM cells are known to vary highly at a molecular level, even within the same tumour [53]. Therefore, we needed to generate a phenotypically diverse population of GBM cell lines that is representative of different potential responses to treatment. For this, sensitivity analyses (summarized in figure 2) were performed on model parameters, revealing rates of cellular proliferation and APNG and MGMT expression as critical drivers of TMZ resistance. Pre-existing variation in the simulated cell populations was subject to constraints reflecting limited cellular capacity to both replicate rapidly and repair DNA damage with high fidelity. We remark that we do not account for mutations in cellular DNA that would result in a cell switching phenotypes in terms of repair enzyme expression. In particular, resistant phenotypes that emerge under selection pressures created by treatment were already present at the time of treatment initiation in our framework. Over the course of these simulated experiments, selection on replicative potential and DNA-repair capacity of cell lines affected the proportions of different clones. Simulations of the response to treatment of polyclonal xenografts further elucidated determinants of resistance to TMZ (figure 3). In these single-dose studies, repair enzyme expression is found to affect cell survival more strongly than doubling time, consistent with the literature [11,13].
Next, cell growth inhibition assays were simulated and a genetic algorithm employed to arrive at optimal dosing and scheduling protocols for each virtual cell line when TMZ was administered together with APNG and MGMT inhibitors. The protocols thus obtained revealed a natural grouping based on cell phenotype, as determined by expression levels of APNG and MGMT. In all cases, alternating the dosing of TMZ, with high doses administered on days 1, 3 and 5 and low doses on days 2 and 4, was predicted to be optimal. Indeed, there is experimental evidence that cells over-expressing MGMT are more susceptible to such a schedule, which may have the additional advantage of reducing dose-limiting haematological toxicity [46]. Against our expectations, APNG inhibition was necessary for maximizing cell kill even in experiments lacking high-APNG-expressing subclones.
Finally, in a virtual pre-clinical trial, strategies optimal for cell lines over-expressing MGMT were predicted to be most successful in treating heteroclonal tumour xenografts when the overall phenotype of the cancer is unknown. We identified the strategy that was optimal for cells over-expressing both MGMT and APNG (strategy D) as the protocol with maximum tumour inhibition potential. A virtual clinical trial was then conducted wherein patients were enrolled in three treatment arms: standard TMZ monotherapy; alternating TMZ monotherapy; and strategy D combination therapy. Kaplan–Meier estimates showed that patients treated with strategy D continue to survive up to three months after treatment has ceased, with an overall improvement of 30% in mean survival time compared with those treated with TMZ alone. Both TMZ monotherapies had similar predicted survival curves, and, therefore, the benefit of alternating TMZ may simply be mitigating side-effects [46]. We note that our model ignores adverse events in patients which may impact predicted survival times. Furthermore, we infer cancer dynamics in human patients from a model calibrated to xenograft data. Byrne et al. [54] noted limitations in this approach. However, owing to ethical constraints, these experiments cannot be carried out in humans. Where parameter values are known in a human context (for example, cancer cell doubling times), human parameter estimates were used.
4.1. Future experiments in cell-based and animal systems
The mathematical model presented here makes testable predictions on the effect of drug timing in cell lines and in animal models. In these experimental systems, biotechnology techniques such as RNAi [55] can be used to inhibit DNA repair enzymes even when drugs are not available; and pre-clinical drugs can be delivered directly to cells even when highly toxic or lacking in bioavailability [56]. Using such approaches, predicted optimal drug combinations and schedules may be tested in vitro by replicating some of the virtual cell growth inhibition assays simulated here. Following the protocol in [35], cell cultures of human GBM cell lines could be established with a range of APNG or MGMT expression. These cell lines could be treated using different schedules of APNG inhibition, MGMT inhibition and TMZ, corresponding to one of the four optimal strategies described above, with cell number tracked over the course of weeks. These experiments could confirm the in silico predictions made here, which would support the biological models of DNA repair and chemotherapy that these models encode. Furthermore, experimental data thus generated would enable fine tuning of mechanistic model parameters and relaxing of modelling assumptions. Using high-dimensional technologies such as Luminex-bead expression assays [57], additional drug targets and potential adjuvant drugs could be identified. While such high-dimensional (omics) experiments are outside the mechanistic scope of the models presented here, an ‘omics’ approach to studying relative drug timings, such as DIGRE [58,59], becomes far more practical when a finite combination of administrations and timings can be proposed.
