Abstract
Introduction:
The present study was done to access the antibacterial activity of clove (Syzygium aromaticum) against extended-spectrum beta-lactamase (ESBL), metallo-beta-lactamase (MBL), and AmpC beta-lactamase-producing gram-negative bacteria causing urinary tract infection.
Methods:
A total of 221 gram-negative uropathogens were isolated and screened for beta-lactamase (ESBL, MBL, and AmpC) production and further tested against ethanolic extract of clove (S. aromaticum) for its antibacterial activity.
Results:
Clove was effective against all gram-negative isolates but the best antibacterial activity was shown against Proteus species with 19 mm zone of inhibition, 0.39 mg/ml minimum inhibitory concentration (MIC) and 0.19 mg/ml minimum bactericidal concentration (MBC).
Conclusions:
Clove extract showed different antibacterial potential against all gram-negative uropathogens. Clove activity for particular strain was found to be similar between isolates producing beta-lactamase and non beta-lactamase.
Keywords: Extended-spectrum β-lactamases, metallo-beta-lactamase, and AmpC beta-lactamase, Syzygium aromaticum (clove), urinary tract infection, uropathogens
Introduction
There are many infectious diseases that occur during a lifetime. One of these is urinary tract infection (UTI), which is experienced by approximately 10% of population and in some cases can lead to morbidity in patients if not treated on time. UTI is caused by many different microorganisms (uropathogens) which include viruses, fungi, and bacteria but the major microorganism responsible for causing UTI in 95% cases is the bacteria.[1,2] Antibiotic resistance against these bacteria causing UTI has been reported by many authors from developed and developing countries. This rapid spread of resistance especially toward beta-lactam antibiotics is a global threat as it possesses a therapeutic challenge which is mediated by different beta-lactamases enzymes such as extended-spectrum beta-lactamase (ESBL), metallo-beta-lactamases (MBLs), and AmpC beta-lactamase. Therefore, it has led to limited choice of antibiotics due to the continuous emergence of these enzymes. Hence, it has become utmost important to find out new antibacterial agents.[3,4] Due to the emergence of resistance pattern currently, medicinal plant extracts have gained interest because of their known antimicrobial nature. Medicinal plants are the richest bioresource of drugs for traditional systems of medicine, nutraceuticals, food supplements, folk medicines, pharmaceutical intermediates, and chemical entities for synthetic drugs.[5] Many spices around the world have been used for several medicinal purposes and as food preservatives, and out of those Syzygium aromaticum (clove) is widely used as it has got anti-inflammatory, antimicrobial, antithrombotic, antioxidant, antimutagenic, and anti-ulcerogenic properties.[6] Considering the importance of clove as an antibacterial agent, the present study was designed to analyze the antibacterial potential of clove against ESBL, MBL, and AmpC Beta-lactamase producing gram-negative uropathogens.
Material and Methods
This study was conducted in the Department of Microbiology, Santosh Medical College and Hospital, Ghaziabad and Department of Microbiology, M. M. Medical College and Hospital, Solan. Approval from the Institutional Ethical Committee was obtained F. No. SU/2017/683 (16) on 26/05/2017. All the urine specimens of clinically suspected patients of UTI were sent to the microbiology laboratory from different clinical departments and processed further. All samples were cultured on the blood agar, cystine-lactose-electrolyte deficient (CLED) agar, and MacConkey agar and were incubated further at 37°C for 18 h.[7] More than 105 cfu/mL colony count for urine specimens was considered as significant bacteriuria for UTI. Bacterial identification for positive urine cultures was performed using standard microbiological tests and was further processed for antibiotic susceptibility testing using Kirby–Bauer technique on Mueller-Hinton agar (MHA) according to the Clinical and Laboratory Standards Institute (CLSI) protocol.[8,9]
ESBL, MBL, and AmpC beta-lactamase detection of all gram-negative uropathogens were performed using phenotypic methods to CLSI guidelines.
