Abstract
Background: The fragmented QRS complex (FQRS) was found to be associated to malignant ventricular arrhythmias and sudden death in patients with hypertrophic cardiomyopathy and other entities. There is scant data available correlating the presence of FQRS with QT interval prolongation in patients with ischemic heart disease (IHD). Methods: A descriptive, retrospective, cross-sectional study was performed in 123 patients with IHD to analyze and correlate the presence of FQRS with QT interval prolongation in the conventional 12-leads electrocardiogram in patients with documented chronic IHD. Results: There were 62% male patients. The mean age was 63.8±12.6 years. Thirty six (44%) patients had fragmented QRS (64% men and 36% women). The duration of QT and QTc, the mean values were 413±59ms, and 463±67ms, respectively. Of the 36 patients with FQRS, 23 patients have prolongation of the QTc interval, and 13 patients did not present it. Of the 45 patients without FQRS, 21 of them have prolongation of the QTc interval, and 24 patients did not have it. These data resulted in a sensitivity of 52% with a moderate SnNout, a specificity of 65% with moderate SpPin, a positive predictive accuracy of 64%, a negative predictive accuracy of 53%. These data resulted in a prevalence of 54%. Conclusion: the presence of FQRS in the ECG has a moderate sensitivity and specificity, as well as, moderate negative and positive predictive value of the existence of QT interval prolongation in patients with ischemic heart disease.
Keywords: Fragmented QRS complex, QT prolongation, ischemic heart disease
Introduction
The prevention of clinical events such as malignant ventricular arrhythmias and sudden cardiac death is the main objective of medicine today. The search for new tools to serve as prognostic factors or predictors of clinical events has lead to the finding of certain electrocardiographic parameters such as fragmented QRS complex (FQRS). The presence of FQRS may be due to multiple causes, being ischemic heart disease (IHD) one of them. FQRS is the manifestation of an intraventricular conduction abnormality caused by the deterioration in the electrical signal propagation and ventricular depolarization associated to the presence of myocardial scars, ischemia and fibrosis [1,2,3,4,5,6].
This was further validated by studies with spectral analysis of high frequency electrograms that revealed increased notches or slurring in the electrograms after myocardial injury [7].
The tissue alterations in structural and functional characteristics of the ventricular myocardium results in changes of the conduction pattern, leading to unilateral blocks and slow conduction of depolarizations in the myocardial scar tissue. This abnormal myocardium may be a substrate for reentrant rhythms leading to malignant ventricular arrhythmias [8,9,10,11].
Despite the increasing growth of IHD worldwide, there is also a survival increase in the general population due to medical advancement in the diagnostic and therapeutic management of cardiovascular diseases. IHD is currently the leading cause of death in most of the countries of the world and still the cause of approximately one third of all deaths in subjects older than 35 years of age [12,13,14,15,16,17,18,19].
The presence of FQRS in the context of IHD is known to be significantly associated with left ventricular dysfunction and impairment of myocardial perfusion, and it was found to predict adverse cardiac events [20,21,22,23,24].
FQRS is a relatively inexpensive, fast, and accessible marker that can help decrease the cost of public health care expenditure by predicting negative clinical outcomes in a high-risk population.
One of the most recognized electrocardiographic parameters potentially identifying ventricular arrhythmias and SCA risk in IHD is QT prolongation [25,26,27,28,29].
The QT interval represents the duration of ventricular depolarization and repolarization. Slower ventricular repolarization manifests as prolongation of the QT interval and indicates an increase in the temporal dispersion of the refractory period of different sites in the ventricular myocardium [25].
With the prolongation of the QT interval there is also a concomitant prolongation of the vulnerable period for arrhythmia induction, resulting in increased susceptibility for developing ventricular arrhythmias [26].
The prolongation of the QT interval is associated with ventricular arrhythmias specially torsade de pointes ventricular tachycardia, syncope, and sudden death due to degeneration into ventricular fibrillation. It is a risk marker both in subjects without structural heart disease or in those with different heart diseases [30,31,32,33,34].
