Abstract
Background:
Our objective was to determine those characteristics associated with reversibility of airflow obstruction and response to maximal bronchodilation in children with severe asthma through the Severe Asthma Research Program (SARP).
Methods:
We performed a cross-sectional analysis evaluating children ages 6–17 years with non-severe (NSA) and severe asthma (SA). Participants underwent spirometry before and after 180 μg of albuterol to determine reversibility (≥12% increase in FEV1). Participants were then given escalating doses up to 720 μg of albuterol to determine their maximum reversibility.
Results:
We evaluated 230 children (n=129 SA, n=101 NSA) from 5 centers across the U.S. in the SARP I and II cohorts. SA (OR 2.08, 95%CI 1.05 to 4.13), second-hand smoke exposure (OR 2.81, 95%CI 1.23 to 6.43), and FeNO (OR 1.97, 95%CI 1.35 to 2.87) were associated with increased odds of airway reversibility after maximal bronchodilation, while higher pre-bronchodilator FEV1% predicted (OR 0.91, 95%CI 0.88 to 0.94) was associated with decreased odds. In an analysis using the SARP III cohort (n=186), blood neutrophils, IgE and FEV1% predicted were significantly associated with bronchodilator reversibility. In addition, children with bronchodilator response have greater healthcare utilization. Bronchodilator reversibility was associated with reduced lung function at enrollment and one-year follow-up though less decline in lung function over one year compared to those without reversibility.
Conclusions:
Lung function, that is FEV1 % predicted, is a predictor of bronchodilator response in children with asthma. Additionally, smoke exposure, higher FeNO or IgE level, and low peripheral blood neutrophils are associated with greater likelihood of bronchodilator reversibility. Bronchodilator response can identify a phenotype of pediatric asthma associated with low lung function and poor asthma control.
Keywords: Asthma, pediatrics, bronchodilator response
Introduction
The presence of reversible airflow obstruction in response to bronchodilators is one major criterion used to diagnose asthma in children. A significant bronchodilator response in asthma is typically considered a 12% increase in FEV1 percent predicted following 2–4 puffs (180–360μg) of albuterol via MDI or 2.5–5mg of nebulized albuterol1. The Severe Asthma Research Program (SARP) has conducted multiple investigations that have helped characterize severe asthma in children2,3. Using a technique of maximal bronchodilator testing with escalating doses of albuterol, children in SARP with severe asthma (SA) had a significant improvement in their FEV1 following albuterol; however, their best FEV1 remained lower than children with mild-to-moderate asthma3. Furthermore, airflow limitation, defined as a reduction in FEV1/FVC, improved after maximal bronchodilation in both participants with SA and mild-to-moderate asthma, but the participants with SA had a larger increase in FEV1/FVC% predicted than the mild-to-moderate group. Despite these studies, the risk factors associated with a significant bronchodilator response within children with SA and its clinical implications have not been fully evaluated.
In this study, we examined data from pediatric participants enrolled in the SARP I and II cohorts (2003–2011) and SARP III cohort (2012-present) who underwent maximal bronchodilation testing in order to determine factors that predict bronchodilator response in children with SA. We hypothesized that children with SA would be more likely to demonstrate bronchodilator reversibility (≥12% improvement in FEV1) of airflow limitation than those with mild-to-moderate asthma and would be less likely to reach a plateau for reversibility following maximal bronchodilation. In addition, we evaluated whether those with a bronchodilator response had greater morbidity and healthcare utilization.
Methods
Subjects
We examined data from children enrolled in the National Heart, Lung and Blood Institute supported SARP I (2003–2006) and II (2006–2011) across 5 centers in the United States (Emory University, University of Pittsburgh, University of Virginia, Wake Forest University and Washington University in Saint Louis) and within 7 centers for SARP III (Emory University, University of Pittsburgh, University of Virginia/Rainbow Babies and Children’s Hospital, University of Wisconsin-Madison, Boston Children’s, University of California San Francisco, and Washington University in Saint Louis). Participants were between 6 and 17 years of age with physician-diagnosed asthma, and had to demonstrate ≥12% FEV1 increase following bronchodilator administration (180 mcg albuterol) or evidence of bronchial hyperresponsiveness by methacholine challenge at time of enrollment. Participants were characterized as having either SA, as defined by 2000 American Thoracic Society (ATS) workshop criteria (SARP I/II)4 and 2014 European Respiratory Society/ATS consensus guidelines (SARP III)5, or non-severe asthma (NSA). For children taking inhaled corticosteroids (ICS), the dose had to be stable for at least 6 months prior to characterization. We defined high-dose ICS as ≥440μg per day of fluticasone or equivalent ICS for children less than 12 years of age and ≥880μg per day of fluticasone or equivalent ICS for children 12 to 17 years of age. Site-specific IRB approval was obtained for all locations and parents/legal guardians provided informed consent prior to enrollment.
Characterization
For the baseline characterization visit, all participants underwent physical examination, provided medical and asthma history, and completed asthma questionnaires6. We obtained peripheral blood to measure total white blood cells, eosinophils, neutrophils, and serum IgE levels. We performed percutaneous skin testing for 16 different allergens (SARP I/II) or serum-specific allergen testing (SARP III; ImmunoCAP assay). Fractional exhaled nitric oxide (FeNO) measurements were obtained via offline (n = 64) or online (n = 144) methods in SARP I/II and online measures were used in SARP III (NIOX or Vero, Circassia, Chicago, IL). Pulmonary function tests were performed during this baseline characterization visit.
Pulmonary function tests
Prior to performing spirometry, participants withheld short-acting bronchodilators for a minimum of 4 hours, long-acting beta-agonists (LABA) for a minimum of 12 hours, and leukotriene antagonists for a minimum of 24 hours. We allowed participants to continue use of ICS therapy prior to spirometry as long as they were not in combination with a LABA. An Aerochamber® (Monaghan Medical Corporation, Plattsburg, NY) or similar device was used with all albuterol administration.
For maximum bronchodilator testing, spirometry was repeated after 180, 360, 540, and up to a maximum 720μg of albuterol sulfate. If FEV1 differed by less than 5% between 360 and 540μg of albuterol, then the final dose of albuterol (720μg) was not given. We calculated percent difference using the following formula: percent difference = (FEV16 puffs–FEV14 puffs)/FEV14 puffs x 100. The participants’ best FEV1, FVC, FEV1/FVC, and FEF25–75 values and percent predicted from 3 reproducible maneuvers were recorded fifteen minutes after each bronchodilation. The percent predicted values were calculated using standard reference equations7.
