Abstract
Objective
Data on the treatment of patients with ovarian cancer (OC) and associated cerebral infarction (CI) are extremely limited. The objectives were to investigate the risk factors for prognosis in patients with OC and associated CI.
Methods
We retrospectively reviewed the electronic medical records of patients with OC from January 2013 to November 2018 in Peking Union Medical Hospital.
Results
In total, 2632 inpatients were diagnosed with malignant ovarian cancer in our institution, and 30 patients (1.1%) were diagnosed with OC-associated CI. The median age was 60 years (range, 37–83). The standard treatment, according to National Comprehensive Cancer Network (NCCN) guidelines, was administered to 19 patients. The median follow-up time was 19.5 months (range, 1–59 months). In total, 17 patients experienced tumor progression, and 16 of them died. In univariate analysis, overall survival was significantly associated with the D-dimer level (P=0.017), FIGO stage (P=0.014), complete cytoreduction (P<0.000) and standard treatment (P<0.000). In multivariate analysis, the standard treatment remained an independent protective factor for death (hazard ratio=0.061, 95% confidence interval=0.007–0.537, P=0.012).
Conclusion
Although the prognosis of patients with OC and associated CI was poor, those who underwent the standard treatment still benefited.
Keywords: cerebral infarction, surgery, ovarian neoplasms, prognosis
Introduction
The association between neoplastic disease and thromboembolic disorders is well known; this association was first recognized by Trousseau in 1865 and cancer-associated thrombosis has been termed Trousseau’s syndrome. The procoagulant mechanisms underlying cancer-associated thrombophilia including cerebral infarction (CI) are complex and multifactorial.1
CI impairs one’s activities of daily living and performance status. Most patients with CI will not be able to continue anticancer treatment. Therefore, the survival of cancer patients with CI is poor.2
Data of association between CI and ovarian cancer (OC) are limited. Recently, Hirokuni Takano et al reported an interesting result. In their study, 827 patients with epithelial ovarian cancer were included and, of these patients, 27 patients were with cancer-associated CI. To find out the risk factors for CI, the clinical and pathological information was retrospectively reviewed. And, univariate analysis and multivariate analysis demonstrated that the histological type of clear cell cancer was a risk factor for CI.3 However, the effect of cancer-associated CI on the prognosis of OC has not been reported. In this study, we retrospectively reviewed the clinical characteristics of patients with OC and associated CI to investigate the association between cancer-associated CI and OC, along with the risk factors for prognosis.
Methods
Study Design
Our study was approved by the ethics committee of Peking Union Medical College Hospital. The need for written informed consent was waived because of the retrospective nature of the study, and the data set was deidentified in order to protect patients’ privacy. Our study was done in compliance with the Declaration of Helsinki. We reviewed the electronic medical records of patient with OC from January 2013 to November 2018 (Supplementary Figure S1).
Patients’ information, including age, serum cancer antigen (CA)-125 level before the initial treatment, extent of surgery, optimal resection or otherwise, staging of OC according to International Federal of Gynecology and Obstetrics (FIGO) system, histological type, treatment modality and outcome, were retrospectively collected.
Enrollment in this study consisted of patients who had experienced a history of CI within 1 year prior to commencement of treatment for malignant disease or who presented with CI during initial clinical treatment for ovarian cancer; these patients were defined as cancer-associated CI for the purpose of this study and were included in the analysis.3 Then, the electronic medical records of patients with CI were reviewed. The diagnosis of CI was corroborated by both the electronic medical records, imaging diagnosis, and consultation with the attending neurologist. The conventional risk factors, including body mass index (BMI), hypertension, diabetes, hyperlipidemia and atrial fibrillation, time of CI onset, chief complaint, the results of hematology examinations, especially the D-dimer level, and Eastern Cooperative Oncology Group performance score (ECOG-PS), were evaluated.