In addition, FQRS was found to be a simple non-invasive ECG marker utilized to identify individuals with high mortality risk in patients with IHD [35,36,37,38,39,40,41].
However, the sensitivity, specificity and predictive value of FQRS for predicting QT interval prolongation in patients with documented chronic IHD remains scantly known. Therefore, we aim to analyze and correlate the presence of FQRS with the prolongation of the QT interval in patients with documented chronic IHD.
Materials and Methods
Study patients
In a descriptive, retrospective, cross-sectional study, a total of 123 patients were admitted to the Cardiology Department of the Clinic Hospital with chronic IHD during the period from March 2016 to February 2017 and studied with noninvasive diagnostic methods and coronary angiography. Although, most of the patients had documented signs of coronary artery disease with noninvasive studies, 81 patients had their IHD corroborated by coronary angiography. The patients were divided in two groups according to the presence or not of fragmented QRS complex and, the respective differences in certain clinical variables were assessed. The presence and location of fragmented QRS complex on the electrocardiogram were investigated, and were correlated to the QT and QTc intervals. The sensitivity, specificity and negative and positive predictive accuracy of the presence of FQRS related to the existence of QT interval prolongation were also analyzed. The studies were conducted in these patients with documented IHD with the approval of the local institutional ethics review board at the Cardiology Department of the Clinic Hospital, Asunción National University in accordance with the Declaration of Helsinki on March 2, 2016. Oral and written informed consent was obtained from all patients.
Study variables and statistics
We analyzed: age, sex, cardiovascular risk factors, symptoms, NYHA functional class. The presence and location of FQRS on the electrocardiogram, heart rate, QT and QTc intervals were investigated. Variables were recorded in the Excel 2007 spreadsheets. The analysis was performed using EPI Info statistical version 7.2.0.1 and Epidat 3.1 software’s. In the descriptive analysis, the qualitative variables were expressed in frequencies and percentages, and the quantitative variables in means and standard deviations (SD); or as medians and interquartile ranges. In the qualitative variables, the sensitivity and specificity were analyzed with 95% confidence intervals.
The 12 leads conventional ECG were taken with an electrocardiographer MAC 600 GE Medical Systems Information Technologies, Inc, Milwake, WI, USA, at a speed of 25mm/s, with automatic standardizations according to voltage. The measurements were made manually, avoiding automated measurements. Regarding the FQRS, the patients who presented it were grouped according to the affected walls in inferior, antero-septal, anterior, lateral, and the combination of any of these. The existence of FQRS on 12-lead ECG was defined according to previous related investigations [2,5,6].
In patients with narrow QRS, namely, QRS less than 120ms, the definition of FQRS comprised the presence of an additional R wave (R’) or notching in the nadir of the R wave or the S wave, or the presence of one R’ (fragmentation) in two contiguous leads. In patients with wide QRS, FQRS was defined as two notches in the R or S wave in two contiguous leads.
The QT interval was measured from the onset of the QRS to the end of the T wave. Each measurement was performed in three successive beats because averaging QT interval reduces the bias. The Bazett formula was utilized for correction of the QT interval for the heart rate, in which the QTc is the ratio of the QT interval and the square root of the R-R interval in milliseconds (QTc=QT/RR1/2). The cut-off value to define abnormal QTc in males was a QTc above 450ms; and, in females, above 470ms. Secondary causes of prolongation of QTc interval, namely dyselectrolithemia, neurological causes, treatment with amiodarone, sotalol or other antiarrhythmics, etc. were excluded.
The ECG were reviewed and measured independently by two researchers (NJA and JMT), and the measurements were entered in duplicate to eliminate interobserver variability. Kappa values were utilized to determine interobserver variability and reliability for categorical variables; values of 0.81-1.0 are indicative of excellent agreement; 0.61-0.80, substantial agreement; 0.41-0.60, moderate agreement; 0.21-0.40, fair agreement; 0-0.20, slight agreement; and values≤0, poor agreement [42].
