Abstract
Background
Unaccustomed eccentric exercise during sport or training may lead to delayed onset muscle soreness (DOMS), which has been demonstrated to influence postural control, potentially resulting in further injury. Afferent sensory input is critical to effective postural control, but little is known about somatosensory changes at the knee following induction of DOMS of the quadriceps muscle. The ‘soreness’ or hyperalgesia associated with DOMS has been postulated to occur because of damage to/inflammation of the exercised muscle, however, effects on central nociceptive mechanisms, which are known to induce altered postural responses, have been less studied.
Purpose/Hypothesis
It was hypothesized that DOMS of the quadriceps muscle would result in widespread hyperalgesia and hypoesthesia at the knee. Therefore, the purpose of this study was to investigate the effects of DOMS on knee somatosensory changes in asymptomatic healthy participants.
Study Design
Quasi-experimental cohort study
Methods
Thirty participants (15 males and 15 females) took part in the study. Eccentric exercise consisted of 10 sets of 10 maximum eccentric quadriceps contractions performed with the dominant knee. Outcome measures consisted of pain intensity (Visual Analog Scale), pressure pain threshold (PPT), vibration perception threshold (VPT) and proprioception, measured via threshold to detection of passive motion (TDPM) at the knee, at three different assessment time points: (1) pre-eccentric exercise; (2) immediately and (3) 48 hours post-eccentric exercise.
Results
Not surprisingly, pain intensity increased and PPT of the vastus medialis and rectus femoris muscles decreased (hyperalgesia) immediately post-exercise on the exercised limb. However, at 48 hours, hyperalgesia was demonstrated at other lower extremity muscles, including bilaterally at the tibialis anterior muscles, and also at the hand. Evidence of hypoesthesia was also demonstrated. VPT and TDPM increased (worsened) ipsilaterally both immediately and 48 hours after exercise, and TDPM increased bilaterally at 48 hours. Females demonstrated greater impairment in TDPM than males at 48 hours. Expanding distribution of hyperalgesia, ipsilaterally impaired VPT and bilaterally impaired proprioception were demonstrated in the presence of DOMS.
Discussion/Conclusion
Inflammation from unaccustomed eccentric exercise may induce neuroplastic changes in nociceptive pathways resulting in wider distribution of pain and hypoesthesia. Futures studies examining the effect of DOMS related somatosensory changes on postural control may be warranted.
Level of evidence
3
Keywords: Eccentric exercise, exercise-induced damage, gender, knee, quantitative sensory
INTRODUCTION
Delayed onset muscle soreness (DOMS) is common in sport, and is defined as a subacute pain state usually arising 24-48 hours after a bout of unaccustomed eccentric muscle contractions.1 DOMS has been attributed to an inflammatory reaction and nociceptive sensitization induced by minor myofibrillar and cytoskeletal damage occurring during eccentric exercise2 and is generally characterized by muscle pain, impaired force production and increased fatigability. Signs and symptoms found with DOMS have been attributed in part to central sensitization of nociceptive pathways.3 Central sensitization, or nociplasticity, has been identified in both chronic4 and acute5 musculoskeletal conditions and is associated with a number of somatosensory changes demonstrated via quantitative sensory testing. For example, widespread pain hyperalgesia,4 vibratory perception deficits and mechanical hypoesthesia6 have been demonstrated in patients with knee osteoarthritis, potentially contributing to functional deficits observed in this patient population.6,7 Somatosensory changes have also been demonstrated following acute knee injury. Individuals at greater than one-year post-anterior cruciate ligament (ACL) reconstruction demonstrated somatosensory deficits in both proprioception (TDPM) and vibratory perception in the surgical knee, when compared to both the opposite limb and to knees of age and sex matched controls.8 Studies of experimentally induced muscle pain may provide a controlled means to examine the sensory changes that occur with musculoskeletal injury.9
In studies with induced DOMS, muscle hyperalgesia has been demonstrated through pressure algometry over the exercised muscle3 and in an expanded distribution regional to the exercised muscle.10 This increased local and regional pain sensitivity potentially implicates alterations in both peripheral and central nociplastic mechanisms in DOMS-induced hyperalgesia. At the quadriceps muscle, Hedayatpour et al found pressure hyperalgesia, i.e., a decrease in pressure pain threshold (PPT), mostly in the distal aspect of the muscle after induction of DOMS.11 Understanding the underlying mechanisms of altered somatosensation and motor control post-injury may aid in developing both management and injury prevention strategies.
Impaired proprioceptive acuity following onset of DOMS has been reported, including alterations in joint position sense12 and force sense.13 Diminished sensory input is believed to adversely affect postural control.14 Correspondingly, altered postural responses to unexpected perturbations,15 and altered quadriceps muscle activation patterns,15 have also been observed after induction of DOMS. Considering that the muscle spindle is thought to provide the main input to proprioception,16 Brockett et al17 proposed that eccentric exercise may damage intrafusal as well as extrafusal muscle fibers leading to proprioceptive deficits; however, this would not explain other sensory phenomena that have been reported with induction of DOMS.18
High-resistance strength training, including components of eccentric exercise, is commonly used by athletes to enhance performance, with DOMS occurring as a common consequence of this type of training. Inadequate recovery from DOMS can lead to overtraining,19 altered motor control and further injury. DOMS of the quadriceps may lead to somatosensory changes at the knee, yet few studies have investigated this hypothesis. Therefore, the purpose of this study was to investigate the effects of DOMS on knee somatosensory changes in asymptomatic healthy participants. It was hypothesized that DOMS elicited in the quadriceps muscle would result in widespread hyperalgesia (demonstrated by lower PPTs in the upper as well as the lower limbs,) and sensory hypoesthesia at the knee measured via vibration perception threshold (VPT) and threshold to detection of passive motion (TDPM).