ESBL-producing isolates were screened in accordance with the zone of inhibition of ≤25 mm for ceftriaxone and ≤22 mm for ceftazidime using disc-diffusion method which was further confirmed by cephalosporin/clavulanate combination disks method.[10]
Phenotypic confirmatory test for ESBL production by cephalosporin/clavulanate combination disks method:
All isolates were inoculated in peptone water and adjusted to 0.5 McFarland unit then isolates were swabbed on to MHA. A 30 μg disk of ceftazidime and 30/10 μg disk of ceftazidime-clavulanic acid were placed on the same plate by keeping a minimum distance of 30 mm between them. Plates were further incubated for overnight at 37°C. Zone size of more than 5 mm around ceftazidime-clavulanic disk compared to ceftazidime disk alone was considered positive for ESBL production. Control strains Escherichia coli ATCC 25922 and Klebsiella pneumoniae ATCC 700603 were used for the procedure.[10]
Screening for AmpC beta-lactamases
AmpC production by the isolates was screened by a disc-diffusion method using cefoxitin disk and the zone size of <18 mm was considered possible producer. It was further confirmed using cefoxitin-cloxacillin double-disc synergy test.[11]
Phenotypic AmpC confirmatory test by cefoxitin-cloxacillin double-disc synergy test:
30 μg disk of cefoxitin and the combination of cefoxitin and 200 μg of cloxacillin were used for this study. All strains of 0.5 McFarland unit were inoculated on MHA and further kept for overnight incubation at 37°C. Equal or more than 4 mm zone size difference between both the disks was indicative of AmpC production.[11]
Screening for metallo-beta-lactamase
All the clinical isolates showing resistance to imipenem disk were considered positive for MBL screening and were further subjected to confirmation by a combined disk test.[12]
Phenotypic confirmatory test by imipenem-ethylene diamine tetraacetic acid (EDTA).
Combined disk test
MBL-screened isolates were swabbed on MHA and two disks of imipenem (10 μg) and imipenem with 10 μL of an EDTA solution were placed on the same plate, further incubated for overnight at 37°C. A zone of inhibition ≥7 mm around with the imipenem-EDTA disk compared to imipenem disk alone indicated MBL production.[12]
Collection and certification of medicinal plants
Clove (S. aromaticum) was obtained and certified (UHF herbarium no. 13632) from the Department of Forestry, Dr. Y. S. Parmar University of Horticulture and Forestry, Nauni, Solan, Himachal Pradesh, India.
Preparation of plant extract
The plant parts (buds of S. aromaticum) were separated, washed, and dried in shade. The plant extract was prepared with 800 g of dry plant powder soaked in 2.5 L of 70% ethanol, for 8–10 days, and stirred every 10 h using a sterilized glass rod. At the end of extraction, it was passed through Whatman filter paper no. 1 (Whatman Ltd., England). This ethanoic filtrate was concentrated using water bath at 40°C till the sticky semisolid mass was obtained and then was stored at 4°C for further use. The crude extract was prepared by dissolving known amount of the dry extract in dimethyl sulfoxide (DMSO) to have a stock solution of 100 mg/ml concentration.[13]
Antibacterial activities of plant extracts
Antibacterial activity of ethanolic clove extract was carried out by disc-diffusion method. The turbidity of the culture was adjusted to 0.5 McFarland standards. Culture suspensions were inoculated on MHA so as to obtain a lawn culture. Sterile paper discs (6 mm, HiMedia, Mumbai) were impregnated with 20 μl of the different concentrations (100, 50, 25, 12.5, and 6.25 mg/ml) of plant extracts and were placed on the inoculated agar. For negative control, discs impregnated with 20 μL of 70% ethanol were placed at the center of inoculated MHA. Culture plates were incubated at 37°C for 24 h. After incubation period, the zones of inhibition were measured.[14]
Determination of minimum inhibitory concentration and minimum bactericidal concentration
The lowest concentration of the antibacterial agent at which there is no visible growth seen is considered as minimum inhibitory concentration (MIC). The broth microdilution method was used for the estimation of MIC. Microplate (96 polystyrene well) was used for the preparation of different concentrations of clove extract (100 mg/ml to 6.25 mg/ml) by serial dilution. The final concentration of each strain suspension was adjusted to 5 × 105 CFU/ml with 10 μL aliquot of bacterial suspension in supplemented MH broth, in a final volume of 100 μL. Positive growth control, negative controls, and color control (wells containing only extracts) were also prepared. The plates were covered with a sterile plate sealer, carefully mixed and incubated at 37°C for 24 h. Bacterial growth was indicated by the turbidity, relative to the negative and positive controls. The minimum bactericidal concentration (MBC) was obtained by subculturing from each well of microplate onto a nutrient agar plate. The well containing the lowest concentration of the extract that failed to show growth, on subculture was considered as MBC for that test strain.[15,16]
Results
The selection of S. aromaticum for this study was based on ethnobotanical data on the traditional use of it in the treatment of bacterial diseases. Antibacterial potential of ethanolic extract of S. aromaticum was tested against a total of 221 gram-negative uropathogens [Table 1].