According to the guidelines of the National Comprehensive Cancer Network (NCCN) Version 2018, for epithelial ovarian cancer (EOC), every effort should be made during the primary cytoreduction procedure to evaluate the patient for occult disease and to achieve maximum cytoreduction of all disease. For FIGO stage IA or IB high-grade EOC and Stage IC EOC, the platinum-based therapy of 3–6 cycles is recommended, and for Stages II–IV, a minimum of 6 cycles of platinum-based therapy is recommended, including at least 3 cycles of adjuvant therapy after interval debulking surgery (IDS). Meeting these requirements was defined as the standard treatment. The outcome information was collected through out-patient medical records or telephone follow-up or public security system.
Statistical Analysis
The duration of the patients’ overall survival (OS) was calculated from the date of EOC diagnosis to the date of death or last contact. Categorical variables are summarized in frequency tables, whereas continuous variables are presented as a median (range). Frequency distributions were compared using the chi-squared test, Fisher’s exact test, or likelihood ratio, and median values were compared by the nonparametric tests by Mann–Whitney U-test. Cox proportion hazards model was utilized for univariate and multivariate regression analysis of survival data. Survival curves were calculated using the Kaplan–Meier method and compared using the log-rank test. All statistics analyses were conducted using SPSS version 23 (IBM Corp, Armonk, NY, USA). A P-value <0.05 was considered statistically significant with the two-tailed hypothesis.
Results
Clinical Characteristics
Between January 2013 and November 2018, 2632 inpatients were diagnosed with OC in our institution. Of these patients, 69 patients had concomitantly CI. Thirty patients (1.1%) were diagnosed as having OC-associated CI (Supplementary Figure S1). The baseline characteristics of all patients with OC and patients with OC-associated CI are presented in Table 1.
Table 1.
The Baseline Characteristics of Ovarian Cancer Patients
| All OC Patients | Patients with OC-Associated CI | Percentagea | P-value (Chi-Square) | |
|---|---|---|---|---|
| Cases | 2632 | 30 | 1.1% | - |
| Age (years) | 50 (0–89) | 60 (37–83) | – | – |
| FIGO stage | ||||
| I | 409 (15.5%) | 5 (16.7%) | 1.2% | >0.999 |
| II | 176 (6.7%) | 4 (13.3%) | 2.3% | 0.282 |
| III | 1118 (42.5%) | 17 (56.7%) | 1.5% | 0.118 |
| IV | 201 (7.6%) | 4 (13.3%) | 3.6% | 0.413 |
| Unknown | 728 (27.7%) | 0 (0.0%) | 0.0% | – |
| Histologic classification | ||||
| Serous | 1752 (66.6%) | 19 (63.3%) | 1.2% | 0.709 |
| Mucinous | 140 (5.3%) | 1 (3.3%) | 0.7% | 0.942 |
| Endometrioid | 227 (8.6%) | 4 (13.3%) | 1.7% | 0.559 |
| Clear cell | 312 (11.9%) | 3 (10.0%) | 1.0% | 0.977 |
| Others or unknown | 201 (7.6%) | 3 (10.0%) | 1.5% | 0.890 |
Notes: Data are shown as median (range) or number (percentage). aPatients with OC-associated CI in all OC patients.
Abbreviations: OC, ovarian cancer; CI, cerebral infarction; FIGO, International Federation of Gynecology and Obstetrics.
The median age of these 30 patients with OC-associated CI was 60 years (range, 37–83 years), which was older than that of the patients with only OC (median age: 50 years; range, 0–89 years). According to the histologic classification, all 30 patients had EOC: 19 cases (63.3%) of high-grade serous carcinoma, 1 case (3.3%) of mucinous carcinoma, 4 cases (13.3%) of endometrioid carcinoma, 3 cases (10.0%) of CCC and 3 cases (10.0%) of unknown type (Table 1). According to FIGO stage, the patients included 5 cases (16.7%) of Stage I, 4 cases (13.3%) of Stage II, 17 cases (56.7%) of Stage III, and 4 cases (13.3%) of Stage IV.