METHODS
Participants
Healthy volunteers (age 18-35) were recruited by local advertisement from the general population and invited to participate in the study. The participants had no previous knee injury, denied pain in any body part, and had not taken any pain-relieving, anti-inflammatory or psychiatric medications in the month prior to the study. Participants were blinded to the results during the testing period and no information on the postulated hypothesis was provided. All participants read and signed a written consent form prior to their participation in the study. The study was approved by Office for the Protection of Research Subjects at the University of Illinois at Chicago.
Eccentric Exercise Protocol
The participants were seated on a Biodex System 3 isokinetic dynamometer (Biodex Medical Systems, Shirley, NY, USA) with the body stabilized by straps over the thighs, waist, and chest and the lateral epicondyle of the femur aligned with the axis of rotation. In order to induce DOMS, one bout of 10 sets of 10 maximum eccentric quadriceps contractions of the dominant knee were performed at 60 °/s with the range of motion set to 10 ° of knee flexion and 90 ° knee flexion and the isokinetic dynamometer set in continuous passive mode.12 Leg dominance was determined by asking the participant which leg they would use to kick a ball.20 The dynamometer passively extended the limb between each eccentric action of the knee extensors. One minute of rest was allotted between sets. Verbal encouragement was given during each set to promote maximal effort. Prior to the exercise session, participants performed a warm-up consisting of eight minutes of cycling on a cycle ergometer (Monark, Vansbro, Sweden) at 70 rpm and 50 watts as previously described.12 Participants were advised to maintain a normal level of activity between data collection time points.
Outcome Measures
Outcome measures were serially assessed at baseline, immediately after the eccentric exercise protocol, and 48 hours post-exercise. A Visual Analog Scale (VAS) was used to assess the perceived maximal intensity of muscle pain 48 hours before, via recall, and 48 hours post-exercise during their regular activities of daily living (e.g. walking, stair climbing and sit to stand), and also immediately after the eccentric exercise protocol during walking.21
Pressure pain threshold was bilaterally assessed over both lower extremities. The tip (area: 1cm2) of an algometer (Model FDX100, Wagner Instruments, CT, USA) was applied perpendicularly to the tissue, at a steady rate of increasing pressure until the individual reported a change from pressure to a pain.22,23 Prior to PPT assessments, the participants were familiarized with algometric testing on their arm to clarify the procedure. The assessments were performed twice, and the mean calculated to determine the PPT which was used for analysis.24 An interval of at least 20 seconds was maintained between each PPT assessment. Participants were blinded to results. All assessments were performed by the same tester (KA) who was not blinded. PPT has shown excellent inter-rater reliability in healthy humans.25 PPT was assessed bilaterally, in a random order, over the muscle belly of the rectus femoris, vastus medialis, and tibialis anterior muscles. In addition, PPT at the webspace between the contra-lateral first and second metacarpal of the hand was also assessed to evaluate for widespread changes (Figure 1). Test areas were marked with an indelible pen to maintain consistent test sites on subsequent assessments. All somatosensory testing was performed with the participant in sitting on the Biodex isokinetic dynamometer.