For purposes of this study, we define bronchodilator (BD) reversibility as a 12% or greater increase in FEV1 from baseline, using the relative difference (FEV1postBD-FEV1preBD/FEV1preBD). The absolute difference (FEV1postBD-FEV1preBD) was also calculated and reported separately in the Supplement for the SARP III cohort.
Statistical Analysis
Independent samples t-tests of continuous variables and Chi-square tests of categorical variables were performed to compare characteristics between asthma groups. Fisher Exact tests were used as appropriate. To compare those with and without a bronchodilator response, we used 2-group t-tests for means and chi-square for proportions. Kruskal-Wallis was used as appropriate. Analysis of covariance (ANCOVA) was performed to determine if there was a difference in the change in lung function between the two asthma severity groups with baseline lung function adjusted as an independent variable. Logarithmic transformation was applied to baseline FEV1/FVC and non-parametric rank ANCOVA was used for the analysis of maximum percent change in FEV1 post-bronchodilator due to non-normal distribution. Spearman’s correlations were used to determine the relationship between variables of interest. When comparing characteristics between those with and without a bronchodilator response, those variables with, P values <0.1 in the univariate analysis were included in the multivariate logistic regression for reversibility. Methacholine PC20 was excluded from multivariate modeling because a large proportion of participants were missing this data. Due to FeNO being collected in both offline and online methods, these two variables were combined and log transformed for use in multivariate analysis. Multivariate analysis was performed with and without log transformed FeNO because of the variability in measurement and that 12 subjects were missing this value. Stepwise procedure was used to identify the factors independently associated with reversibility. Odds ratio (OR) and its corresponding 95% confidence interval were reported. A value of P<0.05 was considered significant. All analyses were performed with the SAS 9 software (SAS Institute, Cary, NC).
Replication Analysis
Given the longitudinal protocol in SARP III, we wanted to replicate the analysis of the SARP I-II cohort to confirm our findings in a large, similar cohort, as well as determine long-term clinical implications of the baseline findings. All analyses performed on the SARP I-II cohort were repeated for the SARP III cohort.
Results
SARP I-II Results
We first analyzed data from 230 participants, 129 SA participants and 101 NSA participants, enrolled in SARP I-II. Baseline demographics and characteristics of children with SA and NSA are compared in Table 1.
Table 1.
Demographics and clinical characteristics of children with asthma enrolled in SARP I-II
| Non-severe Asthma (n=101) |
Severe Asthma (n=129) |
p value | |
|---|---|---|---|
| Demographics | |||
| Age enrollment, yr | 11.5 ± 3.1 | 11.8 ± 3.0 | 0.43 |
| Duration of asthma, yr | 7.6 ± 4.4 | 9.8 ± 3.4 | <0.001 |
| Age of asthma onset, yr | 3.9 ± 3.4 | 2.0 ± 2.3 | <0.001 |
| Female, n (%) | 45 (44.6) | 54 (41.9) | 0.68 |
| African‐American, n (%) | 51 (50.5) | 86 (66.7) | 0.01 |
| Second‐hand smoke exposure, n (%) | 22 (22.0) | 29 (22.5) | 0.93 |
| Medical history | |||
| BMI Z‐score | 0.95 ± 0.99 | 0.88 ± 1.20 | 0.65 |
| History of acute/recurrent sinusitis, n (%) | 42 (42.0) | 60 (46.9) | 0.46 |
| History of GERD, n (%) | 10 (10.5) | 52 (44.8) | <0.001 |
| History of allergies, n (%) | 90 (92.8) | 119 (93.7) | 0.79 |
| History of skin rash, n (%) | 50 (49.5) | 78 (60.5) | 0.097 |
| Family History | |||
| Asthma (1st degree relative), n (%) | 70 (70.7) | 92 (71.9) | 0.85 |
| Eczema (1st degree relative), n (%) | 50 (50.0) | 60 (47.2) | 0.57 |
| Clinical characteristics | |||
| Positive skin reaction to allergens, n (%) | 62 (72.9) | 89 (83.2) | 0.09 |
| # of positive skin reactions | 2.65 ± 2.63 | 3.48 ± 2.73 | 0.02 |
| % neutrophil in peripheral blood | 48.9 ± 12.7 | 44.7 ± 15.1 | 0.12 |
| % eosinophil in peripheral blood | 4.95 ± 3.50 | 6.64 ± 4.85 | 0.004 |
| Total IgE in blood, IU/mL | 544 ± 1334 | 708 ± 1187 | 0.02 |
| Offline eNO ppb [N=64] | 9.62 ± 5.51 | 14.6 ± 9.07 | 0.009 |
| Online eNO ppb [N=144] | 39.6 ± 44.4 | 41.4 ± 35.2 | 0.80 |
| PC20, mg/dl [N=123] | 3.78 ± 5.09 | 2.66 ± 4.66 | 0.03 |
| FEV1 <80% Predicted, n (%) | 6 (6.3) | 42 (33.6) | <0.001 |
| Persistent airway obstruction*, n (%) | 12 (12.0) | 40 (31.0) | 0.001 |
| Healthcare utilization | |||
| On high‐dose ICS, n (%) | 12 (11.9) | 122 (97.6) | <0.001 |
| On systemic corticosteroids, n (%) | 0 | 21 (16.3) | <0.001 |
| More than 3 OCS bursts in last year, n (%) | 15 (14.9) | 74 (57.8) | <0.001 |
| ER/urgent care for asthma in past year, n (%) | 44 (43.6) | 114 (88.4) | <0.001 |
| Admission for respiratory disorder in past year, n (%) | 10 (10.0) | 76 (59.4) | <0.001 |
| ICU for asthma in past year, n (%) | 4 (4.0) | 36 (28.1) | <0.001 |
| History of intubation for asthma, n (%) | 9 (8.9) | 32 (25.0) | 0.002 |
| Use of anti‐IgE therapy, n (%) | 0 | 10 (7.8) | 0.003 |
Persistent airway obstruction is defined by baseline FEV1 <80 % predicted or diurnal PEF variability >20% over 2 weeks.
