Abstract
Background:
Non-alcoholic fatty liver disease (NAFLD) is an important etiology for the development of chronic liver disease worldwide. Its pathophysiology includes chronic low-grade inflammation. There are limited studies on the association of inflammatory markers with NAFLD. Hence, in the present research, we aimed to study the association of one such inflammatory marker hs-CRP with NAFLD in north Indian population.
Materials and Methods:
For this cross-sectional study, 100 subjects of either sex above 18 years of age, being diagnosed as a case of NAFLD on the basis of ultrasonography and age, sex and BMI matched subjects fulfilling the inclusion and exclusion criteria were included. Anthropometric profile, high-sensitivity C-reactive protein (hs-CRP), HbA1c, and hepatic function tests were recorded.
Results:
The baseline variables were matched for age, weight, BMI, waist-hip circumference ratio, and blood pressure. The HbA1c (P < 0.001), alanine aminotransferase (P = 0.002), alkaline phosphatase (0.002), and hs-CRP (P < 0.001) were elevated in subjects with NAFLD. The mean level of hs-CRP was significantly higher in subjects with NAFLD as compared to the control group (3.12 ± 1.42 mg/L vs 1.05 ± 0.44 mg/L, P < 0.001). The mean hs-CRP level was 1.42 ± 0.55 mg/L in grade 1, 0.98 ± 0.72 mg/L in grade 2 with P < 0.001, and 4.5 ± 1.11 mg/L in grade 3 with P < 0.001 when compared to grade 1.The comparative value of hs-CRP in the control group was found to be 1.05 ± 0.44 mg/L. On univariate analysis waist-hip circumference ratio (P = 0.035), HbA1c (P < 0.001), and hs-CRP (P < 0.001), showed a significant association with NAFLD. On logistic regression hs-CRP was found to have significant association with NAFLD even after adjusting waist-hip circumference ratio and HbA1C (odds ratio 1.311, 95% confidence interval 1.146–1.488, P < 0.001).
Conclusion:
In this cohort of north Indian population, hs-CRP showed independent relationships with NAFLD. Thus, hs-CRP may be used as a surrogate marker for the disease severity in NAFLD.
Keywords: hs-CRP, inflammation, non-alcoholic fatty liver disease
Introduction
Fatty liver is a clinical condition characterized by and commonly understood as the accumulation of lipids within hepatocytes. Traditionally, for practical purposes a, hepatic fat content exceeding 5% of the liver weight has been considered as fatty liver.[1] In the past, fatty liver was well-thought-out as a benign and reversible pathology and represented a nonspecific response of the liver to metabolic stress of various origin.[2] However, it is increasingly seen as a part of the spectrum that can be as benign as fatty liver to a fatal condition of hepatocellular carcinoma (HCC). Alcohol consumption has been the most common factor responsible for fatty liver, however, it may also be seen in non-alcoholics and the clinical entity is known as non-alcoholic fatty liver disease (NAFLD). It is commonly associated with various components of metabolic syndrome including obesity, diabetes mellitus, and dyslipidemia.[3] Various reports also suggest that NAFLD may be associated with low-grade inflammation in liver.[4] Various inflammatory markers have been assessed and evaluated in NAFLD. Of the inflammatory markers high-sensitive C-reactive protein (hs-CRP) is known to be associated with inflammation in the liver. In addition, hs-CRP has also been part of the scoring system in a Japanese study which predicts the disease progression in NAFLD. However, studies on the role of hs-CRP as a potential marker of disease progression in NAFLD has been limited in India. The prevalence of NAFLD in India is reported to be 9–32% and is known to increase with various associated factors like diabetes mellitus and dyslipidemia.[5,6] Thus, the study was conducted to assess the levels of hs-CRP in NAFLD patients compared to normal population and its role in disease severity.
