Abstract
Purpose
Microvascular decompression (MVD) and MVD combined with partial sensory rhizotomy (PSR) are effective surgical treatments for idiopathic trigeminal neuralgia (TN). The aim of this study was to compare the long-term outcomes of both MVD and MVD+PSR for the treatment of TN and to identify the factors that may influence the long-term outcomes after MVD or MVD+PSR.
Patients and Methods
From March 2009 to December 2017, 99 patients with idiopathic TN who underwent MVD or MVD+PSR in our hospital (40 MVD, 59 MVD+PSR) were included in the study. The indications for MVD+PSR were as follows: vessels only contacted the nerve root, absence of arterial conflict, or failing to completely decompress from the arteries or veins. All patients were treated by one neurosurgeon and were followed up for at least 1 year. The outcomes were assessed with the Barrow Neurological Institute (BNI) Pain Intensity Scale.
Results
The average follow-up duration was 63.0 months (range, 13.2–118.8 months). Patients in the MVD group were younger than those in the MVD+PSR group (55.1 years and 60.5 years, respectively, P=0.012). A total of 62.5% of the patients in the MVD group and 69.5% of the patients in the MVD+PSR group had favorable long-term outcomes. The Kaplan-Meier survival analysis showed no significant difference in long-term outcomes between the two groups (P=0.202). No factors were associated with long-term outcomes after MVD. For MVD+PSR, a long duration of the disease (odds ratio (OR) 6.967, P=0.016) was associated with unfavorable long-term outcomes, whereas pure arterial compression (OR 0.131, P=0.013) was associated with favorable long-term outcomes.
Conclusion
For patients who are not suitable to undergo pure MVD, MVD+PSR can be used as an effective alternative. For MVD+PSR, patients with a long duration of symptoms may have poor long-term outcomes, while patients with pure arterial compression may have favorable long-term outcomes.
Keywords: trigeminal neuralgia, microvascular decompression, partial sensory rhizotomy, long-term outcome, factors
Introduction
Trigeminal neuralgia (TN) is a disorder characterized by recurrent, brief, abrupt-onset and termination, electric shock-like pains, which are limited to the distribution of one or more divisions of the trigeminal nerve and triggered by innocuous stimuli.1 After being diagnosed with TN, patients are routinely treated with anticonvulsant drugs, such as carbamazepine. However, some patients who have medically refractory pain or cannot tolerate the adverse effects of drugs need surgical treatment. Surgical treatment for TN includes microvascular decompression (MVD), partial sensory rhizotomy (PSR), gamma knife radiosurgery (GKRS), percutaneous balloon compression, radiofrequency thermocoagulation and so on.
Since first proposed by Jannetta2 and after years of development, MVD has become the most effective surgical method for treating TN.3 However, there are still some patients who cannot achieve favorable long-term outcomes after receiving pure MVD. Because of the high incidence of postoperative facial dysesthesia and the high recurrence rate of TN, pure PSR has been used in recent years for patients without obvious intraoperative vascular compression.4,5 Although many neurosurgeons have performed MVD combined with PSR (MVD+PSR) on some TN patients who are not suitable to undergo pure MVD,5–7 only a few studies have reported the differences in postoperative outcomes between MVD and MVD+PSR.5 In addition, while many studies have reported the prognostic factors associated with long-term outcomes after MVD,8–19 few reports have reported the prognostic factors associated with long-term outcomes after MVD+PSR.
We followed up patients who underwent MVD or MVD+PSR at the Department of Neurosurgery of our hospital from March 2009 to December 2017 and collected their data. The purpose of this retrospective study is to provide valuable information for improving the postoperative outcomes of TN patients by comparing the long-term outcomes of both MVD and MVD+PSR in treating TN and to identify the prognostic factors associated with the long-term outcomes after MVD or MVD+PSR.
Methods
Subjects
The subjects were selected from a group of TN patients who underwent surgery at the Department of Neurosurgery, China-Japan Friendship Hospital from March 2009 to December 2017.20 A total of 181 patients with medically refractory TN underwent microneurosurgery via a retrosigmoid approach. All operations were performed by one neurosurgeon. Because the number of patients who underwent pure PSR was small (n=7), this study only included patients who underwent MVD and MVD+PSR. The inclusion criteria were as follows: idiopathic TN according to the diagnostic criteria of the International Classification of Headache Disorders, 3rd edition;1 no previous MVD or PSR; sufficient clinical data; and follow-up duration >1 year. The exclusion criteria were as follows: had secondary TN; had concurrent cranial nerve disorder, such as glossopharyngeal neuralgia; and did not agree to be followed up. All patients underwent magnetic resonance imaging (MRI) to evaluate neurovascular compression (NVC) before the operation. Based on previous studies, we divided the MRI findings of NVC into three categories: no vascular compression, only contact (the vessel touches the nerve without visible alteration of the nerve, and the cerebrospinal fluid cannot be seen between the nerve and the vessel), and obvious compression (nerve root has displacement, distortion, or indentation).21,22 We also judged the type of offending vessel on MRI.
The patients were not randomly treated with the three surgical procedures (MVD, MVD+PSR, PSR). Before surgery, we informed each patient about the advantages and disadvantages of the various surgical procedures. The general principles for selecting the surgical procedure were as follows: Patients with obvious compression (nerve root has displacement, distortion, or indentation) on MRI were recommended for pure MVD [Figure 1A and B]. MVD+PSR was recommended for patients who met the following conditions: vessels only contacted the trigeminal nerve root [Figure 1C], and pure venous compression[Figure 1D]. Before the operation, we informed patients that if conditions we found during operation were not consistent with the preoperative MRI assessment, we might change the surgical plan according to their specific conditions identified during the operation and patients’ wishes. For example, if we were unable to completely decompress arteries or the veins due to technical difficulties (there were still offending vessels, such as veins that affect the brainstem blood flow, or some perforating vessels) or the degree of vascular compression was found intraoperatively to be less severe (only contact) than that of preoperative MRI, patients were recommended to receive MVD+PSR. However, due to the high incidence of facial dysesthesia after PSR, we performed pure MVD for patients who did not want to endure facial dysesthesia. On the other hand, if we found intraoperatively that vessels obviously compress the nerve root, rather than just contacting the nerve root as indicated by preoperative MRI assessment, we would perform pure MVD. Pure PSR was only used for patients without vascular compression.