This method produced an excellent correlation between the two observations with a kappa statistic of 0.85. If there was discrepancy between the two recordings, the original electrocardiogram was retrieved and reassessed by the two researchers and reviewed with a third cardiologist (OC), together until a consensus was reached. We estimated the strength of the associations using 95% confidence intervals and a p-value <0.05 was considered statistically significant.
Results
Of the total 123 patients with ischemic heart disease, 81 had documented coronary artery disease by coronary angiography. These are the patients entered for further analysis. Of these 81 patients, 61.7% were male, and 38% were female. The mean age was 63.8±12.6 years, with a minimum age of 36 years and a maximum age of 94 years of age. Regarding the cardiovascular risk factors, 78% of patients had HBP, 25% DM2, 25% dyslipidemia, 12% obesity, and 11% family history, and 33% were smokers (Table 1).
Table 1.
Comparison of certain clinical variables in IHD patients with and without FQRS. *t test (ANOVA) **Yates correction
|
|
Total n=81 |
FQRS n=36 (44%) |
Normal QRS n=45 (56%) |
p Value* |
|
Age average±SD |
63±12 |
63±12 |
63±12 |
0,9 |
|
Male gender |
50 (62) |
23 (64) |
27 (60) |
0,7 |
|
Female gender |
31 (38) |
13 (36) |
18 (40) |
|
|
Hypertension, n (%) |
63 (78) |
27 (75) |
36 (80) |
0,5 |
|
Diabetes Mellitus, n (%) |
20 (25) |
6 (17) |
14 (31) |
0,1 |
|
Dyslipidemia, n (%) |
20 (25) |
8 (22) |
12 (27) |
0,6 |
|
Obesity, n (%) |
10 (12) |
5 (14) |
5 (11) |
0,7 |
|
CVD, Family history, n (%) |
9 (11) |
2 (5) |
7 (15) |
0,2** |
|
Smoking, n (%) |
27 (33) |
11 (30) |
16 (35) |
0,6 |
|
Myocardial infarction, n (%) |
43 (53) |
22 (61) |
21 (47) |
0,1 |
|
Heart failure, n (%) |
38 (47) |
14 (39) |
24 (53) |
0,1 |
|
Bundle branch Block, n (%) |
7 (9) |
7 (19) |
0 |
0,007** |
|
AV nodal Block, n (%) |
14 (17) |
7 (19) |
7 (15) |
0,6 |
|
QT duration, ms, average±SD |
413±60 |
417±56 |
410±64 |
0,6 |
|
QTc duration, ms, average±DS |
463±56 |
468±56 |
460±56 |
0,5 |
|
Atrial arrhythmias, n (%) |
11 (13) |
4 (11) |
7 (15) |
0,7** |
A total of 36 (44%) patients presented FQRS (64% men and 36% women), being the most frequent location the inferior wall (61%), followed by the antero-septal, and lateral walls (both 14%), then the inferolateral wall 6% and, finally other combined locations with only 3%. A total of 43 (53%) patients had myocardial infarction, and there was no significant difference in the presence or not of FQRS in these patients. There were 37 (46%) patients with multi-vessel disease, 33 (40%) patients with mono-vessel disease, and 11 (14%) patients with only irregularities in the coronary arteries. There was no significant difference in the presence or not of FQRS regarding the number of compromised coronary arteries. In total, only 22 patients presented prolonged QT interval (27%), but remarkably when correcting for HR, we obtained that 44 patients had prolonged QTc (Table 2) (54%). Regarding the analysis of the electrocardiograms, the average heart rate was 77±26 beats per minute, the predominant rhythm was sinus (85.2%) followed by atrial fibrillation (12.3%) and finally atrial flutter (2.5%).
Table 2.