OC-associated CI occurred at a greater incidence among patients with endometrioid carcinoma (1.7%) than among those with other pathological classifications. Moreover, OC-associated CI occurred at a greater incidence among patients with Stage IV OC (3.6%) than among those with other stages of OC.
Among 30 patients with OC-associated CI, the median CA-125 level before initial treatment was 432.00 U/mL (range, 12.63–11,445 U/mL). CI occurred prior to treatment in 20 patients (66.7%) and during treatment in 10 patients (33.3%). The maximum D-dimer of each patient was collected and the median D-dimer level was 4.23 mg/L (range, 0.57–42.75 mg/L; normal range, 0–0.55 mg/L). The D-dimer level was elevated in all the patients. Moreover, we randomly chose 30 ovarian cancer patients without CI in our institution, the median D-Dimer of these patients was 3.29 mg/L (range: 0.33–10.98). No significant difference was found between two groups (P= 0.119). The ECOG-PS in 15 patients (50.0%) was 3–4. And 21 patients (70.0%) had multiple CI lesions (Figure 1).
Figure 1.
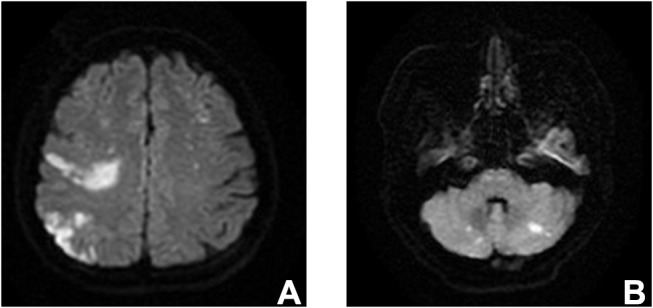
Magnetic resonance imaging.
Notes: Multiple lesions of cerebral infarction in T2-weighted-fluid-attenuated inversion recovery (A and B).
Fifteen patients (50%) had hypertension, 8 patients (26.7%) had diabetes mellitus, 2 patients (6.7%) had hyperlipidemia, 1 patient (3.3%) had atrial fibrillation, 6 patients (20%) had thrombosis in other locations and 0 patients had a history of smoking.
Therapeutic Procedures
With respect to CI, aspirin and/or other agents were administered based on the neurologist’s suggestions. Cytoreductive surgery or ovarian stage surgery was performed in 26 patients, of whom 19 patients attained complete cytoreduction (R0), which was defined as the absence of macroscopic disease. Of 20 patients in whom CI occurred before treatment, 14 patients underwent standard treatment according to the NCCN guidelines. Among 10 patients, CI occurred during the initial treatment in 9 patients and during the recurrent tumor treatment in 1 patient. Of these 10 patients, 5 received standard treatment. Overall, standard treatment was provided to 19 patients (Supplementary Table S1).