Materials and Methods
The study was conducted in the Department of Medicine and Gastroenterology at a teaching tertiary care institute in north India over a period of 18 months. For the study, persons of either sex above 18 years of age and being diagnosed as a case of NAFLD on the basis of ultrasonography were included. Age, sex, and weight-matched individuals without NAFLD constituted the control group. Alcohol consumption, dyslipidemia, coronary artery disease (CAD), any acute inflammatory condition, acute and chronic liver disease, pregnancy, any organ failure, any rheumatological disorder, hypothyroidism, malignancy, obstructive sleep apnea (OSA), those on statins therapy, immunosuppressant, oral contraceptive pills or total parenteral nutrition and after surgery were excluded. Patients with drug-induced steatohepatitis (tamoxifen, glucocorticoids, isoniazid, amiodarone, methotrexate, anti-retroviral drugs, estrogen, sodium valproate, etc.) were also excluded. The study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2000 and approved by the institutional review board, and informed consent was obtained from all study subjects (Institutional ethics committee approval was obtained dated 30/10/2017, IEC/VMMC/SJH/2017-095).
Based on previous studies, the sample size was calculated with 80% power and a 5% level of significance. We enrolled 100 patients in the study group and 100 as the control group.
Standardized methods were used for measurement of height, weight, and blood pressure. Body mass index (BMI) was calculated as weight (kg)/height (m2). A nonstretchable measuring tape was used for the measurement of waist circumference. Blood pressure was measured using an aneroid sphygmomanometer in the right arm in the sitting position. Blood pressure was recorded as a mean of two readings taken 10 min apart.
Serum hs-CRP levels were measured by the latex agglutination method. The kit used in our study has cut-off sensitivity as low as 0.6 mg/dL. HbA1c was measured by a high-performance liquid chromatography method with paper electrophoresis.
Ultrasonographic examination of the liver was performed by a radiologist, using a high-resolution B-mode ultrasonography system. The criteria used for the diagnosis of fatty liver was presence of an ultrasonographic pattern consistent with “bright liver”. They were further graded based on the radiological findings.
Grade I: increased hepatic echogenicity with visible periportal and diaphragmatic echogenicity.
Grade II: increased hepatic echogenicity with imperceptible periportal echogenicity, without obscuration of the diaphragm.
Grade III: increased hepatic echogenicity with imperceptible periportal echogenicity and obscuration of the diaphragm.
Statistical analysis
Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean ± SD and median. The normality of data was tested by the Kolmogorov-Smirnov test. Quantitative variables were compared using the unpaired t-test. Qualitative variables were compared using the Chi-square test. A P value of <0. 05 was considered statistically significant. Univariate regression analysis was carried out to determine the association of NAFLD with various risk factors. Multiple logistic regression analysis was done using the disease state (NAFLD) as the dependent variable and hs-CRP as the independent variable. All analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0.
Results
Table 1 shows the baseline variable in subjects with and without NAFLD. The baseline variables were matched for age, weight, BMI, Waist-hip circumference ratio, and blood pressure. The HbA1c (P < 0.001), alanine aminotransferase (P = 0.002), alkaline phosphatase (P = 0.002), and hs-CRP (P < 0.001) were elevated in subjects with NAFLD compared to the control group. The mean level of hs-CRP was significantly higher in subjects with NAFLD as compared to the control group (3.12 ± 1.42 mg/L vs 1.05 ± 0.44 mg/L, P < 0.001).
Table 1.