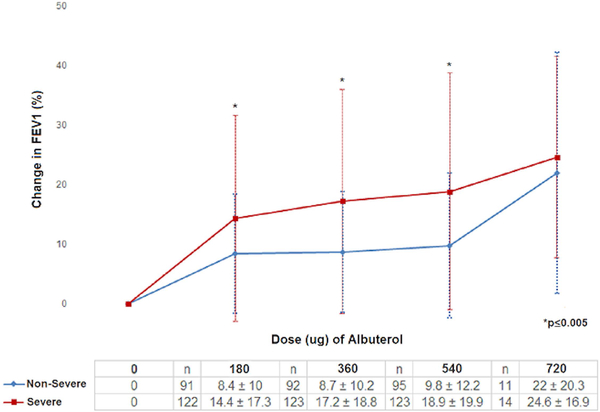
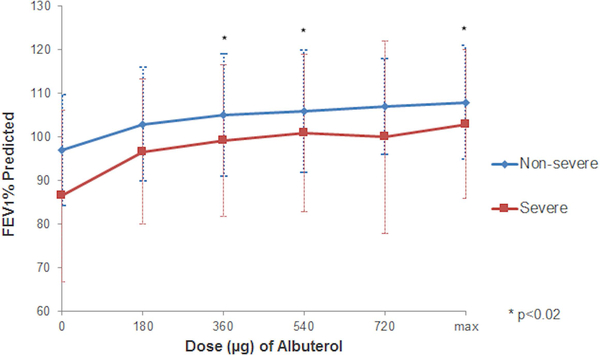
Children with SA had greater pre-bronchodilator (pre-BD) airflow limitation and obstruction compared to children with NSA (E-Table 1). These measures remained lower in SA than NSA despite maximal bronchodilation; however, the maximum relative increase in FEV1 was greater in SA than NSA (FEV1% increase 22.2±20.1 vs 12.8±11.1, p<0.001). For all children (n=220), the average bronchodilator response after 180μg of albuterol was 11.8%, and increased to 18.2% with maximal bronchodilation. After 180μg of albuterol, children with NSA on average increased their FEV1 by 8.4%, while children with SA increased by 14.4% (p=0.005; E-Table 1). A plateau of reversibility was not achieved in many, as both groups continued to increase FEV1 with escalating doses of albuterol; however, the increase was greater in participants with SA than NSA (Figure 1a and 1b). This difference between groups was not statistically significant after the maximal dose, however, only a small number (n=25) of participants required the highest albuterol dose. There was no difference in the dose of albuterol required to reach 12% reversibility among asthma severity groups (p=0.74) (E-Table 2).
Figure1a:
Cumulative percent change in FEV1 for increasing amounts of bronchodilator for patients with asthma enrolled in SARP I-II.
1b: Maximal lung function following bronchodilator in children with asthma enrolled in SARP I-II.
There was a tendency for males to have greater airflow obstruction, but we found no statistically significant difference between males and females in pre-BD FEV1% predicted (89.2±17% vs 93.6±18%, p = 0.07). There was no significant difference in bronchodilator response for maximal FEV1% predicted (103.5±15% vs 107.6±16%, p=0.051) or maximum percent increase in FEV1% predicted (18.1±18%vs 18.2±17%, p=0.89) between males and females. Males, however, demonstrated greater airflow limitation with lower FEV1/FVC ratios before (0.76±0.1 vs 0.78±0.1, p=0.03) and after maximal bronchodilation (0.84±0.08 vs 0.87±0.07, p<0.001).
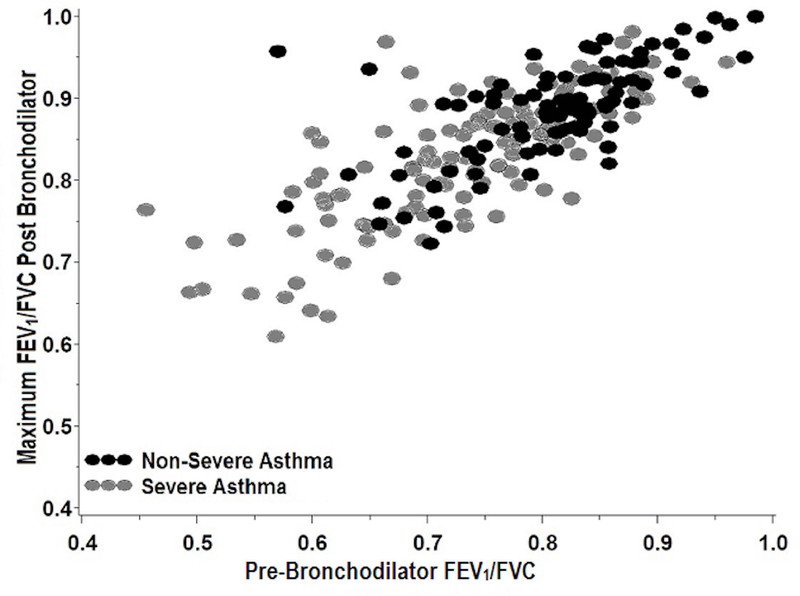
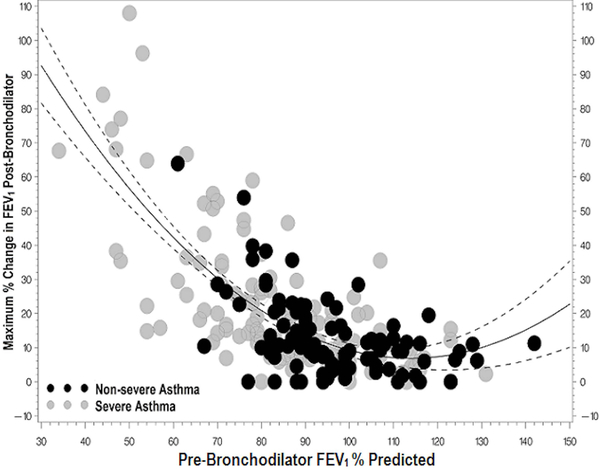
There was a strong positive correlation between pre-BD lung function and both maximum FEV1% predicted and maximal increase in FEV1 (Figure 2). After adjusting for pre-BD FEV1% predicted, gender and presence of a positive skin test, there was a significant association between the maximum increase in FEV1% predicted and SA (p=0.001).
Figure2a.
