Abstract
The purpose of this study was to develop a novel non-contact optical coherence elastography (OCE) approach to measure laterally and axially highly resolved corneal strain distribution at different stages of patterned corneal cross-linking (CXL). Freshly enucleated rat eyes were obtained and prepared for accelerated patterned CXL treatment with distinct ultraviolet (UV) patterns (central, peripheral, bow-tie irradiation). Each cornea was measured repeatedly, in three different conditions: (i) virgin, (ii) after epithelial debridement and 0.5% hypo-osmolar riboflavin instillation for 30 min, and (iii) after patterned CXL at 9 mW cm−2 for 10 min. For biomechanical assessment, the corneal deformation response to an ambient pressure variation of −2 mmHg was recorded by OCE. Strain maps were obtained from phase and magnitude changes in the complex optical coherence tomography signal. Virgin corneas presented negative strain (−2.7 ± 1.1‰) in the anterior cornea and positive strain (1.9 ± 1.3‰) in the posterior cornea. A pronounced shift towards positive strains in the anterior cornea (particularly in UV-irradiated regions) was observed after CXL. Patterned UV irradiation induced localized strain alterations closely matching the geometry of the irradiation pattern. This study demonstrates the possibility of non-contact OCE by ambient pressure modulation, which could substantially improve the early diagnosis of corneal degeneration, advance research in small-animal eyes and refine in vitro mechanical investigation.
Keywords: non-contact, elastography, cross-linking, ambient pressure, strain map, eye
1. Introduction
Corneal tissue is a highly innervated tissue that is extremely sensitive to contact. It is the major refractive component of the eye and plays an important role in the eye's optical performance. Corneal pathologies significantly impeding its refractive functioning, such as corneal ectasia and keratoconus, most likely arise from localized tissue weakening [1,2] due to degenerative diseases and/or after surgical intervention. Determining the mechanical material properties of corneal tissue in a least invasive but still precise manner is an ongoing challenge for diagnostic and preventive efforts of such pathologies. Imaging modalities based on air-puff deformation are used clinically to evaluate the dynamic displacement response of the examined cornea [3], but are confounded by other parameters such as intraocular pressure (IOP) and corneal thickness. As an alternative, Brillouin microscopy has recently been tested in patients with keratoconus [4]. It measures the nonlinear optical shift related to material waves inherent to the molecular structure of corneal tissue, which is indirectly related to its macroscopic elastic properties. Optical coherence elastography (OCE) is another emerging imaging technology allowing for direct measurement of tissue strain [5,6] or shear wave propagation velocity [7], both of which have a clearly defined and more direct relation to inherent material properties such as tissue stiffness, and hence directly assess mechanical characteristics. Unfortunately, all reported OCE approaches so far apply unnatural loading conditions [8] (e.g. micro- air-puff deformation or axial compression) and often are uncomfortable for the patient or difficult to administer (particularly to smaller-animal eyes). Hence, there is an unmet need for a proper, comfortable and clinically accurate approach to measuring the mechanical properties for diagnosis or in order to evaluate treatment efficacy.
Corneal cross-linking (CXL) is a treatment that effectively stops the progression of degenerating corneal pathologies such as keratoconus [9]. Twenty years ago, CXL treatment was introduced as a photochemical technique to stiffen corneal tissue [10]. It requires the application of the photosensitizer riboflavin (vitamin B2) in combination with ultraviolet (UV)-A (365 nm) irradiation. The original Dresden protocol requires 30 min of riboflavin instillation, followed by 30 min of irradiation at 3 mW cm−2. Since then, a series of distinct CXL protocols has been developed that accommodate individual needs, such as shorter duration (accelerated CXL) and reduced pain tolerance (trans-epithelial CXL). Several clinical studies have reported corneal flattening after CXL treatment [11]. In consequence, patterned CXL [12] (or refractive CXL [13]) has been suggested for the correction of mild refractive errors. A drawback of all CXL treatments is the absence of a reliable tool to quantify treatment efficacy and failures early on, which occur in around 8% of treated cases [14].
Optical coherence tomography (OCT) devices are diagnostic tools that are in high demand in ophthalmology and are predominantly used for morphological and angiographic imaging. Over the last 25 years, OCT systems [15] have substantially improved with regard to the imaging speed [16] and image resolution [17]. Nowadays, given the need for in vivo non-contact mechanical characterization, OCT-based elastographic imaging [5–8,18] is gaining more and more interest. Dynamic OCE approaches require expensive high-speed OCT technology. By contrast, quasi-static OCE approaches are more cost-effective and less affected by boundary conditions. Quasi-static in vivo OCE imaging [6] has been demonstrated by analysing corneal displacement maps in response to anterior corneal compression. More recently, corneal strain profiles, as a result of invasively subjecting the eye to physiologically occurring diurnal [19] IOP fluctuations of 5 mmHg, have been reported [5]. A particular advantage of IOP-based load application for corneal elastography is the natural stress distribution, which replicates the mechanical demand of corneal tissue on a daily basis. Overall, quasi-static OCE allows for a high-resolution, cost-effective and less confounded means to measure the mechanical properties of corneal tissue, but so far has required contact and thus is uncomfortable if performed on living subjects.
