Abstract
Background and Aims:
Bone loss around dental implants is generally measured by monitoring changes in marginal bone level using radiographs. After the first year of implantation, an implant should have <0.2 mm annual loss of marginal bone level to satisfy the criteria of success. However, the success rate of dental implants depends on the amount of the crestal bone around the implants. The main aim of this study was to evaluate and compare the crestal bone loss around implants placed with particulate β-Tricalcium Phosphate Bone Graft and platelet concentrates.
Methods:
50 individuals received hundred dental implants. Each individual received one dental implant in the edentulous site filled with β-Tricalcium Phosphate Bone Graft along (β-TCP) with Platelet- Rich Plasma (PRP) (Group A) and another in edentulous site filled only with β-Tricalcium Phosphate Bone Graft (Group B) in the posterior edentulous region. All the 100 implants were prosthetically loaded after a healing period of three months. Crestal bone loss was measured on mesial, distal, buccal and lingual side of each implant using periapical radiographs 3 months, 6 months and 9 months after implant placement.
Results:
The average crestal bone loss 9 months after the implants placement in Group A and Group B was 2.75 mm and 2.23 mm respectively, the value being statistically significant (P < 0.05). In both Group A and Group B, the average crestal bone loss was maximum on the lingual side followed by buccal, distal and mesial sides.
Conclusion:
β-TCP is a promising biomaterial for clinical situations requiring bone augmentation. However, the addition of PRP results in decreased bone loss around the dental implants.
Keywords: Bone loss, dental implants, PRP, TCP
Introduction
Damage to the tooth can be caused by various etiological factors like periodontal disease, abscess formation, trauma, or vertical tooth fracture. Common consequences of tooth loss include progressive resorption of the alveolar bone and decreased masticatory performance. Tooth replacement with dental implants has led to an important revolution in modern clinical dentistry. Branemark first introduced osseointegrated dental implants to allow firm anchorage of titanium implant screws into living bone, a process referred to as osseointegration.[1]
Implant-based treatment is a growing part in the modern dentistry. Loss of alveolar bone around dental implants is revealed in 5-10% of patients. A dental implant is considered to be a failure if it is lost, mobile, or shows peri-implant bone loss of greater than 1.0 mm in the first year and greater than 0.2 mm a year after. Peri-implantitis can result in bone loss around the implant and eventual loss of the implant. Peri-implantitis is a site-specific infectious disease that causes an inflammatory process in soft tissues and bone loss around an osseointegrated implant in function.[2] The long-term clinical success of dental implants depends mainly on the preservation of the bony support around the implant, which is usually evaluated with radiographic images.[3]
In the peri-implantitis treatment together with operative and conservative treatment, bone substitutes are often used to replace the bone defect. One of the materials is Tri calcium phosphate (TCP). TCP is widely used to increase bone after tooth extraction and in dental implantation, also in peri-implantitis treatment. β-tricalcium phosphate (β-TCP), and a combination of HAp/β-TCP are used since they do not evoke adverse cellular reactions, and in time, the material is either replaced by bone or integrated into the body, depending on the degradation properties.[4,5,6]
Platelet concentrates (platelet-rich-fibrin) are obtained by centrifugation of blood, following a method first described by Choukroun and colleagues. These materials contain high concentrations of growth factors (PDGF, TGF-β, IGF and VEGF), and inflammatory molecules (IL-1β, IL-4, IL-6 and TNF-α), and they could enhance the healing process, possibly leading to better bone repair and regeneration. It has been shown that platelet concentrates accelerate the healing of dermal soft tissue and of the oral mucosa in cases of extraction.[7,8,9]
Bone loss often leads to increased chances of implant failure. Possible causes of crestal bone loss could be a local inflammation/infection and mechanical stresses acting on the crestal bone around the implant crest module/collar. Various dental implants with different surface designs are being used for dental rehabilitation of patients. Many of them are two-stage, submerged implants with a 2 mm smooth coronal collar/crest module design. Bone loss with smooth collar has been observed.[10,11,12]
Albrektsson et al.[13] proposed criteria for assessing and evaluating the success of implant survival; these criteria included marginal bone remodeling of less than 2.0 mm in the first year after implant placement and less than 0.2 mm each year thereafter. These changes are usually related to the use of implants with a conventional machined surface and a conventional neck design.