Independent of our model predictions, animal models should be used to assess for synergistic haematological toxicity [46]. The mechanistic models reported here do not address the ratio of GBM killing to haematological toxicity of TMZ plus adjuvant therapy. Experimental results would be of tremendous utility in developing mechanistic models that might identify or predict such adverse synergistic effects.
4.2. Potential for clinical translation
This model also makes testable predictions that could inform either clinical trials or expanded access (formerly known as ‘compassionate use’) of TMZ with adjuvants. There are several clinical trials and studies incorporating inhibition of MGMT, but fewer clinical trials inhibiting APNG [60,61]. While MGMT plays a bigger role than APNG in chemotherapy resistance, we found that, in all cases, optimal treatment always includes APNG inhibitors. At the time of writing, we are unable to find trials that combine both inhibitors with TMZ.
When such trials are conducted, the timing and administration of novel drugs could mark the difference between a successful or failed trial or expanded access intervention. Randomized protocols for TMZ and bortezomib are actively recruiting patients (NIH clinical trial number NCT03643549). This trial incorporates an experimentally motivated schedule, but experimental approaches cannot examine all possible schedules as is done here. Therefore, these trials are not using the optimal schedule identified here. Expanded access would be justified by the lack of long-term effective treatments for this lethal disease [62], and could employ our optimal schedule. Even so, one limitation of our modelling approach should be emphasized: our model incorporates synergistic effectiveness between TMZ and MGMT/APNG inhibition, but does not include synergistic toxicity. Furthermore, our model assumes that pharmacological inhibition of MGMT or APNG is total, while compounds like the MGMT inhibitor bortezomib only achieve partial inhibition in a clinical setting [63].
4.3. Conclusion
In both experimental and clinical contexts, the development and testing of novel treatment strategies in cancer are associated with a steep financial cost and significant human resource investment. Educated predictions about drug doses and schedules most likely to succeed are therefore critical for optimizing experimental design and resource investments on the part of the clinical investigator. In silico experimentation, such as that proposed here, affords the unique opportunity of simulating various treatment protocols over a wide range of parameter values that is not possible in a clinical setting. In our model of TMZ-treated GBM, these simulations give an emergent result which supports both a novel combination therapy targeting APNG and an intermittent schedule of TMZ.
Supplementary Material
Acknowledgements
We are grateful to Prof. Giray Okten, Mr Nicholas Yaeger and Ms. Mayassa Dargham for many helpful discussions.
Data accessibility
Datasets and code are publicly available at https://github.com/hjain-fsu/hjain-fsu. Code to perform identifiability analysis with GENSSI 2.0 was taken from https://github.com/genssi-developer/GenSSI. Code to use eFAST was taken from http://malthus.micro.med.umich.edu/lab/usadata/.
Authors' contributions
I.C.S. and H.V.J. conceived the experiments; I.C.S. conducted the experiments; I.C.S., S.K.H. and H.V.J. analysed the results. All authors reviewed the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Simons Collaboration Grant for Mathematicians 280544 to H.V.J.