In this article, ambient pressure modulation is introduced as a novel non-invasive and contact-free OCE technique to assess corneal strain distribution and evaluate localized differences in mechanical properties. The technique is applied to rodent eyes at different stages of patterned CXL treatment.
2. Methods
2.1. Optical coherence elastography system
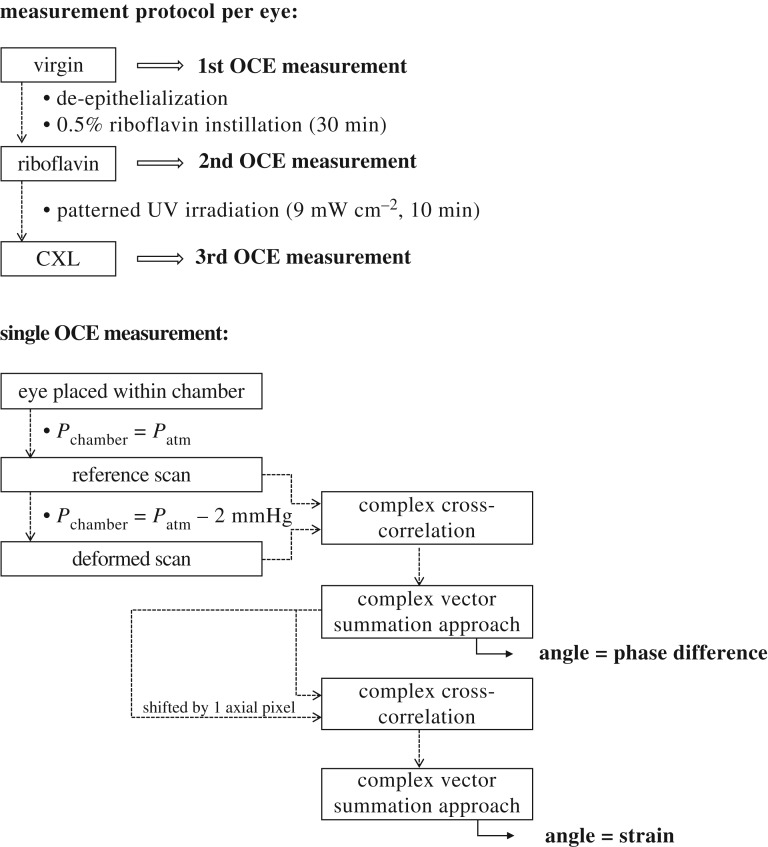
A schematic of the experimental set-up is presented in figure 1. Each eye globe was placed on a silicon mould mounted within a sealed pressure chamber and aligned with the OCT system. For the presented samples, an ambient pressure variation of approximately −2 mmHg was induced by means of a 1 ml syringe attached to the chamber. The other exit of the chamber was connected to a water column to measure the applied pressure. Previously, a series of different pressures (0.5, 1 and 2 mmHg) were evaluated in a separate eye to determine the amount of pressure change that leads to the best signal-to-noise ratio in strain images. OCT volume scans were acquired through the top window, which consisted of a 2 mm acrylic glass plate. The spectral domain OCT operates at a wavelength of 877.8 nm, a bandwidth of 62.5 nm and an output power of 1.62 mW, providing an axial and lateral resolution in tissue of 3.9 µm and 12.5 µm, respectively. A single volume scan of the dimension 6 × 6 mm was acquired within 20 ms with an A-line exposure time of 90 µs.
Figure 1.
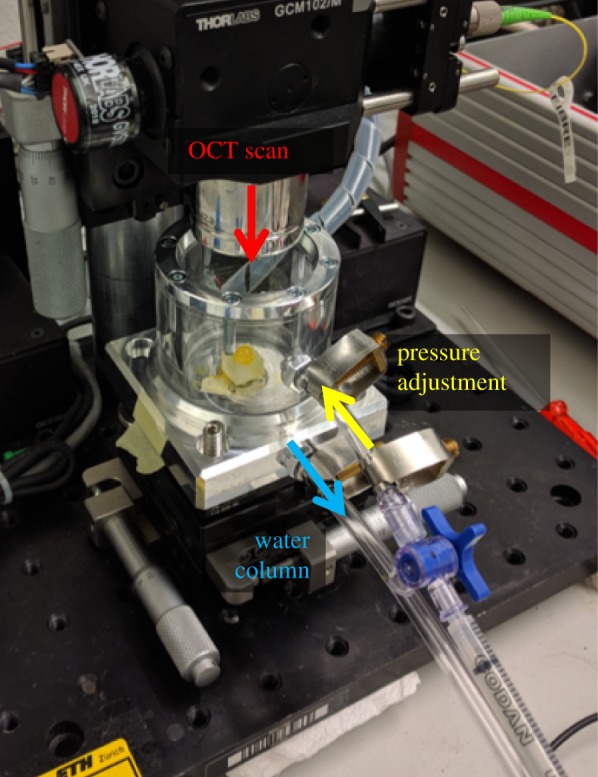
Experimental set-up. The enucleated eye is placed within a sealed pressure chamber. Ambient pressure is decreased by 2 mmHg using a 1 ml syringe, and measured via a water column. Before and after the pressure change, an OCT volume scan of the anterior segment is taken through the top acrylic glass window. (Online version in colour.)
