Abstract
Discrimination of different sound frequencies is pivotal to recognizing and localizing friend and foe. Here, I review the various hair-cell tuning mechanisms employed among vertebrates. Electrical resonance, filtering of the receptor potential by voltage-dependent ion channels, is ubiquitous in all non-mammals, but has an upper limit of about 1 kHz. The frequency range is extended by mechanical resonance of the hair bundles in frogs and lizards, but may need active hair-bundle motion to achieve sharp tuning up to 5 kHz. Tuning in mammals employs somatic motility of outer hair cells, underpinned by the membrane protein prestin, to expand the frequency range. The bird cochlea may also employ prestin at high frequencies, but hair cells below 1 kHz show electrical resonance.
Keywords: hair cells, electrical resonance, prestin, hair bundle, basilar papilla
Hair cell tuning mechanisms and cochlear structure
Hair cells, the sensory receptors of the vertebrate inner ear, convert sound stimuli into electrical signals, and also separate the frequency constituents of the sound, enabling different subsets of hair cells to encode different frequencies. To ensure survival, an animal employs its auditory apparatus both to identify the sound source, whether friend, food or foe, and to spatially localize it. Are the cries within the forest at night those of an offspring or a predator? Crucially, from which direction do they originate? Can you recognize the voice of a friend across a dark room at a crowded party? Accurate classification and localization of sounds depend on their frequency make-up [1, 2]. The mechanisms involved in frequency discrimination differ between the vertebrate classes, reptile, bird or mammal, and importantly depend on the tonal range to be detected. In the evolution of the land vertebrates, there has been a drive to extend the upper frequency limit of hearing from a few hundred Hz in the simplest amphibians or reptiles up to about 100 kHz in small mammals. To this end, changes have occurred in sound transmission through the middle ear, in the structure of the cochlea, and in the roles of the hair cells. The reasons for the frequency extension are not known for certain. They may partly derive from selective pressure for localizing sounds in animals with small heads like the first mammals, or in finding tiny offspring from their high-frequency cries. Another factor driving frequency extension is communication between species members, exemplified by the croaks of frogs, the chirps of geckos and bird songs, which are all composed of kilohertz sound frequencies.
This review describes the evidence for the different cochlear mechanisms, all of which depend upon resonant behavior. A simple illustration of resonance is that generated by a mass, M, suspended on the end of a spring of stiffness K: when the mass is displaced, it oscillates with a resonant frequency, FO, equal to 1//2π.(K/M)1/2, FO increasing with larger stiffness and smaller mass. Resonance refers to the increase in the oscillation’s amplitude if an external force is applied at the resonant frequency (but not if the external force is applied at frequencies far from it). In practice, the oscillations will be damped if the mass is immersed in fluid rather than air. In the cochlea, the resonance can be mechanical or electrical and, to generate a range of resonant frequencies, one or both parameters are graded systematically with position along the cochlea. Thus, each subset of hair cells is associated with a limited tonal set the bounds of which vary according to hair cell location in an arrangement known as the tonotopic map (Figure 1A). The cochlea therefore performs the equivalent of a Fourier analysis on the incoming sound, so that the amplitudes of the component frequencies can be signaled to the brain enabling central categorization of the sound. At least three well-defined frequency selective processes operate in vertebrates: electrical resonance of the hair cell, mechanical amplification by voltage-dependent hair cell contractions, and passive mechanical resonances possibly reinforced by active force generation by the hair bundle. These disparate processes are manifested in different cochlear organization.
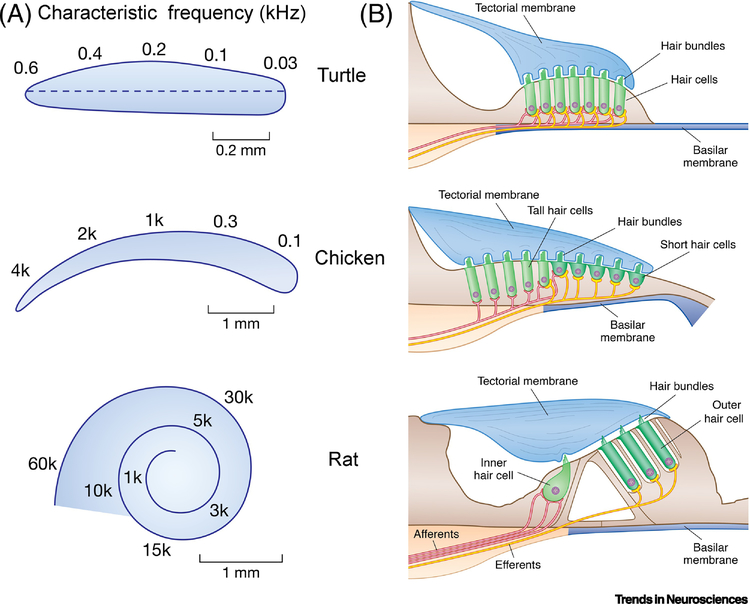
Figure 1.
Structure of three vertebrate cochleas. (A) Top view of the basilar membrane in turtle, chicken and rat showing the tonotopic characteristic frequency mapping form base (left) to apex (right). CFs in kHz. (B) Transverse sections through the cochlear duct showing the hair cells, afferent (red) and efferent (yellow) innervation patterns. In the chicken and rat, there are two hair cell types with different patterns of innervation. For turtle and chicken, each auditory nerve fiber contact a single hair cell, whereas in mammals up to 20 nerve fibers innervate each inner hair cell. For scale, hair cells are 25 μm long in turtle, and hair cells of similar length are tall hair cells in chicken and mid-cochlear OHCs in rat.
In all amniotes, comprising reptiles, birds, and mammals, the cochlea is a blind-ending tube projecting from the saccular division of the inner ear; it varies in length from under 1 mm in turtles to 4 mm in chickens, and in mammals 10 mm in rats, 34 mm in humans and 60 mm in elephants [3]. The cochlear tube is partitioned longitudinally by an elastic basilar membrane surmounted by an epithelium containing the sensory hair cells (Figure 1B). From the top of each hair cell projects a staircase of stereocilia termed the hair bundle, which inserts into an overlying acellular tectorial membrane. Sound-induced motions of the basilar membrane elicit deflections of the hair bundle which are the ultimate stimulus to the hair cell, acting to tension tip links between neighboring stereocilia to apply force on the mechanotransduction channel [4]. Because all tip links course parallel to the hair bundle’s axis of symmetry, the bundles are functionally polarized in that rotations towards the taller edge tension the links and are excitatory, whereas those in the opposite direction relax the links and are inhibitory. In most vertebrate cochleas (apart from some lizards), the bundles all point in one direction, away from the neural limb.
The simplest cochlear structure, epitomized by that of the turtle, is a short epithelium of hair cells of relatively uniform appearance and innervation; in these cells, the receptor potential is tuned by an electrical resonance [5, 6] resulting from activation of voltage-dependent ion channels. The electrical resonance has a limited frequency range extending only to about 1 kHz, and is not employed in mammals [7] needing to hear to much higher frequencies. In contrast, the cochleas of mammals (and birds) possess two types of hair cell with distinct morphology and innervation known as inner (short) hair cells and outer (tall) hair cells (OHCs) [8, 9]; inner hair cells receive the major glutamatergic afferent innervation, whereas outer hair cells are contacted by cholinergic efferents. In mammals, the hair cell epithelium supports a broad mechanical resonance [10] augmented by the contractions of OHCs [11], that perhaps with other unknown processes generates sharp mechanical tuning of the basilar membrane [12, 13]. These two mechanisms, electrical resonance and hair cell somatic motility, represent the extremes, and intermediate processes exist in other vertebrates. For example, both frogs and lizards display electrical resonance at low frequencies supplemented by other mechanical resonances at high frequencies.