Maximum FEV1% predicted following bronchodilators shows a strong positive correlation with pre-BD FEV1% predicted (r=0.81, p < 0.001). Participants with lower pre-BD lung function have lower maximal FEV1% predicted while participants with higher pre-BD lung function maintain higher maximal FEV1% Predicted.
2b. Maximum FEV1/FVC (log transformed) following bronchodilators shows strong correlation with pre-BD FEV1/FVC (log transformed) (r=0.77, p < 0.0001). Participants with greater airflow limitation after maximal bronchodilation have greater pre-BD airflow limitation.
2c. Maximum percent change in FEV1 following bronchodilators shows moderate negative correlation with pre-BD FEV1% predicted using quadratic fit (r=−0.55, p < 0.001). Participants with lower pre-BD lung function had a greater bronchodilator response than participants with higher pre-BD lung function.
Clinical Implications of Bronchodilator Response
Among all children, 112 of 220 (51%) had a significant increase in FEV1 of at least 12% following maximal bronchodilator testing. Only 6 of the 112 (5%) did not achieve a 200mL difference in FEV1, however, we chose to keep them in the analysis (all had a change of at least 130mL and absolute volume change is of less significance in children with smaller lung capacity). Eighty of the 112 participants (71.4%) with bronchodilator reversibility had SA while 32 (28.6%) had NSA. Participants with a 12% relative bronchodilator response had longer duration of asthma, greater atopy, more smoke exposure and greater healthcare utilization (Table 2).
Table 2.
Clinical characteristics associated with bronchodilator reversibility in children with asthma enrolled in SARP I and II
| FEV1 increase <12% (n=108) |
FEV1 increase ≥12% (n=112) |
p-value | |
|---|---|---|---|
| Demographics | |||
| Age enrollment, yr | 11.5 ± 3.1 | 12.0 ± 2.9 | 0.19 |
| Duration of asthma, yr | 8.1 ± 4.3 | 9.7 ± 3.6 | 0.004 |
| Age of asthma onset, yr | 3.3 ± 3.2 | 2.3 ± 2.5 | 0.008 |
| Female, n (%) | 47 (43.5) | 47 (42.0) | 0.82 |
| African‐American, n (%) | 59 (54.6) | 73 (65.2) | 0.11 |
| Second‐hand smoke exposure, n (%) | 15 (14.0) | 35 (31.3) | 0.002 |
| Severe asthma, n (%) | 45 (41.7) | 80 (71.4) | <0.001 |
| Clinical characteristics | |||
| Positive skin reaction to allergens, n (%) | 69 (75.8) | 75 (81.5) | 0.35 |
| # of positive skin reactions | 2.6 ± 2.5 | 3.6 ± 2.9 | 0.01 |
| % eosinophil in peripheral blood | 4.9 ± 3.5 | 6.9 ± 4.9 | 0.002 |
| % neutrophil in peripheral blood | 46.9 ± 14.1 | 45.3 ± 14.7 | 0.48 |
| Total IgE in blood, IU/mL | 607 ± 1476 | 692 ± 1065 | 0.02 |
| Offline eNO ppb [N=64] | 10.5 ± 6.9 | 15.4 ± 8.9 | 0.005 |
| Online eNO ppb [N=144] | 31.9 ± 40.8 | 47.9 ± 38.4 | 0.001 |
| PC20, mg/dl [N=123] | 2.96 ± 3.78 | 2.78 ± 4.77 | 0.04 |
| Pre-bronchodilator FEV1 % predicted | 101 ± 13 | 81 ± 16 | <0.001 |
| Healthcare utilization | |||
| Use of high-dose ICS n (%) | 50 (46.7) | 79 (72.5) | <0.001 |
| More than 3 OCS bursts in last year, n (%) | 33 (30.6) | 51 (46.0) | 0.02 |
| ER/urgent care for asthma in past year, n (%) | 69 (64.5) | 83 (74.8) | 0.098 |
| Admission for respiratory disorder in past year, n (%) | 52 (48.2) | 77 (68.8) | 0.002 |
| ICU for asthma in the past year, n (%) | 19 (17.8) | 19 (17.0) | 0.88 |
| History of intubation for asthma, n (%) | 16 (14.8) | 23 (20.7) | 0.25 |
Among children with SA, those that demonstrated bronchodilator reversibility were older, had lower lung function, higher FeNO and peripheral blood eosinophils, longer duration of asthma, and more second-hand smoke exposure compared to those without a bronchodilator response (E-Table 3).
In a multivariate logistic regression model, SA was associated with significantly increased odds of airway reversibility after maximal bronchodilation, as was second-hand smoke exposure (Table 3a). Higher pre-BD FEV1% predicted was associated with decreased odds of reversibility in children with and without SA (Table 3a and 3c). With addition of FeNO to the model, both FeNO and pre-BD FEV1% were predictive of reversibility (Table 3b and 3c). While FeNO (log transformed) was correlated with asthma severity (r=0.15, p=0.04), bronchodilator reversibility (r=0.34, p<0.0001), and pre-BD FEV1% predicted (r=−0.24, p=0.0007), there was no correlation of FeNO with second-hand smoke exposure (r=0.12, p=0.08). There is a weak but statistically significant correlation of smoke exposure with reversibility (r=0.18, p=0.01); though we found no correlation of the amount of smoke exposure (days/week or hours/day exposed to smoke) with reversibility (p>0.05).
Table 3.