Table 1.
Distribution of uropathogens
| Uropathogens | Total (221) |
|---|---|
| Escherichia coli | 100 |
| Klebsiella pneumoniae | 37 |
| Pseudomonas aeruginosa | 25 |
| Enterobacter species | 20 |
| Citrobacter species | 17 |
| Acinetobacter baumannii | 12 |
| Proteus species | 10 |
Antibacterial activity of Syzygium aromaticum against E. coli
Of the 100 E. coli isolates, 32 (32%) were ESBL producers, 18 (18%) were AmpC producers and 50 were non (ESBL, AmpC, and MBL) producing strains [Table 2].
Table 2.
Distribution of E. coli isolates
| ESBL-producing strains | AmpC-producing strains | Non (ESBL, AmpC, and MBL)-producing strains |
|---|---|---|
| 32 | 18 | 50 |
S. aromaticum was tested against all E. coli isolates for zone of inhibition, MIC, and MBC. The maximum zone of inhibition (17 mm) was shown at 100 mg/ml concentration and minimum zone of inhibition (6 mm) was shown at 6.25 mg/ml. MIC was found to be 0.39 mg/ml, and MBC was 0.19 mg/ml for all E. coli [Table 3].
Table 3.
Antibacterial activity of clove (Syzygium aromaticum) against E. coli
| E. coli: 100 | Average zone of inhibition (mm) at different concentrations (mg/ml) | MIC (mg/ml) | MBC (mg/ml) | ||||
|---|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.25 | |||
| ESBL-producing strains: 32 | 17 | 15 | 11 | 8 | 7 | 0.39 | 0.19 |
| AmpC-producing strains: 18 | 17 | 14 | 11 | 9 | 7 | 0.39 | 0.19 |
| Non (ESBL, AmpC, and MBL) producing strains: 50 | 17 | 15 | 12 | 9 | 6 | 0.39 | 0.19 |
Antibacterial activity of S. aromaticum against Klebsiella pneumoniae
Of the total 37 K. pneumoniae isolates, 14 (38%) were ESBL producers, 07 (19%) were AmpC producers and 16 (43%) were non (ESBL, AmpC, and MBL) producing strains [Table 4].
Table 4.
Distribution of K. pneumoniae isolates
| ESBL-producing strains | AmpC-producing strains | Non (ESBL, AmpC, and MBL)-producing strains |
|---|---|---|
| 14 | 07 | 16 |
All K. pneumoniae isolates were tested against S. aromaticum and maximum zone of inhibitions (16 mm) was shown at 100 mg/ml concentration and minimum zone of inhibitions (09 mm) was shown at 6.25 mg/ml. MIC was 0.78 mg/ml and MBC was 0.39 mg/ml for all K. pneumoniae isolates [Table 5].
Table 5.
Antibacterial activity of clove (S. aromaticum) against K. pneumoniae
| K. pneumoniae: 37 | Average zone of inhibition (mm) at different concentrations (mg/ml) | MIC (mg/ml) | MBC (mg/ml) | ||||
|---|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.25 | |||
| ESBL producing strains: 14 | 16 | 13 | 11 | 10 | 10 | 0.78 | 0.39 |
| AmpC producing strains: 07 | 15 | 12 | 10 | 10 | 09 | 0.78 | 0.39 |
| Non (ESBL, AmpC, and MBL) producing strains: 16 | 16 | 13 | 11 | 10 | 09 | 0.78 | 0.39 |
Antibacterial activity of S. aromaticum against Enterobacter species
Of the 20 Enterobacter speciesisolated, 4 (20%) were ESBL producers and 16 (80%) were non (ESBL, AmpC, and MBL) producing strains [Table 6].