Electrical resonance
Ion channels in electrical tuning
During acoustic stimulation, vibrations of the hair bundle modulate the hair cell membrane potential in an analog fashion. Action potentials are not generated until the spiral ganglion cells, the axons of which relay the auditory message to the brainstem cochlear nucleus. At frequencies less than 1 kHz, the waveform of the hair-cell receptor potential resembles that of the sound stimulus and is graded in amplitude with stimulus intensity. In electrical tuning, the receptor potential is shaped and filtered by voltage-dependent ion channels in the hair cell membrane [5]; in the turtle cochlea, this electrical resonance accounts for almost all of the frequency tuning to sound stimuli [5]. The mechanism can be revealed by injecting extrinsic current pulses into a hair cell through a recording electrode, thus circumventing the transduction apparatus. Extrinsic current pulses elicit damped oscillations in membrane potential at the onset and termination of the step, reminiscent of the ringing of a struck bell (Figure 2). The frequency of the damped oscillation, the resonant frequency, FO, changes systematically with hair cell location along the cochlea to encompass the auditory range of the turtle extending from 30 Hz to 600 Hz at 25°C [14]. However, as discussed below, this range is temperature sensitive.
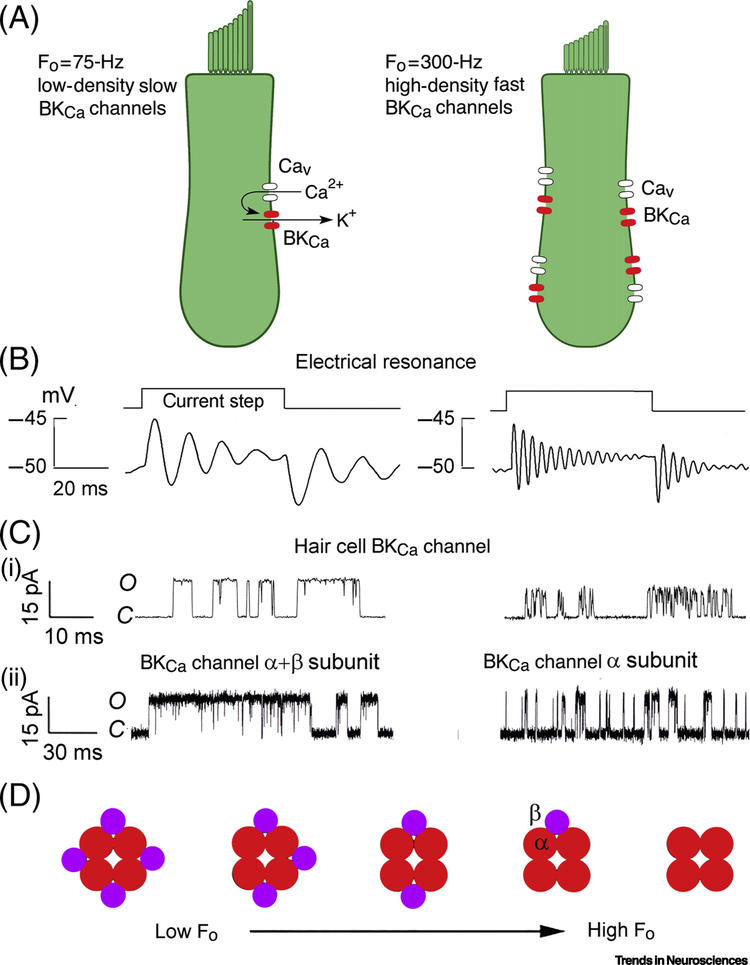
Figure 2.
Mechanisms of electrical tuning in low- frequency and high-frequency turtle hair cells. (A) Resonance arises from voltage-dependent Ca2+ channel (Cav) and Ca2+-activated K+ (KCa) channels. An increase in resonant frequency from 75Hz (left) to 300Hz (right) requires four-fold increase in channel numbers. (B) Examples of voltage ringing induced by current steps. (C) Cell-attached patch recordings from KCa channels in isolated hair cells tuned to 75 and 300 Hz (top); and KCa channels expressed in Xenopus oocytes, for α+β and α subunits (see references 20, 23). (D) Scheme for tonotopy: four KCa channel α subunits associated with one to four β subunits.
Electrical tuning stems from negative feedback between the membrane potential and the current through voltage-dependent K+ channels. In all hair cells, the resting potential is approximately −50 mV, positive to the K+ equilibrium potential (−75 mV), so that the depolarization induced by the current step activates the K+ channels through which an outward repolarizing current flows. Since the K+ channels activate with a delay, the feedback produces several oscillatory cycles. For most of the frequency range, the effective voltage-dependent K+ channel is fashioned [15, 16] from the combined action of voltage-dependent Ca2+ channels (Cav1.3) and large-conductance Ca2+ activated K+ (BKCa) channels (gene KCNMA1). Thus, depolarization opens Ca2+ channels and the Ca2+ influx and depolarization collude to activate nearby BKCa channels; this channel arrangement augments the voltage-sensitivity of the BKCa channels and sharpens the frequency tuning. Other types of voltage-dependent K+ channels, including A-currents (Kv4.2) and inward rectifiers (Kir), may also contribute at low frequencies [17–19], but the principle of their action is similar. At the resonant frequency, FO, the current flowing through the BKCa channels is approximately equal and opposite to the capacitive current, so at FO the membrane impedance is large, maximizing the receptor potential amplitude. FO can be changed by altering the electrical properties of the resonator. On the idea of a negative feedback between membrane potential and current through the BKCa channels, the larger and faster the K+ current, the more rapidly it counters the voltage change to elicit higher-frequency oscillations: a higher density of faster BKCa channels causes an increase in FO [20] (Figure 2A, B). Quantitatively, FO is proportional to the BKCa channel density and to the inverse square of the BKCa channel activation time constant [20], but what mechanisms might govern these channel parameters? One factor that modulates the speed of the BKCa channels is their association with an accessory β-subunit (gene KCNMB1) [21–23] (Figure 2C). The association between the pore-forming and auxiliary subunits can have variable stoichiometry [24], between one and four β-subunits being associated with four pore-forming α-subunits: more β subunits produce incrementally slower BKCa channels (Figure 2D). There is also evidence that the BKCa channel clusters can be regulated by β subunits, in particular β1 and β4, which are preferentially expressed towards the low frequency region of the chick cochlea [25]. However, control of BKCa channel density and kinetics is still not fully understood, and factors other than β-subunits may be involved [26, 27]. There is some evidence in the chicken cochlea (which also employs electrical tuning) that the tonotopic gradient is established in early embryogenesis by gradients in the secreted morphogens Bmp7 and retinoic acid [28, 29].
The frequency limits of electrical tuning
In the auditory organs of all non-mammalian vertebrates, electrical tuning probably acts as the hair-cell tuning mechanism at frequencies below 1 kHz. The mechanism has been shown to occur in the frog amphibian papilla [19, 30], the chicken basilar papilla [31, 32] (Figure 3A, 3B), the alligator basilar papilla [33] and probably in the gecko [34]. It has also been reported in the saccule of the bullfrog [15] and the goldfish [35]. The electrical resonance exhibits distinctive traits, including amplification (due to increased membrane impedance), broadening of the tuning at higher sound levels (due to progressive activation of the BKCa channels) and spontaneous oscillatory activity, in which the hair cell membrane potential displays noisy oscillations around the resonant frequency [14]. One consequence of the spontaneous fluctuations in hair cell membrane potential is a sinusoidal discharge of action potentials in the auditory nerve fibers, with an inter-spike interval approximately equal to the inverse of the fiber’s acoustic characteristic frequency (CF). This auditory nerve phenomenon has been reported in the turtle [14], the pigeon [36] and the gecko [34], but contrasts with the bimodal spontaneous discharge of mammalian auditory nerve fibers [37]. Periodicity in spontaneous auditory nerve firing in the tokay gecko, and temperature dependent CFs (see below), are indirect evidence for electrical tuning in this lizard up to 500 Hz [34, 38].