Predictors of bronchodilator reversibility in children with asthma enrolled in SARP I-II
| a. Predictors of 12% relative bronchodilator reversibility in all children with asthma (n=220) | |||
|---|---|---|---|
| Variables | Odds ratio | 95% CI | p value |
| Exposure to second-hand smoke | 2.81 | 1.23 to 6.43 | 0.014 |
| Severe asthma | 2.08 | 1.05 to 4.13 | 0.036 |
| Pre-bronchodilator FEV1 % predicted | 0.91 | 0.88 to 0.94 | <0.001 |
| b. Predictors of 12% relative bronchodilator reversibility in all children with asthma, including exhaled nitric oxide (n=200) | |||
|---|---|---|---|
| Variables | Odds ratio | 95% CI | p value |
| Log FeNO* | 1.97 | 1.35 to 2.87 | 0.0005 |
| Pre-bronchodilator FEV1 % predicted | 0.91 | 0.88 to 0.94 | <0.001 |
| c. Predictors of 12% relative bronchodilator reversibility in children with severe asthma, including exhaled nitric oxide (n=117) | |||
|---|---|---|---|
| Variables | Odds ratio | 95% CI | p value |
| Log FeNO* | 3.045 | 1.624 to 5.709 | 0.0005 |
| Pre-bronchodilator FEV1 % predicted | 0.91 | 0.87 to 0.95 | <0.001 |
offline and online eNO combined
Variables included: severe asthma, asthma duration, age of asthma onset, #positive skin tests, pre-BD FEV1 (L), pre-BD FEV1% predicted, second-hand smoke exposure, % blood eosinophils, IgE, 3+OCS burst in last year, ER/Urgent care in past year, admission in past year
offline and online eNO combined
Variables included: severe asthma, asthma duration, age of asthma onset, #positive skin tests, pre-BD FEV1 (L), pre-BD FEV1% predicted, second-hand smoke exposure, % blood eosinophils, IgE, 3+OCS burst in last year, ER/Urgent care in past year, admission in past year, FeNO
offline and online eNO combined
Variables included: asthma duration, age at enrollment, pre-BD FEV1 (L), pre-BD FEV1% predicted, second-hand smoke exposure, % blood eosinophils, ICU admission, FeNO
A separate analysis using the absolute change in FEV1 after bronchodilator demonstrated FeNO and pre-BD FEV1% predicted remained significant predictors of bronchodilator response (E-table 5a and 5b). There is a strong correlation between the relative and absolute change in maximal FEV1% predicted (E-Figure 1).
Replication analysis using SARP III Pediatric cohort
We then replicated the analysis of bronchodilator response in 186 children enrolled in SARP III - 109 children with SA and 77 children with NSA. In general, children enrolled in SARP III had less severe asthma than SARP I-II as demonstrated by a lower prevalence of intubation (9.2% vs 25%, p=0.002), ER visits in the prior 12 months (68.8% vs 80.5%, p=0.04), and less exposure to smoke (13.5% vs 22.3%, p=0.022). One exception was more participants in SARP III had at least 3 or more steroid bursts in the prior year (76.4% vs 57.8% p=0.003) compared to SARP I-II.
Differences in characteristics between those with and without a bronchodilator response in SARP III are shown in E-Table 4. Within this cohort, percent blood neutrophils and pre-BD FEV1 % predicted were significant predictors of bronchodilator reversibility in all children and those with SA (Table 4a and 4b), with lower lung function and lower neutrophil percentage indicative of greater bronchodilator response. In addition, IgE was noted as a predictor in all children with asthma (see Table 4a). When using an absolute difference in FEV1 % predicted, significant predictors included SA, aeroallergen sensitivity, FeNO, and hospital admission in the previous year (E-Table 4c and 4d).
Table 4.
Predictors of bronchodilator reversibility in children with asthma enrolled in SARP III
| a. Predictors of 12% relative bronchodilator reversibility in all children with asthma | |||
|---|---|---|---|
| Variables | Odds ratio | 95% CI | p value |
| Blood neutrophil % | 0.97 | 0.94 to 0.997 | 0.031 |
| Total serum IgE | 1.00 | 1.00 to 1.001 | 0.036 |
| Pre-bronchodilator FEV1 % predicted | 0.95 | 0.93 to 0.97 | <0.0001 |
| b. Predictors of 12% relative bronchodilator reversibility in children with severe asthma | |||
|---|---|---|---|
| Variables | Odds ratio | 95% CI | p value |
| Blood neutrophil % | 0.95 | 0.92 to 0.98 | 0.005 |
| Pre-bronchodilator FEV1 % predicted | 0.95 | 0.93 to 0.99 | 0.004 |
Variables included: race, second hand smoke status, asthma severity, % blood eosinophils (log), % blood neutrophils (log), total IgE (log), FeNO, pre-BD FEV1% predicted, ER/Urgent care in past year, ICU admission
Variables included: race, % blood eosinophils (log), % blood neutrophil (log), FeNO, pre-BD FEV1% predicted, admission in past year, history of intubation
Given the prospective nature of the SARP III cohort, we wanted to determine if having a significant bronchodilator response at enrollment (baseline) correlated with clinical outcomes one year later (n=140). For participants with reversibility at baseline, their lung function was lower at baseline (FEV1 % predicted 84.8 ± 15.2 vs 96.3 ± 17.0, p<0.0001) and at one-year follow-up (FEV1%predicted 83.3±15.8 vs 94.0±13.1, p<0.001) compared to those without. Participants with reversibility had less decline in lung function at one-year follow-up compared to those without reversibility (absolute change in FEV1%: 0.3±13.3 vs −5.1±10.1%, p=0.01). There was a negative correlation between having a bronchodilator response at baseline and lung function at one-year (FEV1/FVC r=−0.49, p<0.001 and FEV1 r=−.39, p<0.001), but a positive correlation between baseline reversibility and change in lung function over one year (r=0.29, p=0.0003).
The Asthma Control Test (ACT) score was lower at the one year follow-up for children with a bronchodilator response at baseline compared to those without reversibility (19.1 vs 20.6, p=0.04). However, there were no other differences in measures of asthma control or severity.
Discussion
In this large study of SA in children, we clarify the relationship between pre-BD lung function and the maximal achievable lung function following bronchodilation. Children with SA demonstrated greater pre-BD airflow obstruction and bronchodilator reversibility than NSA. This study expands upon our earlier findings within the pediatric SARP cohort,2,3 in that we found that SA, FeNO, and lung function were significantly associated with maximal bronchodilator reversibility. Interestingly in this prospective longitudinal cohort, bronchodilator reversibility was associated with reduced lung function at baseline and at one-year follow-up though less decline in lung function over one year compared with those without reversibility.
Our study confirms the findings described by Yancey, et al. of an association between lung function and reversibility8. In a retrospective review of 30,816 participants with asthma, baseline FEV1% predicted was inversely related to bronchodilator reversibility. In contrast, Ouksel, et al. determined that the response to bronchodilator was greater when the predicted FEV1 was larger, however, this was only when using absolute values and not percent predicted and only 15 of 30 participants were children9. Furthermore, this study did not address the response to escalating doses of bronchodilator. Our findings along with these previous studies stress the importance of taking into account pre-bronchodilator lung function when examining the bronchodilator response in participants with asthma.