QT Interval measurements in patients with IHD.
|
|
QT Interval |
QTc Interval |
|
|
N |
|
81 |
81 |
|
Media |
413,59 |
463,67 |
|
|
Median |
408,00 |
458,00 |
|
|
Standard D. |
60,762 |
56,376 |
|
|
Range |
336 |
271 |
|
|
Mínimum |
304 |
364 |
|
|
Máximum |
640 |
635 |
|
Considering the relationship of the FQRS with the QTc interval prolongation: Of the patients with FQRS (36 patients), 23 patients had prolongation of the QTc interval, and 13 patients did not present it. Of those without FQRS (45 patients), 21 of them had prolongation of the QTc interval, and 24 patients did not have it. These results gave a sensitivity of 52% with a moderate SnNout, a specificity of 65% with a moderate SpPin, a positive predictive value of 64%, a negative predictive value of 53%, and a prevalence of 54%.
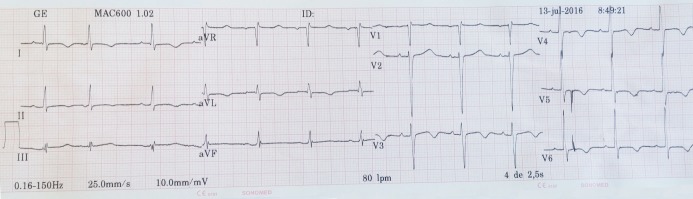
Figure 1 shows FQRS in inferior leads and a normal QTc of 416ms in a male patient with IHD.
Figure 1.
This electrocardiogram shows QRS complex fragmentation in inferior leads and a normal QTc of 416ms in a male patient with ischemic heart disease.
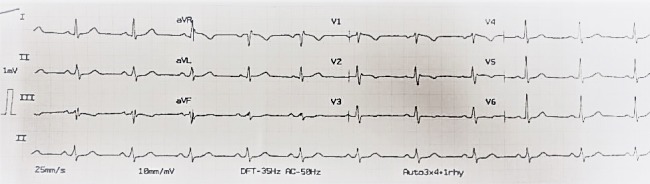
Figure 2 depicts FQRS also in inferior leads and a prolonged QTc of 469ms in a male patient with IHD.
Figure 2.
This electrocardiogram depicts QRS complex fragmentation also in inferior leads and a prolonged QTc of 469ms in a male patient with ischemic heart disease.
Discussion
To the best of our knowledge the present study is the first to report that the presence of FQRS in a conventional electrocardiogram has a moderate sensitivity and specificity, as well as, a moderate negative and positive predictive accuracy of the existence of QT interval prolongation in patients with ischemic heart disease. FQRS recorded on routine 12-lead ECG are proposed as useful indicators for identifying risk of cardiovascular events in patients with IHD and other entities (35-40).
Fragmented QRS complex can be rapidly, easily and simply assessed in an efficient manner with a widely available conventional 12-lead electrocardiogram.
FQRS was proposed as useful marker for identifying risk of ventricular arrhythmias in patients with prolonged QT interval. In this context, there is an interesting research about the association of FQRS with torsades de pointes in patients with acquired long QT syndrome (LQTS) (30). It was evaluated both repolarization (QT components) and depolarization parameters (FQRS) in acquired LQTS patients with markedly prolonged QT interval. The authors studied 70 patients with acquired severe QT prolongation (QTc≥550ms). A total of 32 patients had syncope or torsade des points (syncope group). The other 38 patients did not have any symptoms (asymptomatic group). The existence of FQRS and QT components (QT, QTc, Tpe [interval between peak and end of T wave] intervals, and U-wave voltage) was analyzed. They found that the syncope group had more frequent FQRS (81%) than did the asymptomatic group (21%, P<0.01) and the incidence of FQRS was not different before and after removal of predisposing factors. The incidence of organic heart disease was not different between the two groups. No differences in QTc interval were noted between the syncope and asymptomatic groups, although the syncope group had longer QT and Tpe intervals and higher U wave than the asymptomatic group (P<0.01). Therefore, the authors concluded that acquired predisposing factors promoted repolarization abnormality (especially prolongation of QT and Tpe intervals), and the existence of FQRS had an important role in the development of torsades des points in patients with acquired LQTS. Indeed, when there is a prolonged QT or QTc interval in the electrocardiogram especially with bizarre T wave, namely, notched/flat T wave or negative T wave, physicians should look for the presence of FQRS complex. These electrocardiographic markers are predictors for the occurrence of lethal ventricular arrhythmias.