Outcomes
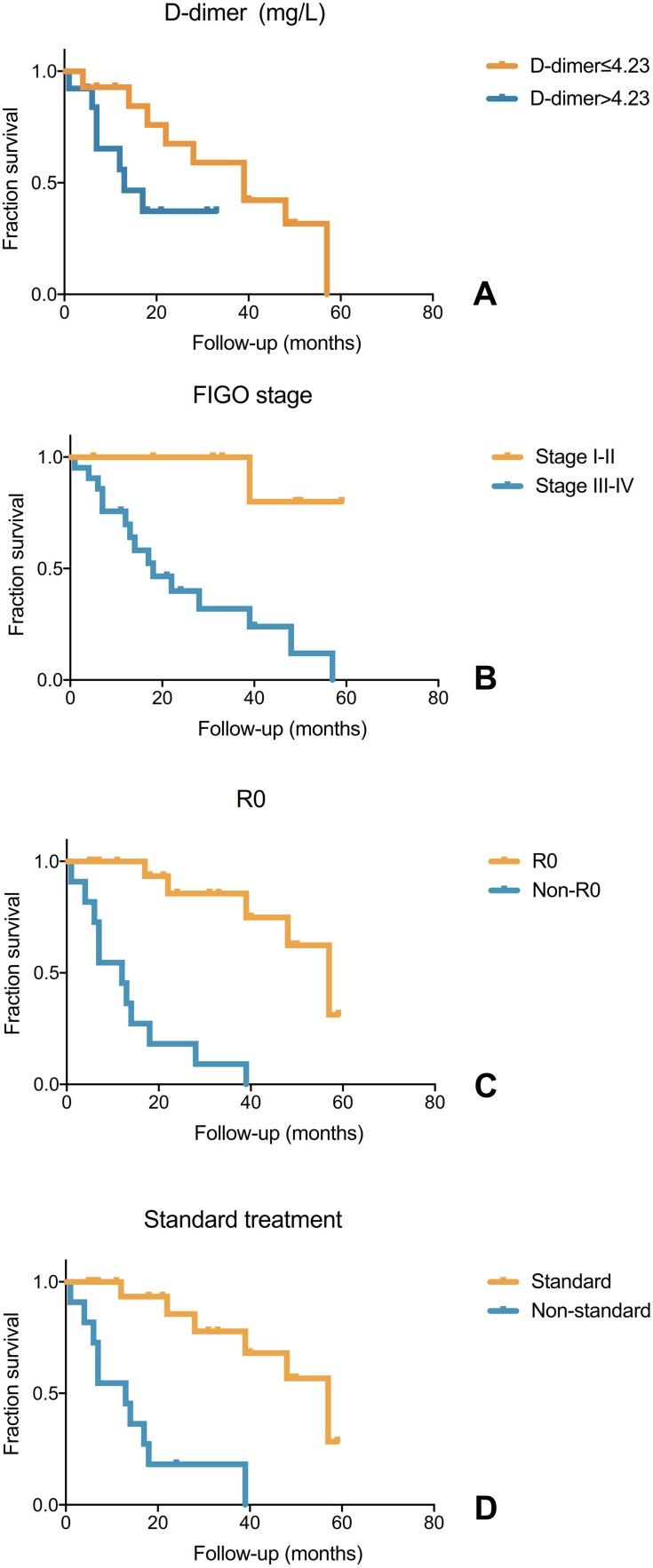
Follow-up information was available for all the 30 patients. The median follow-up time was 19.5 months (range, 1–59 months). Seventeen patients experienced tumor progression, and 16 of them died. Thus, the death rate was 53.3% (16/30). Table 2 shows risk factors for death in OC patients with cancer-associated CI. In univariate analysis, OS was significantly associated with the D-dimer level (P=0.017), FIGO stage (P=0.014), R0 (P<0.000) and standard treatment (P<0.000) (Figure 2). Age, BMI, CA-125 level before initial treatment, ECOG-PS, time of CI onset, number of CI lesions, hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, thrombosis at other locations and histologic classification were not statistically associated with OS. In multivariate analysis, standard treatment remained an independent protective factor for death (hazard ratio=0.061, 95% confidence interval=0.007–0.537, P=0.012).
Table 2.
