Abstract
Anti-γ-aminobutyric acid B receptor (anti-GABABR) encephalitis is a rare type of autoimmune encephalitis (AE). Although it responds well to immunomodulating therapy and has favorable prognosis, anti-GABABR AE has often been misdiagnosed as infectious encephalitis. Herein, we present a case of a 59-year-old female with anti-GABABR AE associated with small cell lung cancer (SCLC) that was once misdiagnosed as infectious encephalitis. Our findings increase the awareness that patients presenting with a clinical trial of cognitive impairment, seizures and SCLC may harbor AE. Our case also highlights the importance of anti-SOX1 antibody in the detection of SCLC.
Keywords: autoimmune encephalitis, anti-GABAB receptor, small cell lung cancer
Introduction
Anti-γ-aminobutyric acid B receptor (anti-GABABR) encephalitis is a rare type of autoimmune encephalitis (AE) associated with anti-neuronal cell surface antibodies and accounts for approximately 5% of patients with autoimmune synaptic encephalitis.1 The most characteristic presentations of this disorder include seizures, cognitive impairment, confusion, and personality changes.2 Nearly 50% of patients with this condition harbor an underlying tumor, particularly a small cell lung cancer (SCLC) or pulmonary neuroendocrine tumor.3 Immunotherapy often results in good outcomes and relapse is uncommon.4 The SOX1 protein is a part of SRY-like high mobility group superfamily of developmental transcription factors and anti-SOX1 antibody was described as immunobiomarker of SCLC.5 Early recognition of anti-SOX1 antibody, identified underlying neoplasm, and prompt initiation of immunotherapy are essential to achieve a better outcome.
As far as we know, only a few cases have been reported to date and its clinical manifestations and treatment have not been investigated systematically. Herein, we reported a 59-year-old woman presenting as rapidly progressive cognitive impairment and seizures diagnosed as AE with anti-SOX1 and anti-GABABR antibody and finally confirmed by biopsy as SCLC.
Case Report
A 59-year-old woman presented at our hospital with memory deficit for 12 days and recurrent convulsions for 8 days. She usually could not remember what she had eaten an hour ago and always complained why her brother did not come to visit her. In fact, her brother had been dead for many years. Four days later, she experienced three convulsions, which lasted about 10 mins every time, manifesting as body stiffness, rolling eyes, foaming at the mouth, urinary incontinence, and consciousness disturbance. She was previously healthy and had no family history of psychiatric disorders. Blood routine examination showed elevated leukocyte count (10.03*109/L, normal range 4-109/L). Considering the possibility of infectious encephalitis, she was treated with ganciclovir 0.25g b.i.d, piperacillin sodium and tazobactam sodium 0.45g t.i.d for 5days, and phenobarbital in the local hospital, but her symptoms did not improve significantly and she came to our hospital for further treatment. Neurological examination revealed a marked decrease in computational ability and memory. The scores of Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) were 18/30 and 6/30, respectively.
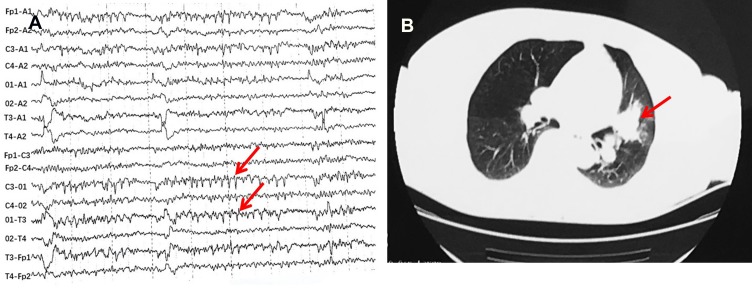
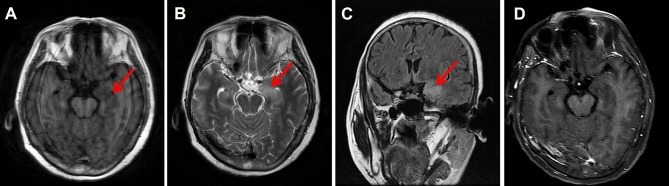
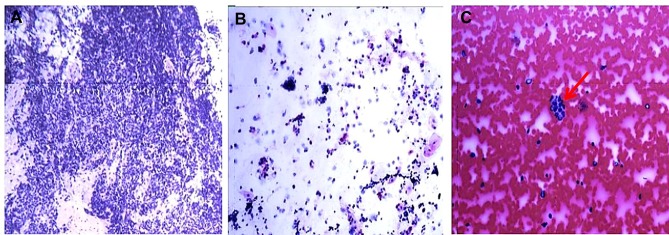
Cerebrospinal fluid (CSF) showed elevated leukocyte (75/uL, normal range 0–5/uL), normal glucose (3.7 mmol/L, normal range 2.5–4.5 mmol/L), lowered chloride (119 mmol/L, normal range 120–130 mmol/L), and normal protein level (37 mg/dL, normal range 20–40 mg/dL). The anti-GABABR antibody was positive both in the serum and CSF. Anti-SOX1 antibody was positive in the serum (the results were from the central laboratory of Beijing Tongren Hospital, and the tested products are from Euroimmun company). However, the other biomarkers of autoimmune encephalitis (NMDAR-Ab, AMPAR1-Ab, AMPAR2-Ab, LGI1-Ab, Caspr2-Ab) and paraneoplastic neuronal antibodies (anti-Hu, -Yo, -Ri, -Ma2/Ta, -Amphiphysin, -CV2, -Tr, -recoverin, -titin, -zic4, -GAD65) were all unremarkable. CSF cultures for bacteria, fungi, and viruses were negative. CSF for cryptococcal antigen, acid-fast bacilli were also negative. Electroencephalogram (EEG) showed epileptiform discharge (Figure 1A). Chest CT showed a tumor in the hilus of the left lung (Figure 1B). Cranial magnetic resonance images (MRI) showed hypointensity in left hippocampus on T1-weighted sequences, corresponding hyperintensitiy on T2-weighted sequences and fluid-attenuated inversion recovery (FLAIR), gadolinium-enhanced cranial MRI revealed no obvious enhancement of the corresponding lesions (Figure 2). Biopsy of Lung showed there were degenerative small cells with nuclear division (Figure 3A). Lung lavage fluid examination revealed heteromorphic cell clusters (Figure 3B). Left bronchial mucosal biopsy showed diffuse infiltration of small blue circle cells in the interstitium of respiratory epithelium (Figure 3C). The pathological examination confirmed the diagnosis of small cell lung cancer (SCLC).