Four years ago, another interesting study (30) investigated the association between FQRS and prolonged QTc duration with occurrence of malignant ventricular arrhythmias or sudden cardiac death in patients with hypertrophic cardiomyopathy. They studied 195 patients with hypertrophic cardiomyopathy. The endpoints comprised sudden cardiac death, documented sustained ventricular tachycardia or fibrillation, or appropriate implantable cardioverter defibrillator therapies. After a median follow-up of 5.7 years, 26 (13%) patients experienced clinical endpoints. Patients with FQRS in 3 or more territories (inferior, lateral, septal and/or anterior) (p=0.004) or QTc≥460ms (p=0.009) had worse cumulative survival free than patients with FQRS in less than 3 territories or QTc<460ms. This two electrocardiographic parameters, FQRS in≥3 territories and QTc≥460ms were independently associated with ventricular arrhythmias and sudden cardiac death.
The authors concluded that both of these electrocardiographic parameters, FQRS in≥3 territories and QTc interval duration are associated with malignant ventricular arrhythmias and sudden cardiac death in patients with hypertrophic cardiomyopathy, independently of and incremental to conventional cardiovascular risk factors [31].
In a different study with patients with hypertrophic cardiomyopathy; Gray B, et al. [43] investigated 164 high risk patients with implantable cardioverter-defibrillators. They analyzed the relation of prolonged QT intervals to predict appropriate device shocks. The authors showed that QTc duration≥439ms independently of the presence of conventional risk factors predicts appropriate device shocks, yielding a more than 3-fold risk increase [43].
Therefore, FQRS is associated to ventricular tachycardias and sudden cardiac deaths in patients prone to develop ventricular arrhythmias. Since FQRS was found to be significantly associated with myocardial scars, it is understandable the association of this arrhythmogenic substrate to the development of reentrant rhythms. In the present study, we found just a moderate sensitivity and specificity, as well as, moderate negative and positive predictive accuracy of FQRS to predict an association to prolonged QT intervals in patients with IHD. Probably, this predictability would have been higher with a greater population. There are limitations with our research. First, this is a retrospective investigation that recruited IHD patients within a single center. Second, the size of our study population was relatively small, hence, our predictability accuracy would have been higher with a greater population.
Therefore, our research may have lacked the statistical power necessary to identify all significant differences and associations. Third, FQRS complex and myocardial fibrosis may be caused by etiologies other than IHD, such as myocarditis, hypertrophic cardiomyopathy, and other cardiomyopathies. However, in this context, all of our patient´s IHD were documented by coronary angiography, and no one had hypertrophic cardiomyopathy.
Conclusion
The presence of fragmented QRS complex in the electrocardiogram has a moderate sensitivity and specificity, as well as, and a moderate negative and positive predictive accuracy of the existence of prolonged QT interval in patients with ischemic heart disease.
Acknowledgments
We thank Dr. Angelica Helga Neumann for her constant support in the making of this manuscript, and Miss Felicita Torales for her help with the literature search. We received no funding, no financial support for this article.
Conflict of Interest
All the authors declare that they have no conflicts of interest related to this article.