Table 6.
Distribution of Enterobacter species
| ESBL-producing strains | Non (ESBL, AmpC, and MBL)-producing strains |
|---|---|
| 04 | 16 |
S. aromaticum showed maximum zone of inhibition (17 mm) at 100 mg/ml concentration and minimum zone of inhibition (08 mm) at 6.25 mg/ml. MIC was 0.78 mg/ml and MBC was 0.39 mg/ml for all Enterobacter isolates [Table 7].
Table 7.
Antibacterial activity of clove (S. aromaticum) against Enterobacter species
| Enterobacter species: 20 | Average zone of inhibition (mm) at different concentrations (mg/ml) | MIC (mg/ml) | MBC (mg/ml) | ||||
|---|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.25 | |||
| ESBL-producing strains: 4 | 17 | 15 | 12 | 10 | 08 | 0.78 | 0.39 |
| Non (ESBL, AmpC, and MBL)-producing strains: 16 | 16 | 15 | 12 | 09 | 08 | 0.78 | 0.39 |
Antibacterial activity of S. aromaticum against Citrobacter species
Of the 17 Citrobacter species, 5 (29%) were ESBL producers and 12 (71%) were non (ESBL, AmpC, and MBL) producing strains [Table 8].
Table 8.
Distribution of Citrobacter species
| ESBL-producing Strains | Non (ESBL, AmpC, and MBL)-producing strains |
|---|---|
| 05 | 12 |
The maximum zone of inhibition (18 mm) was observed at 100 mg/ml concentration and minimum zone of inhibition (08 mm) at 6.25 mg/ml. MIC was 0.39 mg/ml and MBC was 0.19 mg/ml for all Citrobacter isolates [Table 9].
Table 9.
Antibacterial activity of clove (S. aromaticum) against Citrobacter species
| Citrobacter species: 17 | Average zone of inhibition (mm) at different concentrations (mg/ml) | MIC (mg/ml) | MBC (mg/ml) | ||||
|---|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.25 | |||
| ESBL-producing strains: 05 | 18 | 15 | 12 | 12 | 08 | 0.39 | 0.19 |
| Non (ESBL, AmpC, and MBL)-producing strains: 12 | 18 | 16 | 13 | 11 | 08 | 0.39 | 0.19 |
Antibacterial activity of S. aromaticum against Proteus species
Of the10 Proteus species, 03 (30%) were ESBL producers and 07 (70%) were non (ESBL, AmpC, and MBL) producing strains [Table 10].
Table 10.
Distribution of Proteus species
| ESBL-producing strains | Non (ESBL, AmpC, and MBL)-producing strains |
|---|---|
| 03 | 07 |
S. aromaticum revealed maximum zone of inhibition (19 mm) at 100 mg/ml concentration and minimum zone of inhibition (09 mm) at 6.25 mg/ml. MIC was 0.39 mg/ml and MBC was 0.19 mg/ml for all Proteus isolates [Table 11].
Table 11.
Antibacterial activity of clove (S. aromaticum) against Proteus species
| Proteus species: 10 | Average zone of inhibition (mm) at different concentrations (mg/ml) | MIC (mg/ml) | MBC (mg/ml) | ||||
|---|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.25 | |||
| ESBL-producing strains: 03 | 19 | 17 | 12 | 10 | 09 | 0.39 | 0.19 |
| Non-ESBL-producing strains: 07 | 19 | 16 | 12 | 10 | 09 | 0.39 | 0.19 |
Antibacterial activity of S. aromaticum against Pseudomonas aeruginosa
Of the 25 Pseudomonas aeruginosa isolates, 07 (28%) isolates were MBL producers and rest 18 (72%) were Non (ESBL, AmpC, and MBL) producing strains [Table 12].