We found that a diagnosis of SA, second-hand smoke exposure, FeNO and pre-BD lung function were strongly associated with a bronchodilator response in children with asthma. Sorkness, et al. examined maximal bronchodilator response in adults to determine predictors of persistent airflow obstruction10. They found that severe asthma, male gender and increasing age were independent predictors of lower maximal FEV1% predicted. Surprisingly, we did not find that gender or age was a predictor of reversibility in our pediatric cohort. While males demonstrated more airflow limitation, the response to bronchodilator was not different from females.
Several studies have examined the effects of in-utero, perinatal and second-hand smoke exposure on lung function, development of asthma, and response to therapy11–16. Smoke exposure may increase bronchial reactivity11 and has been shown to alter responses to bronchodilators as well as reduce symptoms and need for bronchodilation after treatment with leukotriene receptor antagonists12,13. It is unclear whether smoke exposure alters bronchodilator responsiveness through alterations in airway inflammation, smooth muscle reactivity or remodeling. The association of smoke exposure and bronchodilator response was not replicated in SARP III; however, smoke exposure was significantly lower in SARP III compared to SARP I-II.
As in our study, FeNO has been shown to correlate with bronchodilator response in adults17 as well as a small cohort of children18. Another study showed a relationship between FeNO and lung function in both asthmatic children and children with allergic rhinitis19; however, others have found that this association was only in seen in children with atopic disease20. Indeed, most of our patients were atopic which may explain our ability to detect such a relationship. Addition of FeNO to our model removed the significant effect of smoke exposure on bronchodilator response. Smoke exposure may have an effect on FeNO levels thus there may be some interaction between these variables we did not detect in our correlations (p=0.08)21,22. FeNO is considered a marker of airway inflammation, typically eosinophilic or Type 2 inflammation. Individuals with elevated FeNO as well as IgE levels are more likely to respond with increases in FEV1 upon treatment with ICS23,24. It is possible that FeNO can help identify a phenotype of asthma with less fixed airflow limitation and greater response to asthma treatment. In COPD, neutrophilic inflammation is associated with nonreversible airflow limitation while eosinophilic inflammation, though less common, is associated with improvement in lung function with bronchodilators25,26. In the SARP III cohort, lower peripheral blood neutrophil percent was predictive of a maximal bronchodilator response, perhaps, again, identifying a group less at risk of fixed airflow obstruction.
Several studies have examined the relationship between bronchodilator response and lung function. Vonk et al. showed that fixed airflow obstruction, defined as FEV1 <80% predicted and bronchodilator response <9% following 800μg salbutamol, after 21–33 years of follow-up was more likely when patients had less bronchial hyperreactivity and reversibility at the initial visit, suggesting lack of bronchodilator response may in fact be detrimental for future lung function27. It is unclear whether airway remodeling is associated with fixed airflow obstruction28,29. In biopsies of adults with SA, airway wall area and thickness percentage were positively correlated with a response to bronchodilator, though bronchodilator response itself was associated with baseline lung function and SA30. Tillie-Leblond et al. found that in children with persistent symptoms and obstruction (FEV1 <80% predicted) there was an increase in airway smooth muscle and vascular density compared to children with persistent symptoms but normal lung function28. We are unable to conclude whether a response to bronchodilators, or lack thereof, is related to underlying pathological mechanisms such as airway remodeling in children.
Longitudinal assessment of 1,041 children through the Childhood Asthma Management Program (CAMP) showed that bronchodilator response at baseline was predictive of higher future level of lung function in children with mild-to-moderate asthma, and this association was strongest in those receiving ICS compared to nedocromil or placebo. While this suggests a positive association for bronchodilator response, the conclusions of this study were aimed at the treatment-specific effects and suggests children with bronchodilator response may better respond to ICS than those without31. Indeed, bronchodilator response can be predictive of sustained improvement in FEV1 after initiation of ICS23. Additionally, CAMP demonstrated that those who consistently had a bronchodilator response at each of their yearly follow-up visits over 4 years, had lower baseline FEV1, higher IgE levels, and less treatment with ICS compared to those who did not32. Our data are consistent with these findings, indicating those with a bronchodilator response had lower lung function at baseline and one year; however, they had less decline in lung function over time than those without. While it is possible, this could be related to regression to the mean for lung function, we believe that the bronchodilator response is both an indicator of a more severe asthma phenotype with lower lung function and worse asthma control, as well as an indicator of those more likely to respond to treatment.
We also found that bronchodilator response is associated with increased healthcare utilization, greater oral corticosteroids bursts and hospitalizations at baseline. In CAMP, children with a consistent bronchodilator response over 4 years were more likely to have hospitalizations, prednisone bursts, missed school days and nocturnal awakenings at baseline as well as more exacerbations in the following year compared to those children without32. Hefler, et al found that, in 246 subjects 13 years and older with asthma, a bronchodilator response was associated with lower baseline lung function and ACT scores, and a positive correlation with change in ACT scores and FEV1 after albuterol. We did not find a significant difference in healthcare utilization at one-year follow-up in those with a bronchodilator response, however, asthma control scores were worse in this group.
Finally, the use of a relative 12% change in FEV1 as a threshold for a significant bronchodilator response has been called into question33,34. We found that after 180 μg of albuterol, the average response in SA met the ATS criteria threshold for significance, while that of NSA was lower at 8.4% and only met criteria after maximal bronchodilation. Our study suggests that while a 12% bronchodilator response may be expected in children with SA after 180 μg of albuterol, children with mild-to-moderate asthma may need higher doses to reach that threshold. Alternatively, we could consider a lower threshold in that subgroup, given that those with SA continue to achieve improvements in FEV1 with higher albuterol doses, but the increase in FEV1 is smaller in NSA.
We recognize our study has several limitations. First, we recognize that not all findings between SARP I-II and SARP III were replicated and that may have been driven by differences in the cohorts, such as asthma severity and smoke exposure. Additionally, using an absolute bronchodilator response versus a relative response may be controversial, however we found using the absolute change did not significantly alter our overall conclusions. We recognize the limitations of stepwise logistic regression and the potential to impact the variable selection process35. As our study is inherently exploratory, were are unable to use the preferred causal methods of prediction. Further research with different data sets would be required to validate the results of the stepwise procedure. We also recognize that children’s airways are a dynamic entity and that we cannot make conclusions regarding long-term outcomes. Others have demonstrated the utility of multiple measurements, e.g. FeNO combined with bronchodilator response when finding predictors for loss of asthma control, likely of greater importance in the growing child36.