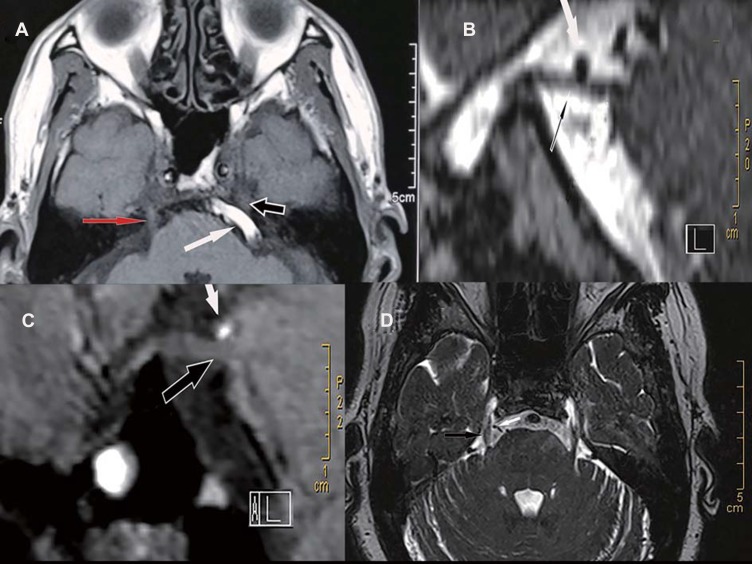
Figure 1.
Preoperative magnetic resonance imaging (MRI) images under different neurovascular compression. (A) The left basilar artery (BA; white arrow) obviously compresses the trigeminal nerve (black arrow) and causes the nerve root to displacement. Note that the trigeminal nerve on the right (red arrow) has no vascular compression and no displacement. (B) The left superior cerebellar artery (SCA; white arrow) obviously compresses the trigeminal nerve (black arrow). (C) The SCA (white arrow) only contacts the trigeminal nerve (black arrow). Note that there is no cerebrospinal fluid visualized between the nerve and the artery. No deformity of the nerve is observed. (D) Only the vein (white arrow) compresses the right trigeminal nerve (black arrow).
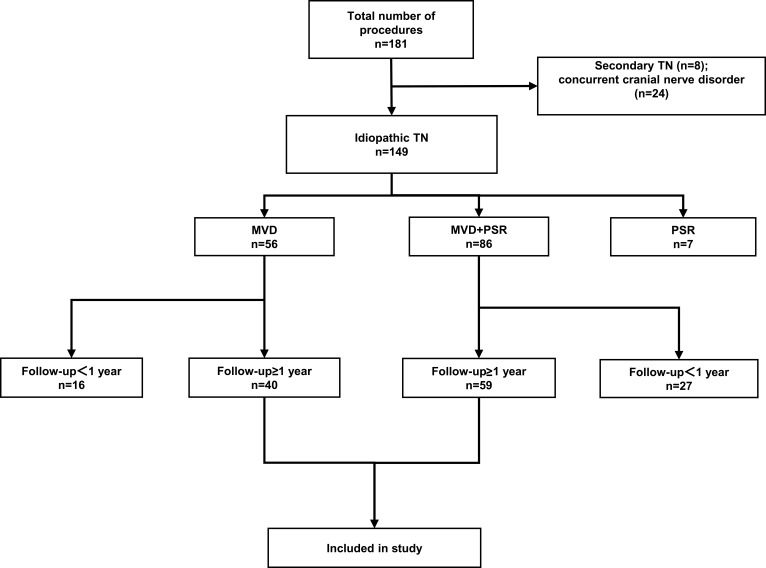
A total of 99 patients met the inclusion criteria of the study: 40 patients underwent MVD, and 59 patients underwent MVD+PSR (Figure 2). The patients signed informed consent forms before undergoing surgery. The study was approved by the Ethics Committee of the China-Japan Friendship Hospital and all patients who participated in the study signed informed consent. This manuscript follows the STROBE reporting guidelines.
Figure 2.
Flowchart showing the inclusion and exclusion criteria of patients in the study.
Abbreviations: TN, trigeminal neuralgia; MVD, microvascular decompression; PSR, partial sensory rhizotomy.
Data Collection and Outcome Measures
When the TN patient underwent surgical treatment at our hospital, the demographic and clinical data of the patient were recorded, including sex, age, duration of disease, affected side, distribution of pain, type of TN (typical or atypical TN), and history of previous ablative procedures (such as percutaneous balloon compression, radiofrequency thermocoagulation, or GRKS). Previous literature has reported that patients older than 60 years had better long-term outcomes after MVD than patients younger than 60 years,9 while patients with a long duration of the disease had unfavorable outcomes after MVD.15 Therefore, the patients were stratified by age (< 60 years or ≥ 60 years) and duration of disease (< 5 years or ≥ 5 years). Atypical TN was characterized by concomitant continuous or near-continuous pain between attacks in the affected trigeminal distribution.1 The intraoperative data of the patient were also recorded, including the posterior fossa volume (normal or small), presence of arachnoid thickening adhesion, type of offending vessel, and decompression degree. Some researchers believe that the small posterior fossa is a risk factor for NVC and may cause TN.23 We assessed the size of the posterior fossa volume (the posterior fossa volume was defined as the region bordered by the tentorium of the cerebellum, the occipital bone, the clivus, and the pyramidal bones24) based on previously reported MRI assessment methods25 or intraoperative conditions. If the posterior fossa volume measured by linear measurement was less than 500 cm3 or if the platybasia or basilar invagination was found during surgery, we considered that the posterior fossa was small. The literature reports that patients with pure arterial compression have better outcomes after MVD than patients without pure arterial compression,8,18 so we divided the type of offending vessel into the following two subgroups: pure arterial compression (only artery) and nonpure arterial compression (artery + vein, pure vein). We classified the intraoperative decompression degree into two categories: complete decompression from arteries or veins (no offending vessels) and incomplete decompression from arteries or veins (there were still offending vessels). The demographic, clinical and intraoperative variables above may be predictors associated with long-term outcomes after surgery. An independent observer followed up all patients with outpatient visits and by telephone. The data collected during the follow-up visits included TN pain description, complications, the impact of facial dysesthesia on life, medication use, and other treatments received. All patients were followed up for at least 1 year.
We used the Barrow Neurological Institute (BNI) Pain Intensity Scale26 to assess the postoperative outcomes (Table 1). According to whether the patient needed medication after surgery, the postoperative outcomes of patients were divided into the following two categories: favorable (BNI score I-II) and unfavorable (BNI score III-V).9 We assessed the immediate outcomes at 1 week after the operation and assessed the long-term outcomes at the last follow-up.
Table 1.