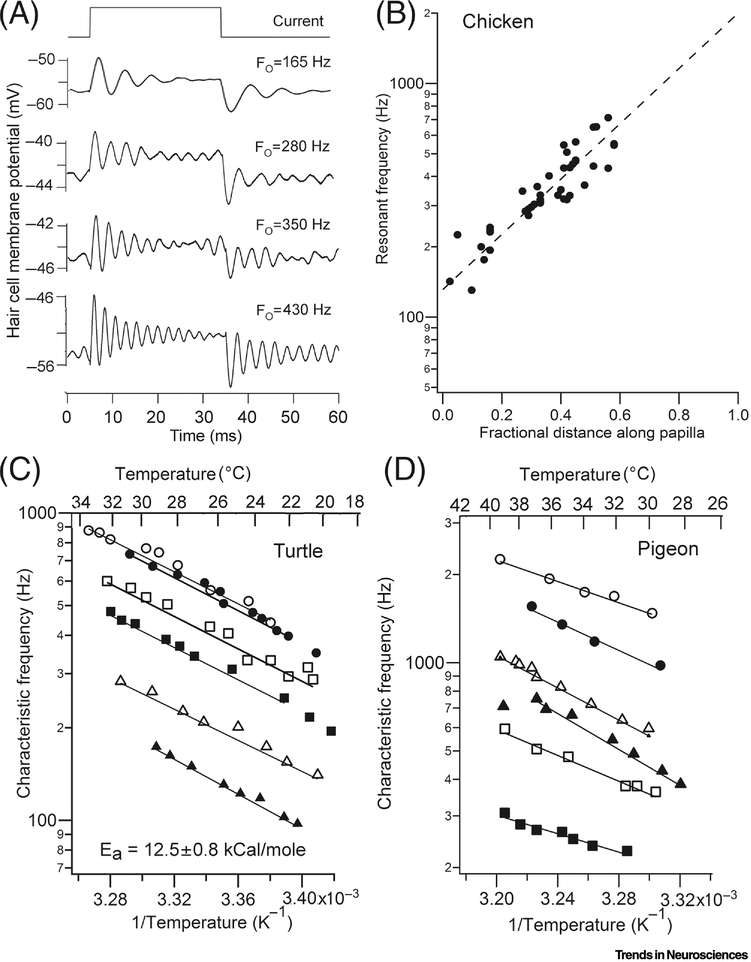
Figure 3.
Electrical tuning in reptile and bird. (A). Example of voltage ringing in four tall hair cells of chicken. (B) Tonotopic map of resonant frequencies in chicken cochlea (T = 33°C); the fit to the resonant frequency measurements is extrapolated to base giving predicted FO of 2 kHz. (C) Arrhenius plots of temperature dependence of characteristic frequencies for sound stimuli in six auditory nerve fibers of turtle. (D) Arrhenius plots of temperature dependence of characteristic frequencies for sound stimuli in six auditory nerve fibers of pigeon; results replotted from reference [40]. Fits giving activation energies Ea have similar slopes in turtle and bird.
The resonant frequencies are also temperature dependent, as might be expected for a mechanism that is limited by ion channel gating kinetics that can have a Q10 of 4.0. For the basilar papilla of the turtle [39] and the pigeon [40], FO roughly doubles for a 10°C rise in temperature. The slopes of Arrhenius equation plots, in which the logarithm of the CF is plotted against the inverse of the absolute temperature (T) can be used to determine the activation energy (Ea) for a reaction. The Arrhenius equation is: log (CF) = A – Ea/RT, where A is a constant and R is the gas constant. These plots (Figure 3C, 3D) yield activation energies for the tuning mechanism of 12.5 ± 0.8 kcal/mole (n=6) for the turtle, 10.3 ± 2.1 kcal/mole (n=6) for the pigeon. For comparison, the activation energy for diffusion, with a Q10 of 1.4, is about 6 kcal/mol. In contrast with the turtle and chicken, there is no evidence for a strong temperature effect on nerve fiber CFs in the mammalian cochlea, which does not employ electrical tuning [41, 42]. Electrical tuning of tall hair cells in the chicken basilar papilla can be recorded up to 700 Hz [32], and extrapolation of the results to the most basal hair cells predicts a resonant frequency of 2 kHz (Figure 3D). If the frequency limit in turtle auditory nerve fibers, 600 Hz at 22°C, is extrapolated to 40°C, this gives an upper limit of 2 kHz for the mechanism. Recordings in vivo give the auditory range in the chicken as 5 kHz [43], suggesting another tuning process may contribute in the kilohertz range.
The hair cell electrical resonance has been well simulated using gating schemes for the underlying ion channels, the voltage-dependent Ca2+ channel and the Ca2+ activated K+ channel [39, 44]. The computed resonant frequency increases with KCa channel density and speed as found experimentally. This approach also enabled prediction, based on simulation of turtle hair cells, of an upper limit which at 40°C would be 2.6 kHz with no other assumptions. A constraint is imposed by shortening of the kinetics of the BKCa channel, which cannot get faster than the activation rate of voltage-dependent Ca2+ channels. To cover the audible range of the chicken, up to 5 kHz, required assuming a kinetic limit for the BKCa channels coupled with an unrealistically large channel density [39]. It therefore seems unlikely that electrical tuning could operate at frequencies above 5 kHz detected by mammals.
Frequency tuning in the mammalian cochlea
The contribution of outer hair cells
The tuning mechanism in the mammalian cochlea has been extensively studied and reviewed [4, 11, 13]. The frequency-tuning curves of the auditory nerve fibers are narrow V-shaped functions of sound frequency, having maximal sensitivity at the CF of the fiber (Figure 4A). Tuning curves are depicted for the gerbil [45] with a frequency range of 0.3–30 kHz [46]. The nerve fiber tuning curves are similar in shape to the vibration patterns of the basilar membrane embodying the mechanical filter ( [13, 47] (Fig. 4C), and differ from the turtle, where basilar membrane vibration contributes no significant frequency selectivity [48]. Mammalian frequency tuning stems from multiple processes in the cochlea. Each sound frequency generates a traveling wave along the basilar membrane, propagating longitudinally from base to apex and growing in magnitude until it attains a peak amplitude at the place specific for frequency of stimulation [10]. Gradations of stiffness and mass along the basilar membrane [10, 49, 50] are thought to create a bank of filters, decreasing in resonant frequency from base to apex. For example, the point stiffness of the gerbil basilar membrane increases 330-fold from apex to base [49]. These gradients generate damped mechanical resonances that are amplified and sharpened by extra force supplied by OHCs which counteracts fluid damping and boosts the motion of the basilar membrane. At low sound levels, the amplification confers a 40 to 60 dB (100- to 1000-fold) increase in sensitivity at frequencies around the CF, but at higher sound levels, the OHC contribution saturates, sensitivity diminishes, and tuning broadens.
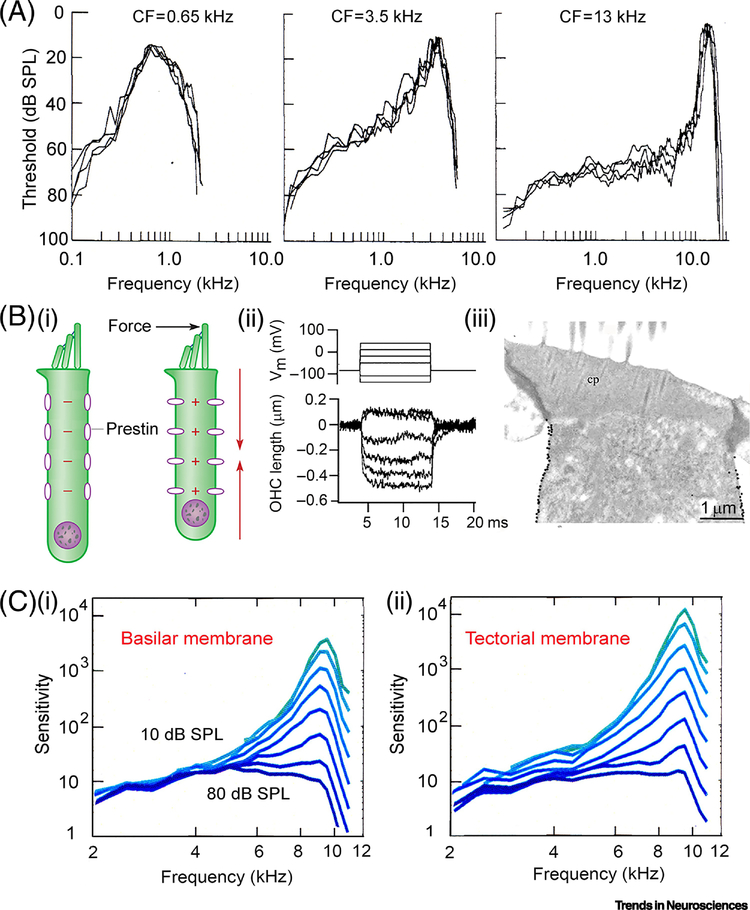
Figure 4.
