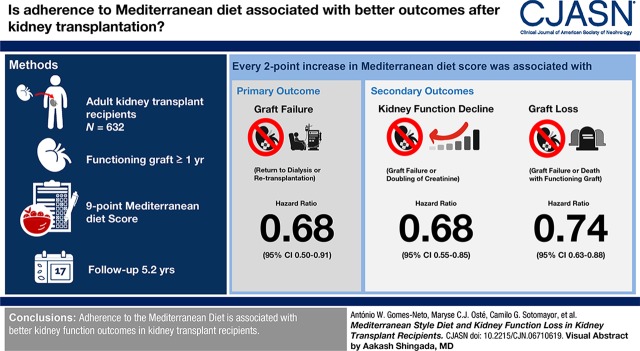
Visual Abstract
Keywords: nutrition, transplant, chronic graft deterioration, renal transplantation, proteinuria, adult, graft survival, kidney transplantation, creatinine, Mediterranean diet, follow-up studies, renal insufficiency, kidney, death, transplants, regression analysis, surveys and questionnaires, humans
Abstract
Background and objectives
Despite improvement of short-term graft survival over recent years, long-term graft survival after kidney transplantation has not improved. Studies in the general population suggest the Mediterranean diet benefits kidney function preservation. We investigated whether adherence to the Mediterranean diet is associated with kidney outcomes in kidney transplant recipients.
Design, setting, participants, & measurements
We included 632 adult kidney transplant recipients with a functioning graft for ≥1 year. Dietary intake was inquired using a 177-item validated food frequency questionnaire. Adherence to the Mediterranean diet was assessed using a nine-point Mediterranean Diet Score. Primary end point of the study was graft failure and secondary end points included kidney function decline (doubling of serum creatinine or graft failure) and graft loss (graft failure or death with a functioning graft). Cox regression analyses were used to prospectively study the associations of the Mediterranean Diet Score with study end points.
Results
During median follow-up of 5.4 (interquartile range, 4.9–6.0) years, 76 participants developed graft failure, 119 developed kidney function decline, and 181 developed graft loss. The Mediterranean Diet Score was inversely associated with all study end points (graft failure: hazard ratio [HR], 0.68; 95% confidence interval [95% CI], 0.50 to 0.91; kidney function decline: HR, 0.68; 95% CI, 0.55 to 0.85; and graft loss: HR, 0.74; 95% CI, 0.63 to 0.88 per two-point increase in Mediterranean Diet Score) independent of potential confounders. We identified 24-hour urinary protein excretion and time since transplantation to be an effect modifier, with stronger inverse associations between the Mediterranean Diet Score and kidney outcomes observed in participants with higher urinary protein excretion and participants transplanted more recently.
Conclusions
Adherence to the Mediterranean diet is associated with better kidney function outcomes in kidney transplant recipients.
Introduction
Kidney transplantation is considered the treatment of choice for patients with ESKD, offering improved quality of life and survival compared with dialysis treatment (1–5). The introduction of novel immunosuppressive drugs has greatly improved the short-term graft survival of kidney transplant recipients; however, improvement of long-term graft survival is lagging behind and graft failure still occurs in 33%–57% of kidney transplant recipients after the first 10 years after transplantation (6). Donor organ quality, recipient age, ethnicity, time on dialysis, HLA incompatibility, recurrence of primary kidney disease, use of nephrotoxic drugs, and cardiovascular comorbidities all have been associated with graft failure. However, to what extent modifiable factors such as diet affect graft survival remains largely unknown. Although adherence to a healthy diet and lifestyle has been acknowledged to be of importance, population-specific dietary recommendations for kidney transplant recipients are lacking (7).
The Mediterranean diet is a dietary pattern traditionally consumed by inhabitants of Mediterranean regions and features a high intake of fish, fruit, vegetables, legumes, nuts, and olive oil, together with low intake of dairy and meat products. To date, the Mediterranean diet is one of the most extensively studied dietary patterns and has demonstrated to reduce the risk of diabetes, cardiovascular disease, and mortality in the general population (8,9). Moreover, epidemiologic studies indicate that the Mediterranean diet might also benefit kidney function preservation (10–13). Previously, we showed that adherence to the Mediterranean diet reduces risk of new-onset diabetes after transplantation and mortality in kidney transplant recipients (14). However, whether the Mediterranean diet is associated with kidney function preservation in kidney transplant recipients is unknown. Therefore, in this study we aimed to evaluate whether adherence to the Mediterranean diet is associated with loss of kidney function in a cohort of stable kidney transplant recipients.