Barrow Neurological Institute (BNI) Pain Intensity Scale
| Score | Description |
|---|---|
| I | No pain, no medication |
| II | Occasional pain, not requiring medication |
| III | Some pain, adequately controlled with medication |
| IV | Some pain, not adequately controlled with medication |
| V | Severe pain/no pain relief |
Note: Reproduced with permission from Rogers CL, Shetter AG, Fiedler JA, Smith KA, Han PP, Speiser BL. Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of The Barrow Neurological Institute. Int J Radiat Oncol Biol Phys. 2000;47(4):1013–1019. © 2000 Elsevier Science Inc. Published by Elsevier Inc. All rights reserved.26
Surgical Methods
All operations were performed by one neurosurgeon. After general anesthesia, the patients were placed in the lateral decubitus position with the affected side facing upward. The suboccipital retrosigmoid approach was used for all operations. The diameter of the bone window was approximately 2 cm. After opening the dura mater, part of the cerebrospinal fluid was suctioned out, and the arachnoid surrounding the trigeminal nerve was carefully separated. The position of the patient’s head and the angle of the microscope were adjusted, and the full length of the trigeminal nerve root was carefully explored to identify the compressive vessels. Different surgical procedures were selected according to the preoperative MRI assessment, patient’s wishes, and the specific conditions identified during the operation. If pure MVD treatment was performed, the compressive vessel was separated, and polyester pads were placed between the vessel and the brainstem. If MVD was combined with PSR treatment, 1/3-2/3 of the trigeminal sensory root was cut according to the distribution of pain (if the pain only affected the V3 division, we cut off 1/3 of the sensory root; if the pain affected the V1 or V2 division, not only the V3 division, we cut off 2/3 of the sensory root). The dura mater was sutured, and the skull defect was repaired. Finally, the muscles and skin were sutured.
Statistical Analysis
All statistical analyses were performed using SPSS version 24.0 (IBM, Armonk, New York, USA). All analyses compared the MVD and MVD+PSR only. The categorical variables are presented as numbers and percentages, and the continuous variables are presented as the mean ± standard deviation. After the continuous variables were tested for normality by the Shapiro–Wilk test, a Student’s t-test or Mann–Whitney U-test was used to compare the continuous variables between the two groups. Categorical variables were compared with Pearson’s χ2 test or Fisher’s exact test. Kaplan-Meier survival analysis was used to assess the outcomes of the two surgical procedures, and the Log rank test was used to compare the long-term outcomes between the two groups. Based on our experience and previous literature, we included the following factors in our study: sex, age, duration of disease, affected side, distribution of pain, type of TN, history of previous ablative procedures, posterior fossa volume, arachnoid thickening adhesion, type of offending vessel, decompression degree, and immediate postoperative outcomes. Univariate analysis was performed using Pearson’s χ2 test or Fisher’s exact test. Multivariate analysis was then performed using binary logistic regression to further assess the association between the prognostic factors and long-term outcome. The association strength was assessed using odds ratios (ORs) and 95% confidence intervals (95% CI). Only factors with a P-value < 0.2 from the univariate analysis were included in the multivariate analysis. In addition, previous literature reported that the duration of disease, the type of offending vessel and the immediate postoperative outcome were factors associated with the long-term outcome,8,15,18 so these three factors were also included in the multivariate analysis. A P-value ≤ 0.05 was considered statistically significant.
Results
Subjects
In this study, a total of 181 patients with TN underwent surgical treatment. Among these patients, 7 patients underwent pure PSR, 8 patients had secondary TN, 24 patients had concurrent cranial nerve disorders, and 44 patients were followed up for less than 1 year (Figure 2). After excluding the above patients, a total of 99 patients were included in the study to comprise the MVD group (n = 40) and the MVD+PSR group (n = 59). The follow-up duration was 13.2 to 118.8 months, with an average of 63.0 months.
The demographic and clinical data of the patients are shown in Table 2. The data showed that the patients in the MVD group (mean age, 55.1 ± 10.7 years) were younger (P = 0.012) than those in the MVD + PSR group (mean age, 60.5 ± 10.1 years). In total, 25 (62.5%) patients in the MVD group were <60 years old, and 24 (40.7%) patients in the MVD+PSR group were <60 years old. In addition, the proportion of patients with complete decompression from arteries or veins in the MVD group was higher than that in the MVD+PSR group (P=0.001). There were no significant differences in sex, duration of disease, affected side, distribution of pain, type of TN, history of previous ablative procedures, posterior fossa volume, presence of arachnoid thickening adhesion, and type of offending vessel between the two groups (P>0.05).
Table 2.
Demographic and Clinical Data of Patients in the MVD and MVD+PSR Groups
| Variable | MVD | MVD+PSR | P value |
|---|---|---|---|
| Sex, n (%) | 0.402a | ||
| Male | 14(35%) | 16(27.1%) | |
| Female | 26(65%) | 43(72.9%) | |
| Age (years), mean±SD | 55.1±10.7 | 60.5±10.1 | 0.012b |
| Age (years), n (%) | 0.033a | ||
| <60 | 25(62.5%) | 24(40.7%) | |
| ≥60 | 15(37.5%) | 35(59.3%) | |
| Duration of disease (years), n (%) | 0.369a | ||
| <5 | 16(40.0%) | 29(49.2%) | |
| ≥5 | 24(60.0%) | 30(50.8%) | |
| Affected side, n (%) | 0.551a | ||
| Left | 18(45.0%) | 23(39.0%) | |
| Right | 22(55.0%) | 36(61.0%) | |
| Distribution of pain, n (%) | 0.802a | ||
| V1 | 1(2.5%) | 0(0.0%) | |
| V2 | 14(35.0%) | 18(30.5%) | |
| V3 | 4(10.0%) | 8(13.6%) | |
| V1+V2 | 6(15.0%) | 6(10.2%) | |
| V2+V3 | 13(32.5%) | 23(39.0%) | |
| V1+V2+V3 | 2(5%) | 4(6.8%) | |
| Type of TN, n (%) | 1.000a | ||
| Typical | 38(95.0%) | 57(96.6%) | |
| Atypical | 2(5.0%) | 2(3.4%) | |
| History of previous ablative procedures, n (%) | 1.000a | ||
| Yes | 5(12.5%) | 7(11.9%) | |
| No | 35(87.5%) | 52(88.1%) | |
| Posterior fossa volume, n (%) | 0.877a | ||
| Normal | 17(42.5%) | 26(44.1%) | |
| Small | 23(57.5%) | 33(55.9%) | |
| Presence of arachnoid thickening adhesion, n (%) | 0.359a | ||
| Yes | 31(77.5%) | 50(84.7%) | |
| No | 9(22.5%) | 9(15.3%) | |
| Type of offending vessel, n (%) | 0.367a | ||
| Pure artery compression | 22(55.0%) | 27(45.8%) | |
| Nonpure artery compression | 18(45.0%) | 32(54.2%) | |
| Decompression degree, n (%) | 0.001a | ||
| Complete decompression from arteries or veins | 38(95.0%) | 39(66.1%) | |
| Incomplete decompression from arteries or veins | 2(5.0%) | 20(33.9%) |
Notes: aP value, Pearson’s χ2 test or Fisher’s exact test; bP value, Student’s t-test.