Frequency selectivity in the mammalian cochlea. (A) Three auditory nerve fiber tuning curves for in gerbil cochlea, threshold plotted against sound frequency, from reference [45]. (B) Somatic motility in OHCs: hair bundle stimulation elicits cell contraction (left); voltage-clamped rat OHC, decreases in length on depolarization (middle); immunogold labeling for prestin of OHC lateral wall in rat (right). (C) Frequency tuning curves measured using OCT for basilar and tectorial membrane of mouse cochlear apex, in eight 10 dB increments. Tuning is sharpest at lowest sound level. Reproduced with permission from reference [47].
This mechanism was initially deduced from experiments demonstrating the crucial requirement of OHCs for cochlear sensitivity, which was diminished when they were preferentially destroyed by gentamicin [51]. The mechanism was bolstered by the unexpected finding that OHCs, when isolated in a dish and electrically stimulated, changed their length by up to 4 percent of their length, 1.0 μm for a 25 μm cell [52–54] (Figure 4B). The contractions and elongations of the cylindrical OHC body are thought to be driven by voltage-dependent conformational changes in prestin, an 80 kDa piezoelectric protein [55] packed at high density in the OHC membrane [56]. Prestin is a modified anion transporter, SLC26A5, requiring chloride ions to support or modulate its voltage-sensitivity [57, 58], and blocked by millimolar concentrations of the anion salicylate extracellularly [59, 60]. Since salicylate is amphiphilic, the binding site may be cytoplasmic, where the blocking concentration is lower [57, 60]. Strong evidence on the importance of prestin for cochlear amplification was obtained using a mouse harboring a mutant prestin that was nonfunctional but still targeted to the OHC lateral membrane. Isolated OHCs from this mutant had much reduced electromotility and 60 dB loss of acoustic sensitivity in vivo [61]. The genetic manipulation argues that OHC electromotility based on prestin is important for cochlear amplification and frequency selectivity. In accord with this conclusion, when the tuning curves of auditory nerve fibers were characterized in gerbils, administration of salicylate caused an elevation of the sound threshold at CF and a reduced sharpness of tuning over the entire frequency range [62]. Moreover, basilar membrane mechanical tuning curves were reversibly abolished by 5 mM salicylate, and by reducing perilymph [Cl−] [63], which underscores prestin’s importance.
Uncertainties regarding prestin
Despite the discovery of prestin and the productive outcomes of the subsequent studies, several areas of contention persist. A key problem is the speed with which the prestin protein can operate as a quasi-piezoelectric element. It was originally argued that OHCs contract and elongate on each cycle of the sound stimulus in order to provide cycle-by-cycle feedback. However, this assumes an ultrafast feedback process in which the membrane time constant is brief and prestin, as a voltage-dependent protein, activates on a microsecond timescale. Original claims that OHCs can undergo cyclical length changes at frequencies of tens of kilohertz [64, 65] have been challenged by recent experiments [66, 67]. Furthermore, measurements of the OHC time constant (which will filter the receptor potential), have yielded variable values [54], the smallest being 25 μs [68], equivalent to a corner frequency of 6 kHz. It has been argued that OHC membrane corner frequencies may be higher when these cells are embedded in the organ of Corti compared to isolated hair cells [69] [70]. A recent in vivo assay of OHC vibrations, applying optical coherence tomography, indicated that motility showed low-pass performance with corner frequencies around 3 kHz [71]. In spite of these apparent kinetic limitations, finite element modeling of the guinea pig cochlea, using existing time constants for OHC electromotility, predicted sharp tuning up to 18 kHz [72, 73]. It has also been shown experimentally that a salicylate-sensitive electromotile process can operate up to 100 kHz at the base of the intact guinea-pig cochlea [74]. There is therefore no general agreement on whether prestin-mediated OHC contractions can underlie cochlear tuning at frequencies of 50–100 kHz used in the ultrasonic hearing of mice and bats [75].
Another area of uncertainty is the origin of tuning at the cochlear apex, at frequencies below a 1 kHz, where auditory nerve fiber tuning curves have a different, more symmetrical, shape (Figure 4A) [37]. These and other observations have led to the proposal that an alternate tuning mechanism may operate at the cochlear apex [76] [73]. It is conceivable that the mass and stiffness of the tectorial membrane, together with the OHC hair bundle stiffness, generate an auxiliary resonance that modifies the mechanical stimuli relayed to the IHC [77] [78]. The stiffness of the tectorial membrane [79] and of the OHC hair bundles [80] both increase progressively towards the base of the cochlea, and together could theoretically generate gradations in the mechanical resonant frequency. The tuning may be enabled by the complex cellular anatomy, notably the pillar and Deiters’ cells, of the organ of Corti [81]. But the difficulty of accessing the organ of Corti and vulnerability of frequency tuning in vivo has hampered systematic investigation of the micromechanics along the cochlea.
Extension of the frequency range in non-mammals
Frogs
A number of non-mammalian species, including frogs, birds and lizards, show extensions of the frequency range above that provided by electrical tuning, which we shall assume operates up to about 1 kHz. The frog inner ear contains two main sound-sensitive end organs, the amphibian papilla and the basilar papilla [82]. Much of the amphibian papilla employs electrical tuning with acoustic CFs ranging from 100 to approximately 1000 Hz at room temperature [83]. Although electrical resonance was not recorded at the highest frequencies (probably due to experimental limitations whereby slight damage to hair cells introducing leak conductance can annihilate the resonance), hair cells across the whole range possessed Ca2+-activated K+ channel currents [19]. However, the other organ, basilar papilla, functions as a single auditory filter, with all auditory nerve fibers having nearly identical shapes and CFs [84]. The spheroidal basilar papilla lacks a basilar membrane but possesses a tectorial membrane over the hair bundles of the sensory hair cells. Sound-induced vibrations of the tectorial membrane were measured in Rana pipiens and found to be tuned to 2 kHz [85], similar to the CFs of the basilar papilla nerve fibers. This observation suggests that the frequency selectivity of the organ is largely attributable to a resonance of basilar end organ [85]. The mechanical coupling between the tectorial membrane and hair bundles may behave as a mechanical resonator, with a resonance frequency dependent on the vibrating mass and the hair bundle stiffness. When compared across species, the anuran basilar papilla is tuned to the principle frequency of the call [86]. More recently, a number of frog species with hearing above 10 kHz have been studied [87]. Recalling that resonant frequency is proportional to the square root of the stiffness divided by the mass, the ultrasonic range can be accounted for by its correlation with modifications of the frog basilar papilla, which will filter the mechanical input to the hair cells [87]. These modifications include a smaller tectorial membrane (reducing the vibrating mass) and shorter hair bundles (increasing their stiffness), which combine to elevate the resonant frequency.