Recently, several studies have shown that implants with a rough surface and a microthreaded-neck design may improve the preservation and stabilization of crestal bone.[14,15,16,17]
The purpose of this in-vivo study was to evaluate and compare the crestal bone loss occurring around the implants using various forms of bone grafts.
Methods
The study was conducted on 50 individuals requiring replacement of 2 missing teeth by implant supported crowns in the posterior region of mandible within the age group of 25-60 years. Each individual received one dental implant in the posterior region of mandible filled with β-Tricalcium Phosphate Bone Graft along (β-TCP) with Platelet- Rich Plasma (PRP) (Group A implants, Nobel Biocare) and another in the posterior region of mandible filled only with β-Tricalcium Phosphate Bone Graft (Group B, Nobel Biocare). Ethical approval obtained on 27-03-2017.
Before the implant placement, platelet concentrates (platelet-rich fibrin) were obtained by centrifugation of blood samples of the patient in 10 ml tubes with no adjuvant anticoagulant, centrifuged at 3000 rpm for 10 minutes. Parts of the centrifuged blood rich in platelets (called buffy-coats) were cut and mixed with particulate of β TCP. Parts rich in fibrin were pressed manually between gauze to obtain autologous rich-in-fibrin membranes.
Fullthickness mucoperiosteal flap was elevated by giving local anesthesia and a mid crestal incision in the edentulous area. Dental implants along with graft materials were placed and flaps were sutured. Patients received postoperative instructions and were advised to rinse with chlorhexidine 0.12% twice a day for 10 days, and sutures were removed 2 weeks after. All implants were inserted until the outer edge of the dental implant reached the marginal bone level, to allow for the apex of the cover screw to be at level with the bone crest during the healing period. Post-operatively, digital radiographs were taken to measure the first crestal bone to implant contact level from the top of the implant along the collar/body surface of each implant on the mesial, distal, buccal and lingual side. These measurements would become the baseline reference levels to measure future bone loss. After 3 months of implant insertion, a second stage surgery was carried out and the healing abutments were placed over the implants. Crestal bone levels were measured on the mesial, distal, buccal and lingual side of each implant using periapical radiographs. Crestal bone loss was calculated by deducting the baseline reference bone levels from present levels, which gave the value of bone loss, occurred after 3 months of placing the implants. Implant level impressions were taken two weeks postoperatively to the healing abutment surgery connection. The permanent metal ceramic crown was delivered two weeks after impressions.
Digital periapical radiographs of the dental implants were recorded at different time points: 3 months after implant insertion, 6 months after implant insertion and 9 months after implant insertion.
The data was presented as means ± standard error and was analyzed by Statistical Package for Scientific Studies for Windows (SPSS 20, IBM, Armonk, NY, USA) at a significance level of P ≤ 0.05. Statistical analysis was carried out using 'Paired t-test' to compare the bone loss along Group-A and B.
The inclusion criteria included patients who had good oral hygiene, non smokers or with smoking history of <3 cigarettes/day, with periodontal healthy teeth adjacent to implant site and without any periapical lesion. Patients with any local or systemic disease, smoking habit >3 cigarettes/day, betel nut or tobacco chewing, alcoholism, pregnancy or breastfeeding, longterm oral medications, oral par function, non-treated periodontal disease, and with inadequate bone volume were excluded.
Results
No significant differences in demographic data were found between the groups. In total, 50 patients (25 men and 25 women) received 100 dental implants in the present study.
Tables 1 and 2 depict the mean values obtained for crestal bone loss at specified time intervals around Group A and Group B implants. The average crestal bone loss around the perimeter of Group A and B implants after 3 months was 1.23 mm and 1.61 mm, 6 months after implant insertion was mm 1.68 mm and 2.01, and 9 months after insertion was 2.23 mm 2.75 mm respectively [Graph 1]. After 9 months of implant insertion, there was statistically significant difference in crestal bone loss among Group A and B implants, the average bone loss being more around Group B implants (P < 0.05).
Table 1.