References
- 1.Kannel WB. Common electrocardiographic markers for subsequent clinical coronary events. Circulation. 1987;75(3 Pt 2):II25–7. [PubMed] [Google Scholar]
- 2.Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495e2501–2495e2501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 3.Morita H, Kusano KF, Miura D, Nagase S, Nakamura K, Morita ST, Ohe T, Zipes DP, Wu J. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118:1697–1704. doi: 10.1161/CIRCULATIONAHA.108.770917. [DOI] [PubMed] [Google Scholar]
- 4.Sakane K, Takaki H, Okamura H. Visualization of intra-QRS fragmented activation in patients with hypertrophic cardiomyopathy and life-threatening ventricular arrhythmia using magnetocardiograph. Eur Heart J. 2011;32:155–155. [Google Scholar]
- 5.Das MK, Suradi H, Maskoun W, Michael MA, Shen C, Peng J, Dandamudi G, Mahenthiran J. Fragmented wide QRS on a 12-lead ECG: A sign of myo¬cardial scar and poor prognosis. Circ Arrhythm Electrophysiol. 2008;1(4):258–268. doi: 10.1161/CIRCEP.107.763284. [DOI] [PubMed] [Google Scholar]
- 6.Das MK, Saha C, El Masry, Peng J, Dandamudi G, Mahenthiran J, McHenry P, Zipes DP. Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4(11):1385–1392. doi: 10.1016/j.hrthm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Schick TD, Powers SR, Jr undefined. Spectral analysis of the high frequency electrocardiogram in contusive myocardial injury. Ann Biomed Eng. 1978;6(2):154–60. doi: 10.1007/BF02584541. [DOI] [PubMed] [Google Scholar]
- 8.Pietrasik G, Zareba W. QRS fragmentation: Diagnostic and prognostic significance. Cardiol J. 2012;19(2):114–21. doi: 10.5603/cj.2012.0022. [DOI] [PubMed] [Google Scholar]
- 9.Simson MB, Untereker WJ, Spielman SR, Horowitz LN, Marcus NH, Falcone RA, Harken AH, Josephson ME. Relation between late potentials on the body surface and directly recorded fragmented electrograms in patients with ventricular tachycardia. Am J Cardiol. 1983;51(1):105–112. doi: 10.1016/s0002-9149(83)80020-4. [DOI] [PubMed] [Google Scholar]
- 10.Das MK, Saha C, El Masry, Peng J, Dandamudi G, Mahenthiran J, McHenry P, Zipes DP. Fragmented QRS on a 12-lead ECG: A predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4(11):1385–1392. doi: 10.1016/j.hrthm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 11.Pietrasik G, Goldenberg I, Zdzienicka J, Moss AJ, Zareba W. Prognostic significance of fragmented QRS complex for predicting the risk of recurrent cardiac events in patients with Q wave myocardial infarction. Am J Cardiol. 2007;100(4):583–586. doi: 10.1016/j.amjcard.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 12.Fuster V, Lewis A. Mechanisms leading to myocardial infarction: Insights from studies of vascular biology. Circulation. 1994;90(4):2126–2146. doi: 10.1161/01.cir.90.4.2126. [DOI] [PubMed] [Google Scholar]
- 13.Rogers WJ, Frederick PD, Stoehr E, Canto JG, Ornato JP, Gibson CM, Pollack CV, Gore JM, Chandra-Strobos N, Peterson ED, French WJ. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non-ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156(6):1026–1034. doi: 10.1016/j.ahj.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd-Jones D, Adams R, Carnethon M, De Simone, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J; Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 16.Kohli P, Cannon CP. Acute coronary syndromes in 2011: walking the tightrope between efficacy and bleeding. Nat Rev Cardiol. 2012;9:69–71. doi: 10.1038/nrcardio.2011.206. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira-González I. Epidemiología de la enfermedad coronaria. Rev Esp Cardiol. 2014;67:139–44. [Google Scholar]
