Abstract
Gastric cancer currently represents one of the most important public health problems. Recent studies have demonstrated the existence of strong correlations between the vegetative nervous system and the role it plays in the initiation of the oncogenetic process and the progression of cancer. The purpose of this paper is to demonstrate the involvement of the sympathetic and parasympathetic vegetative nervous system in the evolution of gastric cancer, according to the stage of tumor differentiation. In this current paper we have included a number of four patients diagnosed with gastric cancer post UGI (Upper Gastrointestinal Endoscopy) and have analyzed relations that exist between the tumor differentiation degree and the metanephrine and normetanephrine serum level in the blood of the patients. Following the research, we have observed an increased value of the metanephrine and normetanephrine serum level in the patient which displayed the lowest degree of differentiation.
Keywords: Gastric cancer, metanephrine, normetanephrine, differentiation degree
Introduction
Although the incidence of gastric cancer is declining at a global level, this disease still remains one of the most frequent cause of death through cancer.
Diagnosed in early stages it is curable, but unfortunately most cases are identified late, in advanced stages.
In 2012, ESMO (European Society for Medical Oncology) has reported approximately one million new cases and about 700,000 deaths worldwide, of which about 140,000 cases were in Europe, with about 104,000 deaths [1].
Currently we can state that the pathogenesis and mechanisms implicated in the evolution of gastric cancer are not fully clarified, therefore we consider that identifying the vegetative nervous system parameters plays an important role in demonstrating the fact that the interaction between nerves and cancer cells goes beyond the invasion and perineural concept [2].
The nervous elements are part of the tumor microenvironment, therefore under physiological conditions, the sympathetic and parasympathetic nervous system together with the enteric nervous system maintain the homeostasis of the tissue they innervate, both directly and also through the neurotransmitters that they release: catecholamines (metanephrine and normetanephrine) and acetylcholine [3].
Through this paper we want to show the fact that the nervous system can influence the cancer initiation process and proliferation, not only through the action exercised by its neurotransmitters, but also of the specific receptors.
Furthermore, we must mention the fact that cancer cells can also influence the nervous elements through the production of tumor growth factors, involved in the cancerous process [3].
The purpose of this paper is to show the correlation between the catecholamines serum values from the peripheral blood of patients diagnosed with gastric cancer in line with the tumor differentiation degree, but also the clinicopathological aspect.
Matherial and Methods
We analyzed the cases of four patients diagnosed with gastric cancer that were subjected to upper gastrointestinal endoscopy at the (CCEH) County Clinical and Emergency Hospital in Craiova, Romania, between November 2018 and April 2019. All patients were presented with a description and purpose of the procedures that we performed for the study and acknowledged in writing their participation. No personal data was collected, all procedures were performed in accordance to current regulations, without interfering with normal diagnostic or treatment procedures.
Blood samples were taken from the patients in order to dose the serum metanephrines and normetanephrines.
The blood samples were taken in special tubes (vacutainer with K3-EDTA, cooled before usage) and analyzed at an analysis laboratory.
Preparing the patient: collection of biological samples-à jeun, without the consumption of alcoholic or caffeinated drinks 24 hours prior collection; also the patient had not taken acetaminophen, tricyclic antidepressants, phenoxybenzamine, monoamine oxidase alpha agonists and inhibitors at least 5 days prior collection; if antihypertensive medication was required, the use of Angiotensin-converting enzyme (ACE) inhibitors, Angiotensin II Receptor Blockers (ARBs) and Alpha 1‐Adrenergic Blockers was recommended, which did not influence the results [4,5].
The venous blood collected in the vacutainer tubes with K3-EDTA, cooled before usage was agitated slowly by flipping and then immediately set on ice. Afterwards, the samples were transported to the laboratory in maximum two hours [4]. Causes for sample rejection: Hemolysis, lipemia, hyperbilirubinemia and heparinization of the specimen.
The processing needed after collection: in a maximum of two hours after collection, the plasma was separated through centrifugation for 15 minutes at 1500 x g, where g=(1118+10-8) x (radius in cm) x (rpm)^2; for the separation of the plasma a plastic dropper was used; if the sample could not be analyzed immediately, it was frozen at-20°C. Separated plasma is stable for 6 hours at 2-8° and for 6 months at-20°C [4].
Method-competitive ELISA.
The free plasmatic metanephrines and normetanephrines are determined quantitatively after a precipitation stage.