Showing bone loss (mean±standard deviation) around Group A implants (TCP with PRP)
| Duration | Mesial | Distal | Buccal | Lingual |
|---|---|---|---|---|
| 3 months after implant placement | 1.0±0.38 | 1.2±0.40 | 1.3±0.42 | 1.4±0.41 |
| 6 months after implant placement | 1.4±0.42 | 1.7±0.43 | 1.80±0.45 | 1.81±0.44 |
| 9 months after implant placement | 2.0±0.46 | 2.21±0.48 | 2.31±0.50 | 2.40±0.49 |
Table 2.
Showing bone loss (mean±standard deviation) around Group B implants (TCP)
| Duration | Mesial | Distal | Buccal | Lingual |
|---|---|---|---|---|
| 3 months after implant placement | 1.4±0.33 | 1.6±0.38 | 1.71±0.37 | 1.72±0.36 |
| 6 months after implant placement | 1.8±0.39 | 2.0±0.40 | 2.11±0.42 | 2.12±0.41 |
| 9 months after implant placement | 2.51±0.41 | 2.71±0.43 | 2.89±0.45 | 2.90±0.44 |
Graph 1.
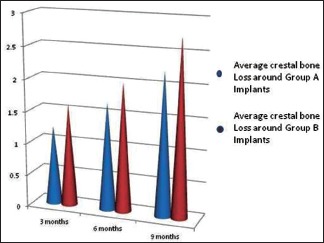
Showing the average crestal bone loss around the perimeter of Group A and B implants
The mean crestal bone loss around Group A implants 3 months after implant insertion was (1.0 ± 0.38 mm on mesial side, 1.20 ± 0.40 mm on the distal side, 1.30 ± 0.42 mm on the buccal side and 1.40 ± 0.41 mm on the lingual side), 6 months after implant insertion was (1.40 ± 0.42 mm on the mesial side, 1.70 ± 0.43 mm on the distal side, 1.80 ± 0.45 mm on the buccal side and 1.81 ± 0.44 mm on the lingual side of dental implants) 9 months after implant insertion was (2.0 ± 0.46 mm on the buccal side, 2.21 ± 0.48 mm on the distal side, 2.31 ± 0.50 mm on the buccal side and 2.40 ± 0.49 on the lingual side of implants)
Statistical analysis showed a statistically significant difference (P < 0.05) between the 3 months and 6 months and between the 3 months and 12 months in all the bone loss measurements around Group A implants [Table 1].
The mean crestal bone loss around Group B implants 3 months after implant placement was (1.40 ± 0.3 mm on mesial side, 1.60 ± 0.35 mm on the distal side, 1.71 ± 0.37 mm on the buccal side and 1.72 ± 0.36 mm on the lingual side), 6 months after implant insertion was (1.80 ± 0.39 mm on the mesial side, 2.0 ± 0.4 mm on the distal side, 2.11 ± 0.42 mm on the buccal side and 2.12 ± 0.41 mm on the lingual side of dental implant) 9 months after implant insertion was (2.51 ± 0.41 mm on the mesial side, 2.71 ± 0.43 mm on the distal side, 2.89 ± 0.42 mm on the buccal side and 2.90 ± 0.44 mm on the lingual side of implants)
Statistical analysis showed a statistically significant difference (P < 0.05) between 3 months and 6 months and between the 3 months and 9 months in all the bone loss measurements around Group B implants [Table 2].