Figure 1.
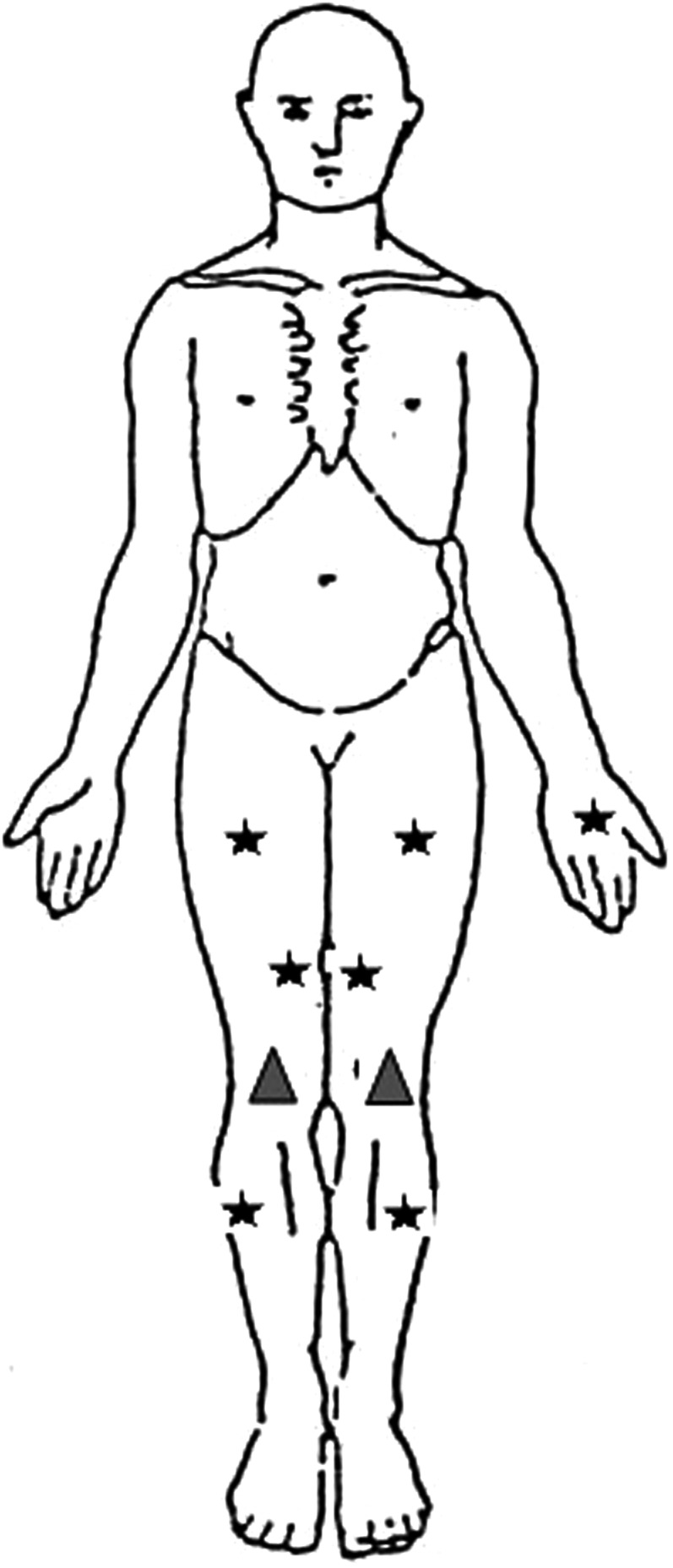
Sites for sensory assessment (Star indicates site of pressure pain threshold; Triangle indicates vibration perception threshold).
VPT was assessed bilaterally using a Biothesiometer (Bio-Medical, OH, USA) applied at the center of the patella. Excellent intra-rater and test-retest reliability has been reported.26 The vibratory tip (13 mm cylinder) oscillates at a frequency of 100 Hz at the site of application and the vibration amplitude of vibration (expressed in “biothesiometer units”) was increased until the participant perceived an initial vibration sensation.27 Three trials of this measurement were taken and the mean was used in the main analysis.8
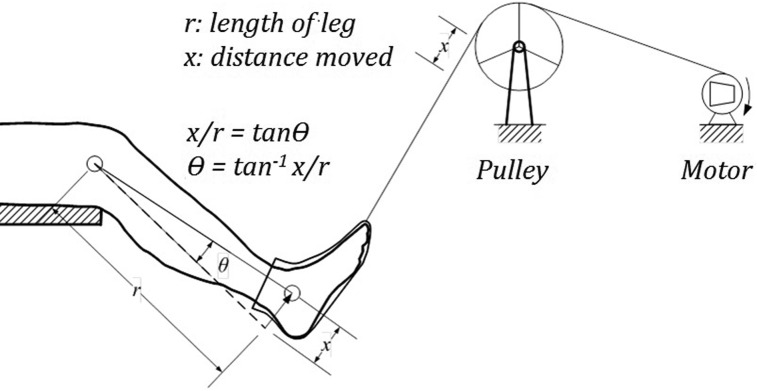
Proprioception was examined using TDPM. This method has been shown in a previous study by these authors to be reliable28 and has been used previously in athletes and patients.28-30 Participants were tested in the seated position (hip flexed at 70˚, knee flexed at 45˚, ankle in neutral) on the Biodex System 3 (Biodex Medical Systems Inc, Shirley, NY, USA). A custom-made device, utilizing a motor and pulley system, moved the extremity passively into either flexion or extension at a slow rate (0.5˚/s) after a random delay (Figure 2). The direction of movement and limb to be tested was randomly assigned. The participant was provided a handheld switch and was instructed to push the switch upon detecting change of joint position. An air splint inflated to 20 mm Hg was placed on the foot to minimize cutaneous input. The participant was blindfolded and listened to white noise to minimize visual and auditory inputs, respectively. Before every trial the participant was asked to co-contract the knee for 10 s to minimize the effects of thixotropy which can alter TDPM.31 A pretest trial was followed by three actual trials, the mean of which was used for data analysis. The amount of linear movement of the pulley (x) was documented and used to obtain the angle of threshold to detection of motion (θ in degrees) using the formula θ = tan −1 (x/r) where r = shank length.
Figure 2.
Measurement of threshold to detection of passive motion.
Sample Size Calculation
The sample size determination was conducted with an appropriate software (Tamaño de la Muestra, 1.1©, Spain). The determinations were based on detecting significant differences of 20% on PPTs between sessions with an alpha level of 0.05, and a desired power of 80%. This generated a sample size of at least 16 participants.
Statistical Analysis
Statistical analysis was performed using SPSS software, version 24.0 (Chicago, IL, USA). Mean, standard deviations and/or 95% confidence intervals were calculated for each variable. The Kolmogorov-Smirnov test revealed a normal distribution of the variables (p>0.05). Measurements (before, immediately after and 48 hours after eccentric exercise) and side (ipsi-lateral or contra-lateral) were introduced as within-subject factors and sex was introduced a covariate in a full-factorial repeated measure analysis of covariance for the VAS, PPT, VPT and TDPM. Bonferroni adjustment for multiple comparisons was used as post hoc test. In all tests, p<0.05 was considered significant.