2.2. Sample preparation
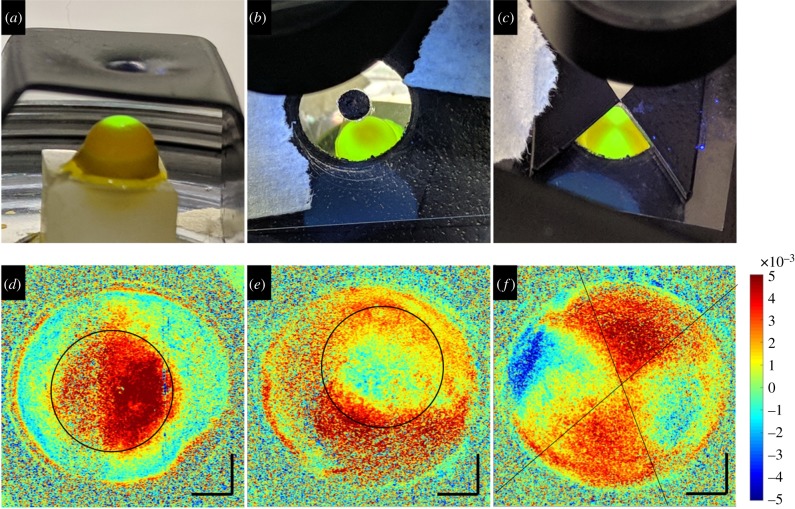
Ex vivo rat eyes were obtained from the phenomics centre (ETH Zurich, Switzerland) after termination of an unrelated preceding experiment. Directly after euthanasia, eye globes were enucleated and stored in Dulbecco's modified Eagle's medium for transport. Each eye was measured in three subsequent conditions (study design in figure 2). In the virgin condition, eyes were left intact. In the riboflavin condition, the epithelium was removed by applying 35% ethanol for 2 min followed by gentle rubbing with a cotton swab. Then the eye was submerged in 0.5% riboflavin (Streuli Pharma AG, Switzerland) solution for 30 min. A higher riboflavin concentration than for human application was applied in order to adapt the absorption profile for the thinner [20] rat cornea. In the CXL condition, the riboflavin pre-treated eye was taken and exposed to patterned UV-A irradiation (365 nm) at 9 mW cm−2 for 10 min. To achieve central, peripheral and bow-tie patterns, UV light was restricted by a customized shield. For peripheral and bow-tie patterns, the shield included a microscopic cover plate, which reduced UV irradiance at the sample by 10%. In consequence, UV irradiance of the source was increased by this amount.
Figure 2.
Study design. Schematic of the experimental protocol and signal processing.
2.3. Data acquisition and processing
Each eye was measured at three different stages of CXL treatment: virgin, after riboflavin instillation for 30 min and after patterned UV irradiation for 10 min. Care was taken that the orientation of the eye globe remained the same between the two latter conditions. For one OCE measurement of a given eye and condition, two individual OCT volume scans were required (figure 2): first, a ‘reference scan’ at standard pressure was taken. Subsequently, ambient pressure was decreased by −2 mmHg and the second ‘deformed scan’ was acquired. The overall measurement time took less than 60 s. To measure an eye in three conditions, a total of three OCE measurements were performed, each consisting of a reference–deformed scan pair. The decrease in ambient pressure induced an expansion of the eye globe. Axial strain, defined in the direction of the optical axis of the OCT system, was computed from phase differences between the raw interference signals of the reference and deformed scans, as described previously [5,18]. Briefly, phase differences corresponding to the angle CIOP were computed by an amplitude-weighted complex cross-correlation,
| 2.1 |
where represents the complex OCT interference signal recorded at axial location z, lateral location x and chamber pressure p, with amplitude and phase . wz = 3 and wx = 3, indicating the size of the applied phase-processing windows.