Table 12.
Distribution of P. aeruginosa
| MBL-producing strains | Non (ESBL, AmpC, and MBL)-producing strains |
|---|---|
| 07 | 18 |
S. aromaticum revealed maximum zone of inhibition (14 mm) at 100 mg/ml concentration and minimum zone of inhibition (09 mm) at 6.25 mg/ml. MIC was 1.56 mg/ml and MBC was 0.78 mg/ml for all P. aeruginosa [Table 13].
Table 13.
Antibacterial activity of clove (S. aromaticum) against P. aeruginosa
| P. aeruginosa: 25 | Average zone of inhibition (mm) at different concentrations (mg/ml) | MIC (mg/ml) | MBC (mg/ml) | ||||
|---|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.25 | |||
| MBL-producing strains: 07 | 14 | 13 | 12 | 10 | 09 | 1.56 | 0.78 |
| Non-MBL-producing strains: 18 | 14 | 13 | 11 | 10 | 09 | 1.56 | 0.78 |
Antibacterial activity of S. aromaticum against Acinetobacter baumannii
Of the 12 Acinetobacter baumannii isolates, 04 (33%) were MBL producers and 08 (67%) were non (ESBL, AmpC, and MBL) producing strains [Table 14].
Table 14.
Distribution of A. baumannii
| MBL-producing strains | Non (ESBL, AmpC, and MBL)-producing strains |
|---|---|
| 04 | 08 |
S. aromaticum showed maximum zone of inhibition (18 mm) at 100 mg/ml concentration and minimum zone of inhibition (09 mm) at 6.25 mg/ml. MIC was 0.78 mg/ml and MBC was 0.39 mg/ml for all A. baumannii isolates [Table 15].
Table 15.
Antibacterial activity of clove (S. aromaticum) against A. baumannii
| A. baumannii: 12 | Zone of inhibition (mm) at different concentrations (mg/ml) | MIC (mg/ml) | MBC (mg/ml) | ||||
|---|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.25 | |||
| MBL-producing strains: 04 | 18 | 15 | 12 | 10 | 09 | 0.78 | 0.39 |
| Non-MBL-producing strains: 08 | 18 | 15 | 11 | 10 | 09 | 0.78 | 0.39 |
Discussion
Infections caused by beta-lactamase-producing bacteria continue to pose serious health problems in the world and particularly in developing countries. The use of medicinal plant extracts is nowadays essential in the search for new active antibacterial biomolecules against antibiotics. Clove extract acts through the presence of potentially bioactive components such as eugenol (2-methoxy-4-(2-propenyl) phenol), glycosides, flavonoids, saponins and tannins, and essential oils. These bioactive components have shown several bioactivities like antipyretic, antispasmodic, anticarcinogenic, inhibition of 5-LOX enzyme activity in human polymorphonuclear leukocytes cells, antioxidant, protection against peroxynitrite-mediated tyrosine nitration and lipid peroxidation, antifungal activity of essential oil, antimicrobial, and, antibacterial. Hence, in vitro evaluation of antibacterial activity of clove was screened against seven bacterial strains: E. coli, K. pneumoniae, Enterobacter species, Citrobacter species, Proteus species, P. aeruginosa, and A. baumannii.
Our studyshowed a range of6–17 mm zone of inhibitions against E. coli at different concentrations of clove. Mostafa et al.[17] reported a 12 mm zone of inhibition at 15 mg/ml, however, the same zone of inhibitions came at 25 mg/ml in our study. Other studies also revealed the zone of inhibition of E. coli ranging from 17–19 mm against clove which are in accordance with our study.[18,19,20] Clove demonstrated its best antibacterial activity against E. coli and Proteus species as they had maximum inhibition zones and minimum MIC and MBC values [Figures 1 and 2]. MIC and MBC for E. coli turned out to be 0.39 and 0.19 mg/ml, respectively whereas in a study done by Noumedem et al.[21] they reported 0.51–1.02 mg/ml MIC. Another study by Pundir et al. reported 10 mg/ml MIC value, which was higher compared to the present study against E. coli.[22]
Figure 1.