The antibodies contained in the kit recognize only the L biologically relevant forms of the metanephrines [4].
Reference values:
Metanephrine: <65pg/mL
Normetanephrine: <196pg/mL3
Detection limit:
Metanephrine: 5pg/mL
Normetanephrine: 10pg/mL3
Metanephrine and normetanephrine are degradation products (metabolites) of epinephrine (adrenaline) and norepinephrine (noradrenaline).
Epinephrine and norepinephrine are hormones called catecholamines, which are released in the blood as a response to physical and emotional stress and help with the adjustment of blood flow and pressure in the organism.
Catecholamines are synthetized in the brain, adrenal medulla, chromaffin cells dispersed in other tissues and in the nervous endings of the sympathetic nervous system.
Later, the biological samples taken through upper gastrointestinal endoscopy were analyzed histopathological. The tissue samples were processed in paraffin and the HE stained slides were analyzed in the Pathological Anatomy Laboratory of the CCE Hospital in Craiova.
Following the results obtained we have managed to make a correlation concerning the tumor differentiation degree and the serum value of the metanephrines and normetanephrines.
Results
The paper includes a number of four patients, of which three male and one female, with ages between 51 and 82 years old (Tables 1 , 2).
Table 1.
Individual characteristics of the patients.
Table 2.
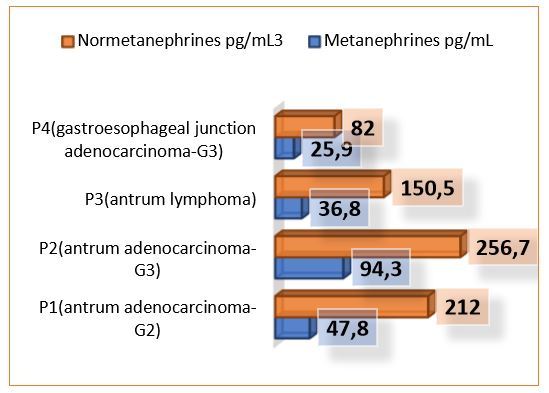
Metanephrines and normetanephrines serum values according to clinical characteristics of the patients included in the study.
|
|
G |
Location |
HP aspect |
Metanephrines (pg/mL) |
Normetanephrines (pg/mL3) |
|
P 1 |
G2 |
Antrum |
Adeno-carcinoma |
47.8 |
212 |
|
P 2 |
G3 |
Antrum |
Adeno-carcinoma |
94.3 |
256.7 |
|
P 3 |
Lymphoma |
Antrum |
Lymphoma |
36.8 |
150.5 |
|
P 4 |
G3 |
Gastro Esophageal junction |
Adeno-carcinoma |
25.9 |
82 |
Of these, only three have had the gastric adenocarcinoma diagnosis confirmed.
The fourth was diagnosed with lymphoma.
The first patient included in the study was a 79-year-old female, which, at the moment she was diagnosed, at the endoscopic exam, presented a vegetation formation, friable and with spontaneous bleeding during biopsy. At the moment of the diagnosis, from an imagistic point of view, the patient displayed hepatic dissemination. Histologically, a gastric mucosa microscopic structure with invasive papillary adenocarcinoma islands in the muscularis mucosa and with moderate diffuse lymphoplasmacytic infiltrate (Figures 1 and 2).
Figure 1.
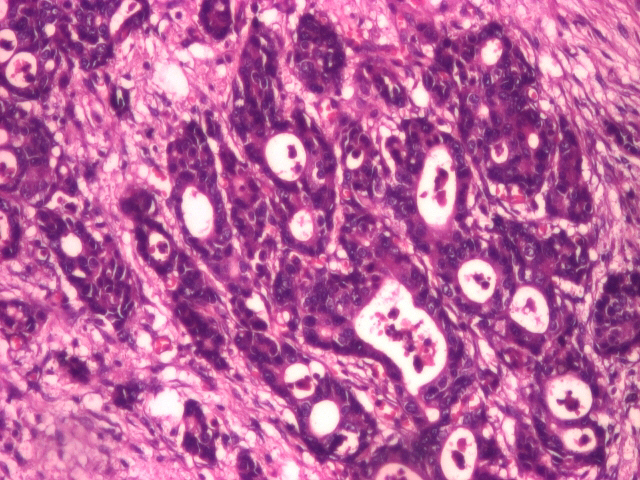
Well differentiated adenocarcinoma (HE staining), 20x.