Chickens
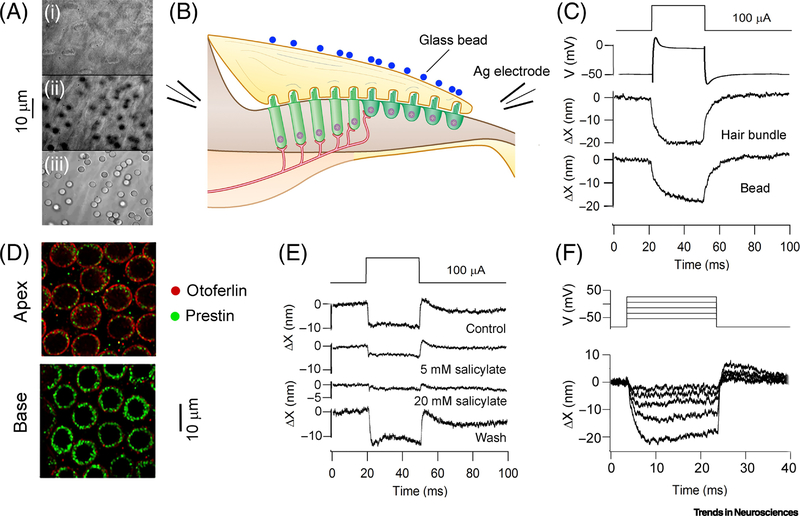
Birds such as chickens and pigeons have an audible range extending to 5 kHz [43, 88]. Auditory hair cells that are electrically tuned will have acoustic CFs higher than the turtle due to the increased body temperature of 40°C. The avian basilar papilla, like the mammalian organ of Corti, contains two types of hair cell with different innervation (Figure 1), suggesting that other processes operate. Tall hair cells overlying the cartilaginous limbus contact afferents, whereas short hair cells surmounting the basilar membrane have little or no afferent innervation. Another mammalian similarity is the presence of a traveling wave on the pigeon basilar membrane propagating from base to apex, but with broad frequency tuning up to a cut-off at about 6 kHz [89]. Furthermore, auditory nerve fiber tuning is susceptible to intra-cochlear perfusion of up to 20 mM Na salicylate. The agent causes threshold elevation, particularly in mid to high frequencies (0.8 – 4 kHz), which, similar to mammals, suggest that prestin may be involved in amplification and tuning [90]. Although there is no direct evidence for electromotility of chicken prestin [91], the protein is present in the chicken basilar papilla [92]. In addition, electrical stimulation when applied across the papilla, or to individual hair cells, evokes significant motion of the hair bundles and tectorial membrane [92]. Hair cell depolarization produces bundle deflections towards the neural limbus (Figure 5), which can be abolished with extracellular salicylate at millimolar concentrations (Figure 5E), similar to those that block prestin [59]; salicylate at these concentrations has no effect on mechanotransduction [92]. Taken together, the results argue that receptor potentials in the short hair cells are amplified and mechanically relayed via the tectorial membrane to the tall hair cells on the neural limbus. Antibody labeling demonstrates that prestin is more heavily concentrated in high-frequency hair cells compared to low frequency cells (Figure 5D). Such a gradient in prestin concentration accords with the frequency dependent effects of salicylate in the pigeon, the drug preferentially elevating thresholds above 1 kHz [90]. It seems that prestin may confer amplification and frequency tuning in the bird basilar papilla too. It is possible that residual (low quality factor) electrical tuning in the short hair cells augments the mechanical amplification [93]. Despite the presence of prestin, and the high body temperature, most bird species have experienced no selective pressure to extend the upper limit of hearing beyond 5 kHz. An exception is the owl, for which CFs exceed 9 kHz [94], though it is unclear how this is accomplished. The frequency range of song-birds may be matched to the composition of their song, which is usually restricted to the low kilohertz range [95]. An example is the white-crowned sparrow, whose song comprises an initial 4 kHz whistle followed by a 3 to 5 kHz trill [95, 96].
Figure 5.
Prestin in the chicken cochlea. (A) Images of hair bundles (top), middle of tectorial membrane (middle) and top of tectorial membrane showing 3μm glass beads (bottom). (B). Schematic of experiment to polarize hair cells with electrodes on either side of chicken basilar papilla. (C). Displacements evoked by 100 μA extracellular current pulse: intracellular voltage record from tall hair cell, deflections of hair bundle and of bead, both towards neural limb. (D). Labeling of short hair cells with prestin antibody shows more at the basal than apical regions of papilla. (E) Na salicylate inhibits hair bundle motion to 100 μA current pulse across papilla. (F) Motion of hair bundle is graded with depolarization of voltage-clamped short hair cell.
Lizard
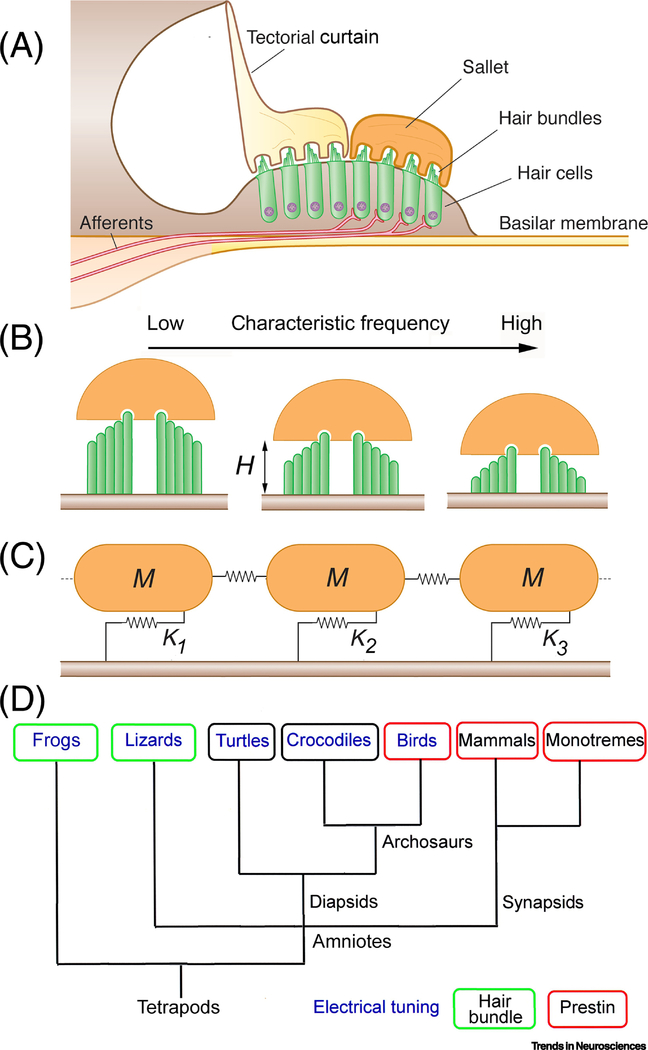
Frequency tuning in the lizard auditory periphery shows principles similar to that of the frog, but differs in that the low- and high-frequency mechanisms are united in a single papilla. The frequency range extends from about 150 Hz to 5 kHz (e.g., tokay gecko, Gekko gecko [34]; bobtail skink, Tiliqua rugosa [97]). The gecko papilla consists of two parts. One third of the papilla resembles that of the turtle, encodes frequencies below 1 kHz, and is most likely served by an electrical resonance (see above). The other two thirds cover frequencies above 1 kHz [98], and this part is demarcated by a change in the organization of the papilla, which is split longitudinally into two parallel sections with distinct tectorial coats. Hair cells located near the neural limb are overlain with a continuous tectorial curtain, whereas abneural hair cells are topped by discrete tectorial domes known as sallets [99–101] (Figure 6A). The two parallel strips also differ in their innervation. All afferent fibers project solely to the abneural salletal hair cells, and neural hair cells surprisingly appear to lack innervation, based upon antibody labeling for neurofilament-associated protein 200 [102], casting their role into doubt. In the gecko, there are 170 sallets, each covering a single transverse row of between four and eight hair cells. Auditory nerve tuning due to tectorial sallets has been proposed to stem from a mechanical resonance created by the mass of the sallet, M, and the stiffness of the attached hair bundles [101] [103, 104]. Morphology suggests a low to high frequency gradient in stiffness because of a decrease in hair bundle height (H = 16 – 4.6 μm) (Figure 6B), and increases in stereociliary complement (NS = 32–48) and in numbers of bundles devoted to each sallet (NHB = 4 to 8) [99, 100, 103]. Thus high frequency sallets are predicted to have a higher resonant frequency because they envelope more bundles that are shorter and contain more stereocilia. The relationship can be quantified as FO = 1/2π (α. NS.NHB/ H2.M)1/2, where α is the rotational stiffness of a single stereocilium, 0.026 × 10−14 N.m/rad [105]. This analysis predicts passive resonant frequencies from 1.2 to 7.4 kHz, having similar range and extent to those observed experimentally (1 to 5 kHz).In practice, the sharpness of tuning and the resonant frequencies will be reduced by damping from the fluid endolymph [103], and by the fibrous connections between neighboring sallets [99], which are represented as weak springs in Figure 6C [106].
Figure 6.