In this study, we demonstrated that lung function, in particular FEV1 % predicted, is a significant predictor of the response to bronchodilator in children with asthma, and that smoke exposure, higher FeNO and IgE level, and lower peripheral blood neutrophils, may also identify those most likely to have reversibility of airflow obstruction following maximal bronchodilation. Maximal bronchodilator response is associated with more asthma exacerbations and hospitalizations at baseline as well as worse lung function and asthma control at one year of follow-up. We have identified a bronchodilator phenotype of pediatric asthma that is associated with worse health outcomes. Further prospective evaluation with ultra-low dose CT chest imaging37 may help determine the pathophysiologic role of airway remodeling in these children with and without a bronchodilator response. Newer asthma therapies including biologics might alter this process in children with SA but this remains to be proven.
Supplementary Material
Acknowledgments
Funding: This project is supported by the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program NIH/NHLBI U10 HL109257, U10 HL109250, U10 HL109152, U10 HL109164, U10 HL109146, U10 HL109172, U10 HL109168, U10 HL109086 and NIH/NCATS 5UL1 TR000448. In addition, SARP has received support from the following companies: AstraZeneca, Boehringer-Ingelheim, Genentech, GSK, Sanofi-Genzyme-Regeneron, and TEVA.
LBB reports personal fees from GlaxoSmithKline, Genentech/Novartis, Merck, DBV Technologies, Teva, Boehringer Ingelheim, AstraZeneca, WebMD/Medscape, Sanofi/Regeneron, Vectura, and Circassia. WP has received funding NIH (Institutional), Genentech, Novartis, Alk Abello, GSK, Monaghen, Lincoln Diagnostics, Thermo Fisher, Regeneron, Sanofi, Merck, Circassia, Astra Zeneca; she has consulted for Genentech, Novartis, GSK, Regeneron, Sanofi and has received speaker’s fees from Genentech, GSK. SEW has received financial support for SARP operations from Boehringer Ingelheim; she has consulted for AstraZeneca, GSK, Sanofi Genzyme and has been local PI on multicenter trials for AstraZeneca, GSK, Sanofi Genzyme, Novartis. ERB has received funding for clinical trials from AstraZeneca, MedImmune, Boehringer Ingelheim, Genentech, Novartis, Regeneron, and Sanofi Genzyme; he has consulted for ALK-Abello, AztraZeneca, MedImmune, Glaxo Smith Kline, Novartis, Regeneron, Sanofi Genzyme, and TEVA. NPY has received grant support from Vertex, Gilead, CFF, NSF, and NIH. DTM has received grant support for SARP from AstraZeneca, Boehringer-Ingelheim, Genentech, GSK, Sanofi-Genzyme-Regeneron, and TEVA. MC has received University Grant Funding from NIH, American Lung Association, PCORI and Pharmaceutical Grant Funding from AstraZeneca, Chiesi, Novartis, GSK, Sanofi-Aventis; he has consulted for Genentech, Theravance, VIDA, Teva, Sanofi-Aventis; he has received speaker’s fees for AstraZeneca, Genentech, GSK, Regeneron, Sanofi, Teva and Royalties from Elsevier. The remaining authors have no relevant conflicts of interest or financial disclosures.
References
- 1.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J. et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948–68. [DOI] [PubMed] [Google Scholar]
- 2.Fitzpatrick AM, Teague WG, National Institutes of Health/National Heart L, Blood Institute’s Severe Asthma Research Program. Progressive airflow limitation is a feature of children with severe asthma. J Allergy Clin Immunol 2011;127:282–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzpatrick AM, Teague WG. Severe Asthma in Children: Insights from the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Pediatr Allergy Immunol Pulmonol 2010;23:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. Am J Respir Crit Care Med 2000;162:2341–51. [DOI] [PubMed] [Google Scholar]
- 5.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43:343–73. [DOI] [PubMed] [Google Scholar]
- 6.Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax 1992;47:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG Jr., Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol 1993;15:75–88. [DOI] [PubMed] [Google Scholar]
- 8.Yancey SW, Ortega HG. Retrospective characterization of airway reversibility in patients with asthma responsive to bronchodilators. Curr Med Res Opin 2007;23:3205–7. [DOI] [PubMed] [Google Scholar]
- 9.Ouksel H, Meslier N, Badatcheff-Coat A, Racineux JL. Influence of predicted FEV1 on bronchodilator response in asthmatic patients. Respiration 2003;70:54–9. [DOI] [PubMed] [Google Scholar]
- 10.Sorkness RL, Teague WG, Penugonda M, Fitzpatrick AM, National Institutes of Health NHL, Blood Institute’s Severe Asthma Research P. Sex dependence of airflow limitation and air trapping in children with severe asthma. J Allergy Clin Immunol 2011;127:1073–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubus JC, Oddoze C, Badier M, Guillot C, Bruguerolle B. Possible interaction between exposure to environmental tobacco smoke and therapy in children with asthma. Clin Sci (Lond) 1998;95:143–9. [PubMed] [Google Scholar]
- 12.Rabinovitch N, Silveira L, Gelfand EW, Strand M. The response of children with asthma to ambient particulate is modified by tobacco smoke exposure. Am J Respir Crit Care Med 2011;184:1350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein AB, Castile RG, Davis SD, Filbrun DA, Flucke RL, McCoy KS, Tepper RS. Bronchodilator responsiveness in normal infants and young children. Am J Respir Crit Care Med 2001;164:447–54. [DOI] [PubMed] [Google Scholar]
- 14.Tamimi A, Serdarevic D, Hanania NA. The effects of cigarette smoke on airway inflammation in asthma and COPD: therapeutic implications. Respir Med 2012;106:319–28. [DOI] [PubMed] [Google Scholar]