Figure 2.
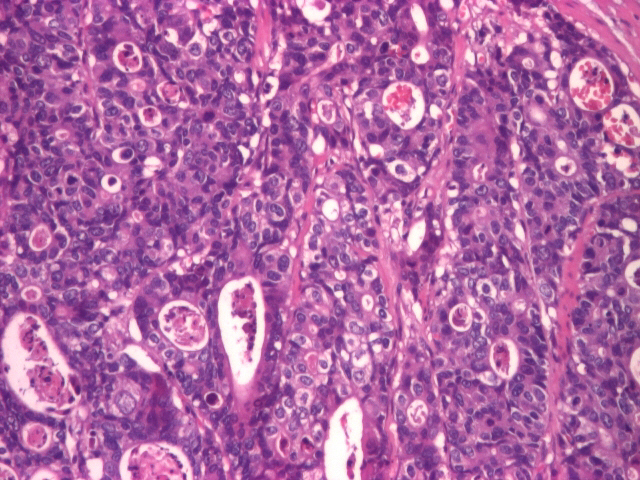
Moderately differentiated adenocarcinoma (HE staining), 20x.
Following the blood tests results, the patient displayed a Metanephrines value of 47.8pg/mL and a Normetanephrines value of 212pg/mL3.
The second patient included in the study was a male with de age of 82 years, diagnosed with low differentiated gastric cancer (G3-G4). The histopathological exam of the smear described an ulcerated gastric mucosa with hyperplasia of the parietal cells and areas of epithelial cell proliferation, with a pronounced inflammatory infiltrate of polymorphic character. Endoscopically, distal from the esogastric junction, an elevated formation with granular covering mucosa, which bled easily when touched with the endoscope. The CT scan showed that the patient displayed not only dissemination located in the apical segment of the right upper pulmonary lobe but also lymphadenopathy localized paratracheal lower left and lower right. At the peritoneal level, the CT scan showed a small amount of liquid describing peritoneal carcinomatosis. Following the blood tests results, the patient displayed a Metanephrines value of 94.3pg/mL and a Normetanephrines value of 256.7pg/mL3.
The third patient included in the study, a 51-year-old man, displayed a histopathological appearance of ulcerated gastric mucosa with the distortion of the architecture with an area of malignant cell proliferation, with medium and large cells with nucleolate nuclei and lymphoepithelial lesions. This histopathological appearance suggested the presence of a malign gastric lymphoma (Figure 3).
Figure 3.
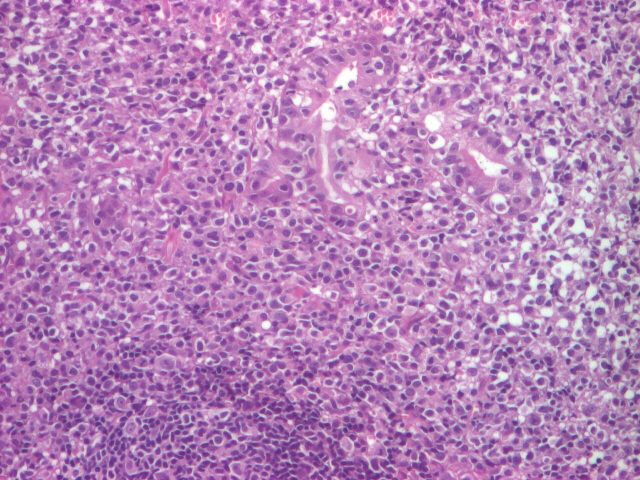
Gastric lymphoma (malignant proliferation of medium and large cells with nucleolate nuclei and lymphoepithelial lesions present) (HE staining), 10x.
Endoscopically the stomach displayed numerous antrum and prepyloric ulcerations, and a friable gastric body mucosa with multiple ulcerations with a perilesional infiltrative appearance. The CT scan sowed that the patient displayed lymphadenopathy at several levels: left gastroepiploic, superior pyloric, and left gastric. Other changes of oncological interest could not be detected. We must mention that the metanephrines and normetanephrines serum level of this patient was the lowest documented, in comparison with the other patients diagnosed with gastric adenocarcinoma. For this patient the results were within normal limits: a Metanephrines value of 36.8pg/mL and a Normetanephrines value of 150.5pg/mL3.