RESULTS
Thirty age and sex matched participants, 15 males (mean age: 24.4 ± 3.8 years; BMI: 23.4 ± 3.3 kg/m2) and 15 females (mean age: 24.5 ± 3.7; BMI: 19.8 ± 1.3 kg/m2), aged 19 to 34 years (mean age: 25 ± 4 years; height: 169 ± 9 cm; BMI: 21.6 ± 3.1 kg/m2) participated in this study. All were right dominant; therefore, DOMS was induced on the right lower extremity.
The analysis showed a significant measurement × side interaction (F(2,60) = 48.902; p<0.001) for induced muscle pain, without influence of sex (F(2,30) = 2.615; p = 0.112). Pain was mostly induced on the ipsilateral quadriceps muscle immediately and 48 hours after eccentric exercise (Table 1).
Table 1.
Induced muscle pain and pressure pain thresholds (PPT) before and after high intensity eccentric exercise.
| Variable | Mean | SD | Effect Size |
|---|---|---|---|
| Trained Muscle Induced Pain (VAS, 0-10 cm) | |||
| Baseline | 0.0 | 0.1 | |
| Post-intervention | 3.1* | 1.6 | 2.06 |
| 48 hours after | 3.0* | 1.5 | 1.85 |
| Untrained Muscle Induced Pain (VAS, 0-10 cm) | |||
| Baseline | 0.0 | 0.1 | |
| Post-intervention | 0.4 | 0.5 | 0.39 |
| 48 hours after | 0.7 | 0.9 | 0.47 |
| PPTs Trained Rectus Femoris (kPa) | |||
| Baseline | 463.1 | 142.2 | |
| Post-intervention | 389.3* | 132.1 | 0.81 |
| 48 hours after | 290.2* | 149.4 | 1.14 |
| PPTs Untrained Rectus Femoris (kPa) | |||
| Baseline | 477.3 | 114.2 | |
| Post-intervention | 469.6 | 108.2 | 0.21 |
| 48 hours after | 420.9 | 114.3 | 0.37 |
| PPTs Trained Vastus Medialis (kPa) | |||
| Baseline | 378.0 | 114.1 | |
| Post-intervention | 290.1* | 118.7 | 0.81 |
| 48 hours after | 252.8* | 136.4 | 1.04 |
| PPTs Untrained Vastus Medialis (kPa) | |||
| Baseline | 365.7 | 112.5 | |
| Post-intervention | 335.7 | 99.4 | 0.35 |
| 48 hours after | 308.2 | 117.0 | 0.45 |
| PPTs Trained Tibialis Anterior (kPa) | |||
| Baseline | 526.1 | 150.6 | |
| Post-intervention | 506.3 | 131.3 | 0.25 |
| 48 hours after | 439.8* | 107.2 | 0.92 |
| PPTs Untrained Tibialis Anterior (kPa) | |||
| Baseline | 524.8 | 136.4 | |
| Post-intervention | 518.0 | 144.9 | 0.22 |
| 48 hours after | 448.4* | 123.6 | 0.77 |
| PPTs Webspace of 1st/2nd Metacarpal (kPa) | |||
| Baseline | 383.8 | 159.2 | |
| Post-intervention | 349.3 | 111.4 | 0.38 |
| 48 hours after | 301.4* | 98.6 | 0.73 |
Statistically significant differences (RM-ANCOVA, p<.001)
The RM-ANCOVA also revealed significant measurement × side interactions for PPT over the rectus femoris (F(2,60) = 14.832; p<0.001) and vastus medialis (F(2,60) = 13.792; p<0.001), but not for tibialis anterior (F(2,60) = 0.157; p = 0.693). The ipsilateral rectus femoris and vastus medialis muscles exhibited pressure pain hyperalgesia, i.e., expressed as decreased PPTs, immediately after and 48 hours after eccentric exercise (Table 1). No influence of sex was found for either rectus femoris (F(2,30) = 1.681; p = 0.191), vastus medialis (F(2,30) = 0.660; p = 0.420) or tibialis anterior (F(2,30) = 1.072; p = 0.305). A significant effect of measurement was shown for PPT in the tibialis anterior muscle (F(2,60) = 43.239; p<0.001), suggesting a bilateral decrease in PPT over the tibialis anterior 48 hours after eccentric exercise (Table 1). Finally, a significant decrease in PPTs at the hand (F(1,30) = 15.057; p<0.001) was also found 48 hours after eccentric exercise, potentially indicating widespread hyperalgesia to pressure pain. In this point, a significant effect of sex (F(2,30) = 17.061, p<0.001) was found. Females exhibited a greater decrease in PPT, i.e., higher pressure pain hyperalgesia, as compared to men.
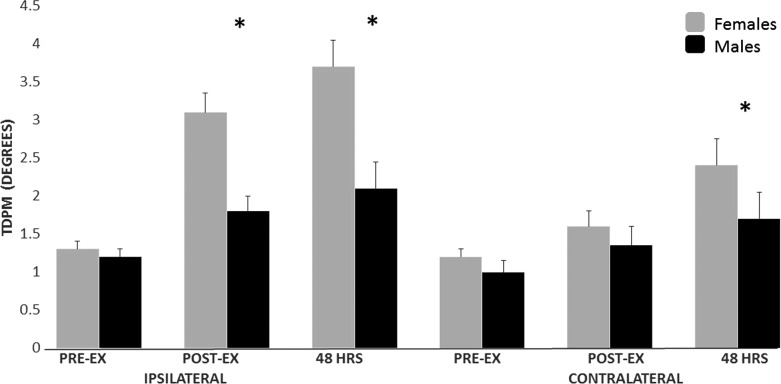
Finally, significant measurement × side interactions for VPT (F(2,60) = 19.281; p<0.001) and TDPM (F(2,60) = 4.577; p = 0.037) were also shown (Table 2)\. The exercised side exhibited an increase in VPT and TDPM, suggesting vibration hypoesthesia and decreased proprioceptive acuity 48 hours after eccentric exercise. A significant effect of sex was found for TDPM (F(2,30) = 9.743 p = 0.003) but not for VPT (F(2,30) = 0.468, p = 0.497). Females exhibited more loss of proprioceptive acuity than men (Figure 3).