- 18.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111(2):383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 19.Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med. 1978;89(2):157–161. doi: 10.7326/0003-4819-89-2-157. [DOI] [PubMed] [Google Scholar]
- 20.Chew DS, Wilton SB, Vaid HM, Southern DA, Ellis L, Howarth AG, White JA, Exner DV. Fragmented QRS complexes after acute myocardial infarction are independently associated with unfavorable left ventricular remodeling. J Electrocardiol. 2018;51(4):607–612. doi: 10.1016/j.jelectrocard.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Tangwiwat C, Kaolawanich Y, Krittayaphong R. Electrocardiographic predictors of myocardial fibrosis and apical hypertrophic cardiomyopathy. Ann Noninvasive Electrocardiol. 2019;24(2):e12612–e12612. doi: 10.1111/anec.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanjanahattakij N, Rattanawong P, Riangwiwat T, Prasitlumkum N, Limpruttidham N, Chongsathidkiet P, Vutthikraivit W, Crossey E. Fragmented QRS and mortality in patients undergoing percutaneous intervention for ST-elevation myocardial infarction: Systematic review and meta‐analysis. Ann Noninvasive Electrocardiol. 2018;23(6):e12567–e12567. doi: 10.1111/anec.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gungor undefined, B undefined, Ozcan KS, Karatas MB, Sahin I, Ozturk R, Bolca O. Prognostic value of QRS fragmentation in patients with acute myocardial infarction: a meta‐analysis. Annals of Noninvasive Electrocardiology. 2016;21(6):604–612. doi: 10.1111/anec.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.anga A, Kocaman SA, Durakoğlugil ME, Cetin M, Erdoğan T, Kırış T, Erden M. Relationship between fragmented QRS complexes and left ventricular systolic and diastolic functions. Herz. 2013;38(6):665–670. doi: 10.1007/s00059-012-3739-1. [DOI] [PubMed] [Google Scholar]
- 25.Abildskov JA. Adrenergic effects on the QT interval of the electrocardiogram. Am Heart J. 1976;92(2):210–216. doi: 10.1016/s0002-8703(76)80256-6. [DOI] [PubMed] [Google Scholar]
- 26.Han J, Goel BG. Electrophysiologic precursors of ventricular tachyarrhythmias. Arch Intern Med. 1972;129(5):749–755. [PubMed] [Google Scholar]
- 27.Locati E, Schwartz PJ. Prognostic value of QT interval prolongation in post myocardial infarction patients. Eur Heart J. 1987;8:121–126. doi: 10.1093/eurheartj/8.suppl_a.121. [DOI] [PubMed] [Google Scholar]
- 28.Pohjola-Sintonen S, Siltanen P, Haapakoski J. Usefulness of QTc interval on the discharge electrocardiogram for predicting survival after acute myocardial infarction. Am J Cardiol. 1986;57(13):1066–1068. doi: 10.1016/0002-9149(86)90675-2. [DOI] [PubMed] [Google Scholar]
- 29.Zabel M, Klingenheben T, Franz MR, Hohnloser SH. Assessment of QT dispersion for prediction of mortality or arrhythmic events after myocardial infarction: results of a prospective, long-term follow-up study. Circulation. 1998;97(25):2543–2550. doi: 10.1161/01.cir.97.25.2543. [DOI] [PubMed] [Google Scholar]