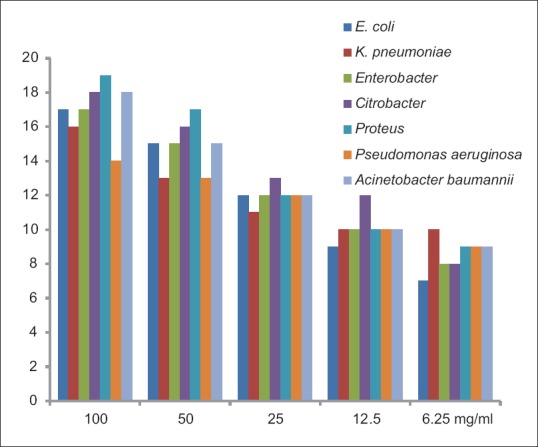
Maximum zone of inhibition (mm) of clove against uropathogens
Figure 2.
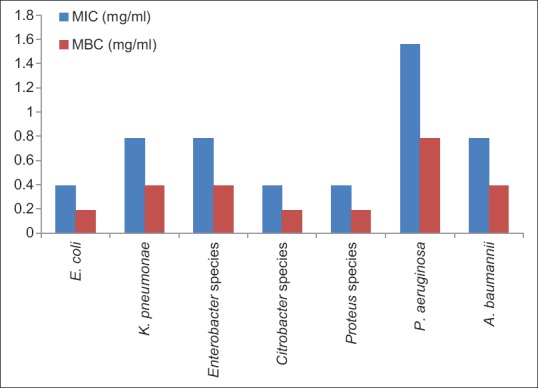
Average minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values of clove against uropathogens
K. pneumoniae showed 9–16 mm inhibitory zones in our study. Sethi et al.[18] also reported 17 mm maximum zone of inhibition while Gupta et al.[19] reported 15 mm zone of K. pneumoniae against clove which was close to our study. Researchers from University of Dschang reported MIC 1.02 mg/ml which was in concordance to our study.[21] Sethi et al. and Nascimento et al. demonstrated high values of MIC against K. pneumoniae which isin contrast to present study.[18,23] Sharmeen et al. have also concluded that clove extract had significant antibacterial activity against K. pneumoniae.[24]
Although, very few studies have been done on antibacterial activity of clove against Enterobacter species, Citrobacter species, Proteus species, and A. baumannii. The present study revealed that all these clinicalisolates responded good sensitivity to clove. Noumedem et al.'s study also reported significant antibacterial property against different Enterobacter strains which was quite similar to our results.[21] On the other hand, researchers from Brazil did not observe any antibacterial activity against Enterobacter aerogenes.[23] Sethi et al. stated that 100% concentration of clove showed best antibacterial activity (19 mm) against Citrobacter of the seven different pathogenic strains.[18] Clove demonstrated best activity against Proteus species in our study which was supported by studies done by other researchers from different countries.[18,23] A study done in Iraq showed 28 mm inhibition zone at 10% clove concentration whereas our study showed maximum 18 mm zone at 100 mg/ml concentration.[25]
Mostafa et al. applied different concentrations (1.25–15.0 mg/ml) against P. aeruginosa and they observed maximum zone of inhibition of 17.5 ± 0.35 mm at highest concentration.[17] Other authors from different countries, Sulieman et al. and Nascimento et al. also demonstrated range a of 7–20 mm inhibition zones at different concentrations which were in concordance to present study.[20,23] In contrast to our findings, Mehrotra et al. and Noumedem et al. reported MIC value of 0.025 mg/ml and 0.2 mg/ml, respectively.[21,26]
Medicinal plants and primary care physicians
The global prevalence of the use of medicinal plants or its product continues to rise as patients self-medicate with or without informing their physicians. In India, physicians generally do not ask the patients about having used herbal preparations while taking their history. But it is very important that primary care physicians must ask the patient about any prior use of medicinal plant or preparation especially when presenting with unusual signs and symptoms while prescribing allopathic medicine as they may cause side effects and adverse reactions. On the other hand, Traditional Medicines Programme by WHO encourages countries to identify aspects of traditional medicine from medicinal plants that provide safe and effective remedies and utilize these aspects in primary health care. Medicinal plants are one aspect of the traditional medicine, should be incorporated into primary health care because many individuals already use medicinal plants, they could be an effective way to alleviate problems caused by the high demand and limited availability of modern medicines in primary health care setting in developing countries. Before inclusion into national policies and protocols, medicinal plants must be studied at local levels for effectiveness.[27,28]
Conclusion
Thus, ethanolic extracts of clove at all concentrations were found to be effective against all clinical isolates and provided baseline information for the potential use of clove to treat bacterial infections. Hence, our study concluded that clove extracts can be used to develop new antimicrobial drug which is the need of the hour. However, further research is required for the identification and characterization of bioactive molecules present in the clove extract and their in vivo antibacterial activities against human pathogens.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
I humbly thank the Department of Forestry, Dr. Y. S. Parmar University of Horticulture and Forestry, Solan, Himachal Pradesh for clove plant authentication and certification.