The Prognostic Factors of Overall Survival in Univariate and Multivariate Analysis
| Factors | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| p-value | HR (95% CI) | p-value | HR (95% CI) | |
| Age (years) | 0.210 | 1.033 (0.982–1.087) | ||
| BMI (kg/m2) | 0.170 | 0.886 (0.745–1.053) | ||
| CA125 (U/mL) | ||||
| <1000 | 1 | |||
| ≥1000 | 0.176 | 2.243 (0.696–7.229) | ||
| D-dimer (mg/L) | 0.017 | 1.062 (1.011–1.116) | 0.257 | 1.029 (0.980–1.080) |
| ECOG-PS | ||||
| 1–2 | 1 | |||
| 3–4 | 0.717 | 1.200 (0.449–3.208) | ||
| Onset of CI | ||||
| Prior treatment | 1 | |||
| During treatment | 0.321 | 1.718 (0.591–4.995) | ||
| CI lesions | ||||
| Single | 1 | |||
| Multiple | 0.371 | 1.684 (0.537–5.279) | ||
| FIGO stage | ||||
| I–II | 1 | 1 | ||
| III–IV | 0.014 | 12.78 (1.665–98.124) | 0.127 | 8.691 (0.541–139.734) |
| Hypertension | ||||
| No | 1 | |||
| Yes | 0.898 | 0.931 (0.314–2.763) | ||
| Diabetes mellitus | ||||
| No | 1 | |||
| Yes | 0.871 | 0.915 (0.312–2.683) | ||
| Hyperlipidemia | ||||
| No | 1 | |||
| Yes | 0.219 | 2.584 (0.569–11.728) | ||
| Atrial fibrillation | ||||
| No | 1 | |||
| Yes | 0.497 | 0.043 (0.000–372.579) | ||
| Thrombosis on other locations | ||||
| No | 1 | |||
| Yes | 0.580 | 0.654 (0.145–2.939) | ||
| Histologic classification | ||||
| High-grade serous | 0.243 | 1.968 (0.632–6.132) | ||
| Others | 1 | |||
| R0 | ||||
| No | 1 | 1 | ||
| Yes | 0.000 | 0.073 (0.020–0.268) | 0.076 | 0.240 (0.050–1.158) |
| Standard treatment | ||||
| No | 1 | 1 | ||
| Yes | 0.000 | 0.108 (0.031–0.361) | 0.012 | 0.061 (0.007–0.537) |
Abbreviations: BMI, body mass index; ECOG-PS, Eastern Cooperative Oncology Group performance score; CI, cerebral infarction; FIGO, International Federal of Gynecology and Obstetrics; R0, complete cytoreduction.
Figure 2.
The prognostic factors of overall survival.
Notes: Overall survival according to D-dimer (A), International Federal of Gynecology and Obstetrics (FIGO) stage (B), complete cytoreduction (R0) (C) and standard treatment (D).
Discussion
Traditionally, evaluation of the association between thrombosis and malignancy has been focused on the occurrence of venous thromboembolism. Arterial thrombosis in patients with cancer has received much less attention.1 Values of the global burden of arterial thrombosis in the general population for the two common artery acute diseases, i.e., acute myocardial infarction and ischemic stroke, have been estimated to be 139.3 and 114.3 per 100 000 people, respectively, accounting for an overall incidence rate of 0.25%.4 Male sex, age, hypertension, smoking, lung cancer, and kidney cancer were associated with a higher risk of arterial thromboembolism.5 The brain is frequently injured by this pathophysiology because of the rich distribution of the procoagulation factor, thromboplastin, combined with the low levels of the anticoagulation factor, thrombomodulin, in the epithelium of brain.6
Cancer and CI are the second and third causes of death worldwide, respectively.7 A total of 10.8% of patients with CI were affected by cancer.8 OC is the leading cause of death from gynecologic cancer. In 2018, it is estimated that 22,240 new diagnoses and 14,070 deaths from this neoplasm will occur in the United States.9 Surveillance, Epidemiology, and End Results Program (SEER) Cancer Statistic Review revealed that the 5-year survival of OC is about 46.5%. In patients with cancer, the development of arterial thromboembolism has been reported to be associated with a 3-fold to 5-fold increased risk of death.5,10 Data regarding the prognosis of OC patients with cancer-associated CI patients are limited.
To our knowledge, this is the first larger study discussing the prognosis of patients with OC-associated CI. We found that the incidence of OC-associated CI was greater in endometrioid carcinoma than that in other pathologic classifications. In another study, cancer-associated CI occurred at a greater incidence among patients with CCC.3 It needs to be further investigated whether OC-associated CI is more likely to occur in endometriosis-associated ovarian carcinoma. Herein, we also found that the incidence of OC-associated CI was greatest in patients with FIGO Stage IV cancer. This finding may be easily understood because with more extensive lesions, the impact of the tumor on coagulation may be greater.