- 15.Cohen RT, Raby BA, Van Steen K, Fuhlbrigge AL, Celedon JC, Rosber BA, Strunk RC, Zeiger RS, Weiss ST, Childhood Asthma Management Program Research, Group. In utero smoke exposure and impaired response to inhaled corticosteroids in children with asthma. J Allergy Clin Immunol 2010;126:491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oddoze C, Dubus JC, Badier M, Thirion X, Pauli AM, Pastor J, Bruguerolle B. Urinary cotinine and exposure to parental smoking in a population of children with asthma. Clin Chem 1999;45:505–9. [PubMed] [Google Scholar]
- 17.Malinovschi A, Gislason T, Olivieri M, Olin A-C, Jarvis D, and Janson C. Bronchodilator response and previous lung function decline in relation with exhaled nitric oxide levels in asthma. Eur Resp J 2017;50:OA3216. [Google Scholar]
- 18.Colon-Semidey AJ, Marshik P, Crowley M, Katz R, Kelly HW. Correlation between reversibility of airway obstruction and exhaled nitric oxide levels in children with stable bronchial asthma. Pediatr Pulmonol 2000;30:385–92. [DOI] [PubMed] [Google Scholar]
- 19.Ciprandi G, Tosca MA, Capasso M. High exhaled nitric oxide levels may predict bronchial reversibility in allergic children with asthma or rhinitis. J Asthma 2013;50:33–8. [DOI] [PubMed] [Google Scholar]
- 20.Silvestri M, Sabatini F, Sale R, Defilippi AC, Fregonese L, Battistini E, Biraghi MG, Rossi GA. Correlations between exhaled nitric oxide levels, blood eosinophilia, and airway obstruction reversibility in childhood asthma are detectable only in atopic individuals. Pediatr Pulmonol 2003;35:358–63. [DOI] [PubMed] [Google Scholar]
- 21.Jacinto T, Malinovschi A, Janson C, Fonseca J, and Alving K. Differential effect of cigarette smoke exposure on exhaled nitric oxide and blood eosinophils in healthy and asthmatic individuals. J Breath Res 2017;11:036006. [DOI] [PubMed] [Google Scholar]
- 22.Nerpin E, Jarvis D, Olivieri M, Gislason T, Olin A-C, Janson C, Malinovschi A. Different relation between exhaled nitric oxide and lung function with regard to current smoking. Eur Resp J 2018;52:PA4488. [Google Scholar]
- 23.Kerstjens HAM, Overbeek SE, Schouten JP, Brand PLP, Postma DS and the Dutch CNSLD Study Group. Airways hyperresponsiveness, bronchodilator response, allergy and smoking predict improvement in FEV1 during long-term inhaled corticosteroid treatment. Eur Resp J 1993;6:868–876. [PubMed] [Google Scholar]
- 24.Price DB, Buhl R, Chan A, Freeman D, Gardener E, Godley C, Gruffydd-Jones K, McGarvey L, Ohta K, Ryan D, et al. Fractional exhaled nitric oxide as a predictor of response to inhaled corticosteroids in patients with non-specific respiratory symptoms and insignificant bronchodilator reversibility: a randomised controlled trial. Lancet Respir Med 2018;6:29–39. [DOI] [PubMed] [Google Scholar]
- 25.Perng D-W, Huang H-Y, Chen H-M, Lee Y-C, and Perng R-P. Characteristics of airway inflammation and bronchodilator reversibility in COPD. Chest 2004;126:375–381. [DOI] [PubMed] [Google Scholar]
- 26.Sitkauskiene B, Sakalauskas R, Malakauskas K, Lotvall J. Reversibility to β2-agonist in COPD: relationship to atopy and neutrophil function. Respir Med 2003;97:591–598. [DOI] [PubMed] [Google Scholar]
- 27.Vonk JM, Jongepier H, Panhuysen CI, Schouten JP, Bleecker ER, Postma DS. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax 2003;58:322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tillie-Leblond I, de Blic J, Jaubert F, Wallaert B, Scheinmann P, Gosset P. Airway remodeling is correlated with obstruction in children with severe asthma. Allergy 2008;63:533–41. [DOI] [PubMed] [Google Scholar]
- 29.Fish JE, Peters SP. Airway remodeling and persistent airway obstruction in asthma. J Allergy Clin Immunol 1999;104:509–16. [DOI] [PubMed] [Google Scholar]
- 30.Aysola RS, Hoffman EA, Gierada D, Wenzel S, Cook-Granroth J, Tarsi J, Zheng J, Schechtman KB, Ramkumar TP, Cochran R et al. Airway remodeling measured by multidetector CT is increased in severe asthma and correlates with pathology. Chest 2008;134:1183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tantisira KG, Fuhlbrigge AL, Tonascia J, Van Natta M, Zeiger RS, Strunk RC, Szefler SJ, Weiss ST, Childhood Asthma Management Program Research, Group. Bronchodilation and bronchoconstriction: predictors of future lung function in childhood asthma. J Allergy Clin Immunol 2006;117:1264–71. [DOI] [PubMed] [Google Scholar]
- 32.Sharma S, Litonjua AA, Tantisira KG, Fuhlbrigge AL, Szefler SJ, Strunk RC, Zeiger RS, Murphy AJ, Weiss ST, Childhood Asthma Management Program Research, Group. Clinical predictors and outcomes of consistent bronchodilator response in the childhood asthma management program. J Allergy Clin Immunol 2008;122:921–8 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tse SM, Gold DR, Sordillo JE, Hoffman EB, Gillman MW, Rifas-Shiman SL, Fuhlbrigge AL, Tantisira KG, Weiss ST, Litonjua AA. Diagnostic accuracy of the bronchodilator response in children. J Allergy Clin Immunol 2013;132:554–9 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward H, Cooper BG, Miller MR. Improved criterion for assessing lung function reversibility. Chest 2015;148:877–86. [DOI] [PubMed] [Google Scholar]
- 35.Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, Ost DE, Punjabi NM, Schatz M, Smyth AR, et al. Control of Confounding and Reporting of Results in Casual Inference Studies. Ann Am Thorac Soc 2019;16:22–28. [DOI] [PubMed] [Google Scholar]
- 36.Kim J-K, Jung J- Y, Kim H, Eom S- Y, and Hahn Y- S. Combined use of fractional exhaled nitric oxide and bronchodilator response in predicting future loss of asthma control among children with atopic asthma. Respirology 2017;22:466–472. [DOI] [PubMed] [Google Scholar]
- 37.Berair R, Hartley R, Mistry V, Sheshadri A, Gupta S, Singapuri A, Gonem S, Marshall RP, Sousa AR, Shikotra A, et al. Associations in asthma between quantitative computed tomography and bronchial biopsy-derived airway remodelling. Eur Respir J 2017;49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.