Abbreviations: MVD, microvascular decompression; PSR, partial sensory rhizotomy, TN, trigeminal neuralgia; V1, the first division of the trigeminal nerve; V2, the second division of the trigeminal nerve; V3, the third division of the trigeminal nerve.
The most common offending vessels were the superior cerebellar artery (SCA) in 71 cases (71.7%, MVD: 33 cases, MVD+PSR: 38 cases), followed by the superior petrosal vein (SPV) in 50 cases (50.5%, MVD: 18 cases, MVD+PSR: 32 cases) and the anterior inferior cerebellar artery (AICA) in 36 cases (36.4%, MVD: 13 cases, MVD+PSR: 23 cases). A total of 56.6% of patients were found to have two or more offending vessels during surgery (60.0% in the MVD group and 54.2% in the MVD+PSR group) (Table 3).
Table 3.
Type of Offending Vessel
| Vessel | All | MVD | MVD+PSR |
|---|---|---|---|
| SCA, n (%) | 71(71.7%) | 33(82.5%) | 38(64.4%) |
| AICA, n (%) | 36(36.4%) | 13(32.5%) | 23(39.0%) |
| PICA, n (%) | 1(1.0%) | 1(2.5%) | 0(0.0%) |
| BA, n (%) | 5(5.1%) | 2(5.0%) | 3(5.1%) |
| SPV, n (%) | 50(50.5%) | 18(45.0%) | 32(54.2%) |
| Multiple offending vessels, n (%) | 56(56.6%) | 24(60.0%) | 32(54.2%) |
Abbreviations: SCA, superior cerebellar artery; AICA, anterior inferior cerebellar artery; PICA, posterior inferior cerebellar artery; BA, basilar artery; SPV, super petrosal vein.
Postoperative Outcomes
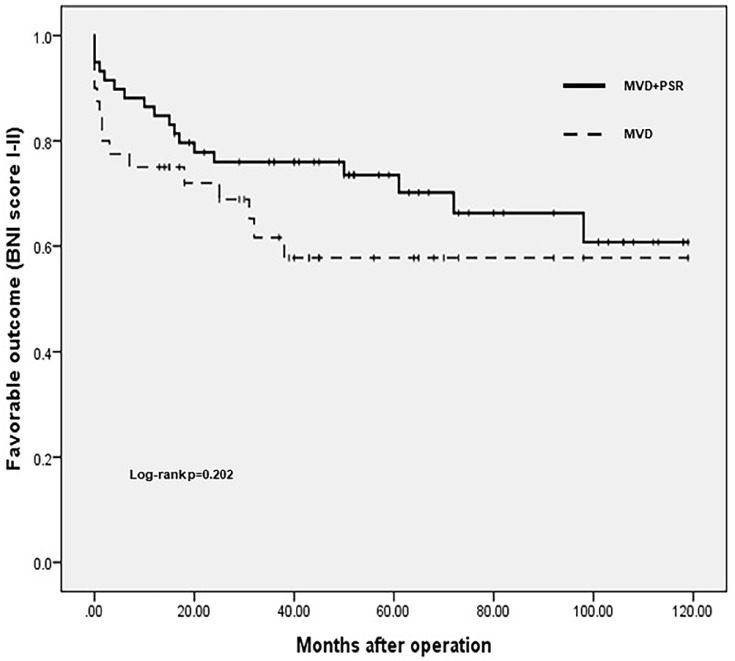
One week after the operation, 34 (85%) patients in the MVD group achieved favorable outcomes, and 55 (93.2%) patients in the MVD+PSR group achieved favorable outcomes. There was no significant difference between the two groups (P=0.321). The average follow-up duration was 48.4 months (13.2–118.8 months) in the MVD group and 72.9 months (16.8–118.8 months) in the MVD+PSR group. There was a statistically significant difference between the two groups (P<0.001). At the last follow-up, 25 (62.5%) of the patients who underwent MVD achieved favorable long-term outcomes, and 15 (37.5%) had unfavorable long-term outcomes. In the MVD+PSR group, 41 (69.5%) patients achieved favorable long-term outcomes, and 18 (30.5%) patients had unfavorable long-term outcomes. To compare the differences in long-term outcomes between the two groups, we performed a Kaplan-Meier survival analysis (Figure 3). The duration of favorable outcomes in the MVD group was 74.2 ± 9.0 months, and the duration of favorable outcomes in the MVD + PSR group was 86.0 ± 6.5 months. However, the Log rank test showed no significant difference in long-term outcomes between the two groups (P=0.202).
Figure 3.
Postoperative outcomes: MVD versus MVD+PSR. The Kaplan-Meier survival curves for the MVD and MVD+PSR groups are shown.
Abbreviations: MVD, microvascular decompression; PSR, partial sensory rhizotomy.
Factors Influencing the Postoperative Outcomes
We analyzed the association between the following factors and long-term outcomes after MVD or MVD+PSR: sex, age, duration of disease, affected side, distribution of pain, type of TN, history of previous ablative procedures, posterior fossa volume, arachnoid thickening adhesion, type of offending vessel, decompression degree, and immediate postoperative outcomes.
In the MVD group, the univariate analysis showed no significant associations between any of the above factors and the long-term outcomes after MVD (Table 4). Because no factors met the criteria of P < 0.2, we only included the duration of disease (P=0.505), type of offending vessel (P=0.870) and immediate postoperative outcomes (P=0.253). The multivariate analysis showed that the duration of disease (OR 1.212, 95% CI 0.295–4.972, P=0.790), type of offending vessel (OR 0.663, 95% CI 0.166–2.652, P=0.561) and immediate postoperative outcomes (OR 4.853, 95% CI 0.707–33.299, P=0.108) were not significantly associated with the long-term outcomes of patients.
Table 4.