A complex vector summation approach was applied [21] to retrieve the phase gradient, which represents axial strain εz,
| 2.2 |
where λmean = 888 nm is the central wavelength, asu = 4.48 µm is the axial sampling unit and wz′ = 3 and wx′ = 1 are the applied strain-processing windows. A complex vector summation approach was applied [21] to retrieve the phase gradient, which represents strain. The resolution of strain is determined by the size of the processing windows and was 45 µm axially and 49 µm laterally. The minimal strain that can be reliably detected depends on the phase noise of the light source and was 2.44 × 10−5 in the current study. No upper limit of detectable strain was found within the previously [18] investigated range of up to 3.85‰ induced axial strain. To better analyse lateral strain distribution, the anterior corneal surface was segmented and the image subsequently flattened for enface slicing. Because of the higher noise levels in en face versus cross-sectional images, the latter were subjected to three-pixel vertical averaging and subsequent lateral median filtering with a kernel size of 3 × 3 pixels.
2.4. Statistical analysis
A one-sample two-sided t-test was applied to test whether differences in corneal strain between irradiated and non-irradiated regions were significantly different from zero. p-values of less than 0.05 were considered to indicate significance.
3. Results
Minimal ambient pressure variation by −2 mmHg (approx. 266 Pa) induced a clearly measurable deformation response in freshly enucleated rat eyes. The eyes were measured within a sealed pressure chamber that isolated the eye fully from its original environment, so that pressure variation was limited to a small volume of approximately 66 ml. To achieve the desired pressure change of −2 mmHg, 200 µl of air needed to be withdrawn from the chamber. Patterned CXL treatment was investigated, as it brings the advantage of having an intra-specimen sham control. Only regions that are irradiated with UV light are expected to have an increased stiffness. The untreated regions hence serve as a control for hydration effects and post-mortem time.
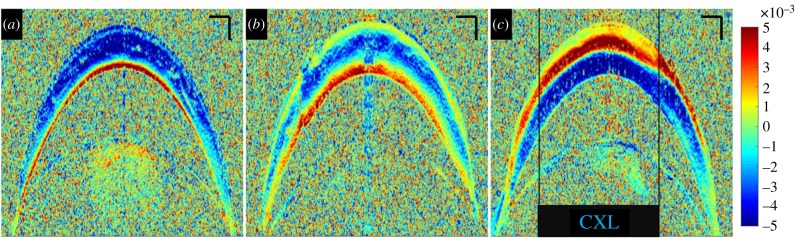
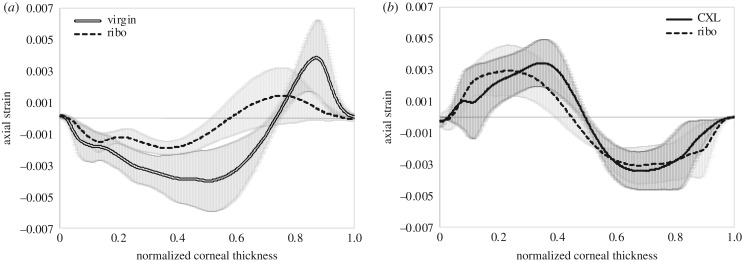
Cross-sectional imaging of corneal tissue is the standard way of examining corneal morphology. Corneal strain distribution hence was first examined from this perspective. Figure 3 shows the cross-sectional corneal strain distribution at different stages of CXL treatment. Virgin corneas presented a consistent negative strain of −2.7 ± 1.1‰ across 73% of the anterior cornea, indicating compression upon ambient pressure decrease. The remaining 27% of the posterior stroma showed positive strain (on average 1.9 ± 1.3‰), indicating expansion. Riboflavin corneas had an increased thickness (476 ± 8 versus 420 ± 16 µm, p = 0.012) and a more inhomogeneous strain distribution. The general trend to negative strain in the anterior and positive strain in the posterior cornea remained unchanged. On average, strain was lower in riboflavin-treated than in virgin corneas. The anterior 58% of stroma had a mean negative strain of −1.2 ± 0.6‰ and the remaining posterior stroma had a mean positive strain of 0.7 ± 0.5‰. After CXL, strain distribution switched sign in the entire cornea: in irradiated regions, the anterior 50% of cornea indicated a mean positive strain of 1.9 ± 1.1‰ (expansion) and the posterior half a mean negative strain of −2.1 ± 1.2‰ (compression). Similarly in non-irradiated regions, the anterior 46% of cornea indicated a mean positive strain of 1.8 ± 1.1‰ (expansion) and the posterior part a mean negative strain of −2.1 ± 1.0‰ (compression). A localized effect of the UV pattern can hardly be recognized in the cross-sectional view (figure 3c). The inversion of strain distribution in the entire cornea after CXL treatment seemed to mask localized differences in the lateral direction. Figure 4 presents the axial strain distribution across corneal depth, averaged within an optical zone of 5.4 mm diameter. A shift of the anterior positive strain peak towards the posterior cornea can be perceived in irradiated versus non-irradiated regions.
Figure 3.
Cross-sectional corneal strain distribution. (a) In a virgin cornea, (b) after 0.5% hypo-osmolar riboflavin instillation and (c) after central CXL treatment. Negative strains indicate compression and positive strains elongation along the vertical axis. Size bars have a dimension of 200 µm axially and 500 µm laterally. (Online version in colour.)