Lizard basilar papilla. (A) Transverse section of gecko papilla in high frequency region shows two types of tectorial structure. Hair bundles of non-innervated neural hair cells covered with a tectorial membrane that forms ‘curtain’ above papilla, whereas hair bundles of innervated abneural hair cells are surmounted by a tectorial ‘sallet’. (B). Schematic of hair bundles inserted into sallets for progressive distance from low- to high-frequency positions. Note the hair bundles are bidirectional and their height, H, decreases (16 μm to 4.6 μm; [100]) with increase in frequency. (C) Model of longitudinal section through salletal region depicting three sallets of mass M attached to hair bundles represented by springs of stiffness K1, K2, and K3, decreasing with increase in ‘H’. M was calculated by taking each sallet as a semicircular slice, 40 μm diameter, 3 μm thick and density 1000 kg/m3, giving a total mass, M = 1.9 × 10−12 kg. (D) Evolutionary tree indicating tuning mechanisms shown to operate in auditory hair cells of different vertebrate classes. Tree based on reference [112].
Evidence also exists for an amplification mechanism attributable to active force generation by the hair bundle, albeit much weaker than prestin [105] [107]. An active force generator may, depending on its phase, counteract the viscous damping imposed by the surrounding fluid, and hence increase the sharpness of tuning. The mechanoelectrical transducer channels in the hair bundle are activated by force delivered via tip-links connecting adjacent stereocilia [4]. Active hair bundle motility is proposed to reflect a bidirectional coupling between tip-link tension and transducer channel gating: increased tension opens the channels, but conversely, channel closure, as occurs during transducer adaptation, exerts a force that moves the bundle [93]. This mechanism has been extensively characterized in frog saccular hair cells, where it can drive spontaneous oscillations at a specific frequency [107, 108]. It may also operate in the salletal hair cells of the gecko papilla, where each hair bundle has been hypothesized to be mechanically active and generate sustained oscillations that might drive spontaneous otoacoustic emissions [109, 110]. Such emissions are faint tones continuously radiated from the external ear in the absence of a stimulus, and occur in many vertebrates, but those in the tokay gecko are evident as a dozen or more equally spaced peaks between 1 and 4 kHz [106, 110]. However, hair cells covered by a single sallet possess hair bundles pointing in both neural and abneural directions (Figure 6A). Since active force production will be unidirectional, the bidirectional hair bundles linked to one sallet will not cooperate as an efficient force generator. Further experiments are required to clarify how active bundle motility might function in these cells.
Concluding remarks
At least three distinct mechanisms are employed to produce frequency-selective tuning in the vertebrate inner ear (Figure 6D). A fairly ubiquitous electrical resonance, filtering of the receptor potential by voltage-dependent ion channels, has an upper limit of about 1 kHz. A selective drive to extend the frequency range in all vertebrate classes employs a mechanical resonance stemming from stiffness of the hair bundles coupled with the mass of attached tectorial covering. The sharpness of tuning, the quality factor of the resonance, may in such cases be amplified by force generation from the hair bundle motion linked to transducer channel gating. To widen the frequency range, an unusual mechanism operates in mammals. Here, one class of hair cell, the OHC, generates amplification by somatic contractions mediated by a biologically-piezoelectric protein prestin, though whether it underlies frequency tuning at the mouse upper limit of 70 kHz [111] is unclear. The basilar papilla in birds also comprises two classes of hair cell and may also employ prestin over a limited frequency band. It has become clear that there are multiple designs for achieving frequency tuning in the vertebrate ear, each having particular limitations on the operating range of frequencies, and each appropriate to the animal’s behaviour.
Outstanding questions.
Can the upper frequency limit of electrical resonance in the inner ear be precisely defined, what determines that limit, and how are the resonant properties set up developmentally?
What underlies frequency-tuning in the salletal hair cells of the gecko lizards, and is the tuning reinforced by active hair bundle motility?
Can prestin be gated on a cycle-by-cycle basis to provide amplification up to 100 kHz, and if not, how does prestin produce amplification at the highest frequencies?
What are the voltage-dependent changes in the tertiary structure of prestin that underlie OHC electromotility?
A different mechanism is employed for frequency tuning in the apical low-frequency region of the mammalian cochlea compared to the high-frequency region. What is the mechanism operating in the low-frequency region?
Highlights.
The vertebrate cochlea contains a bank of filters to separate the sound frequency components in the sensory hair cells. This computation enables auditory nerve fibers to relay spectral information to the brain, which aids with communication and sound localization.
In non-mammals, the hair cell receptor potential is electrically tuned by Ca2+-activated K+ channels, generating band-pass filters with center frequencies less than 1 kHz. This mechanism is limited by the intrinsic kinetics of the K+ channel.
Evolutionary pressure for higher frequency hearing has recruited two other processes. One, a mechanical resonance of the sensory hair bundles, encompasses frequencies up to 10 kHz, and is employed in frogs and lizards. In mammals, a separate mechanism creates narrow-band filters extending up to 100 kHz. For all or part of the range, this involves contractions of the outer hair cell body, produced by the voltage-sensitive membrane protein prestin.
The cochlea of the bird, similar to the mammal, contains two types of hair cell probably due to convergent evolution. Bird cochlear outer hair cells may also employ prestin over a limited high frequency range, but auditory hair cells are electrically tuned below 1 kHz.
Acknowledgements
This work was supported by grant RO1 DC01362 from the National Institute of Deafness and other Communication Disorders to RF. I thank Maryline Beurg for comments on the manuscript and Karl Grosh for useful discussions about modeling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grothe B et al. (2010) Mechanisms of sound localization in mammals. Physiol Rev 90 (3), 983–1012. [DOI] [PubMed] [Google Scholar]
- 2.Yin TCT et al. (2019) Neural Mechanisms of Binaural Processing in the Auditory Brainstem. Compr Physiol 9 (4), 1503–1575. [DOI] [PubMed] [Google Scholar]
- 3.West CD (1985) The relationship of the spiral turns of the cochlea and the length of the basilar membrane to the range of audible frequencies in ground dwelling mammals. J Acoust Soc Am 77 (3), 1091–101. [DOI] [PubMed] [Google Scholar]
- 4.Fettiplace R (2017) Hair Cell Transduction, Tuning, and Synaptic Transmission in the Mammalian Cochlea. Compr Physiol 7 (4), 1197–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford AC and Fettiplace R (1981) An electrical tuning mechanism in turtle cochlear hair cells. J Physiol 312, 377–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fettiplace R and Fuchs PA (1999) Mechanisms of hair cell tuning. Annu Rev Physiol 61, 809–34. [DOI] [PubMed] [Google Scholar]
- 7.Kros CJ and Crawford AC (1990) Potassium currents in inner hair cells isolated from the guinea-pig cochlea. J Physiol 421, 263–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirokawa N (1978) The ultrastructure of the basilar papilla of the chick. J Comp Neurol 181 (2), 361–74. [DOI] [PubMed] [Google Scholar]
- 9.Lim DJ (1986) Functional structure of the organ of Corti: a review. Hear Res 22, 117–46. [DOI] [PubMed] [Google Scholar]
- 10.Bekesy G.v. (1960) Experiments in Hearing, McGraw-Hill. [Google Scholar]
- 11.Guinan JJ Jr. et al. (2012) Progress in cochlear physiology after Bekesy. Hear Res 293 (1–2), 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhode WS (1971) Observations of the vibration of the basilar membrane in squirrel monkeys using the Mossbauer technique. J Acoust Soc Am 49 (4), Suppl 2:1218+. [DOI] [PubMed] [Google Scholar]
- 13.Robles L and Ruggero MA (2001) Mechanics of the mammalian cochlea. Physiol Rev 81 (3), 1305–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawford AC and Fettiplace R (1980) The frequency selectivity of auditory nerve fibres and hair cells in the cochlea of the turtle. J Physiol 306, 79–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis RS and Hudspeth AJ (1983) Voltage- and ion-dependent conductances in solitary vertebrate hair cells. Nature 304 (5926), 538–41. [DOI] [PubMed] [Google Scholar]