General Characteristics of Subjects with and without Non-alcoholic Fatty Liver Disease
| Variable | Cases (n=100) | control (n=100) | P |
|---|---|---|---|
| Age (years) | 39.19±9.67 | 39.07±10.68 | 0.467 |
| BMI (kg/m2) | 25.75±2.7 | 25.31±3.28 | 0.148 |
| Waist-hip Circumference ratio | 0.99±0.1 | 0.99±0.1 | 0.454 |
| Systolic Blood pressure (mmHg) | 120±15 | 121±13 | 0.38 |
| Diastolic Blood pressure (mmHg) | 72±12 | 74±11 | 0.36 |
| Weight (kg) | 69.55±11.7 | 69.28±9.5 | 0.429 |
| HbA1C | 7.39±1.86 | 6.67±0.94 | <0.001 |
| Aspartate aminotransferase (IU/L) | 24.6±6.8 | 21.0±8.2 | 0.144 |
| Alanine aminotransferase (IU/L) | 28.9±10.1 | 21.5±13.0 | 0.002 |
| Alkaline phosphatase (IU/L) | 224.1±56.1 | 195.2-54.6 | 0.002 |
| hs-CRP level | 3.12±1.42 | 1.05±0.44 | <0.001 |
Data are mean–SD values. BMI=Body mass index, hs-CRP=high-sensitivity C-reactive protein
Figure 1 compares the levels of hs-CRP levels in different grades of fatty liver. The mean hs-CRP level was 1.42 ± 0.55 mg/L in grade 1, whereas in grade 2 it was 2.98 ± 0.72 mg/L with P < 0.001. In grade 3 the mean hs-CRP level was found to be 4.5 ± 1.11 mg/L with P < 0.001 when compared to grade 1. On comparison of hs-CRP levels in grade 2 and grade 3, the difference was highly significant with P < 0.001. The comparative value of hs-CRP in the control group was found to be 1.05 ± 0.44 mg/L.
Figure 1.
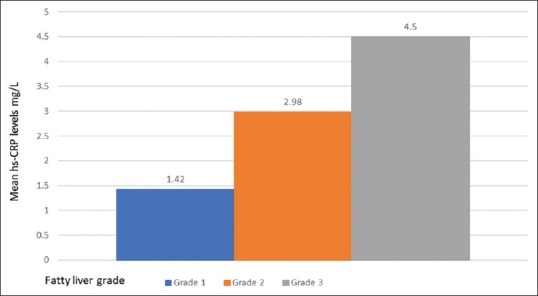
Mean high-sensitivity C-reactive protein (hs-CRP) levels in relation to severity of nonalcoholic fatty liver disease (NAFLD)
Table 2 depicts the results of the univariate analysis of NAFLD with various variables. Waist-hip circumference ratio (P = 0.035), HbA1c (P < 0.001), and hs-CRP (P < 0.001), showed a significant association with NAFLD, whereas age and BMI did not show any significant association.
Table 2.
Univariate Analysis with Non-alcoholic Fatty Liver Disease as the Dependent Variable
| Variable | Odds ratio | 95% Confidence interval | P |
|---|---|---|---|
| Age | 1.003 | 0.981-1.025 | 0.931 |
| BMI | 1.005 | 1.001-1.005 | 0.181 |
| Waist-hip circumference ratio | 1.030 | 0.989-1.046 | 0.035 |
| hs-CRP | 1.467 | 1.302-1.613 | <0.001 |
| HbA1c | 1.451 | 1.221-1.712 | <0.001 |
BMI=Body mass index; hs-CRP=high-sensitivity C-reactive protein
We also performed logistic regression analyses to identify risk factors that were independently associated with NAFLD as shown in Table 3. hs-CRP was found to have a significant association with NAFLD even after adjusting waist-hip circumference ratio and HbA1C (odds ratio 1.311, 95% confidence interval 1.146–1.488, P < 0.001).
Table 3.
Logistic Regression Analysis with Non-alcoholic Fatty Liver Disease as the Dependent Variable
| Independent Variable | Odds ratio | 95% Confidence interval | P |
|---|---|---|---|
| hs-CRP unadjusted | 1.467 | 1.302-1.613 | <0.001 |
| hs-CRP adjusted for waist hip circumference ratio, HbA1C | 1.311 | 1.146-1.488 | <0.001 |
hs-CRP=high-sensitivity C-reactive protein
Discussion
Non-alcoholic fatty liver disease (NAFLD) has become an important health hazard and is amongst the commonest causes of chronic liver disease. Various epidemiological studies have estimated the prevalence of NAFLD to be in the range from 18% to 46% in the general population.[7,8] Initially considered to be a benign disease, it has now become amongst the leading causes of chronic liver disease.