Table 2.
Vibration perception thresholds (VPT) and proprioception via threshold to detection of passive movement (TDPM) before and after high intensity eccentric exercise.
| Variable | Mean | SD | Effect Size |
|---|---|---|---|
| Trained Limb Vibration (VPT) | |||
| Baseline | 15.3 | 3.2 | |
| Post-intervention | 22.8* | 5.9 | 1.68 |
| 48 hours after | 20.68 | 4.9 | 1.32 |
| Untrained Limb Vibration (VPT) | |||
| Baseline | 15.2 | 2.6 | |
| Post-intervention | 15.8 | 3.0 | 0.25 |
| 48 hours after | 16.4 | 3.4 | 0.40 |
| Trained Limb TDPM | |||
| Baseline | 1.25 | 0.5 | |
| Post-intervention | 2.5* | 1.3 | 1.35 |
| 48 hours after | 2.9* | 1.5 | 1.40 |
| Untrained Limb TDPM | |||
| Baseline | 1.1 | 0.4 | |
| Post-intervention | 1.5 | 0.8 | 0.14 |
| 48 hours after | 2.1# | 1.4 | 0.43 |
Statistically significant differences (RM-ANCOVA, p<0.001) # Statistically significant differences (RM-ANCOVA, p<0.05)
Figure 3.
Sex differences in proprioception. (* Asterisk indi-cates signi? cant differences between males and females).
DISCUSSION
In the present study, a comprehensive battery of tests was used to examine the effects of eccentric exercise (DOMS) on quantitative sensory testing measures. Diminished thresholds of pressure pain were found at a site distant from the quadriceps (the hand) at 48 hours, but not immediately after eccentric exercise. Vibration perception was impaired demonstrating ipsilateral hypoesthesia both immediately and 48 hours after eccentric exercise, while proprioception, measured via TDPM, was impaired ipsilaterally immediately after eccentric exercise and also bilaterally 48 hours later. Further, females demonstrated greater deficits in proprioception and had increased hyperalgesia at a site remote to the quadriceps (hand). These findings have both mechanistic and clinical implications.
Muscle hyperalgesia is a hallmark of DOMS. The pain experienced following a bout of high intensity eccentric exercise typically occurs during physical activity or with applied pressure stimuli, and not at rest,32 supporting the notion that mechanical hyperalgesia is a main consequence of DOMS.33 Peripheral sensitization is likely the main mechanism responsible for hyperalgesia to pressure of the exercised muscle in DOMS.34 This study found evidence of regional expansion of hyperalgesia, termed secondary hyperalgesia, which may occur due to peripheral and central changes in nociceptive processing.35 While consistent with results of previous DOMS research of the tibialis anterior muscle,36 the findings of secondary hyperalgesia in individuals with DOMS in the quadriceps may be critical in understanding persistent weakness in this muscle group following knee injury/disease. This is particularly relevant considering recent work which demonstrated force deficits in both the ipsilateral and contralateral quadriceps muscles following induction of DOMS.37
In addition, this study demonstrated evidence of possible widespread hyperalgesia, indicated by significantly diminished PPTs at the contralateral hand at 48 hours when compared to pre-exercise. DOMS is believed to occur due to minor myofibrillar and cytoskeletal damage causing an inflammatory reaction and nociceptive sensitization.2 Inflammatory mediators cause neuroplastic changes of nociceptors resulting in primary hyperalgesia of the eccentrically exercised muscle.34 Continued nociceptive input, particularly from a large muscle group such as the quadriceps, may result in facilitation of nociceptive processing at spinal and potentially supraspinal levels, which produces heightened intensity and expanded distribution of pain.4 This may be particularly salient considering recent research that suggests a ‘repeated bout effect’ (i.e., less physiological disturbance) with subsequent bouts of induced DOMS.38
Of importance, females demonstrated lower PPTs at the hand than men. This potentially indicates that females may be predisposed to widespread hyperalgesia following musculoskeletal insult as compared to males. Several factors may be responsible for these differences. A propensity to widespread hyperalgesia may occur due to impaired inhibitory mechanisms39 as well as factors such as impaired sleep, psychosocial factors and sedentary lifestyle. Future studies which more closely examine these factors in relation to DOMS may be indicated.