- 16.Hudspeth AJ and Lewis RS (1988) Kinetic analysis of voltage- and ion-dependent conductances in saccular hair cells of the bull-frog, Rana catesbeiana. J Physiol 400, 237–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs PA and Evans MG (1990) Potassium currents in hair cells isolated from the cochlea of the chick. J Physiol 429, 529–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman MB and Art JJ (1996) Variations in the ensemble of potassium currents underlying resonance in turtle hair cells. J Physiol 497 ( Pt 2), 395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smotherman MS and Narins PM (1999) The electrical properties of auditory hair cells in the frog amphibian papilla. J Neurosci 19 (13), 5275–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Art JJ and Fettiplace R (1987) Variation of membrane properties in hair cells isolated from the turtle cochlea. J Physiol 385, 207–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramanathan K et al. (1999) A molecular mechanism for electrical tuning of cochlear hair cells. Science 283 (5399), 215–7. [DOI] [PubMed] [Google Scholar]
- 22.Ramanathan K and Fuchs PA (2002) Modeling hair cell tuning by expression gradients of potassium channel beta subunits. Biophys J 82 (1 Pt 1), 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones EM et al. (1999) The role of Ca2+-activated K+ channel spliced variants in the tonotopic organization of the turtle cochlea. J Physiol 518 ( Pt 3), 653–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YW et al. (2002) Consequences of the stoichiometry of Slo1 alpha and auxiliary beta subunits on functional properties of large-conductance Ca2+-activated K+ channels. J Neurosci 22 (5), 1550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai JP et al. (2011) beta4-subunit increases Slo responsiveness to physiological Ca2+ concentrations and together with beta1 reduces surface expression of Slo in hair cells. Am J Physiol Cell Physiol 300 (3), C435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai JP et al. (2012) CDK5 interacts with Slo and affects its surface expression and kinetics through direct phosphorylation. Am J Physiol Cell Physiol 302 (5), C766–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surguchev A et al. (2012) Hair cell BK channels interact with RACK1, and PKC increases its expression on the cell surface by indirect phosphorylation. Am J Physiol Cell Physiol 303 (2), C143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mann ZF et al. (2014) A gradient of Bmp7 specifies the tonotopic axis in the developing inner ear. Nat Commun 5, 3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiede BR et al. (2014) Retinoic acid signalling regulates the development of tonotopically patterned hair cells in the chicken cochlea. Nat Commun 5, 3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitchford S and Ashmore JF (1987) An electrical resonance in hair cells of the amphibian papilla of the frog Rana temporaria. Hear Res 27 (1), 75–83. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs PA et al. (1988) Electrical tuning in hair cells isolated from the chick cochlea. J Neurosci 8 (7), 2460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan X et al. (2013) Electrical tuning and transduction in short hair cells of the chicken auditory papilla. J Neurophysiol 109 (8), 2007–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuchs PA and Evans MG (1988) Voltage oscillations and ionic conductances in hair cells isolated from the alligator cochlea. J Comp Physiol A 164 (2), 151–63. [DOI] [PubMed] [Google Scholar]
- 34.Eatock RA et al. (1981) Auditory nerve fibre activity in the Tokay Gecko. J Comp Physiol 142, 203–218. [Google Scholar]
- 35.Sugihara I and Furukawa T (1989) Morphological and functional aspects of two different types of hair cells in the goldfish sacculus. J Neurophysiol 62 (6), 1330–43. [DOI] [PubMed] [Google Scholar]
- 36.Temchin AN (1988) Unusual discharge patterns of single fibers in the pigeon’s auditory nerve. J Comp Physiol A 163 (1), 99–115. [DOI] [PubMed] [Google Scholar]
- 37.Liberman MC (1978) Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am 63 (2), 442–55. [DOI] [PubMed] [Google Scholar]
- 38.Eatock RA and Manley GA (1981) Auditory nerve fibre activity in the Tokay Gecko: II Temperature effect on tuning. J. Comp Physiol 142, 219–226. [Google Scholar]
- 39.Wu YC et al. (1995) A kinetic description of the calcium-activated potassium channel and its application to electrical tuning of hair cells. Prog Biophys Mol Biol 63 (2), 131–58. [DOI] [PubMed] [Google Scholar]
- 40.Schermuly L and Klinke R (1985) Change of characteristic frequency of pigeon primary afferetns with temperature. J Comp Physiol 156, 209–211. [Google Scholar]
- 41.Gummer AW and Klinke R (1983) Influence of temperature on tuning of primary-like units in the guinea pig cochlear nucleus. Hear Res 12 (3), 367–80. [DOI] [PubMed] [Google Scholar]
- 42.Ohlemiller KK and Siegel JH (1994) Cochlear basal and apical differences reflected in the effects of cooling on responses of single auditory nerve fibers. Hear Res 80 (2), 174–90. [DOI] [PubMed] [Google Scholar]
- 43.Jones TA et al. (2006) Emergence of hearing in the chicken embryo. J Neurophysiol 96 (1), 128–41. [DOI] [PubMed] [Google Scholar]
- 44.Hudspeth AJ and Lewis RS (1988) A model for electrical resonance and frequency tuning in saccular hair cells of the bull-frog, Rana catesbeiana. J Physiol 400, 275–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohlemiller KK and Echteler SM (1990) Functional correlates of characteristic frequency in single cochlear nerve fibers of the Mongolian gerbil. J Comp Physiol A 167 (3), 329–38. [DOI] [PubMed] [Google Scholar]
- 46.Muller M (1996) The cochlear place-frequency map of the adult and developing Mongolian gerbil. Hear Res 94 (1–2), 148–56. [DOI] [PubMed] [Google Scholar]
- 47.Lee HY et al. (2015) Noninvasive in vivo imaging reveals differences between tectorial membrane and basilar membrane traveling waves in the mouse cochlea. Proc Natl Acad Sci U S A 112 (10), 3128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Neill MP and Bearden A (1995) Laser-feedback measurements of turtle basilar membrane motion using direct reflection. Hear Res 84 (1–2), 125–38. [DOI] [PubMed] [Google Scholar]
- 49.Emadi G et al. (2004) Stiffness of the gerbil basilar membrane: radial and longitudinal variations. J Neurophysiol 91 (1), 474–88. [DOI] [PubMed] [Google Scholar]
- 50.Teudt IU and Richter CP (2014) Basilar membrane and tectorial membrane stiffness in the CBA/CaJ mouse. J Assoc Res Otolaryngol 15 (5), 675–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dallos P and Harris D (1978) Properties of auditory nerve responses in absence of outer hair cells. J Neurophysiol 41 (2), 365–83. [DOI] [PubMed] [Google Scholar]
- 52.Brownell WE et al. (1985) Evoked mechanical responses of isolated cochlear outer hair cells. Science 227 (4683), 194–6. [DOI] [PubMed] [Google Scholar]
- 53.Ashmore JF (1987) A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J Physiol 388, 323–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ashmore J (2008) Cochlear outer hair cell motility. Physiol Rev 88 (1), 173–210. [DOI] [PubMed] [Google Scholar]
- 55.Zheng J et al. (2000) Prestin is the motor protein of cochlear outer hair cells. Nature 405 (6783), 149–55. [DOI] [PubMed] [Google Scholar]
- 56.Mahendrasingam S et al. (2010) The ultrastructural distribution of prestin in outer hair cells: a post-embedding immunogold investigation of low-frequency and high-frequency regions of the rat cochlea. Eur J Neurosci 31 (9), 1595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oliver D et al. (2001) Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science 292 (5525), 2340–3. [DOI] [PubMed] [Google Scholar]
- 58.Gorbunov D et al. (2014) Molecular architecture and the structural basis for anion interaction in prestin and SLC26 transporters. Nat Commun 5, 3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tunstall MJ et al. (1995) Action of salicylate on membrane capacitance of outer hair cells from the guinea-pig cochlea. J Physiol 485 ( Pt 3), 739–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kakehata S and Santos-Sacchi J (1996) Effects of salicylate and lanthanides on outer hair cell motility and associated gating charge. J Neurosci 16 (16), 4881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dallos P et al. (2008) Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron 58 (3), 333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muller M et al. (2003) Auditory nerve fibre responses to salicylate revisited. Hear Res 183 (1–2),37–43. [DOI] [PubMed] [Google Scholar]