The fourth patient included in the study was a 67-year-old man. The histopathological diagnosis described a gastric mucosa with areas of poorly differentiated adenocarcinoma with large areas of necrosis (Figure 4).
Figure 4.
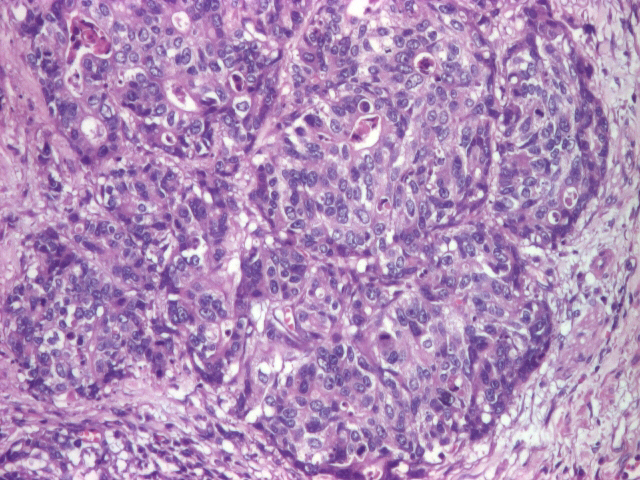
Poorly differentiated adenocarcinoma (HE staining), 20x.
We must mention that, for this patient, the endoscopic examination described a stenosing and bleeding vegetation formation at the level of the cardia and the fornix (gastroesophageal junction).
From an imagistic point of view the patient also displayed hepatic dissemination (Figures 5 and 6).
Figure 5.
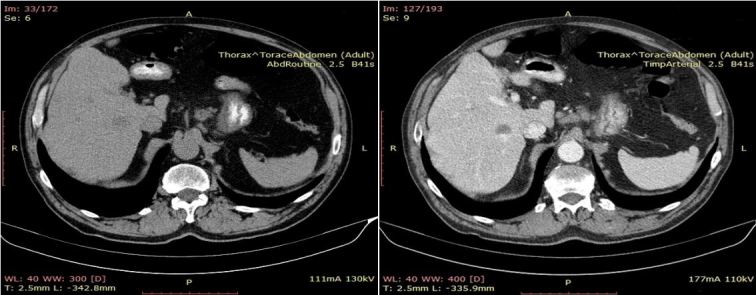
CT image suggestive for secondary hepatic determinations (Liver with non-homogeneous structure due to the presence of spontaneous hypodense, weakly iodophile oval round formations).
Figure 6.
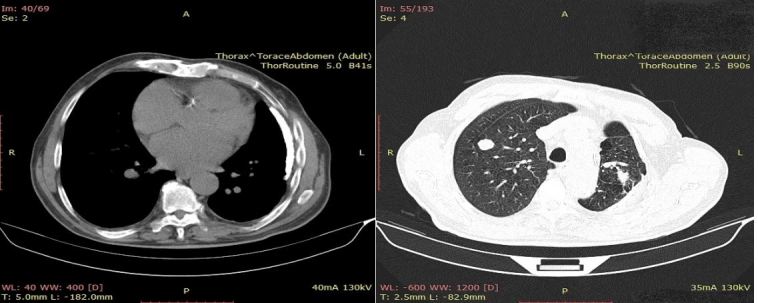
CT image suggestive for secondary pulmonary determinations (round tissue formations, discrete iodophile, well delimited).
Following the blood tests results, the patient displayed values within normal limits: a Metanephrines value of 25.9pg/mL and a Normetanephrines value of 82pg/mL3.
Following the analysis of the four cases included in the paper, we observed an increased metanephrines and normetanephrines serum value for the patients with a tumor formation located strictly at the gastric level, considering that for the patient with the tumor located at the gastroesophageal junction, the catecholamines values was within normal limits.
Regarding the pathological results, the patients with adenocarcinomas presented high catecholamines values, in comparison with the patient diagnosed with lymphoma.
The differentiation degree can be considered an influencing factor concerning the analysis of the serum metanephrines and normetanephrines level, considering that the patient with the lowest differentiation degree had the highest catecholamines serum value.
Discussion
Over the past decades there have been many studies that have shown the fact that the sympathetic nervous system activation leads to the release of catecholamine neurotransmitters from the adrenal glands into the blood through the SAM (Sympathetic-Adrenal-Medullary) axis. Nevertheless, the role of the adrenal gland in the control of malign tumors is not yet fully understood, fact which has determined us to thoroughly study the effect that the sympathetic nervous system has in the development of cancer through the local release of adrenergic neurotransmitters in peripheral tissues [6].