The present study demonstrated deficits in VPT acuity of the exercised limb. Other studies have reported vibratory perceptual deficits associated with musculoskeletal pain conditions, including patellofemoral pain40 and knee osteoarthritis,22,27 and suggested that these changes may be due to central nociplasticity, although peripheral neuropathy could not be ruled out. This study, in contrast, demonstrated sensory deficits that were clearly not present prior to induction of DOMS. The phenomenon of pain inhibiting sensory input has been referred to as a ‘reverse pain gate,’ or ‘touch-gate,’ suggesting that pain may inhibit non-nociceptive sensation.41 Apkarian et al41 proposed that perceptual deficits associated with pain or inflammation may be due to central neural mechanisms rather than impairment at the receptor level in line with previous studies.42,43
The present study also demonstrated somatosensory deficits in proprioception measured via TDPM, indicating that the limb was passively moved a significantly greater distance through range before the individual was able to perceive it. This finding is supported by a previous study reporting an attenuation of position sense of the lower limbs in presence of DOMS.12 TDPM may be a more appropriate measure of proprioceptive hypoesthesia since it measures the quantity of joint motion that must occur before movement is perceived. Hypoesthesia of TDPM has also been observed following musculoskeletal injury like anterior cruciate ligament injury29,30 and knee osteoarthritis.44,45 Considering that muscle fatigue has been implicated as a factor in certain ACL injuries,46 understanding the relationship between muscle fatigue and/or muscle soreness may be critical. In most instances, deficits in proprioception following knee injury have been attributed to loss of afferent input due to joint damage.29,47 However, in this study joint damage was not sustained, underscoring the major contribution of the muscle spindle to proprioception. Hypoesthesia was found bilaterally even with unilateral induction of DOMS, pointing to a spinal or supraspinal influence on sensory afferents. It is generally accepted that motor control and enhanced function is reliant on somatosensory input.48 Thus, hypoesthesia following a bout of eccentric exercise may predispose athletes to traumatic injury. Eccentric exercise is commonly used by athletes to enhance performance, with DOMS a common consequence of this training. With rates of lower extremity injury increasing in recent years and particularly ACL injury in adolescent females,49 understanding the effects of muscle soreness on somatosensation may be important for prevention of traumatic joint injury.
Some sex differences were demonstrated regarding DOMS related altered somatosensation. While hyperalgesia was increased in females at a site distant from the quadriceps (hand), pain intensity reported at 48 hours after eccentric exercise was similar for males and females, which is similar to previous research.50 Interestingly, sex differences were also noted in TDPM but not in VPT, suggesting these two modalities, mediated via different neural pathways, may be affected by muscle soreness in distinct ways. The higher deficit in proprioception observed in females compared to males was consistent with the results of previous research51 underlining the potential higher risk for musculoskeletal disorders or injuries. Interestingly, time course recovery after eccentric exercise has been reported to be similar between males and females for soreness intensity but different for muscle strength and thickness with longer recovery time for females.52 The present study provides new findings concerning sex similarities and differences when both males and females are exposed to the eccentric exercise protocol. It confirmed that the experimental approach used to induce DOMS, the targeted body region and the methodology used most likely explain the presence or not of sex differences in DOMS. Future studies should keep these aspects in mind and plan sex-specific training programs including, e.g. different recovery time to avoid risk of overloading and injuries.
The impact of DOMS related signs and symptoms on function, balance and postural control was not explored. Future work examining the effect of DOMS related somatosensory changes on function may be beneficial. DOMS is a common sequela of sports training. Considering its potential effect on somatosensation, it is possible that it may predispose athletes to injury. This study serves as important preliminary research for the development of a future larger scale investigation on the effects on eccentric exercise on somatosensation, motor control and function.
CONCLUSION
In conclusion, widespread hyperalgesia, ipsilaterally impaired vibration perception and bilaterally impaired proprioception were demonstrated at 48 hours after eccentric exercise. Moreover, this study revealed sex differences in the extent of hyperalgesia and proprioception deficit that may explain the higher prevalence of musculoskeletal disorders and injuries in females compared to males. These results may provide some explanation for previous findings of altered postural control and muscle activation patterns following induction of DOMS. Athletes performing strenuous lower limb eccentric exercise in training leading to DOMS may experience increased pain and hypoesthesia and be predisposed to lower quarter joint trauma.