Discussion
The success rate of dental implant mainly depends on its design and has long been established through various studies. PRP can be defined as a blood derivate where platelets have a higher concentration above baseline levels. In clinical practice PRP has been applied in musculoskeletal treatment, with results reported on cartilage, bone, muscle, tendon and ligament regeneration, and also as an augmentation procedure to favour implant healing, although this aspect has not been largely documented in the literature. The first evidence of the clinical benefits of PRP in implant osseointegration was reported in 1998 by Marx et al.,[18] who studied 88 patients with mandibular defects treated with platelet concentrate and cancellous cellular marrow bone graft. Results showed that PRP allowed a radiographic graft maturation rate of 1.62-2.16 times higher than that without PRP at 6 months, and also showed greater bone density. Since then the use of PRP has been broadened as an augmentation procedure for several applications.[19,20,21]
Crest module is the transosteal portion of a two-piece metal dental implant that creates a transition zone to the load bearing implant body and is designed to hold the prosthetic components in place.[22]
Collar is usually designed to minimize plaque accumulation; hence many implants have a polished smooth collar of varying lengths. The tissue height above the implant is on an average 2.5 mm and usually, the toothbrush bristles cannot enter a sulcus more than one mm. Thus, on the contrary, this smooth collar may contribute to bone loss. Crestal bone is weakest against shear forces and strongest against compressive forces. A smooth parallel collar results in shear forces in the crestal bone region. Resulting bone loss may be due to the lack of mechanical stimulation in the crest region. An angled crest module of more than 20 degrees with a surface texture that increases bone contact might result in compressive and tensile components, thus reducing crestal bone loss. The modified rough collar design with microthreads results in decreased crestal bone loss.[23]
There is insufficient literature available to support the hypothesis that implants incorporated with β-Tricalcium Phosphate Bone Graft along (β-TCP) along Platelet- Rich Plasma (PRP) results in lesser crestal bone loss as compared to an implant incorporated with β-Tricalcium Phosphate Bone Graft along (β-TCP).
Elkarargy Amr conducted a study on 12 patients with ridge defect at the maxillary anterior region and divided the patients into two groups; each group consists of six patients; group A the patients received ridge expansion then immediate implant therapy with, β-TCP and PRP. Group B consisted of 6 patients received ridge expansion then immediate implant therapy with and β-TCP. They concluded that both the groups showed gain of alveolar bone width and increase in bone density around dental implants, there was no significant difference between the two groups in the relative amounts of newly generated tissue and mineralized bone generated around the implants.[24]
Block et al.[25] in 2002 placed 22 implants, of which 3 were inserted immediately after the extraction of single rooted tooth with a human mineralized cancellous bone complement and the remainder of which were placed in a tooth socket preserved with an allograft (human mineralized cancellous bone). The radiological measurements at 4 months after the implant placement revealed an average bone loss of 0.51 ± 0.41 mm at the mesial level and 0.48 ± 0.53 mm at the distal level.
Vadims Klimecs et al.[2] conducted a study using biphasic calcium phosphate ceramic granules in 12 patients to prevent bone loss. After 5 years at the minimum, clinical and 3D cone-beam computed tomography control was done. Clinical situation confirmed good stability of implants without any signs of inflammation around. Radiodensity of the previous gap and alveolar bone horizontally from middle point of dental implants showed similar radiodensity as in normal alveolar bone.
An X et al. performed flapless crestal sinus augmentation surgery using BMP-2-loaded Bio-Oss collagen, with non-functional implants immediately loaded after surgery. The bone height was assessed using preoperative and postoperative cone beam computed tomography (CBCT). Bone density of the sinus graft sites and implant stability (after 3 months) along with marginal bone loss were evaluated using postoperative CBCT scans and Periotest, respectively. The results indicated high level of bone density and good implant stability, showing minimal marginal bone loss after 37.3 months.[26]
Both types of implants were placed in the same jaw of the subjects in similar regions i.e. mandibular posterior region, so that their bone beds have similar bone qualities and they receive similar occlusal forces during function.[27]
From the results, it can be observed that crestal bone loss around Group A and Group B implants 3 months after implant insertion was 1.23 mm and 1.61 mm, respectively. The difference in bone loss with both types of implants at this stage was statistically not significant. Surgical trauma and lack of positive stimulation due to occlusal forces may have caused this observed bone loss.
In the present study it was observed that 6 months after implant insertion the overall average crestal bone loss around Group A and B implants was 1.68 mm and 2.01 mm, respectively. This difference in the bone loss was found to be statistically non significant though the average difference was 0.33.
After 12 months of implant insertion, there was statistically significant difference in crestal bone loss among Group A and B implants 2.23 mm and 2.75 mm, respectively. The average bone loss being more around Group B implants (P < 0.05).
According to the study by Barone et al.[28] in 2012, the average peri-implant bone loss at 3 years of follow-up was 1.02 ± 0.3 mm for the group without alveolar conservation and 1.00 ± 0.2 mm for the group with tooth sockets preserved using xenograft (pig bone). Furthermore, the authors did not observe significant differences in the marginal bone loss between the two groups at 1 year, 2 years or 3 years.