Figure 1.
EEG showed epileptiform discharge (red arrows) and Chest CT showed a tumor in the hilus of the left lung (red arrow).
Notes: (A) EEG. (B) Chest CT.
Abbreviations: EEG, Electroencephalogram; CT, computed tomography.
Figure 2.
Images from the magnetic resonance imaging after admission.
Notes: (A) T1-weighted sequences showed hypointensity in left hippocampus (red arrow). (B) T2-weighted sequences showed hyperintensitiy in left hippocampus (red arrow). (C) FLAIR showed hyperintensitiy in left hippocampus (red arrow). (D) Postcontrast enhanced image revealed no obvious enhancement of lesions.
Abbreviation: FLAIR, fluid-attenuated inversion recovery.
Figure 3.
Pathological examination.
Notes: (A) Biopsy of Lung (Wright staining, magnificationx40). (B) Cytological examination of lung lavage fluid (Wright staining, magnificationx100). (C) Biopsy of left bronchial mucosal (Wright staining, magnificationx400), small tumor circle cells (red arrow).
Based on the patient’s history, physical signs, and auxiliary examination, she was diagnosed with anti-GABABR AE accompanied anti-SOX1 antibody. With the treatment of intravenous immunoglobulin (IVIg) for 5 days and levetiracetam 0.5g b.i.d. orally, she had no additional seizures occurred and her memory impairment improved, finally discharged from our hospital. As for the treatment of SCLC, we failed to follow up.
This case study was approved by the Ethics Committee of Beijing Chaoyang Hospital, Capital Medical University. Written informed consent was obtained from the patient to publish the case details.
Discussion
The field of autoimmune neurology has evolved rapidly in recent years. As more neural autoantibodies have been discovered, cases of encephalitis previously presumed to be viral or idiopathic have been determined to be autoimmune in aetiology.6 Anti-GABABR AE was first described on the literature in 2010 by Lancaster et al.7 Although symptoms of anti-GABABR AE (seizures, confusion, and memory loss) were characteristics originally proposed, additional associated features have become apparent, such as: status epilepticus, ataxia, epileptiform electroencephalogram (EEG) findings.8 Imaging features are also variable. In clinical work, it is easy to ignore the disease, resulting in misdiagnosis. Herein, we reported a rare case of a 59-year-old woman with anti-GABABR AE associated with SCLC, initially misdiagnosed as infectious encephalitis. The patient we reported presented with significant memory impairment and seizures, which was consistent with previously reported cases. The pathogenesis is still unclear. There is evidence that a lack of normal GABAB receptors structure and function can lead to spontaneous seizures.9 In animal models, pharmacological or genetic knockout of GABAB receptors can result in disorders of memory, learning, and behavior.10 Cranial MRI abnormalities reported in patients are mostly hyperintensities in the medial temporal lobes on T2-weighted or fluid-attenuated inversion recovery (FLAIR) images.7 In our case, cranial MRI revealed specific unilateral hippocampal lesions, which was consistent with prior reports. The clinical features, imaging findings, positive anti-GABABR antibody in CSF and serum, and favorable prognosis eventually contributed to the diagnosis of anti-GABABR AE. The lack of recognition of clinical manifestations and imaging features may be the major cause of misdiagnosis. Thorough differential diagnosis of AEs should be considered in patients with presentation of symptoms. The recognition of anti-GABABR AE has essential clinical implications for patient outcomes, as untreated patients are more likely to die or progress to coma.8