References
- 1.Preusser M, De Ribaupierre S, Wöhrer A, Erridge SC, Hegi M, Weller M, Stupp R. 2011. Current concepts and management of glioblastoma. Ann. Neurol. 70, 9–21. ( 10.1002/ana.22425) [DOI] [PubMed] [Google Scholar]
- 2.Louis DN. et al. 2016. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131, 803–820. ( 10.1007/s00401-016-1545-1) [DOI] [PubMed] [Google Scholar]
- 3.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. 2014. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 64, 83–103. ( 10.3322/caac.21219) [DOI] [PubMed] [Google Scholar]
- 4.Bleeker FE, Molenaar RJ, Leenstra S. 2012. Recent advances in the molecular understanding of glioblastoma. J. Neurooncol. 108, 11–27. ( 10.1007/s11060-011-0793-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newlands E, Stevens M, Wedge S, Wheelhouse R, Brock C. 1997. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat. Rev. 23, 35–61. ( 10.1016/S0305-7372(97)90019-0) [DOI] [PubMed] [Google Scholar]
- 6.Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. 2017. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 19, v1–v88. ( 10.1093/neuonc/nox158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SY. 2016. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 3, 198–210. ( 10.1016/j.gendis.2016.04.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan CH, Liu WL, Cao H, Wen C, Chen L, Jiang G. 2013. O6-methylguanine DNA methyltransferase as a promising target for the treatment of temozolomide-resistant gliomas. Cell Death Dis. 4, e876 ( 10.1038/cddis.2013.388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakada M, Furuta T, Hayashi Y, Minamoto T, Hamada J-i. 2012. The strategy for enhancing temozolomide against malignant glioma. Front. Oncol. 2, 98 ( 10.3389/fonc.2012.00098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haskell CM, Berek JS, Haskell CM. 1985. Cancer treatment. New York, NY: Saunders. [Google Scholar]
- 11.Happold C, Stojcheva N, Silginer M, Weiss T, Roth P, Reifenberger G, Weller M. 2018. Transcriptional control of O6-methylguanine DNA methyltransferase expression and temozolomide resistance in glioblastoma. J. Neurochem. 144, 780–790. ( 10.1111/jnc.14326) [DOI] [PubMed] [Google Scholar]
- 12.Kitange GJ, Carlson BL, Schroeder MA, Grogan PT, Lamont JD, Decker PA, Wu W, James CD, Sarkaria JN. 2009. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro Oncol. 11, 281–291. ( 10.1215/15228517-2008-090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Tu Y, Yuan J, Mao X, He S, Wang L, Fu G, Zong J, Zhang Y. 2012. Aberrant expression of n-methylpurine-DNA glycosylase influences patient survival in malignant gliomas. BioMed. Res. Int. 2012, 760679 ( 10.1155/2012/760679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hegi ME. et al. 2005. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 352, 997–1003. ( 10.1056/NEJMoa043331) [DOI] [PubMed] [Google Scholar]
- 15.Fosmark S, Hellwege S, Dahlrot RH, Jensen KL, Derand H, Lohse J, Sørensen MD, Hansen S, Kristensen BW. 2017. APNG as a prognostic marker in patients with glioblastoma. PLoS ONE 12, e0178693 ( 10.1371/journal.pone.0178693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahlrot RH. et al. 2014. Novel approaches for quantifying protein biomarkers in gliomas: benefits and pitfalls. CNS Oncol. 3, 287–298. ( 10.2217/cns.14.30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su Y-T. et al. 2018. Novel targeting of transcription and metabolism in glioblastoma. Clin. Cancer Res. 24, 1124–1137. ( 10.1158/1078-0432.CCR-17-2032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivasan A, Gold B. 2012. Small-molecule inhibitors of DNA damage-repair pathways: an approach to overcome tumor resistance to alkylating anticancer drugs. Future Med. Chem. 4, 1093–1111. ( 10.4155/fmc.12.58) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frieboes HB, Lowengrub JS, Wise S, Zheng X, Macklin P, Bearer EL, Cristini V. 2007. Computer simulation of glioma growth and morphology. Neuroimage 37, S59–S70. ( 10.1016/j.neuroimage.2007.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swanson KR, Bridge C, Murray J, Alvord EC. 2003. Virtual and real brain tumors: using mathematical modeling to quantify glioma growth and invasion. J. Neurol. Sci. 216, 1–10. ( 10.1016/j.jns.2003.06.001) [DOI] [PubMed] [Google Scholar]