Various studies have found that obese subjects have a prevalence of 40% for non-alcoholic steatohepatitis (NASH), 23% for advanced fibrosis, and 5.8% for cirrhosis.[9] The progression of NASH to cirrhosis is seen in as many as 15–25% of patients and is now considered a very important cause of cryptogenic cirrhosis.[10]
It has a wide range of manifestations which include mild-fatty infiltration, steatohepatitis, and cirrhosis of the liver.[11] NAFLD has been associated with various components of metabolic syndrome including diabetes, obesity, dyslipidemia, hypertension, and insulin resistance.[11,12] Various authors consider NAFLD as the hepatic manifestation of metabolic syndrome.
Metabolic syndrome has been known to have chronic low-grade inflammation with the involvement of various pro-inflammatory cytokines including IL-6, IL-1β, and TNF-α.[13] The role of inflammation in NAFLD and its progression has not been clearly elucidated. However, a systematic review by Argo et al. done on liver biopsy studies, has demonstrated that the presence of both acute and/or chronic acinar and/or portal inflammation and its duration on the initial biopsy were independently associated with development of advanced fibrosis in NASH.[14] These data imply that inflammation is the key predictor of eventual histological progression to fibrosis and cirrhosis
Low-grade systemic inflammation is associated with elevated levels of various inflammatory markers including hs-CRP.[15,16,17] Consequently, hs-CR has been commonly evaluated for the pathogenesis study of atherosclerotic vascular disease and various therapeutic agents are being investigated which may alter the levels of inflammatory markers including hs-CRP.[18,19] We conducted this study to evaluate the level of hs-CRP in NAFLD and its association with various grades of fatty liver.
The current study showed a rise in levels of inflammatory marker i.e. hs-CRP in patients with NAFLD as compared to subjects without it. The levels were related to the severity of the fatty liver. The levels of hs-CRP were significant after adjusting the waist-hip circumference ratio and HbA1C levels on multivariate regression analysis. Similar findings were noted in a study by Yeniova et al. wherein hs-CRP levels were found to be higher in patients with NAFLD as compared to the control group (0.68 mg/dL vs. 0.34 mg/dL, respectively; P < 0.05). There was no significant difference between patients with simple fatty liver and NASH in terms of hs-CRP levels (P > 0.05). They also performed logistic regression analysis which revealed that hs-CRP was a strong predictor of NAFLD (odds ratio: 6.04; 95% confidence interval: 2.08–17.74).[20] In our study on logistic regression analysis we also found hs-CRP to be independently associated with NAFLD with odds ratio of 1.311; 95% confidence interval 1.146–1.488, P < 0.001. A lower odds ratio may be due to the difference in the race, dietary habits, and the genetic makeup of the enrolled subjects which are known to be important predictors in the development of NAFLD.[20] In another case-control study in north India the median (range) of hs-CRP (mg/dL) in NAFLD [2.6 (0.2–13.4)] was significantly higher than controls [1.4 (0.03–11.4), P value = 0.01]. On multivariate logistic regression analysis hs-CRP (P = 0.003) was found to be independently associated with NAFLD.[21]
A recent follow-up study was done by Lee et al. to address whether hs-CRP levels within the normal range can predict the development of NAFLD in healthy male subjects. In the 7-years follow-up, it was found that the risk for NAFLD increased as the hs-CRP level increased (P < 0.001). As the hs-CRP level increased within the healthy cohort, the risk of developing NAFLD increased.[22]
The strengths of the study are that careful inclusion/exclusion criteria were used for the enrollment of subjects. Unlike other studies, we excluded patients with dyslipidemia which in itself is associated with low-grade inflammation and may alter the levels of inflammatory markers. Our study had few limitations. Diagnosis of fatty liver and NAFLD was based on clinical history and ultrasound examination. Although ultrasound is not a gold standard, conducting liver biopsy which is the gold standard for hepatic steatosis identification, on normal population was not feasible. Moreover, we did not follow-up the patients to ascertain the progression in the grades of the fatty liver.