Figure 4.
Strain profile. Comparison between (a) virgin and riboflavin conditions in subsequent measurements, averaged across the entire cornea and (b) riboflavin and cross-linking conditions, averaged across, respectively, the irradiated and non-irradiated region within the same corneas. Mean values across n = 3 samples are presented. Normalized corneal thickness corresponds to zero at the anterior surface and to 1 at the posterior surface.
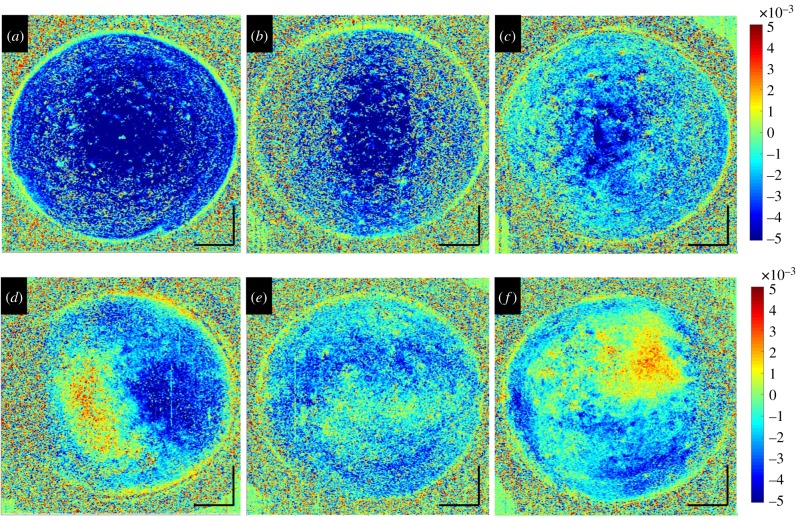
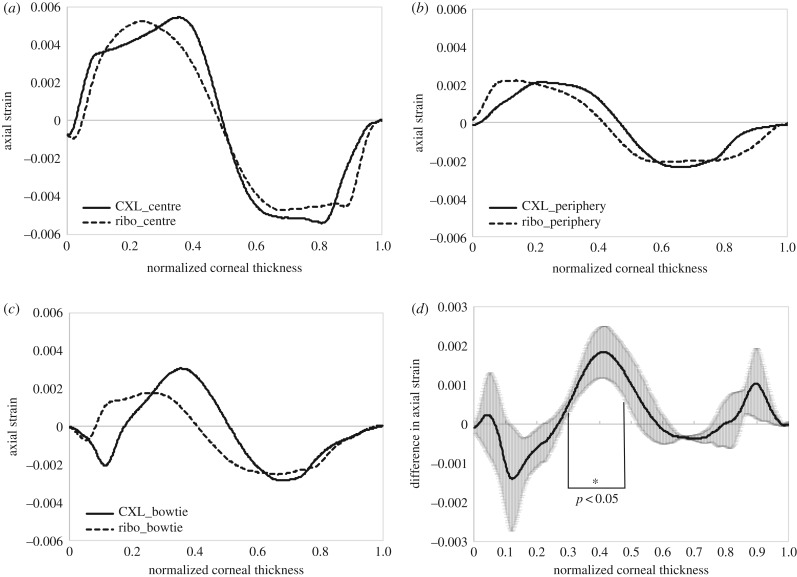
The diagnostic value of strain maps is directly related to their power of identifying spatially resolved mechanical characteristics. Hence, for more precise lateral strain interpretation, the cross-sectional images were flattened and en face OCT slices examined. Figure 5 presents the en face strain distribution in the mid-stroma of a set of three virgin and subsequently riboflavin-treated corneas. Similar to before, virgin corneas demonstrated a more homogeneous strain distribution than after riboflavin application. Figure 6 presents the same corneas as in figure 3, but after CXL treatment with distinct UV patterns. Strain distribution in the mid-stroma matched the geometry of the applied UV irradiation pattern extraordinarily well. Interestingly, the area demonstrating an altered strain distribution was larger than the area that actually had been irradiated. This effect was more obvious with peripheral and bow-tie patterns than with central irradiation. CXL treatment is expected to be effective up to two-thirds of corneal depth. In line with this, the posterior cornea did not show any localized pattern-related strain modifications (data not shown). By contrast, the anterior cornea had an unexpected irregular strain distribution, which was unrelated to the irradiation pattern. Figure 7a–c presents the axial strain distribution after CXL treatment in irradiated versus non-irradiated regions. Figure 7d shows the corresponding mean difference of cross-linked minus riboflavin strain distribution. A significant shift towards positive strains in the irradiated region was found between 30% and 47% depth of corneal tissue (p < 0.05). Overall, the proposed non-contact OCE approach was able to identify localized strain modifications resulting from patterned CXL in the en face analysis, with surprisingly good geometrical correspondence to the irradiation pattern.