Thus addition of PRP in TCP bed around the implant prevents the excess of bone loss as compared to TCP used alone around dental implants.
Implications for clinical practice
Proper selection of bone graft materials and implant design are the key components of managing the crestal bone loss. Several bone substitutes are currently being applied in clinical practice, but none meets all the requirements of an ideal implant bone graft. Ideally, to obtain good osseointegration, bone implants should provide four elements: structural integrity; an osteoconductive matrix as a scaffold that permits bone ingrowth; osteogenic cells, which offer the potential to differentiate and facilitate the various stages of bone regeneration; and osteoinductive factors, the mediators that induce the various stages of bone regeneration and repair. However, knowledge on this topic is still preliminary with the presence mainly of low quality studies. Many aspects still have to be understood, such as the biomaterials that can benefit most from PRP and the best protocol for PRP both for production and application.[28,29]
Therefore, this concept must be taken into consideration before selecting the type and combination of bone graft in clinical practice.
Conclusion
The results of the present study revealed that the crestal bone loss around the implants surrounded by TCP and PRP showed lesser bone loss than the implants inserted with TCP alone. However, considering the limitations of the current study, the results should be interpreted cautiously.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pye AD, Lockhart DEA, Dawson MP, Murray CA, Smith AJ. A review of dental implants and infection. J Hosp Infect. 2009;72:104–10. doi: 10.1016/j.jhin.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Klimecs V, Grishulonoks A, Salma I, Neimane L, Locs J, Saurina E, et al. Bone loss around dental implants 5 years after implantation of biphasic calcium phosphate (HAp/βTCP) granules. J Healthc Eng. 2018:1–7. doi: 10.1155/2018/4804902. doi: 101155/2018/4804902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verhoeven JW, Cune MS, De Putter C. Reliability of some clinical parameters of evaluation in implant dentistry. J Oral Rehabil. 2000;27:211–6. doi: 10.1046/j.1365-2842.2000.00524.x. [DOI] [PubMed] [Google Scholar]
- 4.Vamze J, Pilmane M, Skagers A. Biocompatibility of pure and mixed hydroxyapatite and α-tricalcium phosphate implanted in rabbit bone. J Mater Sci Mater Med. 2015;26:73–7. doi: 10.1007/s10856-015-5406-6. [DOI] [PubMed] [Google Scholar]
- 5.Al-Sanabani JS, Madfa AA, Al-Sanabani FA. Application of calcium phosphate materials in dentistry? Int J Biomater. 2013:1–12. doi: 10.1155/2013/876132. doi: 10.1155/2013/876132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheikh Z, Abdallah MN, Hanafi AA, Misbahuddin S, Rashid H, Glogauer M. Mechanisms of in vivo degradation and resorption of calcium phosphate based biomaterials. Materials. 2015;8:7913–25. doi: 10.3390/ma8115430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Hom DB, Linzie BM, Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007;9:174–83. doi: 10.1001/archfaci.9.3.174. [DOI] [PubMed] [Google Scholar]
- 9.Quoc JB, Vang A, Evrard L. Peri-implant bone loss at implants placed in preserved alveolar bone versus implants placed in native bone: A retrospective radiographic study. Open Dent J. 2018;12:529–45. doi: 10.2174/1874210601812010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jovanovic SA. Diagnosis and treatment of peri implant complications. In: Newman MG, Takei HH, Carranza FA, editors. Carranza's Clinical Periodontology. 9th ed. Philadelphia: WB Saunders; 2003. pp. 931–42. [Google Scholar]
- 11.Vaillancourt H, Pilliar RM, McCammond D. Factors affecting crestal bone loss with dental implants partially covered with porous coating: A finite element analysis. Int J Oral Maxillofac Implants. 1996;11:351–9. [PubMed] [Google Scholar]