Notably, the patient we reported with the coexistence of anti-SOX1 and anti-GABABR antibodies associated with SCLC, which is an extremely rare condition. About parallel detection of anti-GABABR antibodies and other coexisting antibodies, Chung et al11 firstly described the case of a patient with coexisting anti-GABABR antibodies as well as anti-IgLON5 and predominant clinical features of anti-IgLON5 disease (severe sleep disorder, bulbar symptoms, and gait abnormalities). Interestingly, the patient’s clinical syndrome is clearly dominated by “classical” symptoms of antiIgLON5 disease without distinct features of limbic encephalitis, which suggested that more antibodies expressed, the clinical manifestation of the patient may be more complicated. The association of different antibody expression with clinical symptoms is helpful for us to better understand the disease. Anti-SOX1 antibodies have been reported to be quite prevalent in patients with Lambert-Eaton myasthenic syndrome (LEMS) and a specific marker for SCLC-LEMS.5 Although the pathogenic role of Anti-SOX1 antibodies remains unclear, their strong association with underlying neoplastic disease (mainly small-cell lung cancer) has designated them as onconeural antibodies.12 Therefore, positive anti-SOX1 antibodies in patients with encephalitis may further indicate the high risk of SCLC. Early, continuous and regular tumor screening are vital for the diagnosis of the disorder. For the treatment of anti-GABABR AE, both immunomodulating therapy and cancer treatment in the presence of malignancy are necessary. One of the suggested guidelines is using high doses of corticosteroid, intravenous immunoglobulin and plasmapheresis (plasma exchange, immunoadsorption) as first-line therapy and adding rituximab and cyclophosphamide as second-line therapies in refractory cases.1 McKay et al have reported 86.3% of patients with GABAB encephalitis treated either demonstrating partial or complete recovery,8 which indicated the disorder has a robust response to immunotherapy or cancer treatment.
Conclusion
This rare case suggests that patients presenting with a clinical trial of memory changes, seizures association with SCLC may harbor autoimmune encephalitis. Our case increases the awareness of anti-GABABR encephalitis and related differential diagnosis. Our case also highlights the importance of anti-SOX1 antibody in the detection of SCLC. Recognition of these conditions is crucial as with prompt diagnosis and treatment the majority have favourable outcomes.
Author Contributions
Wei Qin and Xiao Wang were co-first authors. Wei Qin and Xiao Wang examined, evaluated the patient, collected data and drafted the manuscript. Jing Yang revised it critically for important intellectual content. Wenli Hu participated in the design of the case-report and helped to draft the manuscript. All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology. 2011;77(2):179–189. doi: 10.1212/WNL.0b013e318224afde [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalmau J, Rosenfeld MR. Autoimmune encephalitis update. Neuro Oncol. 2014;16(6):771–778. doi: 10.1093/neuonc/nou030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gozzard P, Woodhall M, Chapman C, et al. Paraneoplastic neurologic disorders in small cell lung carcinoma: a prospective study. Neurology. 2015;85(3):235–239. doi: 10.1212/WNL.0000000000001721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoftberger R, Titulaer MJ, Sabater L, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. 2013;81(17):1500–1506. doi: 10.1212/WNL.0b013e3182a9585f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipka AF, Verschuuren JJ, Titulaer MJ. SOX1 antibodies in Lambert-Eaton myasthenic syndrome and screening for small cell lung carcinoma. Ann N Y Acad Sci. 2012;1275:70–77. doi: 10.1111/j.1749-6632.2012.06772.x [DOI] [PubMed] [Google Scholar]
- 6.Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California encephalitis project. Clin Infect Dis. 2012;54(7):899–904. doi: 10.1093/cid/cir1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9(1):67–76. doi: 10.1016/S1474-4422(09)70324-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKay JH, Dimberg EL, Lopez CA. A systematic review of gamma-aminobutyric acid receptor Type B autoimmunity. Neurol Neurochir Pol. 2019;53(1):1–7. doi: 10.5603/PJNNS.a2018.0005 [DOI] [PubMed] [Google Scholar]
- 9.Schuler V, Luscher C, Blanchet C, et al. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)). Neuron. 2001;31(1):47–58. doi: 10.1016/S0896-6273(01)00345-2 [DOI] [PubMed] [Google Scholar]
- 10.Prosser HM, Gill CH, Hirst WD, et al. Epileptogenesis and enhanced prepulse inhibition in GABA(B1)-deficient mice. Mol Cell Neurosci. 2001;17(6):1059–1070. doi: 10.1006/mcne.2001.0995 [DOI] [PubMed] [Google Scholar]
- 11.Chung HY, Wickel J, Voss A, et al. Autoimmune encephalitis with anti-IgLON5 and anti-GABAB-receptor antibodies: A case report. Medicine (Baltimore). 2019;98(20):e15706. doi: 10.1097/MD.0000000000015706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alessandro L, Schachter D, Farez MF, Varela F. Cerebellar ataxia with extreme photophobia associated with Anti-SOX1 antibodies. Neurohospitalist. 2019;9(3):165–168. doi: 10.1177/1941874418802130 [DOI] [PMC free article] [PubMed] [Google Scholar]