References
- 1.Moroh J-LA, Fleury Y, Tia H, Bahi C, Lietard C, Coroller L, et al. Diversity and antibiotic resistance of uropathogenic bacteria from Abidjan. Afr J Urol. 2014;20:18–24. [Google Scholar]
- 2.Farajnia S, Alikhani MY, Ghotaslou R, Naghili B, Nakhlband A. Causative agents and antimicrobial susceptibilities of urinary tract infections in the northwest of Iran. Int J Infect Dis. 2009;13:140–4. doi: 10.1016/j.ijid.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Baral P, Neupane S, Marasini BP, Ghimire KR, Lekhak B, Shrestha B. High prevalence of multidrug resistance in bacterial uropathogens from Kathmandu, Nepal. BMC Res Notes. 2012;5:38. doi: 10.1186/1756-0500-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sultan A, Rizvi M, Khan F, Sami H, Shukla I, Khan HM. Increasing antimicrobial resistance among uropathogens: Is fosfomycin the answer? Urol Ann. 2015;7:26–30. doi: 10.4103/0974-7796.148585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad I, Beg AZ. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol. 2001;74:113–23. doi: 10.1016/s0378-8741(00)00335-4. [DOI] [PubMed] [Google Scholar]
- 6.Okmen G, Mammadhkanli M, Vurkun M. The antibacterial activities of Syzygium aromaticum (L) Merr and Perry against oral bacteria and its antioxidant and antimutagenic activities. Int J Pharm Sci Res. 2018;9:4634–41. [Google Scholar]
- 7.Sewify M, Nair S, Warsame S, Murad M, Alhubail A, Behbehani K, et al. Prevalence of urinary tract infection and antimicrobial susceptibility among diabetic patients with controlled and uncontrolled glycemia in Kuwait. J Diabetes Res. 2016;2016:6573215. doi: 10.1155/2016/6573215. doi: 10.1155/2016/6573215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehrishi P, Faujdar SS, Kumar S, Solanki S, Sharma A. Antibiotic susceptibility profile of uropathogens in rural population of Himachal Pradesh, India: Where we are heading? Biomed Biotechnol Res J. 2019;3:171–5. [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twentieth Informational Supplement M100-S20. Wayne, PA: Clinical and Laboratory Standards Institute (CLSI); 2010. pp. 32–43. [Google Scholar]
- 10.Malik N, Bisht D, Faujdar SS. Extended spectrum β-lactamases and metallo-β-lactamases production in Klebsiella pneumoniae isolates causing pneumonia in rural population of Uttar Pradesh. Int J Curr Microbiol App Sci. 2019;8:1732–8. [Google Scholar]
- 11.Polsfuss S, Bloemberg GV, Giger J, Meyer V, Böttger EC, Hombach M. Practical approach for reliable detection of AmpC beta-lactamase-producing Enterobacteriaceae. J Clin Microbiol. 2011;49:2798–803. doi: 10.1128/JCM.00404-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panchal CA, Oza SS, Mehta SJ. Comparison of four phenotypic methods for detection of metallo-β-lactamase-producing Gram-negative bacteria in rural teaching hospital. J Lab Physicians. 2017;9:81–3. doi: 10.4103/0974-2727.199624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad I, Aqil F. In vitro efficacy of bioactive extracts of 15 medicinal plants against ESbL-producing multidrug-resistant enteric bacteria. Microbiol Res. 2007;162:264–75. doi: 10.1016/j.micres.