The Univariate Analysis of Factors Associated with Long-Term Outcomes in MVD Group
| Variable | BNI Score 1-2 | BNI Score 3-5 | P value |
|---|---|---|---|
| Sex, n (%) | 0.608 | ||
| Male | 8(32.0%) | 6(40.0%). | |
| Female | 17(68.0%) | 9(60.0%) | |
| Age (years), n (%) | 0.800 | ||
| <60 | 16(64.0%) | 9(60.0%) | |
| ≥60 | 9(36.0%) | 6(40.0%) | |
| Duration of disease (years), n (%) | 0.505 | ||
| <5 | 11(44.0%) | 5(33.3%) | |
| ≥5 | 14(56.0%) | 10(66.7%) | |
| Affected side, n (%) | 0.412 | ||
| Left | 10(40.0%) | 8(53.3%) | |
| Right | 15(60.0%) | 7(46.7%) | |
| Distribution of pain, n (%) | 0.498 | ||
| V1 | 0(0.0%) | 1(6.7%) | |
| V2 | 7(28.0%) | 7(46.7%) | |
| V3 | 3(12.0%) | 1(6.7%) | |
| V1+V2 | 5(20.0%) | 1(6.7%) | |
| V2+V3 | 8(32.0%) | 5(33.3%) | |
| V1+V2+V3 | 2(8.0%) | 0(0.0%) | |
| Type of TN, n (%) | 1.000 | ||
| Typical | 24(96.0%) | 14(93.3%) | |
| Atypical | 1(4.0%) | 1(6.7%) | |
| History of previous ablative procedures, n (%) | 0.711 | ||
| Yes | 4(16.0%) | 1(6.7%) | |
| No | 21(84.0%) | 14(93.3%) | |
| Posterior fossa volume, n (%) | 0.804 | ||
| Normal | 11(44.0%) | 6(40.0%) | |
| Small | 14(56.0%) | 9(60.0%) | |
| Presence of arachnoid thickening adhesion, n (%) | 0.494 | ||
| Yes | 18(72.0%) | 13(86.7%) | |
| No | 7(28.0%) | 2(13.3%) | |
| Type of offending vessel, n (%) | 0.870 | ||
| Pure artery compression | 14(56.0%) | 8(53.3%) | |
| Nonpure artery compression | 11(44.0%) | 7(46.7%) | |
| Decompression degree, n (%) | 1.000 | ||
| Complete decompression from arteries or veins | 24(96.0%) | 14(93.3%) | |
| Incomplete decompression from arteries or veins | 1(4.0%) | 1(6.7%) | |
| Immediate postoperative outcomes, n (%) | 0.253 | ||
| Favorable | 23(92.0%) | 11(73.3%) | |
| Unfavorable | 2(8.0%) | 4(26.7%) |
Note: p values: Pearson’s χ2 test or Fisher’s exact test.
Abbreviations: MVD, microvascular decompression; TN, trigeminal neuralgia; V1, the first division of the trigeminal nerve; V2, the second division of the trigeminal nerve; V3, the third division of the trigeminal nerve; BNI, Barrow Neurological Institute.
We also analyzed the effects of the above factors on the long-term outcomes of MVD+PSR patients. The univariate analysis showed that the duration of disease (P=0.001), posterior fossa volume (P=0. 025) and type of offending vessel (P=0. 016) were associated with long-term outcomes (Table 5). To further evaluate the impact of these factors on the long-term outcomes, we included these three variables and the immediate postoperative outcomes (P=0.150) in a binary logistic regression for multivariate analysis. The multivariate analysis showed that a duration of disease ≥ 5 years (OR 6.967, 95% CI 1.444–33.626, P=0.016) was significantly associated with unfavorable postoperative long-term outcomes (Table 6), while pure arterial compression (OR 0.131, 95% CI 0.026–0. 647, P=0. 013) was significantly associated with favorable long-term outcomes.
Table 5.
The Univariate Analysis of Factors Associated with Long-Term Outcomes in MVD+PSR Group
| Variable | BNI Score 1-2 | BNI Score 3-5 | P value |
|---|---|---|---|
| Sex, n (%) | 1.000 | ||
| Male | 11(26.8%) | 5(27.8%) | |
| Female | 30(73.2%) | 13(72.2%) | |
| Age (years), n (%) | 0.334 | ||
| <60 | 15(36.6%) | 9(50.0%) | |
| ≥60 | 26(63.4%) | 9(50.0%) | |
| Duration of disease (years), n (%) | 0.001 | ||
| <5 | 26(63.4%) | 3(16.7%) | |
| ≥5 | 15(36.6%) | 15(83.3%) | |
| Affected side, n (%) | 0.569 | ||
| Left | 15(36.6%) | 8(44.4%) | |
| Right | 26(63.4%) | 10(55.6%) | |
| Distribution of pain, n (%) | 0.988 | ||
| V1 | 0(0.0%) | 0(0.0%) | |
| V2 | 13(31.7%) | 5(27.8%) | |
| V3 | 6(14.6%) | 2(11.1%) | |
| V1+V2 | 4(9.8%) | 2(11.1%) | |
| V2+V3 | 15(36.6%) | 8(44.4%) | |
| V1+V2+V3 | 3(7.3%) | 1(5.6%) | |
| Type of TN, n (%) | 1.000 | ||
| Typical | 39(95.1%) | 18(100.0%) | |
| Atypical | 2(4.9%) | 0(0.0%) | |
| History of previous ablative procedures, n (%) | 0.233 | ||
| Yes | 3(7.3%) | 4(22.2%) | |
| No | 38(92.7%) | 14(77.8%) | |
| Posterior fossa volume, n (%) | 0.025 | ||
| Normal | 22(53.7%) | 4(22.2%) | |
| Small | 19(46.3%) | 14(77.8%) | |
| Presence of arachnoid thickening adhesion, n (%) | 0.847 | ||
| Yes | 34(82.9%) | 16(88.9%) | |
| No | 7(17.1%) | 2(11.1%) | |
| Type of offending vessel, n (%) | 0.016 | ||
| Pure artery compression | 23(56.1%) | 4(22.2%) | |
| Nonpure artery compression | 18(43.9%) | 14(77.8%) | |
| Decompression degree, n (%) | 0.592 | ||
| Complete decompression from arteries or veins | 28(68.3%) | 11(61.1%) | |
| Incomplete decompression from arteries or veins | 13(31.7%) | 7(38.9%) | |
| Immediate postoperative outcomes, n (%) | 0.150 | ||
| Favorable | 40(97.6%) | 15(83.3%) | |
| Unfavorable | 1(2.4%) | 3(16.7%) |
Note: p values: Pearson’s χ2 test or Fisher’s exact test.
Abbreviations: MVD, microvascular decompression; PSR, partial sensory rhizotomy, TN, trigeminal neuralgia; V1, the first division of the trigeminal nerve; V2, the second division of the trigeminal nerve; V3, the third division of the trigeminal nerve; BNI, Barrow Neurological Institute.
Table 6.
The Binary Logistic Regression Analysis of Factors Associated with Long-Term Outcomes in MVD+PSR Group
| Variable | OR (95% CI) | P value |
|---|---|---|
| Duration of disease (years) | ||
| <5 | 1 | |
| ≥5 | 6.967(1.444–33.626) | 0.016 |
| Posterior fossa volume | ||
| Normal | 1 | |
| Small | 3.703(0.724–18.938) | 0.116 |
| Type of offending vessel | ||
| Nonpure artery compression | 1 | |
| Pure artery compression | 0.131 (0.026–0.647) | 0.013 |
| Immediate postoperative outcomes | ||
| Favorable | 1 | |
| Unfavorable | 29.692(0.905–974.694) | 0.057 |
Note: All the factors were analyzed by binary logistic regression analysis, including the duration of disease, posterior fossa volume, type of the offending vessel and the immediate postoperative outcomes.