REFERENCES
- 1.Friden J. Delayed onset muscle soreness. Scand J Med Sci Sports. 2002;12(6):327-328. [DOI] [PubMed] [Google Scholar]
- 2.Tegeder L Zimmermann J Meller ST Geisslinger G. Release of algesic substances in human experimental muscle pain. Inflamm Res. 2002;51(8):393-402. [DOI] [PubMed] [Google Scholar]
- 3.Nie H Kawczynski A Madeleine P Arendt-Nielsen L. Delayed onset muscle soreness in neck/shoulder muscles. Eur J Pain. 2005;9(6):653-660. [DOI] [PubMed] [Google Scholar]
- 4.Arendt-Nielsen L Nie H Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149(3):573-581. [DOI] [PubMed] [Google Scholar]
- 5.Courtney CA Durr RK Emerson-Kavchak AJ Witte EO Santos MJ. Heightened flexor withdrawal responses following ACL rupture are enhanced by passive tibial translation. Clin Neurophysiol. 2011;122(5):1005-1010. [DOI] [PubMed] [Google Scholar]
- 6.Kavchak AJ Fernandez-de-Las-Penas C Rubin LH, et al. Association between altered somatosensation, pain, and knee stability in patients with severe knee osteoarthrosis. Clin J Pain. 2012;28(7):589-594. [DOI] [PubMed] [Google Scholar]
- 7.Shakoor N Felson DT Niu J, et al. The association of vibratory perception and muscle strength with the incidence and worsening of knee instability: The multicenter osteoarthritis study. Arthritis Rheumatol. 2017;69(1):94-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courtney CA Atre P Foucher KC Alsouhibani AM. Hypoesthesia after anterior cruciate ligament reconstruction: The relationship between proprioception and vibration perception deficits in individuals greater than one year post-surgery. Knee. 2019;26(1):194-200. [DOI] [PubMed] [Google Scholar]
- 9.Lund H Vestergaard-Poulsen P Kanstrup IL Sejrsen P. Isokinetic eccentric exercise as a model to induce and reproduce pathophysiological alterations related to delayed onset muscle soreness. Scand J Med Sci Sports. 1998;8(4):208-215. [DOI] [PubMed] [Google Scholar]
- 10.Domenech-Garcia V Palsson TS Herrero P Graven-Nielsen T. Pressure-induced referred pain is expanded by persistent soreness. Pain. 2016;157(5):1164-1172. [DOI] [PubMed] [Google Scholar]
- 11.Hedayatpour N Falla D Arendt-Nielsen L Farina D. Sensory and electromyographic mapping during delayed-onset muscle soreness. Med Sci Sports Exerc. 2008;40(2):326-334. [DOI] [PubMed] [Google Scholar]
- 12.Paschalis V Nikolaidis MG Giakas G Jamurtas AZ Pappas A Koutedakis Y. The effect of eccentric exercise on position sense and joint reaction angle of the lower limbs. Muscle Nerve. 2007;35(4):496-503. [DOI] [PubMed] [Google Scholar]
- 13.Proske U Gregory JE Morgan DL Percival P Weerakkody NS Canny BJ. Force matching errors following eccentric exercise. Hum Mov Sci. 2004;23(3-4):365-378. [DOI] [PubMed] [Google Scholar]
- 14.Diener HC Dichgans J Guschlbauer B Mau H. The significance of proprioception on postural stabilization as assessed by ischemia. Brain Res. 1984;296(1):103-109. [DOI] [PubMed] [Google Scholar]
- 15.Hedayatpour N Hassanlouei H Arendt-Nielsen L Kersting UG Falla D. Delayed-onset muscle soreness alters the response to postural perturbations. Med Sci Sports Exerc. 2011;43(6):1010-1016. [DOI] [PubMed] [Google Scholar]
- 16.Proske U Gandevia SC. The kinaesthetic senses. J Physiol. 2009;587(Pt 17):4139-4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brockett C Warren N Gregory JE Morgan DL Proske U. A comparison of the effects of concentric versus eccentric exercise on force and position sense at the human elbow joint. Brain Res. 1997;771(2):251-258. [DOI] [PubMed] [Google Scholar]
- 18.Nie H Arendt-Nielsen L Madeleine P Graven-Nielsen T. Enhanced temporal summation of pressure pain in the trapezius muscle after delayed onset muscle soreness. Exp Brain Res. 2006;170(2):182-190. [DOI] [PubMed] [Google Scholar]
- 19.Raeder C Wiewelhove T Simola RA, et al. Assessment of fatigue and recovery in male and female athletes after 6 days of intensified strength training. J Strength Cond Res. 2016;30(12):3412-3427. [DOI] [PubMed] [Google Scholar]
- 20.van Melick N Meddeler BM Hoogeboom TJ Nijhuis-van der Sanden MWG van Cingel REH. How to determine leg dominance: The agreement between self-reported and observed performance in healthy adults. PLoS One. 2017;12(12):e0189876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hjermstad MJ Fayers PM Haugen DF, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: A systematic literature review. J Pain Symptom Manage. 2011;41(6):1073-1093. [DOI] [PubMed] [Google Scholar]
- 22.Courtney CA Steffen AD Fernandez-de-Las-Penas C Kim J Chmell SJ. Joint mobilization enhances mechanisms of conditioned pain modulation in individuals with osteoarthritis of the knee. J Orthop Sports Phys Ther. 2016;46(3):168-176. [DOI] [PubMed] [Google Scholar]
- 23.Rolke R Baron R Maier C, et al. Quantitative sensory testing in the German research network on neuropathic pain (DFNS): Standardized protocol and reference values. Pain. 2006;123(3):231-243. [DOI] [PubMed] [Google Scholar]
- 24.Joergensen TS Henriksen M Danneskiold-Samsoee B Bliddal H Graven-Nielsen T. Experimental knee pain evoke spreading hyperalgesia and facilitated temporal summation of pain. Pain Med. 2013;14(6):874-883. [DOI] [PubMed] [Google Scholar]
- 25.Balaguier R Madeleine P Vuillerme N. Is one trial sufficient to obtain excellent pressure pain threshold reliability in the low back of asymptomatic individualsϿ. A test-retest study. PLoS One. 2016;11(8):e0160866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Deursen RW Sanchez MM Derr JA Becker MB Ulbrecht JS Cavanagh PR. Vibration perception threshold testing in patients with diabetic neuropathy: Ceiling effects and reliability. Diabet Med. 2001;18(6):469-475. [DOI] [PubMed] [Google Scholar]