Figure 5.
En face corneal strain distribution in the mid-stroma: (a–c) in virgin corneas and (d–f) after 0.5% hypo-osmolar riboflavin instillation. Negative strains indicate compression and positive strains elongation along the vertical axis. Size bars have a dimension of 1 mm axially and laterally. (Online version in colour.)
Figure 6.
Patterned CXL. (a–c) UV light pattern used for localized CXL treatment. (d–f) Corresponding strain maps of the mid-stroma (approx. 55% depth). Negative strains indicate compression and positive strains elongation along the vertical axis. Black lines indicate the frontier between irradiated and non-irradiated areas. Size bars have a dimension of 1 mm axially and laterally. (Online version in colour.)
Figure 7.
Change in strain profile after CXL. Comparison of the corneal strain profile between irradiated and non-irradiated regions (a) in the central cornea, (b) in the peripheral cornea and (c) with a bow-tie pattern. (d) Mean difference across n = 3 samples between riboflavin-only and cross-linked regions.
4. Discussion
The results described above clearly demonstrate that non-contact OCE by ambient pressure modulation is capable of quantifying axial corneal strain distribution upon minimal pressure changes. A decrease of 2 mmHg corresponds to the atmospheric pressure at 22 m above sea level. This pressure difference is also lower than diurnal physiological IOP fluctuations [19], and hence can be considered harmless.
Ambient pressure modulation is an ideal non-contact and sterile approach to provoke corneal expansion similar to invasively increasing IOP by injecting water [22]. Given its non-contact nature and the fact that only small amounts of strain are induced, measurements can be performed repeatedly in the same eye without expecting any influence from the preceding measurement. In contrast with previously applied techniques to assess corneal biomechanics, such as air-puff deformation or anterior compression by a lens, the loading condition in the current setting closely matches the natural situation of the eye in a wide range of specimens, given that atmospheric pressure changes occur on a daily basis.
Despite the rather small size of the rat eye, which amounts to approximately one-third of a human eye [23], strain images of good quality were retrieved. The resolution of the retrieved strain images was limited by the size of the processing windows, which may introduce artefacts, particularly at tissue borders. However, as the thicknesses of positive and negative corneal strain layers were larger than the size of the accumulated processing windows, the bi-layered strain distribution is very likely to be an inherent tissue characteristic. Biomechanical assessment of rodent eyes has previously only been possible by destructive stress–strain extensometry [20]. Rodent strains with a huge variety of distinct genetic modifications are available for research. In this regard, non-invasively assessing biomechanical properties in rodent eyes is of particular relevance for long-term studies to reduce both the number of animals needed and inter-animal variability.
The effect of patterned CXL treatment could be captured well with the novel non-contact OCE approach. A more irregular strain distribution after riboflavin instillation compared with virgin corneas probably results from the mechanical insult during epithelial removal, as well as from inevitable minor differences in riboflavin diffusion that may have an effect on local tissue hydration. Compared with the pronounced changes in strain distribution after CXL, the effects of riboflavin instillation were small. In both cross-sectional and en face images, it was clearly visible that cross-linking led to a shift towards positive strains. In particular, the shift from negative to positive strains after CXL may directly relate to the molecular changes induced by the photochemical cross-linking reaction. Positive strains observed under compression typically indicate a negative Poisson's ratio, and hence auxetic material properties. Auxetic characteristics arise in materials with highly ordered micro-structures. Typically, such materials are manufactured because of their unique properties, including increased resistance to indentation and shear, better shock and vibration absorption properties (in comparison with conventional materials) and the tendency to form double-curved (i.e. dome-shaped) surfaces. Interestingly, all such properties would be relevant to develop and maintain corneal shape. Auxetic behaviour of corneal collagen fibrils has been recently suggested from X-ray crystallography [24,25], but could not be confirmed on a macroscopic deformation scale [24].
Given the limitations of the current experimental setting (only axial and not lateral strain distribution can be assessed; corneal stress distribution can only be theoretically estimated but not experimentally measured), further investigation by means of uniaxial mechanical characterization is required to confirm the presence of auxetic properties after CXL.
Seen from a functional point of view, a layer of positive strain upon posterior pressurization may contribute to keeping corneal refraction stable during diurnal physiological IOP fluctuations. A thicker cornea experiences lower stress values at a given IOP than a thin cornea. The fact that the dimension of this layer increased from 27% thickness in virgin corneas to 42% after riboflavin instillation demonstrates its dependency on hydration level and probably its different capability of compensation. After cross-linking, the positive and negative strain layers switched position and presented overall larger strain amplitudes, indicating a global modification of mechanical mechanisms. Overall larger strains do not necessarily imply a softer tissue, given that compression and expansion are counteracting processes. In fact, the thickness of the positive strain layer was largest (50%) after cross-linking, suggesting that the mechanism counteracting corneal thinning during pressurization was largest. When considering the entire eye globe, a larger layer of positive strain will result in a lower radial expansion in response to the increase in IOP. Macroscopically, this results in a more stable geometry and an apparently stiffer tissue. The latter is in line with prior literature reporting smaller curvature changes after cross-linking in whole-eye inflation experiments [22].