Abbreviations: OR, odds ratio; CI, confidence interval.
Postoperative Complications
The postoperative complications of the two groups of patients are shown in Table 7. In MVD group, complications included facial dysesthesia in 3 cases, intracranial infection in 4 cases, hearing loss in 2 cases, and intracranial bleeding in 1 case. In MVD+PSR group, complications included facial dysesthesia in 48 cases, intracranial infection in 2 cases, hearing loss in 4 cases, facial continuous pain in 3 cases, facial paresis in 1 case, wound infection in 1 case, and dizziness in 2 cases. There were no perioperative deaths in either group. There were no significant differences in postoperative complications between the two groups, except for facial dysesthesia. For the impact of facial dysesthesia on life, only 1 of the 4 people with facial dysesthesia in the MVD group thought that facial dysesthesia had an impact on life. In MVD+PSR group, only 2 of the 48 people with facial dysesthesia considered that facial dysesthesia had an impact on life.
Table 7.
Postoperative Complications of Patients in the MVD and MVD+PSR Groups
| Postoperative Complications | MVD | MVD+PSR | P value |
|---|---|---|---|
| Facial dysesthesia | 3(7.5%) | 48(81.4%) | <0.001 |
| Intracranial infection | 4(10.0%) | 2(3.4%) | 0.356 |
| Hearing loss | 2(5.0%) | 4(6.8%) | 1.000 |
| Intracranial bleeding | 1(2.5%) | 0(0.0%) | 0.404 |
| Facial continuous pain | 0(%) | 3(5.1%) | 0.395 |
| Facial paresis | 0(%) | 1(1.7%) | 1.000 |
| Wound infection | 0(%) | 1(1.7%) | 1.000 |
| Dizziness | 0(%) | 2(3.4%) | 0.514 |
Note: p values: Pearson’s χ2 test or Fisher’s exact test.
Abbreviations: MVD, microvascular decompression; PSR, partial sensory rhizotomy.
Discussion
We retrospectively analyzed 99 patients who underwent MVD or MVD+PSR and found no statistically significant difference in long-term outcomes between the two groups. In addition, we found that no factors were associated with long-term outcomes after MVD. For patients who underwent MVD+PSR, a long duration of the disease (≥5 years) was an independent predictor of unfavorable long-term outcomes, while pure arterial compression was an independent predictor of favorable long-term outcomes.
We evaluated the postoperative outcomes with the BNI Pain Intensity Scale. The postoperative outcomes were divided into the following two categories according to whether the patients needed to take medication after surgery: favorable (BNI score I-II) and unfavorable (BNI score III-V).9 Among patients who underwent MVD, 62.5% of the patients maintained favorable outcomes at the last follow-up. This result was similar to the MVD outcomes reported in many other studies.3,8,14,17 For MVD+PSR, 69.5% of the patients had favorable long-term outcomes. In the study by Zhang et al5 after 2 years of follow-up, the pain-free rate in the MVD+PSR group was 95.7%. The longer follow-up duration (an average of 6.1 years) in this study may be the cause of the above differences. According to our experiences and previous literature,8,10–12,16,18 pure MVD leads to poor outcomes in patients who had the following conditions: vessels only contacted the trigeminal nerve root, pure venous compression, and incomplete decompression from arteries or veins. Therefore, we recommend that these patients receive MVD+PSR treatment. Patients who underwent MVD+PSR in this study were older than those who underwent MVD, and we thought it might be because older patients were more likely to accept postoperative facial dysesthesia, so they were willing to choose MVD+PSR. In our study, although the average follow-up duration in the MVD+PSR group was significantly longer than that in the MVD group, the proportion of patients with favorable long-term outcomes in the MVD+PSR group was slightly higher than that in the MVD group, but there was no statistically significant difference. Therefore, the long-term outcomes of MVD+PSR on patients who had the above conditions and can tolerate postoperative facial dysesthesia are positive.
For patients without clear vascular compression, there are other alternative surgical treatments in addition to PSR, such as GKRS and internal neurolysis (IN). For GKRS, in one previous study, the patients with NVC had better responses to GKRS than those without NVC.27 In another study, after long-term follow-up, although the proportion of patients with NVC who had good outcomes was higher than that of patients without NVC, there was no statistical difference between the two groups (86.4% and 68.0%,respectively).28 Chang et al29 found that there was no significant difference in successful pain control rates (BNI score I-III) among patients with different NVC conditions after GKRS treatment (successful pain control rate, no NVC 85%, small vessel NVC 75%, large vessel NVC 88%, respectively). However, a large proportion of patients will experience facial numbness after receiving GKRS.17,30 For IN, it is also a safe and effective option for the treatment of TN without vascular compression. Jie et al31 found that IN has better efficacy in patients without vascular compression than patients with vascular compression. As reported in previous studies, for patients without NVC, 47%–72.7% of patients receiving IN can achieve good long-term results, but the incidence of facial dysesthesia and numbness after IN is 76.5%-96%.32,33 However, there is currently no study comparing the efficacy of PSR, IN, and GKRS in the treatment of TN without clear NVC.
Some studies have noted that a long duration of TN had an adverse effect on the long-term outcomes of MVD. A study by Barker et al8 reported that patients who had TN for less than 8 years were more likely to have good long-term outcomes. Sarsam et al15 found that the risk of recurrence after MVD increased by 0.3% for each additional month of preoperative pain. However, many studies have shown that the duration of TN was not associated with long-term outcomes after MVD.9,14,16,18,19 Our study also found that the duration of the disease was not associated with long-term outcomes after MVD; however, for patients who underwent MVD+PSR, patients with a longer duration of disease are less likely to obtain favorable long-term outcomes. We believe that the reason for the above results is as follows: patients with a longer duration of the disease have more severe nerve damage. For patients who are suitable to undergo pure MVD, vascular compression may be the only cause of nerve damage. After complete decompression, nerve function can be gradually recovered. However, for the above patients who are not suitable to undergo pure MVD, vascular compression may not be the only cause of pain. MVD+PSR cannot solve other potential pathological mechanisms, and patients with a longer duration of the disease have more severe nerve damage. Therefore, the postoperative outcomes of this population of patients are poor.