- 27.Shakoor N Agrawal A Block JA. Reduced lower extremity vibratory perception in osteoarthritis of the knee. Arthritis Rheum. 2008;59(1):117-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courtney CA Rine R Jenk DT Collier PD Waters A. Enhanced proprioceptive acuity at the knee in the competitive athlete. J Orthop Sports Phys Ther. 2013;43(6):422-426. [DOI] [PubMed] [Google Scholar]
- 29.Barrack RL Skinner HB Buckley SL. Proprioception in the anterior cruciate deficient knee. Am J Sports Med. 1989;17(1):1-6. [DOI] [PubMed] [Google Scholar]
- 30.Courtney C Rine RM Kroll P. Central somatosensory changes and altered muscle synergies in subjects with anterior cruciate ligament deficiency. Gait Posture. 2005;22(1):69-74. [DOI] [PubMed] [Google Scholar]
- 31.Wise AK Gregory JE Proske U. The effects of muscle conditioning on movement detection thresholds at the human forearm. Brain Res. 1996;735(1):125-130. [DOI] [PubMed] [Google Scholar]
- 32.Proske U Morgan DL. Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537(Pt 2):333-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proske U. Muscle tenderness from exercise: Mechanisms? J Physiol. 2005;564(Pt 1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graven-Nielsen T Arendt-Nielsen L. Peripheral and central sensitization in musculoskeletal pain disorders: An experimental approach. Curr Rheumatol Rep. 2002;4(4):313-321. [DOI] [PubMed] [Google Scholar]
- 35.Bajaj P Bajaj P Graven-Nielsen T Arendt-Nielsen L. Osteoarthritis and its association with muscle hyperalgesia: An experimental controlled study. Pain. 2001;93(2):107-114. [DOI] [PubMed] [Google Scholar]
- 36.Hosseinzadeh M Samani A Andersen OK Nosaka K Arendt-Nielsen L Madeleine P. Ipsilateral resistance exercise prevents exercise-induced central sensitization in the contralateral limb: A randomized controlled trial. Eur J Appl Physiol. 2015;115(11):2253-2262. [DOI] [PubMed] [Google Scholar]
- 37.Hedayatpour N Izanloo Z Falla D. The effect of eccentric exercise and delayed onset muscle soreness on the homologous muscle of the contralateral limb. J Electromyogr Kinesiol. 2018;41:154-159. [DOI] [PubMed] [Google Scholar]
- 38.Coratella G Chemello A Schena F. Muscle damage and repeated bout effect induced by enhanced eccentric squats. J Sports Med Phys Fitness. 2016;56(12):1540-1546. [PubMed] [Google Scholar]
- 39.Sluka KA Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience. 2016;338(3):114-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen R Hystad T Kvale A Baerheim A. Quantitative sensory testing of patients with long lasting patellofemoral pain syndrome. Eur J Pain. 2007;11(6):665-676. [DOI] [PubMed] [Google Scholar]
- 41.Apkarian AV Stea RA Bolanowski SJ. Heat-induced pain diminishes vibrotactile perception: A touch gate. Somatosens Mot Res. 1994;11(3):259-267. [DOI] [PubMed] [Google Scholar]
- 42.Roberts D Friden T Stomberg A Lindstrand A Moritz U. Bilateral proprioceptive defects in patients with a unilateral anterior cruciate ligament reconstruction: A comparison between patients and healthy individuals. J Orthop Res. 2000;18(4):565-571. [DOI] [PubMed] [Google Scholar]
- 43.Ben Moussa Zouita A Zouita S Dziri C Ben Salah FZ Zehi K. Isokinetic, functional and proprioceptive assessment of soccer players two years after surgical reconstruction of the anterior cruciate ligament of the knee. Ann Readapt Med Phys. 2008;51(4):248-256. [DOI] [PubMed] [Google Scholar]
- 44.Hewitt BA Refshauge KM Kilbreath SL. Kinesthesia at the knee: The effect of osteoarthritis and bandage application. Arthritis Rheum. 2002;47(5):479-483. [DOI] [PubMed] [Google Scholar]
- 45.Barrack RL Skinner HB Cook SD Haddad RJ Jr. Effect of articular disease and total knee arthroplasty on knee joint-position sense. J Neurophysiol. 1983;50(3):684-687. [DOI] [PubMed] [Google Scholar]
- 46.Benjaminse A Webster KE Kimp A Meijer M Gokeler A. Revised approach to the role of fatigue in anterior cruciate ligament injury prevention: A systematic review with meta-analyses. Sports Med. 2019;49(4):565-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hurley MV. The effects of joint damage on muscle function, proprioception and rehabilitation. Man Ther. 1997;2(1):11-17. 10.1054/math.1997.0281. [DOI] [PubMed] [Google Scholar]
- 48.Horak FB. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35 Suppl 2:ii7-ii11. [DOI] [PubMed] [Google Scholar]
- 49.Beck NA Lawrence JTR Nordin JD DeFor TA Tompkins M. ACL tears in school‐aged children and adolescents over 20 years. Pediatrics. 2017;139(3):10.1542/peds.2016‐1877. [DOI] [PubMed] [Google Scholar]
- 50.Cramer JT Housh TJ Weir JP, et al. Gender, muscle, and velocity comparisons of mechanomyographic and electromyographic responses during isokinetic muscle actions. Scand J Med Sci Sports. 2004;14(2):116-127. [DOI] [PubMed] [Google Scholar]
- 51.Vafadar AK Cote JN Archambault PS. Sex differences in the shoulder joint position sense acuity: A cross-sectional study. BMC Musculoskelet Disord. 2015;16:273-015-0731-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flores DF Gentil P Brown LE Pinto RS Carregaro RL Bottaro M. Dissociated time course of recovery between genders after resistance exercise. J Strength Cond Res. 2011;25(11):3039-3044. [DOI] [PubMed] [Google Scholar]