Despite the limitations of the study, we were able to demonstrate higher levels of hs-CRP in NAFLD subjects as compared to the control group. The levels showed a rising trend with increasing disease severity. We could also demonstrate it to be independently associated with NAFLD by logistic regression analysis. As NAFLD has been associated with lifestyle diseases and various components of metabolic syndrome, its potential reversal by lifestyle and dietary modifications becomes an important component of the treatment by the primary care and family physicians who form an integral part of the management of such population which, in turn, would help to reduce the burden of chronic liver diseases.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sherlock S, Dooley J. Diseases of the Liver and Biliary System. Oxford: Blackwell Science; 2002. [Google Scholar]
- 2.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–8. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Hernandez H, Simental-Mendia LE, Rodriguez-Ramirez G, Reyes-Romero MA. Obesity and inflammation: Epidemiology, risk factors, and markers of inflammation. Int J Endocrinol. 2013;2013:678159. doi: 10.1155/2013/678159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–49. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 6.Wong VW. Nonalcoholic fatty liver disease in Asia: A story of growth. J Gastroenterol Hepatol. 2013;28:18–23. doi: 10.1111/jgh.12011. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Takada Y, Fujimoto Y, Haga H, Oike F, Kobayashi N, et al. Nonalcoholic steatohepatitis in donors for living donor liver transplantation. Transplantation. 2007;15(83):257–62. doi: 10.1097/01.tp.0000250671.06456.3f. [DOI] [PubMed] [Google Scholar]
- 8.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 9.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(Suppl 1):S5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 10.Falck-Ytter Y, Younossi ZM, Marchesini G, McCullough AJ. Clinical features and natural history of nonalcoholic steatosis syndromes. Semin Liver Dis. 2001;21:17–26. doi: 10.1055/s-2001-12926. [DOI] [PubMed] [Google Scholar]
- 11.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: A feature of the metabolic syndrome. Diabetes. 2001;50:1844–50. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 13.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:1372–9. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 14.Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371–9. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Chiang CH, Huang CC, Chan WL, Chen JW, Leu HB. The severity of non-alcoholic fatty liver disease correlates with high sensitivity C-reactive protein value and is independently associated with increased cardiovascular risk in healthy population. Clin Biochem. 2010;43:1399–404. doi: 10.1016/j.clinbiochem.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Kuppan G, Anjana RM, Deepa M, Paramasivam P, Chandrakumar S, Kaliyaperumal V, et al. Inflammatory markers in relation to nonalcoholic fatty liver disease in urban South Indians. Diabetes Technol Ther. 2012;14:152–8. doi: 10.1089/dia.2011.0213. [DOI] [PubMed] [Google Scholar]
- 17.Ndumele CE, Nasir K, Conceicao RD, Carvalho JA, Blumenthal RS, Santos RD. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1927–32. doi: 10.1161/ATVBAHA.111.228262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saadati S, Sadeghi A, Mansour A, Yari Z, Poustchi H, Hedayati M, et al. Curcumin and inflammation in non-alcoholic fatty liver disease: A randomized, placebo controlled clinical trial. BMC Gastroenterol. 2019;19:133. doi: 10.1186/s12876-019-1055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang B, Li F, Guo J, Wang C, Xu D, Li C. Effects of liver function, insulin resistance and inflammatory factors on vascular endothelial dilation function and prognosis of coronary heart disease patients complicated with NAFLD. Exp Ther Med. 2019;17:1306–11. doi: 10.3892/etm.2018.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeniova AO, Küçükazman M, Ata N, Dal K, Kefeli A, Başyiǧit S, et al. High-sensitivity C-reactive protein is a strong predictor of non-alcoholic fatty liver disease. Hepatogastroenterology. 2014;61:422–5. [PubMed] [Google Scholar]
- 21.Nigam P, Bhatt SP, Misra A, Vaidya M, Dasgupta J, Chadha DS. Non-alcoholic fatty liver disease is closely associated with sub-clinical inflammation: A case-control study on Asian Indians in North India. PLoS One. 2013;8:e49286. doi: 10.1371/journal.pone.0049286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Yoon K, Ryu S, Chang Y, Kim HR. High-normal levels of hs-CRP predict the development of non-alcoholic fatty liver in healthy men. PLoS One. 2017;12:e0172666. doi: 10.1371/journal.pone.0172666. [DOI] [PMC free article] [PubMed] [Google Scholar]