- 30.Haraoka K, Morita H, Saito Y, Toh N, Miyoshi T, Nishii N, Nagase S, Nakamura K, Kohno K, Kusano KF, Kawaguchi K, Ohe T, Ito H. Fragmented QRS is associated with torsades de pointes in patients with acquired long QT syndrome. Heart Rhythm. 2010;7(12):1808–1814. doi: 10.1016/j.hrthm.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Debonnaire P, Katsanos S, Joyce E, Van Den Brink OV, Atsma DE, Schalij MJ, Bax JJ, Delgado V, Marsan NA. QRS Fragmentation and QTc Duration Relate to Malignant Ventricular Tachyarrhythmias and Sudden Cardiac Death in Patients with Hypertrophic Cardiomyopathy. J Cardiovasc Electrophysiol. 2015;26(5):547–555. doi: 10.1111/jce.12629. [DOI] [PubMed] [Google Scholar]
- 32.Zareba W, Moss AJ, le Cessie. Dispersion of ventricular repolarization and arrhythmic cardiac death in coronary artery disease. Am J Cardiol. 1994;74(6):550–553. doi: 10.1016/0002-9149(94)90742-0. [DOI] [PubMed] [Google Scholar]
- 33.Gray B, Ingles J, Medi C. Prolongation of the QTc Interval Predicts Appropriate Implantable Cardioverter-Defibrillator Therapies in Hypertrophic Cardiomyopathy. JACC Heart Fail. 2013;1:149–155. doi: 10.1016/j.jchf.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Johnson JN, Grifoni C, Bos JM, Saber-Ayad M, Ommen SR, Nistri S, Cecchi F, Olivotto I, Ackerman MJ. Prevalence and clinical correlates of QT prolongation in patients with hypertrophic cardiomyopathy. Eur Heart J. 2011;32(9):1114–1120. doi: 10.1093/eurheartj/ehr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan G, Wang M, Yiu KH, Lau CP, Zhi G, Lee SW, Siu CW, Tse HF. Subclinical left ventricular Dysfunction Revealed by Circumferential 2D Strain Imaging in Coronary Artery Disease Patients with ECG Fragmented QRS Complex. Heart Rhythm. 2012;9(6):928–935. doi: 10.1016/j.hrthm.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Dinakrisma AA, Wijaya IP, Nasution SA, Dewiasty E. The Role of Fragmented QRS (fQRS) As A Predictor of Major Adverse Cardiac Event within 30 days in Acute Coronary Syndrome Patients: A Retrospective Cohort Study. Acta Med Indones-Indones J Intern Med. 2019;51(1):3–9. [PubMed] [Google Scholar]
- 37.Centurión OA, Aquino-Martinez NJ, Torales-Salinas JM, Miño LM, Sequeira-Villar OR, Scavenius-Aguilera KE. Role of QRS Complex Fragmentation in Patients at High Risk of Cardiovascular Events. M J Cardiol. 2017;2(1):009–009. [Google Scholar]
- 38.Michael MA, Das MK. Fragmented QRS on a 12-lead ECG is a Sign of Acute or Recent Myocardial Infarction. Circulation. 2006;114:II 512–II 512. [Google Scholar]
- 39.Das MK, Michael MA, Suradi H. Fragmented QRS Complex on 12-lead ECG Developed During the First 48 Hours after Acute Myocardial Infarction Predicts Mortality. Circulation. 2008;118:S 1059–S 1059. [Google Scholar]
- 40.Das MK, Maskoun W, Shen C, Michael MA, Suradi H, Desai M, Subbarao R, Bhakta D. Fragmented QRS on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm. 2010;7(1):74–80. doi: 10.1016/j.hrthm.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 41.Ciftci O, Keskin S, Karaçağlar E, Yılmaz KC, Aktaş A, Sezer S, Moray G, Müderrisoğlu İH, Haberal M. Fragmented QRS on 12-Lead Electrocardiogram Is Correlated With Severe Coronary Artery Disease and Abnormal Myocardial Perfusion Scintigraphy Results in Renal Transplant Candidates. Experim Clin Transpl. 2018;16(6):690–695. doi: 10.6002/ect.2017.0263. [DOI] [PubMed] [Google Scholar]
- 42.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 43.Gray B, Ingles J, Medi C. Semsarian C. Prolongation of the QTc Interval Predicts Appropriate Implantable Cardioverter-Defibrillator Therapies in Hypertrophic Cardiomyopathy. JACC Heart Fail. 2013;1(2):149–155. doi: 10.1016/j.jchf.2013.01.004. [DOI] [PubMed] [Google Scholar]