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Efstratiou E, Hussain AI, Nigam PS, Moore JE, Ayub MA, Rao JR. Antimicrobial activity of Calendula officinalis petal extracts against fungi, as well as Gram-negative and Gram-positive clinical pathogens. Complement Ther Clin Pract. 2012;18:173–6. doi: 10.1016/j.ctcp.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6:71–9. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soberón JR, Sgariglia MA, Sampietro DA, Quiroga EN, Vattuone MA. Antibacterial activity of plant extracts from northwestern Argentina. J Appl Microbiol. 2007;102:1450–61. doi: 10.1111/j.1365-2672.2006.03229.x. [DOI] [PubMed] [Google Scholar]
- 17.Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci. 2018;25:361–6. doi: 10.1016/j.sjbs.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sethi S, Dutta A, Gupta BL, Gupta S. Antimicrobial activity of spices against isolated food borne pathogens. Int J Pharm Pharm Sci. 2013;5:260–2. [Google Scholar]
- 19.Gupta C, Garg AP, Uniyal RC, Kumari A. Antimicrobial activity of some herbal oils against common food-borne pathogens. Afr J Microbiol Res. 2008;2:258–61. [Google Scholar]
- 20.Sulieman AME, El Boshra IMO, El Khalifa EAA. Nutrient value of clove (Syzygium aromaticum) and detective of antimicrobial effects of its bud oil. Res J Microbiol. 2007;2:266–71. [Google Scholar]
- 21.Noumedem JA, Mihasan M, Kuiate JR, Stefan M, Cojocaru D, Dzoyem JP, et al. In vitro antibacterial and antibiotic-potentiation activities of four edible plants against multidrug-resistant gram-negative species. BMC Complement Altern Med. 2013;13:190. doi: 10.1186/1472-6882-13-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pundir RK, Jain P, Sharma C. Antimicrobial activity of ethanolic extracts of Syzygium aromaticum and Allium sativum against food associated bacteria and fungi. Ethnobotanical Leaflets. 2010;14:344–60. [Google Scholar]
- 23.Nascimento GGF, Locatelli J, Freitas PC, Silva GL. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz J Microbiol. 2000;31:247–56. [Google Scholar]
- 24.Sharmeen R, Hossain MN, Rahman MM, Foysal MJ, Miah MF. In-vitro antibacterial activity of herbal aqueous extract against multi-drug resistant Klebsiella sp. isolated from human clinical samples. Int Curr Pharm J. 2012;1:133–7. [Google Scholar]
- 25.Abdullah BH, Hatem SF, Jumaa W. A comparative study of the antibacterial activity of clove and rosemary essential oils on multidrug resistant bacteria. UK J Pharm Biosci. 2015;3:18–22. [Google Scholar]
- 26.Mehrotra S, Srivastava AK, Nandi SP. Comparative antimicrobial activities of neem, amla, aloe, assam tea and clove extracts against Vibrio cholerae, Staphylococcus aureus and Pseudomonas aeruginosa. J Med Plants Res. 2010;4:2393–8. [Google Scholar]
- 27.Al-Omaim DA, Al-Arfaj G, Abbas MAF. Knowledge and attitude of primary care physicians toward use of herbs as medicine in Al-wazarat health center, prince sultan military medical city, Riyadh, Saudi Arabia. Int J Pharm Sci Res. 2018;9:2859–68. [Google Scholar]
- 28.Lazarou R, Heinrich M. Herbal medicine: Who cares? The changing views on medicinal plants and their roles in British lifestyle. Phytother Res. 2019;33:2409–20. doi: 10.1002/ptr.6431. [DOI] [PubMed] [Google Scholar]