- 21.Tracqui P, Cruywagen GC, Woodward DE, Bartoo GT, Murray JD, Alvord EC. 1995. A mathematical model of glioma growth: the effect of chemotherapy on spatio-temporal growth. Cell Prolif. 28, 17–31. ( 10.1111/j.1365-2184.1995.tb00036.x) [DOI] [PubMed] [Google Scholar]
- 22.Sorribes IC, Moore MN, Byrne HM, Jain HV. 2019. A biomechanical model of tumor-induced intracranial pressure and edema in brain tissue. Biophys. J. 116, 1560–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alfonso JCL, Talkenberger K, Seifert M, Klink B, Hawkins-Daarud A, Swanson KR, Hatzikirou H, Deutsch A. 2017. The biology and mathematical modelling of glioma invasion: a review. J. R. Soc. Interface 14, 20170490 ( 10.1098/rsif.2017.0490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Lin Y, Gerson SL. 2012. MGMT: a critical DNA repair gene target for chemotherapy resistance and for stem cell protection. In DNA repair in cancer therapy (ed. Kelley MR.), pp. 17–28. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 25.Filippi-Chiela EC, Thomé MP, Pelegrini AL, Ledur PF, Garicochea B, Zamin LL, Lenz G. 2013. Resveratrol abrogates the temozolomide-induced G2 arrest leading to mitotic catastrophe and reinforces the temozolomide-induced senescence in glioma cells. BMC Cancer 13, 147 ( 10.1186/1471-2407-13-147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson JR, Gilmartin A, Imburgia C, Winkler JD, Marshall LA, Roshak A. 2000. An indolocarbazole inhibitor of human checkpoint kinase (Chk1) abrogates cell cycle arrest caused by DNA damage. Cancer Res. 60, 566–572. [PubMed] [Google Scholar]
- 27.Hirose Y, Berger MS, Pieper RO. 2001. Abrogation of the Chk1-mediated G2 checkpoint pathway potentiates temozolomide-induced toxicity in a p53-independent manner in human glioblastoma cells. Cancer Res. 61, 5843–5849. [PubMed] [Google Scholar]
- 28.Sausville EA. et al. 2001. Phase I trial of 72-hour continuous infusion UCN-01 in patients with refractory neoplasms. J. Clin. Oncol. 19, 2319–2333. ( 10.1200/JCO.2001.19.8.2319) [DOI] [PubMed] [Google Scholar]
- 29.Roos WP, Kaina B. 2006. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 12, 440–450. ( 10.1016/j.molmed.2006.07.007) [DOI] [PubMed] [Google Scholar]
- 30.Eisenberg MC, Jain HV. 2017. A confidence building exercise in data and identifiability: modeling cancer chemotherapy as a case study. J. Theor. Biol. 431, 63–78. ( 10.1016/j.jtbi.2017.07.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lecourtier Y, Walter E, Bertrand P. 1982. Identifiability testing for state-space models. IFAC Proc. Volumes 15, 887–892. ( 10.1016/S1474-6670(17)63106-9) [DOI] [Google Scholar]
- 32.Ligon TS, Fröhlich F, Chis OT, Banga JR, Balsa-Canto E, Hasenauer J. 2017. GenSSI 2.0: multi-experiment structural identifiability analysis of SBML models. Bioinformatics 34, 1421–1423. ( 10.1093/bioinformatics/btx735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehrara E, Forssell-Aronsson E, Ahlman H, Bernhardt P. 2007. Specific growth rate versus doubling time for quantitative characterization of tumor growth rate. Cancer Res. 67, 3970–3975. ( 10.1158/0008-5472.CAN-06-3822) [DOI] [PubMed] [Google Scholar]
- 34.Christmann M, Verbeek B, Roos WP, Kaina B. 2011. O6-Methylguanine-DNA methyltransferase (MGMT) in normal tissues and tumors: enzyme activity, promoter methylation and immunohistochemistry. Biochim. Biophys. Acta, Rev. Cancer 1816, 179–190. ( 10.1016/j.bbcan.2011.06.002) [DOI] [PubMed] [Google Scholar]