The literature reports that patients with pure arterial compression can achieve favorable long-term outcomes after MVD.8,18 In our study, there was no significant difference in the long-term outcomes between patients with pure arterial compression and patients with nonpure arterial compression (artery+vein, pure vein) after MVD treatment. However, in the MVD+PSR group, patients with pure artery compression were more likely to have good long-term outcomes after the operation than those with nonpure arterial compression. Compared to decompressing arteries, decompressing veins is more difficult, because the risk of bleeding is higher, and it is not possible to perform electrocoagulation or other decompression operations on parts of offending veins that affect the brainstem blood flow. Therefore, it is more difficult to perform complete decompression in patients with venous compression than in those with pure arterial compression. Moreover, because we mostly performed MVD+PSR on patients who could not be completely decompressed, there were more patients in the MVD+PSR group than in the MVD group who could not be decompressed completely due to nonpure arterial compression. This may be why the results of our study are different from those of previous studies. In addition, 50.5% of the patients in our study had venous compression, which was consistent with the venous involvement rates (17.5%–68%) reported in other studies.8,19,34,35 We believe that the adverse effect of venous compression on the long-term outcomes may be caused by incomplete decompression, so careful intraoperative exploration should be performed to avoid missing veins, and patients should be treated with MVD or MVD+PSR according to the degree of intraoperative decompression.
Many studies have reported some prognostic factors associated with long-term outcomes after MVD, but the conclusions are different. These factors included sex,8,9 age,9,15,18 distribution of pain,9 types of TN,13,15,18,19 history of previous ablative procedures,12 and immediate postoperative outcomes.8 However, in our study, we did not find that these factors were associated with long-term outcomes after MVD or MVD+PSR.
Patients who underwent MVD+PSR had a significantly higher probability of facial dysesthesia than patients who underwent pure MVD, but only a few people thought that facial dysesthesia had an impact on life.
Limitations
There are several limitations to our study. First, this study was a retrospective study, which forces us to exclude patients with incomplete follow-up data. Second, the patients in this study were not randomly assigned to the different treatment groups (MVD or MVD+PSR) so their treatment choice was subject to bias. We cannot rule out the possibility that the differences in the observed outcomes in this study were due to differences in the baseline characteristics between the two groups. Third, the sample size of this study was small, and we hope to conduct a study with a larger sample size in the future. Finally, our study only included patients with primary TN, so our results are not applicable to patients with secondary TN and patients with concurrent cranial nerve disorders.
Conclusion
In our study, some patients who were not suitable to undergo pure MVD were treated with MVD+PSR, and these patients were able to achieve similar outcomes to the patients who underwent MVD. No factors were associated with long-term outcomes after MVD. For MVD+PSR, patients with a long duration of symptoms may have poor long-term outcomes, while patients with pure arterial compression may have favorable long-term outcomes. We suggest that the appropriate surgical procedure should be selected based on the opinions of the patients, preoperative MRI evaluation and the specific conditions identified during the operation. For patients who are not suitable to undergo pure MVD, MVD+PSR can be considered an effective alternative.
Funding Statement
No funding was received for this research.
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of the China-Japan Friendship Hospital and all patients who participated in the study signed informed consent.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Arnold M. Headache classification committee of the International Headache Society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. [DOI] [PubMed] [Google Scholar]
- 2.Jannetta PJ. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg. 1967;26(1 Suppl):159–162. doi: 10.3171/jns.1967.26.1part2.0159 [DOI] [PubMed] [Google Scholar]
- 3.Tatli M, Satici O, Kanpolat Y, Sindou M. Various surgical modalities for trigeminal neuralgia: literature study of respective long-term outcomes. Acta Neurochir (Wien). 2008;150(3):243–255. doi: 10.1007/s00701-007-1488-3 [DOI] [PubMed] [Google Scholar]
- 4.Young JN, Wilkins RH. Partial sensory trigeminal rhizotomy at the pons for trigeminal neuralgia. J Neurosurg. 1993;79(5):680–687. doi: 10.3171/jns.1993.79.5.0680 [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Zhang Y, Li C, Zhu S. Surgical treatment of primary trigeminal neuralgia: comparison of the effectiveness between MVD and MVD+PSR in a series of 210 patients. Turk Neurosurg. 2012;22(1):32–38. doi: 10.5137/1019-5149.JTN.4447-11.2 [DOI] [PubMed] [Google Scholar]
- 6.Bederson JB, Wilson CB. Evaluation of microvascular decompression and partial sensory rhizotomy in 252 cases of trigeminal neuralgia. J Neurosurg. 1989;71(3):359–367. doi: 10.3171/jns.1989.71.3.0359 [DOI] [PubMed] [Google Scholar]
- 7.Theodosopoulos PV, Marco E, Applebury C, Lamborn KR, Wilson CB. Predictive model for pain recurrence after posterior fossa surgery for trigeminal neuralgia. Arch Neurol. 2002;59(8):1297–1302. doi: 10.1001/archneur.59.8.1297 [DOI] [PubMed] [Google Scholar]
- 8.Barker FG 2nd, Jannetta PJ, Bissonette DJ, Larkins MV, Jho HD. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. 1996;334(17):1077–1083. doi: 10.1056/NEJM199604253341701 [DOI] [PubMed] [Google Scholar]
- 9.Bick SK, Huie D, Sneh G, Eskandar EN. Older patients have better pain outcomes following microvascular decompression for trigeminal neuralgia. Neurosurgery. 2019;84(1):116–122. doi: 10.1093/neuros/nyy011 [DOI] [PubMed] [Google Scholar]
- 10.Cheng J, Meng J, Liu W, Zhang H, Hui X, Lei D. Nerve atrophy in trigeminal neuralgia due to neurovascular compression and its association with surgical outcomes after microvascular decompression. Acta Neurochir (Wien). 2017;159(9):1699–1705. doi: 10.1007/s00701-017-3250-9 [DOI] [PubMed] [Google Scholar]