- 63.Santos-Sacchi J et al. (2006) Control of mammalian cochlear amplification by chloride anions. J Neurosci 26 (15), 3992–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gale JE and Ashmore JF (1997) An intrinsic frequency limit to the cochlear amplifier. Nature 389 (6646), 63–6. [DOI] [PubMed] [Google Scholar]
- 65.Frank G et al. (1999) Limiting dynamics of high-frequency electromechanical transduction of outer hair cells. Proc Natl Acad Sci U S A 96 (8), 4420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santos-Sacchi J and Tan W (2018) The Frequency Response of Outer Hair Cell Voltage-Dependent Motility Is Limited by Kinetics of Prestin. J Neurosci 38 (24), 5495–5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santos-Sacchi J et al. (2019) Outer hair cell electromotility is low-pass filtered relative to the molecular conformational changes that produce nonlinear capacitance. J Gen Physiol 151 (12), 1369–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson SL et al. (2011) Prestin-driven cochlear amplification is not limited by the outer hair cell membrane time constant. Neuron 70 (6), 1143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rabbitt RD et al. (2009) Power efficiency of outer hair cell somatic electromotility. PLoS Comput Biol 5 (7), e1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mistrik P et al. (2009) Three-dimensional current flow in a large-scale model of the cochlea and the mechanism of amplification of sound. J R Soc Interface 6 (32), 279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vavakou A et al. (2019) The frequency limit of outer hair cell motility measured in vivo. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meaud J and Grosh K (2012) Response to a pure tone in a nonlinear mechanical-electrical-acoustical model of the cochlea. Biophys J 102 (6), 1237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sasmal A and Grosh K (2019) Unified cochlear model for low- and high-frequency mammalian hearing. Proc Natl Acad Sci U S A 116 (28), 13983–13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grosh K et al. (2004) High-frequency electromotile responses in the cochlea. J Acoust Soc Am 115 (5 Pt 1), 2178–84. [DOI] [PubMed] [Google Scholar]
- 75.Fay RR (1988) Hearing in Vertebrates: a Psychophysics Databook, Hill-Fay Associates. [Google Scholar]
- 76.Temchin AN and Ruggero MA (2010) Phase-locked responses to tones of chinchilla auditory nerve fibers: implications for apical cochlear mechanics. J Assoc Res Otolaryngol 11 (2), 297–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zwislocki JJ and Kletsky EJ (1979) Tectorial membrane: a possible effect on frequency analysis in the cochlea. Science 204 (4393), 639–41. [DOI] [PubMed] [Google Scholar]
- 78.Gummer AW et al. (1996) Resonant tectorial membrane motion in the inner ear: its crucial role in frequency tuning. Proc Natl Acad Sci U S A 93 (16), 8727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Richter CP et al. (2007) Tectorial membrane stiffness gradients. Biophys J 93 (6), 2265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beurg M et al. (2008) The actions of calcium on hair bundle mechanics in mammalian cochlear hair cells. Biophys J 94 (7), 2639–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Motallebzadeh H et al. (2018) Cochlear amplification and tuning depend on the cellular arrangement within the organ of Corti. Proc Natl Acad Sci U S A 115 (22), 5762–5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feng AS et al. (1975) Three populations of primary auditory nerve fibers in bullfrog (Rana catesbeiana): their peripheral origins and frequency selectivity. J Comp Physiol 100 221–229. [Google Scholar]
- 83.Ronken DA (1991) Spike discharge properties that are related to the characteristic frequency of single units in the frog auditory nerve. J Acoust Soc Am 90 (5), 2428–40. [DOI] [PubMed] [Google Scholar]
- 84.Van Dijk P et al. (2011) Mechanics of the frog ear. Hear Res 273 (1–2), 46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schoffelen RL et al. (2009) Tuning of the tectorial membrane in the basilar papilla of the northern leopard frog. J Assoc Res Otolaryngol 10 (3), 309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilczynski W and Capranica RR (1984) The auditory system of anuran amphibians. Prog Neurobiol 22 (1), 1–38. [DOI] [PubMed] [Google Scholar]
- 87.Arch VS et al. (2012) Inner ear morphological correlates of ultrasonic hearing in frogs. Hear Res 283 (1–2), 70–9. [DOI] [PubMed] [Google Scholar]
- 88.Sachs MB et al. (1974) Discharge patterns of single fibers in the pigeon auditory nerve. Brain Res 70 (3), 431–47. [DOI] [PubMed] [Google Scholar]
- 89.Gummer AW et al. (1987) Basilar membrane motion in the pigeon measured with the Mossbauer technique. Hear Res 29 (1), 63–92. [DOI] [PubMed] [Google Scholar]
- 90.Shehata-Dieler WE et al. (1994) Effects of endolymphatic and perilymphatic application of salicylate in the pigeon. I: Single fiber activity and cochlear potentials. Hear Res 74 (1–2), 77–84. [DOI] [PubMed] [Google Scholar]
- 91.Tang J et al. (2013) Lizard and frog prestin: evolutionary insight into functional changes. PLoS One 8 (1), e54388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beurg M et al. (2013) A prestin motor in chicken auditory hair cells: active force generation in a nonmammalian species. Neuron 79 (1), 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hudspeth AJ et al. (2000) Putting ion channels to work: mechanoelectrical transduction, adaptation, and amplification by hair cells. Proc Natl Acad Sci U S A 97 (22), 11765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koppl C (1997) Frequency tuning and spontaneous activity in the auditory nerve and cochlear nucleus magnocellularis of the barn owl Tyto alba. J Neurophysiol 77 (1), 364–77. [DOI] [PubMed] [Google Scholar]
- 95.Kroodsma D (2005) The singing life of birds, Houghton Mifflin. [Google Scholar]
- 96.Margoliash D (1983) Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J Neurosci 3 (5), 1039–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manley GA et al. (1990) Peripheral auditory processing in the bobtail lizard, Tiliqua rugosa. I Frequency tuning of auditory nerve fibres. J Comp Physiol 167, 89–99. [Google Scholar]
- 98.Manley GA et al. (1999) Reversed tonotopic map of the basilar papilla in Gekko gecko. Hear Res 131 (1–2), 107–16. [DOI] [PubMed] [Google Scholar]
- 99.Miller MR (1973) A scanning electron microscope study of the papilla basilaris of Gekko gecko. Z Zellforsch Mikrosk Anat 136 (3), 307–28. [DOI] [PubMed] [Google Scholar]
- 100.Koppl C and Authier S (1995) Quantitative anatomical basis for a model of micromechanical frequency tuning in the Tokay gecko, Gekko gecko. Hear Res 82 (1), 14–25. [DOI] [PubMed] [Google Scholar]
- 101.Wever EG (1978) The Reptile Ear. pp. 464–572, Princeton University Press. [Google Scholar]
- 102.Chiappe ME et al. (2007) The structural and functional differentiation of hair cells in a lizard’s basilar papilla suggests an operational principle of amniote cochleas. J Neurosci 27 (44), 11978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Authier S and Manley GA (1995) A model of frequency tuning in the basilar papilla of the Tokay gecko, Gekko gecko. Hear Res 82 (1), 1–13. [DOI] [PubMed] [Google Scholar]
- 104.Manley GA et al. (1989) Micromechanical basis of high-frequency tuning in bobtail lizard In Cochlear Mechanism: structure, function and modles. (Wilson JP and Kempo DT eds), pp. 143–151, Plenum Press. [Google Scholar]
- 105.Crawford AC and Fettiplace R (1985) The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol 364, 359–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gelfand M et al. (2010) Interactions between hair cells shape spontaneous otoacoustic emissions in a model of the tokay gecko’s cochlea. PLoS One 5 (6), e11116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martin P and Hudspeth AJ (1999) Active hair-bundle movements can amplify a hair cell’s response to oscillatory mechanical stimuli. Proc Natl Acad Sci U S A 96 (25), 14306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martin P et al. (2003) Spontaneous oscillation by hair bundles of the bullfrog’s sacculus. J Neurosci 23 (11), 4533–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vilfan A and Duke T (2008) Frequency clustering in spontaneous otoacoustic emissions from a lizard’s ear. Biophys J 95 (10), 4622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Manley GA and Koppl C (2008) What have lizard ears taught us about auditory physiology? Hear Res 238 (1–2), 3–11. [DOI] [PubMed] [Google Scholar]
- 111.Taberner AM and Liberman MC (2005) Response properties of single auditory nerve fibers in the mouse. J Neurophysiol 93 (1), 557–69. [DOI] [PubMed] [Google Scholar]
- 112.Irisarri I et al. (2017) Phylotranscriptomic consolidation of the jawed vertebrate timetree. Nat Ecol Evol 1 (9), 1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]