In addition, cancer-associated CI has been considered as one of the etiologies of a cryptogenic stroke.11 Active cancer was present in 4.4% of patients with ischemic stroke.12 In our study, CI occurred before the initiation of treatment in 20 patients (66.7%). The identification of factors associated with the presence of occult cancer in patients with ischemic stroke patients should be adequately investigated in future appropriately designed studies.12 Further research is needed to define the subset of patients who could benefit from cancer screening.13
In line with the results from previous studies, all the patients had an elevated D-dimer level in our study. Ishikawa et al found that all patients, with Trousseau’s syndrome associated with malignant gynecological tumors, presented with significantly elevated D-dimer levels.14 Ito et al reported that the D-dimer level may be a good biomarker for cancer-associated CI.15 In our study, the D-dimer level was significantly associated with OS in univariate analysis. Kikuchi et al also reported that serial D-dimer levels may be a useful predictor of the prognosis of patients with cancer-associated CI.15 Interestingly, the D-dimer level may be an indispensable marker before and during the treatment of OC and CI.
Cancer-associated thrombosis is one of the disseminated intravascular coagulopathy (DIC) complications occurring in the course of malignancy.16 Cancer-associated stroke is more frequently with multiple territorial infarcts.17 In line with previous studies, 21 patients (70.0%) in our study had multiple CI lesions. Therefore, patients with CI but without known cancer and multiple territorial infarcts and/or with DIC should be screened for cancer.17
Prognosis was poor in CI patients with cancer.18 And the survival of cancer patients with CI was poor.2 Despite the treatment of cancer before the onset of CI, a CI may lead to palliative care in many patients with cancer.19 Consistent with the results of previous studies, 11 patients (36.7%) in our study underwent non-standard treatment in our study.
For OC patients without severe complications, it is obvious that patients benefit from the standard treatment and would be more likely to have a better prognosis. However, for OC patients with CI, it is unclear whether they still benefit from the standard treatment is unclear. Surgery, the use of central venous catheters and chemotherapeutic agents such as platinum compounds, growth factors (granulocyte colony-stimulating factor) may lead to thrombosis.6 It is unclear whether surgery and/or anti-cancer therapy are detrimental to OS. As far as we know, this is the first study to focus on this issue in patients with OC. In univariate analysis, R0 and the standard treatment were protective factors for OS, and in multivariate analysis, the standard treatment remained a protective factor. Therefore, if possible, the standard treatment is preferred for OC patients with OC and cancer-associated CI.
The limitations of this study are the inherent shortcomings of a retrospective study design. First, as 11 patients did not receive the standard treatment, progress-free survival was not estimated. Second, the data for the D-dimer level and ECOG-PS were collected at admission in 20 patients with CI prior to treatment. And the details of acute CI treatment were unclear relatively. Whereas, it may be better to collect all the data when CI occurs. Thirdly, the specific cause of death was not detailed, in other words, we cannot identify whether the patient died of OC or CI. However, this does not influence our results; no matter what is the specific cause of death, the standard treatment of OC would improve OS. A prospective, multidisciplinary study on this topic would be informative.