- 11.Jo KW, Kong DS, Hong KS, Lee JA, Park K. Long-term prognostic factors for microvascular decompression for trigeminal neuralgia. J Clin Neurosci. 2013;20(3):440–445. [DOI] [PubMed] [Google Scholar]
- 12.Mendoza N, Illingworth RD. Trigeminal neuralgia treated by microvascular decompression: a long-term follow-up study. Br J Neurosurg. 1995;9(1):13–19. doi: 10.1080/02688699550041692 [DOI] [PubMed] [Google Scholar]
- 13.Miller JP, Magill ST, Acar F, Burchiel KJ. Predictors of long-term success after microvascular decompression for trigeminal neuralgia. J Neurosurg. 2009;110(4):620–626. doi: 10.3171/2008.9.17660 [DOI] [PubMed] [Google Scholar]
- 14.Nunta-Aree S, Patiwech K, Sitthinamsuwan B. Microvascular decompression for treatment of trigeminal neuralgia: factors that predict complete pain relief and study of efficacy and safety in older patients. World Neurosurg. 2018;110:e979–e988. doi: 10.1016/j.wneu.2017.11.147 [DOI] [PubMed] [Google Scholar]
- 15.Sarsam Z, Garcia-Finana M, Nurmikko TJ, Varma TR, Eldridge P. The long-term outcome of microvascular decompression for trigeminal neuralgia. Br J Neurosurg. 2010;24(1):18–25. doi: 10.3109/02688690903370289 [DOI] [PubMed] [Google Scholar]
- 16.Sindou M, Leston J, Decullier E, Chapuis F. Microvascular decompression for primary trigeminal neuralgia: long-term effectiveness and prognostic factors in a series of 362 consecutive patients with clear-cut neurovascular conflicts who underwent pure decompression. J Neurosurg. 2007;107(6):1144–1153. doi: 10.3171/JNS-07/12/1144 [DOI] [PubMed] [Google Scholar]
- 17.Wang DD, Raygor KP, Cage TA, et al. Prospective comparison of long-term pain relief rates after first-time microvascular decompression and stereotactic radiosurgery for trigeminal neuralgia. J Neurosurg. 2018;128(1):68–77. doi: 10.3171/2016.9.JNS16149 [DOI] [PubMed] [Google Scholar]
- 18.Wei Y, Pu C, Li N, Cai Y, Shang H, Zhao W. Long-term therapeutic effect of microvascular decompression for trigeminal neuralgia: Kaplan-Meier analysis in a consecutive series of 425 patients. Turk Neurosurg. 2018;28(1):88–93. doi: 10.5137/1019-5149.JTN.18322-16.1 [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Lei D, You C, Mao BY, Wu B, Fang Y. The long-term outcome predictors of pure microvascular decompression for primary trigeminal neuralgia. World Neurosurg. 2013;79(5–6):756–762. doi: 10.1016/j.wneu.2012.01.040 [DOI] [PubMed] [Google Scholar]
- 20.Deng Z, Liu R, Liu Y, Wang Z, Yu Y, Zhang L. Factors that may affect delayed relief of trigeminal neuralgia after microneurosurgery and the long-term outcomes associated with delayed relief. J Pain Res. 2019;12:2817–2823. doi: 10.2147/JPR.S222467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinzeu A, Drogba L, Sindou M. Reliability of MRI for predicting characteristics of neurovascular conflicts in trigeminal neuralgia: implications for surgical decision making. J Neurosurg. 2018;130(2):1–11. [DOI] [PubMed] [Google Scholar]
- 22.Hughes MA, Frederickson AM, Branstetter BF, Zhu X, Sekula RF Jr. MRI of the trigeminal nerve in patients with trigeminal neuralgia secondary to vascular compression. AJR Am J Roentgenol. 2016;206(3):595–600. doi: 10.2214/AJR.14.14156 [DOI] [PubMed] [Google Scholar]
- 23.Cheng J, Meng J, Liu W, Zhang H, Hui X, Lei D. Primary trigeminal neuralgia is associated with posterior fossa crowdedness: a prospective case-control study. J Clin Neurosci. 2018;47:89–92. doi: 10.1016/j.jocn.2017.10.032 [DOI] [PubMed] [Google Scholar]
- 24.Horinek D, Brezova V, Nimsky C, et al. The MRI volumetry of the posterior fossa and its substructures in trigeminal neuralgia: a validated study. Acta Neurochir (Wien). 2009;151(6):669–675. doi: 10.1007/s00701-009-0283-8 [DOI] [PubMed] [Google Scholar]
- 25.Macielak RJ, Harris MS, Kirsch CF, Prevedello LM, Adunka OF. Influence of posterior fossa volume on clinical outcomes after vestibular schwannoma resection. Otol Neurotol. 2016;37(8):1155–1161. doi: 10.1097/MAO.0000000000001128 [DOI] [PubMed] [Google Scholar]
- 26.Rogers CL, Shetter AG, Fiedler JA, Smith KA, Han PP, Speiser BL. Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of the barrow neurological institute. Int J Radiat Oncol Biol Phys. 2000;47(4):1013–1019. doi: 10.1016/S0360-3016(00)00513-7 [DOI] [PubMed] [Google Scholar]
- 27.Erbay SH, Bhadelia RA, Riesenburger R, et al. Association between neurovascular contact on MRI and response to gamma knife radiosurgery in trigeminal neuralgia. Neuroradiology. 2006;48(1):26–30. doi: 10.1007/s00234-005-0008-5 [DOI] [PubMed] [Google Scholar]
- 28.Jung HH, Park CK, Jung NY, Kim M, Chang WS, Chang JW. Gamma knife radiosurgery for idiopathic trigeminal neuralgia: does the status of offending vessels influence pain control or side effects? World Neurosurg. 2017;104:687–693. doi: 10.1016/j.wneu.2017.05.058 [DOI] [PubMed] [Google Scholar]
- 29.Chang C-S, Huang C-W, Chou -H-H, Lin L-Y, Huang C-F. Outcome of Gamma Knife radiosurgery for trigeminal neuralgia associated with neurovascular compression. J Clin Neurosci. 2018;47:174–177. doi: 10.1016/j.jocn.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 30.Inoue T, Hirai H, Shima A, et al. Long-term outcomes of microvascular decompression and Gamma Knife surgery for trigeminal neuralgia: a retrospective comparison study. Acta Neurochir (Wien). 2017;159(11):2127–2135. doi: 10.1007/s00701-017-3325-7 [DOI] [PubMed] [Google Scholar]
- 31.Jie H, Xuanchen Z, Deheng L, et al. The long-term outcome of nerve combing for trigeminal neuralgia. Acta Neurochir (Wien). 2013;155(9):1703–1708; discussion 1707. doi: 10.1007/s00701-013-1804-z [DOI] [PubMed] [Google Scholar]
- 32.Yang DB, Wang ZM. The efficacy and safety of nerve combing for trigeminal neuralgia without neurovascular compression. Acta Neurol Belg. 2019;119:439–444. doi: 10.1007/s13760-019-01099-2 [DOI] [PubMed] [Google Scholar]
- 33.Ko AL, Ozpinar A, Lee A, Raslan AM, McCartney S, Burchiel KJ. Long-term efficacy and safety of internal neurolysis for trigeminal neuralgia without neurovascular compression. J Neurosurg. 2015;122(5):1048–1057. doi: 10.3171/2014.12.JNS14469 [DOI] [PubMed] [Google Scholar]
- 34.Dumot C, Brinzeu A, Berthiller J, Sindou M. Trigeminal neuralgia due to venous neurovascular conflicts: outcome after microvascular decompression in a series of 55 consecutive patients. Acta Neurochir (Wien). 2017;159(2):237–249. doi: 10.1007/s00701-016-2994-y [DOI] [PubMed] [Google Scholar]
- 35.Zhong J, Zhu J, Sun H, et al. Microvascular decompression surgery: surgical principles and technical nuances based on 4000 cases. Neurol Res. 2014;36(10):882–893. doi: 10.1179/1743132814Y.0000000344 [DOI] [PubMed] [Google Scholar]