- 35.Agnihotri S. et al. 2012. Alkylpurine–DNA–N-glycosylase confers resistance to temozolomide in xenograft models of glioblastoma multiforme and is associated with poor survival in patients. J. Clin. Invest. 122, 253–266. ( 10.1172/JCI59334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marino S, Hogue IB, Ray CJ, Kirschner DE. 2008. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol. 254, 178–196. ( 10.1016/j.jtbi.2008.04.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagy JD, Armbruster D. 2012. Evolution of uncontrolled proliferation and the angiogenic switch in cancer. Math. Biosci. Eng. 9, 843–876. ( 10.3934/mbe.2012.9.843) [DOI] [PubMed] [Google Scholar]
- 38.Pan Y, Jing R, Pitre A, Williams BJ, Skalli O. 2008. Intermediate filament protein synemin contributes to the migratory properties of astrocytoma cells by influencing the dynamics of the actin cytoskeleton. FASEB J. 22, 3196–3206. ( 10.1096/fj.08-106187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jing R, Pizzolato G, Robson RM, Gabbiani G, Skalli O. 2005. Intermediate filament protein synemin is present in human reactive and malignant astrocytes and associates with ruffled membranes in astrocytoma cells. Glia 50, 107–120. ( 10.1002/glia.20158) [DOI] [PubMed] [Google Scholar]
- 40.Guo M, Roman RJ, Falck JR, Edwards PA, Scicli AG. 2005. Human U251 glioma cell proliferation is suppressed by HET0016 [N-hydroxy-N′-(4-butyl-2-methylphenyl) formamidine], a selective inhibitor of CYP4A. J. Pharmacol. Exp. Ther. 315, 526–533. ( 10.1124/jpet.105.088567) [DOI] [PubMed] [Google Scholar]
- 41.dos Reis Vasques L, Pujiz RS, Strauss BE, Krieger JE. 2010. Knockdown of E2F1 by RNA interference impairs proliferation of rat cells in vitro. Genet. Mol. Biol. 33, 17–22. ( 10.1590/S1415-47572009005000104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunapuli P, Chitta KS, Cowell JK. 2003. Suppression of the cell proliferation and invasion phenotypes in glioma cells by the LGI1 gene. Oncogene 22, 3985 ( 10.1038/sj.onc.1206584) [DOI] [PubMed] [Google Scholar]
- 43.Fernandes C, Costa A, Osório L, Lago RC, Linhares P, Carvalho B, Caeiro C. 2017. Current standards of care in glioblastoma therapy. In Glioblastoma (ed. S De Vleeschouwer), pp. 197–242. Brisbane, Australia: Codon Publications. [PubMed]
- 44.Holland JH. 1992. Adaptation in natural and artificial systems: an introductory analysis with applications to biology, control, and artificial intelligence. New York, NY: MIT Press. [Google Scholar]
- 45.Martínez-Garcia M. et al. 2018. SEOM clinical guidelines for diagnosis and treatment of glioblastoma (2017). Clin. Transl. Oncol. 20, 22–28. ( 10.1007/s12094-017-1763-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wick W, Platten M, Weller M. 2009. New (alternative) temozolomide regimens for the treatment of glioma. Neuro Oncol. 11, 69–79. ( 10.1215/15228517-2008-078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ballesta A, Zhou Q, Zhang X, Lv H, Gallo J. 2014. Multiscale design of cell-type–specific pharmacokinetic/pharmacodynamic models for personalized medicine: application to temozolomide in brain tumors. CPT: Pharmacometrics Syst. Pharmacol. 3, 1–11. ( 10.1038/psp.2014.9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stensjøen AL, Solheim O, Kvistad KA, Håberg AK, Salvesen Ø, Berntsen EM. 2015. Growth dynamics of untreated glioblastomas in vivo. Neuro Oncol. 17, 1402–1411. ( 10.1093/neuonc/nov029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiesa-Vottero AG, Rybicki LA, Prayson RA. 2003. Comparison of proliferation indices in glioblastoma multiforme by whole tissue section vs tissue microarray. Am. J. Clin. Pathol. 120, 902–908. ( 10.1309/8UAUKFK3NBDMVTNU) [DOI] [PubMed] [Google Scholar]
- 50.Temodar (temozolomide): side effects, interactions, warning, dosage & uses. See www.rxlist.com/temodar-drug.htm#medguide.