Social factors or stress factors can predict the effects on health among cancer patients [7], fact demonstrated by recent epidemiological data, which has shown that social and psychological stress can be associated with the onset of cancer [8]. Similarly, animal studies from decades ago have shown that neuronal stimulation associated with chronic or prolonged stress not only promotes tumor growth and cancer cell dissemination, but also decreases the chances of survival [9,10].
Recently, following thorough research, it was shown that individuals suffering from chronic stress had increased neutrophils, monocytes and lymphocytes values, caused by the activation of hematopoietic proliferation in the bone marrow, associated with an increase in the expression of adrenergic neurotransmitters [11].
A possible explanation for the fact that tumor development can be associated with stress relies on the activation of the sympathetic nervous system (SNS) through the sympathetic-adrenal-medullary (SAM) axis, which controls the adrenal release of adrenergic neurotransmitters in the blood flow, such as epinephrine or norepinephrine, in support of the fight-or-flight response. The implication of adrenergic neurotransmitters in cancer has been revealed by recent studies on the monitoring of tumor formation through pharmacological manipulation of the β-adrenergic signaling pathway [12,13].
Proving the fact that the autonomic nervous system stimulates the development and progression of gastric cancer can describe the role of neurogenesis in the development of new cancer targeted therapies [14,15].
Sympathetic nervous fibers play an interesting role in aiding tumor growth and development through the interaction with β2-and β-adrenergic receptors on the stromal cells.
Convincing proof that the autonomic nerves contribute to the progression of cancer through adrenergic or cholinergic signaling, raise questions concerning the mechanisms that control cancer-related neurogenesis and vice versa mechanisms that could be controlled by the nervous systems through these systems and biological pathways. Understanding the specific mechanisms of cancer and sympathetic nervous system interactions is essential for the development of new cancer therapies [16,17].
The gastric cancer patient is a patient submitted to a severe stress form. The experiences generated by the stress of the disease are founded on the threat represented by the oncological disease-gastric cancer. The cancer diagnosis is associated with a disabling disease, with death, with mutilation after surgical procedures. Exposal to such a process disturbs the adjustment patterns with which the patient is used to on a daily basis. The adjustment pattern of each individual can be defined as being a system of beliefs and behaviors that maintains the biological and emotional side of an individual in harmony with the environmental factors with which they interact. Each time this balance is disturbed or threatened, the patient generates anxious and depressive attitudes regarding the disturbing event. These experiences relate to the fact that the individual feels that he is losing control over the meaning of life, sees himself incapable of adjusting to the new environment requirements and becomes vulnerable in front of the disease’s uncertainty [18].
Gastric cancer is an oncological disease whose diagnosis is delayed because of the nonspecific symptoms which don’t lead to a fast diagnosis. The initial symptoms of this type of disease are quite vague in the beginning and very easily confused with other, less serious diseases. This is the reason for which confirming such a diagnosis generates a high stress level, assimilated by the respective patient as an emotional shock [19].
By highlighting the fact that the nervous system plays an important role in the malignancy process through the interaction between the cancer cell and the nerve, fact not only explained through the fact that in gastric cancer the neurotransmitters lead to cancer progression, but also through the fact that in the tumor microenvironment nervous elements are permanently recruited by the cancer cell, fact which leads to the initiation and progression of malignancy [20], the purpose of this paper is to try and improve the early diagnostic means of gastric cancer and also the possibility of discovering new targeted therapies for this aggressive oncological disease.
Conclusions
Through this paper we have tried to show the involvement of the vegetative nervous system in the evolution and prognosis of patients with gastric cancer, depending on its location, differentiation degree and stage.
We have shown the fact that the metanephrines and normetanephrines serum values are increased in the case of low differentiation adenocarcinoma patient, in comparison with the patient that presented a different cancer form.
Simultaneously we highlighted the fact that the metanephrines and normetanephrines serum values of the patients diagnosed with gastric adenocarcinoma are higher for the patient with a low differentiation degree (G3) in comparison with the patient with well differentiated adenocarcinoma.
According to tumor location we showed the fact that the catecholamines serum values are lower for the patient with gastroesophageal junction cancer.
Taking into consideration the fact that gastric cancer is a highly aggressive cancer, the differentiation degree plays an important role in the extension of the disease.