The geometric shape of the UV pattern and the zone of modified strain did match extraordinarily well. Yet, the size of the irradiated zone was somewhat smaller than the zone that presented a shift towards the positive strain. On the one hand, this may result from the initial positioning for UV irradiation, which was challenging because of the small size of the rat eye. On the other hand, a larger stiffness zone compared with the UV-irradiated zone has already previously been reported [26] and ascribed to result from light scattering within the corneal tissue, leading to an augmentation of the effective treatment zone. This effect has been shown to go along with increased post-mortem time [26], which can be confirmed in the current study, given that central irradiation was performed first and showed the least discrepancy between the irradiated and strain-modified zones.
Recently, we have reported that initial IOP values affect the amplitude of corneal strain [18] and that a higher IOP results in a lower strain amplitude. In this study, initial IOP values of the rat eyes were not evaluated at any stage, as invasive measurement would lead to leakage and non-invasive measurement would be impossible. This limitation may explain minor differences in strain amplitude between subsequently measured eyes. However, it does not affect the spatial strain distribution within a given cornea. The initial IOP may also define the ambient pressure change that is most suitable for non-contact OCE imaging, as even smaller displacements result in noisier strain maps and excessively large displacements negatively affect the speed of displacement tracking.
This study has shown that corneal strain distribution is a sensitive measure for localized changes in tissue stiffness and the proposed non-contact OCE approach is likely to be a promising tool for diagnosing corneal degeneration, for example in pathologies such as keratoconus, and as a screening tool before and after refractive surgery. In addition, maps of corneal strain distribution are an important input parameter for patient-specific numerical simulations in ophthalmology. A particular benefit of the proposed technique is its applicability to different sizes of eye globes. The size of the pressure chamber can be increased to enclose entire anaesthetized rodents, permitting repeated in vivo mechanical characterization. Moreover, the chamber can be adapted to cover the human eye (e.g. swimming goggles isolate the human eye from its environment). By connecting the eye chamber to an external pressure control, the ambient pressure can be modified locally in front of the eye, while simultaneously tissue deformation can be recorded by the OCT system through the transparent front window. When applying the proposed OCE approach to living specimens, care needs to be taken to perform the reference and deformed scans within a period shorter than the interval of saccadic eye movements. Given its non-contact and sterile operating mechanism, the proposed OCE technique may also be of interest for in vitro application in cell, tissue and organ cultures—particularly in the area of mechanotransduction.
Acknowledgements
The author thanks Dr Moritz P. Günther for scientific discussions and advice.
Data accessibility
This article has no additional data.
Competing interests
The author is the co-inventor of patent application Method and Apparatus for Investigating a Sample (No. EP19193554.3).
Funding
Financial support was received from the Swiss National Science Foundation (Ambizione PZ00P2_174113).
References
- 1.Scarcelli G, Besner S, Pineda R, Kalout P, Yun SH. 2015. In vivo biomechanical mapping of normal and keratoconus corneas. JAMA Ophthalmol. 133, 480–482. ( 10.1001/jamaophthalmol.2014.5641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreassen TT, Simonsen AH, Oxlund H. 1980. Biomechanical properties of keratoconus and normal corneas. Exp. Eye Res. 31, 435–441. ( 10.1016/S0014-4835(80)80027-3) [DOI] [PubMed] [Google Scholar]
- 3.Vinciguerra R, Ambrósio R, Elsheikh A, Roberts CJ, Lopes B, Morenghi E, Azzolini C, Vinciguerra P. 2016. Detection of keratoconus with a new biomechanical index. J. Refract. Surg. 32, 803–810. ( 10.3928/1081597X-20160629-01) [DOI] [PubMed] [Google Scholar]