- 51.Malmström A. et al. 2012. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 13, 916–926. ( 10.1016/S1470-2045(12)70265-6) [DOI] [PubMed] [Google Scholar]
- 52.Bolstad BM, Irizarry RA, Åstrand M, Speed TP. 2003. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193. ( 10.1093/bioinformatics/19.2.185) [DOI] [PubMed] [Google Scholar]
- 53.Parker NR, Khong P, Parkinson JF, Howell VM, Wheeler HR. 2015. Molecular heterogeneity in glioblastoma: potential clinical implications. Front. Oncol. 5, 55 ( 10.3389/fonc.2015.00055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Byrne AT. et al. 2017. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat. Rev. Cancer 17, 254 ( 10.1038/nrc.2016.140) [DOI] [PubMed] [Google Scholar]
- 55.Mansoori B, Shotorbani SS, Baradaran B. 2014. RNA interference and its role in cancer therapy. Adv. Pharm. Bull. 4, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patrizii M, Bartucci M, Pine SR, Sabaawy HE. 2018. Utility of glioblastoma patient-derived orthotopic xenografts in drug discovery and personalized therapy. Front. Oncol. 8, 23 ( 10.3389/fonc.2018.00023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subramanian A. et al. 2017. A next generation connectivity map: L1000 platform and the first 1 000 000 profiles. Cell 171, 1437–1452. ( 10.1016/j.cell.2017.10.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bansal M. et al. 2014. A community computational challenge to predict the activity of pairs of compounds. Nat. Biotechnol. 32, 1213 ( 10.1038/nbt.3052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madani Tonekaboni SA, Soltan Ghoraie L, Manem VSK, Haibe-Kains B. 2016. Predictive approaches for drug combination discovery in cancer. Brief. Bioinform. 19, 263–276. ( 10.1093/bib/bbw104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fishel ML, He Y, Smith ML, Kelley MR. 2007. Manipulation of base excision repair to sensitize ovarian cancer cells to alkylating agent temozolomide. Clin. Cancer Res. 13, 260–267. ( 10.1158/1078-0432.CCR-06-1920) [DOI] [PubMed] [Google Scholar]
- 61.Montaldi AP, Sakamoto-Hojo ET. 2013. Methoxyamine sensitizes the resistant glioblastoma T98G cell line to the alkylating agent temozolomide. Clin. Exp. Med. 13, 279–288. ( 10.1007/s10238-012-0201-x) [DOI] [PubMed] [Google Scholar]
- 62.Rahman MA. et al. 2018. Abstract 2928: Bortezomib sensitizes glioblastoma with unmethylated MGMT promoter to temozolomide-chemotherapy through MGMT depletion and abrogated autophagy flux. Cancer Res. 78, 2928–2928. ( 10.1158/0008-5472.CAN-17-2897) [DOI] [Google Scholar]
- 63.Raizer JJ. et al. 2016. A phase II trial evaluating the effects and intra-tumoral penetration of bortezomib in patients with recurrent malignant gliomas. J. Neurooncol. 129, 139–146. ( 10.1007/s11060-016-2156-3) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets and code are publicly available at https://github.com/hjain-fsu/hjain-fsu. Code to perform identifiability analysis with GENSSI 2.0 was taken from https://github.com/genssi-developer/GenSSI. Code to use eFAST was taken from http://malthus.micro.med.umich.edu/lab/usadata/.