Acknowledgments
Alina-Maria Balea and Roxana Cruce share first authorship.
Conflict of interests
None to declare.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Jobling P, Pundavela J, Oliveira SM, Roselli S, Walker MM, Hondermarck H. Nerve Cancer Cell Cross-talk: A Novel Promoter of Tumor Progression. Cancer Res. 2015;75(9):1777–1781. doi: 10.1158/0008-5472.CAN-14-3180. [DOI] [PubMed] [Google Scholar]
- 3.Florescu C, Istrătoaie O, Târtea GC, Pirici D, Streba TC, Bogdan C, Puiu I, Târtea AE, Caragea DC, Ghiluşi MC, Comănescu MV, Rogoveanu I, Vere CC. Neuro-neoplastic interrelationships in colorectal level-immunohistochemical aspect in three cases and review of the literature. Rom J Morphol Embryol. 2016;57(2 suppl):639–650. [PubMed] [Google Scholar]
- 4.Eisenhofer G, Walther M, Keiser HR, Lenders JW, Friberg P, Pacak K. Plasma Metanephrines: A Novel and Cost-Effective Test for Pheocromocytoma. Braz J Med Biol Res. 2000;33(10):1157–1169. doi: 10.1590/s0100-879x2000001000005. [DOI] [PubMed] [Google Scholar]
- 5. McPetersen R, Pincus M . Henry’s Clinical Diagnosis and Management by Laboratory Methods . 21. Sauders Elsevier; 2007 . Evaluation of Endocrine Function ; pp. 342 – 344 . [Google Scholar]
- 6.Peters LJ, Kelly H. The influence of stress and stress hormones on the transplantability of a non-immunogenic syngeneic murine tumor. Cancer. 1977;39:1482–1488. doi: 10.1002/1097-0142(197704)39:4<1482::aid-cncr2820390420>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 7.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 8.Penninx BW, Guralnik JM, Pahor M, Ferrucci L, Cerhan JR, Wallace RB, Havlik RJ. Chronically depressed mood and cancer risk in older persons. J Nat Cancer Inst. 1998;90:1888–1893. doi: 10.1093/jnci/90.24.1888. [DOI] [PubMed] [Google Scholar]
- 9.Riley V. Mouse mammary tumors: alteration of incidence as apparent function of stress. Science. 1975;189:465–467. doi: 10.1126/science.168638. [DOI] [PubMed] [Google Scholar]
- 10.Hermes GL, Delgado B, Tretiakova M, Cavigelli SA, Krausz T, Conzen SD, McClintock MK. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc Nat Acad Sci USA. 2009;106:22393–22398. doi: 10.1073/pnas.0910753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 13.Shahzad MM, Arevalo JM, Armaiz-Pena GN, Lu C, Stone RL, Moreno-Smith M, Nishimura M, Lee JW, Jennings NB, Bottsford-Miller J, et al. Stress effects on FosB-and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J Biol Chem. 2010;285:35462–35470. doi: 10.1074/jbc.M110.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361–1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 15.Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, Flatberg A, Johannessen H, Friedman RA, Renz BW, Sandvik AK, Beisvag V, Tomita H, Hara A, Quante M, Li Z, Gershon MD, Kaneko K, Fox JG, Wang TC, Chen D. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6(250):250ra115–250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnon C. Role of the autonomic nervous system in tumorigenesis and metastasis. Mol Cell Oncol. 2015;2(2):e975643–e975643. doi: 10.4161/23723556.2014.975643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vere CC, Streba CT, Streba LM, Ionescu AG, Sima F. Psychosocial stress and liver disease status. World J Gastroenterol. 2009;15(24):2980–2986. doi: 10.3748/wjg.15.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord. 1988;14(1):61–68. doi: 10.1016/0165-0327(88)90072-9. [DOI] [PubMed] [Google Scholar]
- 19.Borkovec T, Costello E. Efficacy of applied relaxation and cognitive behavioral therapy in the treatment of generalized anxiety disorder. J Consult Clin Psychol. 1993;61(4):611–619. doi: 10.1037//0022-006x.61.4.611. [DOI] [PubMed] [Google Scholar]
- 20.Monje M. Settling a Nervous Stomach: The Neural Regulation of Enteric Cancer. Cancer Cell. 2017;31(1):1–2. doi: 10.1016/j.ccell.2016.12.008. [DOI] [PubMed] [Google Scholar]