- 4.Scarcelli G, Besner S, Pineda R, Yun SH. 2014. Biomechanical characterization of keratoconus corneas ex vivo with Brillouin microscopy. Invest. Ophthalmol. Vis. Sci. 55, 4490–4495. ( 10.1167/iovs.14-14450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kling S, Khodadadi H. 2019. Optical coherence elastography based corneal strain mapping during low-amplitude intraocular pressure modulation. Invest. Ophthalmol. Vis. Sci. 60, 1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Stefano VS, Ford MR, Seven I, Dupps WJ Jr. 2018. Live human assessment of depth-dependent corneal displacements with swept-source optical coherence elastography. PLoS ONE 13, e0209480 ( 10.1371/journal.pone.0209480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang S, Larin KV. 2014. Noncontact depth-resolved micro-scale optical coherence elastography of the cornea. Biomed. Opt. Exp. 5, 3807–3821. ( 10.1364/BOE.5.003807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Larin KV. 2015. Optical coherence elastography for tissue characterization: a review. J. Biophotonics 8, 279–302. ( 10.1002/jbio.201400108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. 2008. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J. Cataract Refract. Surg. 34, 796–801. ( 10.1016/j.jcrs.2007.12.039) [DOI] [PubMed] [Google Scholar]
- 10.Spoerl E, Huhle M, Seiler T. 1998. Induction of cross-links in corneal tissue. Exp. Eye Res. 66, 97–103. ( 10.1006/exer.1997.0410) [DOI] [PubMed] [Google Scholar]
- 11.Ziaei M, et al. 2015. Reshaping procedures for the surgical management of corneal ectasia. J. Cataract Refract. Surg. 41, 842–872. ( 10.1016/j.jcrs.2015.03.010) [DOI] [PubMed] [Google Scholar]
- 12.Seven I, Roy AS, Dupps WJ Jr. 2014. Patterned corneal collagen crosslinking for astigmatism: computational modeling study. J. Cataract Refract. Surg. 40, 943–953. ( 10.1016/j.jcrs.2014.03.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elling M, Kersten-Gomez I, Dick HB. 2018. Photorefractive intrastromal corneal crosslinking for treatment of myopic refractive error: findings from 12-month prospective study using an epithelium-off protocol. J. Cataract Refract. Surg. 44, 487–495. ( 10.1016/j.jcrs.2018.01.022) [DOI] [PubMed] [Google Scholar]
- 14.Koller T, Mrochen M, Seiler T. 2009. Complication and failure rates after corneal crosslinking. J. Cataract Refract. Surg. 35, 1358–1362. ( 10.1016/j.jcrs.2009.03.035) [DOI] [PubMed] [Google Scholar]
- 15.Huang D, et al. 1991. Optical coherence tomography. Science. 254, 1178–1181. ( 10.1126/science.1957169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein T, Huber R. 2017. High-speed OCT light sources and systems. Biomed. Opt. Exp. 8, 828–859. ( 10.1364/BOE.8.000828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werkmeister RM, et al. Ultrahigh-resolution OCT imaging of the human cornea . Biomed. Opt. Exp. 8, 1221–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kling S, Khodadadi H, Göksel O. In press. Optical coherence elastography based corneal strain imaging during low-amplitude intraocular pressure modulation. Front. Bioeng. Biotechnol. [DOI] [PMC free article] [PubMed]
- 19.David R, Zangwill L, Briscoe D, Dagan M, Yagev R, Yassur Y. 1992. Diurnal intraocular pressure variations: an analysis of 690 diurnal curves. Br. J. Ophthalmol. 76, 280–283. ( 10.1136/bjo.76.5.280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammer A, Kling S, Boldi M-O, Richoz O, Tabibian D, Randleman JB, Hafezi F. 2015. Establishing corneal cross-linking with riboflavin and UV-A in the mouse cornea in vivo: biomechanical analysis. Invest. Ophthalmol. Vis. Sci. 56, 6581–6590. ( 10.1167/iovs.15-17426) [DOI] [PubMed] [Google Scholar]
- 21.Matveyev A, Matveev L, Sovetsky A, Gelikonov G, Moiseev A, Zaitsev V. 2018. Vector method for strain estimation in phase-sensitive optical coherence elastography. Laser Phys. Lett. 15, 065603 ( 10.1088/1612-202X/aab5e9) [DOI] [Google Scholar]
- 22.Kling S, Remon L, Pérez-Escudero A, Merayo-Lloves J, Marcos S. 2010. Corneal biomechanical changes after collagen cross-linking from porcine eye inflation experiments. Invest. Ophthalmol. Vis. Sci. 51, 3961–3968. ( 10.1167/iovs.09-4536) [DOI] [PubMed] [Google Scholar]
- 23.Lozano DC, Twa MD. 2013. Development of a rat schematic eye from in vivo biometry and the correction of lateral magnification in SD-OCT imaging. Invest. Ophthalmol. Vis. Sci. 54, 6446–6455. ( 10.1167/iovs.13-12575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell J, et al. 2018. The hierarchical response of human corneal collagen to load. Acta Biomater. 65, 216–225. ( 10.1016/j.actbio.2017.11.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patten K, Wess T. 2013. Suprafibrillar structures of collagen, evidence for local organization and auxetic behaviour in architectures. J. Biophys. Chem. 4, 103 ( 10.4236/jbpc.2013.43014) [DOI] [Google Scholar]
- 26.Webb JN, Langille E, Hafezi F, Randleman JB, Scarcelli G. 2019. Biomechanical impact of localized corneal cross-linking beyond the irradiated treatment area. J. Refract. Surg. 35, 253–260. ( 10.3928/1081597X-20190304-01) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.