In conclusion, as data on treatment of OC patients with cancer-associated CI are extremely limited, this study offers new aspects to consider in patients with OC and associated CI. Although the prognosis of patients with OC and associated CI was poor, those who underwent the standard treatment still obtain a benefit.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (81572576 [Shen Keng]) and CAMS Initiative for Innovative Medicine (CAMS-2018-12M-1-002 [Keng Shen]).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.De Stefano V. Arterial thrombosis and cancer: the neglected side of the coin of Trousseau syndrome. Haematologica. 2018;103(9):1419. doi: 10.3324/haematol.2018.197814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kato M, Shukuya T, Mori K, et al. Cerebral infarction in advanced non-small cell lung cancer: a case control study. BMC Cancer. 2016;16:203. doi: 10.1186/s12885-016-2233-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takano H, Nakajima K, Nagayoshi Y, et al. Clinical associations of Trousseau’s syndrome associated with cerebral infarction and ovarian cancer. J Gynecol Oncol. 2018;29(5). doi: 10.3802/jgo.2018.29.e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. 2016;118(9):1340–1347. doi: 10.1161/CIRCRESAHA.115.306841 [DOI] [PubMed] [Google Scholar]
- 5.Grilz E, Konigsbrugge O, Posch F, et al. Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica. 2018;103(9):1549–1556. doi: 10.3324/haematol.2018.192419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikushima S, Ono R, Fukuda K, Sakayori M, Awano N, Kondo K. Trousseau’s syndrome: cancer-associated thrombosis. Jpn J Clin Oncol. 2016;46(3):204–208. doi: 10.1093/jjco/hyv165 [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umemura T, Yamamoto J, Akiba D, Nishizawa S. Bilateral cerebral embolism as a characteristic feature of patients with Trousseau syndrome. J Clin Neurosci. 2017;42:155–159. doi: 10.1016/j.jocn.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD, Jemal AA-O. Cancer statistics 2018 . CA Cancer J Clin. 2018;68(1):7–30. (1542–4863 (Electronic)). [DOI] [PubMed] [Google Scholar]
- 10.Navi BB, Reiner AS, Kamel H, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70(8):926–938. doi: 10.1016/j.jacc.2017.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navi BB, Singer S, Merkler AE, et al. Cryptogenic subtype predicts reduced survival among cancer patients with ischemic stroke. Stroke. 2014;45(8):2292–2297. doi: 10.1161/STROKEAHA.114.005784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grazioli S, Paciaroni M, Agnelli G, et al. Cancer-associated ischemic stroke: a retrospective multicentre cohort study. Thromb Res. 2018;165:33–37. (1879–2472 (Electronic)). doi: 10.1016/j.thromres.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 13.Qureshi AI, Malik AA, Saeed O, Adil MM, Rodriguez GJ, Suri MFK. Incident cancer in a cohort of 3,247 cancer diagnosis free ischemic stroke patients. Cerebrovascular Dis. 2015;39(5–6):262–268. doi: 10.1159/000375154 [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa M, Nakayama K, Ishibashi T, et al. Case series of cerebral infarction with Trousseau’s syndrome associated with malignant gynecological tumors. Mol Clin Oncol. 2016;5:138–142. (2049–9450 (Print)). doi: 10.3892/mco.2016.888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kikuchi K, Ueda A, Nagao R, et al. Changes in serial d-dimer levels predict the prognoses of trousseau’s syndrome patients. Front Neurol. 2018;9(undefined):528. doi: 10.3389/fneur.2018.00528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Conde I, Bharwani LD, Dietzen DJ, Pendurthi U, Thiagarajan P, Lopez JA. Microvesicle-associated tissue factor and Trousseau’s syndrome. J Thromb Haemost. 2007;5(1):70–74. doi: 10.1111/j.1538-7836.2006.02301.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeşilot N, Ekizoğlu E, Çoban O. Clinical features of cancer associated ischemic stroke. Noro psikiyatri ars. 2018;55(2):113–117. doi: 10.29399/npa.22999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kneihsl M, Enzinger C, Wünsch G, et al. Poor short-term outcome in patients with ischaemic stroke and active cancer. J Neurol. 2016;263(1):150–156. doi: 10.1007/s00415-015-7954-6 [DOI] [PubMed] [Google Scholar]
- 19.Naito H, Nezu T, Hosomi N, et al. Antithrombotic therapy strategy for cancer-associated ischemic stroke: a case series of 26 patients. J Stroke Cerebrovasc Dis. 2018;27(9):. doi: 10.1016/j.jstrokecerebrovasdis.2018.04.021 [DOI] [PubMed] [Google Scholar]