- 12.Wiskott HW, Belser UC. Lack of integration of smooth titanium surface: A working hypothesis based on strains generated in the surrounding bone. Clin Oral Implants Res. 1999;10:429–44. doi: 10.1034/j.1600-0501.1999.100601.x. [DOI] [PubMed] [Google Scholar]
- 13.Albrektsson T, Zarb G, Worthington P, Eriksson RA. The long-term efficacy of currently used dental implants: A review and proposed criteria for success. Int J Oral Maxillofac Implants. 1986;1:11–25. [PubMed] [Google Scholar]
- 14.Rocci A, Martignoni M, Gottlow J. Immediate loading of Branemark System TiUnite and machined-surface implants in the posterior mandible: A randomized open-ended clinical trial. Clin Implant Dent Relat Res. 2003;5:57–63. doi: 10.1111/j.1708-8208.2003.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 15.Friberg B, Jemt T. Rehabilitation of edentulous mandibles by means of five TiUnite implants after one-stage surgery: A 1-year retrospective study of 90 patients. Clin Implant Dent Relat Res. 2008;10:47–54. doi: 10.1111/j.1708-8208.2007.00060.x. [DOI] [PubMed] [Google Scholar]
- 16.Al-Thobity AM, Kutkut A, Almas K. Microthreaded implants and crestal bone loss: A systematic review. J Oral Implantol. 2017;XLIII:157–66. doi: 10.1563/aaid-joi-D-16-00170. [DOI] [PubMed] [Google Scholar]
- 17.Goswami MM. Comparison of crestal bone loss along two implant crest module designs. MJAFI. 2009;65:319–22. doi: 10.1016/S0377-1237(09)80091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 19.Kon E, Filardo G, Di Martino A, Marcacci M. Platelet-rich plasma (PRP) to treat sports injuries: Evidence to support its use. Knee Surg Sports Traumatol Arthrosc. 2011;19:516–27. doi: 10.1007/s00167-010-1306-y. [DOI] [PubMed] [Google Scholar]
- 20.Tschon M, Fini M, Giardino R, Filardo G, Dallari D, Torricelli P, et al. Lights and shadows concerning platelet products for musculoskeletal regeneration. Front Biosci. 2011;3:96–107. doi: 10.2741/e224. [DOI] [PubMed] [Google Scholar]
- 21.Roffi A, Filardo G, Kon E, Marcacci M. Does PRP enhance bone integration with grafts, graft substitutes, or implants? A systematic review. BMC Musculoskelet Disord. 2013;14:330. doi: 10.1186/1471-2474-14-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aparna IN, Dhanasekar B, Lingeshwar D, Gupta L. Implant crest module: A review of biomechanical considerations. Indian J Dent Res. 2012;23:257–63. doi: 10.4103/0970-9290.100437. [DOI] [PubMed] [Google Scholar]
- 23.Misch CE, Bidez MW. A scientific rationale for dental implant design. In: Misch CE, editor. Contemporary Implant Dentistry. 2nd ed. St Louis: Mosby; 1999. pp. 329–43. [Google Scholar]
- 24.Elkarargy A. The use of beta-tricalcium phosphate with platelet-rich plasma in maxillary anterior ridge expansion with simultaneous implant placement. Egyptian Dent J. 2009:55. [Google Scholar]
- 25.Block MS, Finger I, Lytle R. Human mineralized bone in extraction sites before implant placement: Preliminary results. J Am Dent Assoc. 2002;133:1631–8. doi: 10.14219/jada.archive.2002.0112. [DOI] [PubMed] [Google Scholar]
- 26.An X, Lee C, Fang Y, Choi BH. Immediate nonfunctional loading of implants placed simultaneously using computer-guided flapless maxillary crestal sinus augmentation with bone morphogenetic protein-2/collagen matrix. Clin Implant Dent Relat Res. 2019 doi: 10.1111/cid.12831. doi: 101111/cid 12831. [DOI] [PubMed] [Google Scholar]
- 27.Barone A, Orlando B, Cingano L, Marconcini S, Derchi G, Covani U. A randomized clinical trial to evaluate and compare implants placed in augmented versus non-augmented extraction sockets: 3-year results. J Periodontol. 2012;83:836–46. doi: 10.1902/jop.2011.110205. [DOI] [PubMed] [Google Scholar]
- 28.Gazdag AR, Lane JM, Glaser D, Forster RA. Alternatives to autogenous bone graft efficacy and indications. J Am Acad Orthop Surg. 1995;3:1–8. doi: 10.5435/00124635-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002;84-A:1032–44. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]