Materials and Methods
Study Design and Population
For this study we used data from the TransplantLines Food and Nutrition Biobank and Cohort Study (ClinicalTrials.gov; #NCT02811835). In summary, all kidney transplant recipients (aged ≥18 years) without known malignancies or active infections and a functioning allograft for ≥1 year who visited the outpatient clinic of the University Medical Center Groningen (UMCG) between 2008 and 2011 were invited to participate as described previously (15). Written consent was obtained from 707 (87%) of the 817 invited kidney transplant recipients. We excluded participants missing dietary data, leaving 632 individuals eligible for analysis. The study was conducted according to the guidelines settled in the Declaration of Helsinki and the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. The Institutional Review Board of the UMCG approved the study protocol (METc 2008/186).
Kidney Transplant Characteristics
All participants were transplanted in the UMCG and had no history of drug or alcohol addiction according to medical records. Patients were treated with standard antihypertensive and immunosuppressive therapy. Except for discouraging excess sodium intake and encouraging losing weight in overweight individuals, no specific dietary advice was conferred. Relevant transplantation characteristics such as transplantation date, donor status, pre-emptive transplantation (i.e., no dialysis treatment before transplantation), history of acute rejection, hepatitis C status, and cytomegalovirus infection were retrieved from the UMCG Renal Transplantation Database. Information regarding smoking behavior was inquired using a questionnaire.
Mediterranean Diet Score
Dietary intake was assessed at baseline using a validated food frequency questionnaire (FFQ) (16,17). The questionnaire was self-administered and filled out at home. The FFQ inquired about intake of 177 food items during the last month, taking into account seasonal variations. Frequency was recorded in times per day, week, or month and servings were expressed as natural units or household measures. All questionnaires were checked for completeness by a trained researcher and inconsistent answers were verified with the participant. Dietary data were converted into energy and nutrient intake by research dieticians and nutritionists using the Dutch Food Composition Table (18).
Adherence to the Mediterranean diet was assessed using a Mediterranean Diet Score according to the method described by Trichopoulou et al. (19). Food items were divided over nine food components (Supplemental Table 1) (14). On the basis of the sex-specific median intake, participants were assigned either one or zero points per food component. For each food component typically consumed in higher amounts in the Mediterranean diet (high ratio of monounsaturated to saturated fatty acids, legumes, cereals, vegetables, fruit, and fish), one point was attributed to those who consumed more than the sex-specific median intake. For food components consumed in lower amounts in the Mediterranean diet (meat and dairy), one point was attributed if the patient’s consumption was lower than the sex-specific median intake. Moderate alcohol consumption was scored by assigning one point to men who consumed between 10 and 50 g/d, and to women who consumed between 5 and 25 g/d. These scores were summarized to obtain the Mediterranean Diet Score with varying scores between 0 (lowest adherence) to 9 (highest adherence).
Assessment of Covariates
All measurements were performed once at baseline during a morning visit to the outpatient clinic. Body weight and height was measured with participants wearing indoor clothing without shoes. Body mass index was calculated as weight divided by height in meters squared and body surface area (BSA) was estimated in meters squared (20). BP and heart rate were measured by a semiautomatic device (Dinamap 1846; Critikon, Tampa, FL) following a strict protocol. Information on smoking behavior was inquired using a questionnaire. Physical activity was assessed using the Short Questionnaire to Assess Health-Enhancing Physical Activity score in time multiplied by intensity (21). Weight gain within the first year after baseline was retrieved from medical records.
Blood was drawn in the morning after an 8- to 12-hour hour fasting period and participants were asked to complete 24-hour urine collection. Serum creatinine was measured using an enzymatic, isotope dilution mass spectrometry–traceable assay on a Roche P-Modulator automated analyzer (Roche Diagnostics) and eGFR was calculated using the CKD Epidemiology Collaboration creatinine-based formula (22). Other laboratory measurements, including glucose homeostasis parameters, lipids, and electrolytes, were measured according to routine laboratory methods.
Clinical End Points
The primary end point was graft failure, defined as return to dialysis or retransplantation. Secondary end points were the composite end points kidney function decline defined as doubling of serum creatinine or graft failure, and graft loss defined as graft failure or death with a functioning graft. For the end points graft failure and kidney function decline, patients who died with a functioning graft were censored at time of death. All participants received medical care at the UMCG alone or medical care shared with a secondary referral hospital. In accordance with the Kidney Disease: Improving Global Outcomes guidelines, follow-up visits the first year after transplantation were performed every 3 months (7). Data on graft failure and death were retrieved from medical records and verified with the corresponding nephrologist or the Municipal Personal Records Database in case of death. End points were recorded until September 30, 2015.
Statistical Analyses
Normally distributed data are presented as mean±SD, whereas skewed data are presented as median (interquartile range [IQR]) and percentages are used to summarize categorical data. Associations between baseline characteristics and the Mediterranean Diet Score were analyzed by linear regression for continuous data or chi-squared test for categorical data. Skewed distributed data were log-transformed to meet the assumption of linearity for linear regression analysis.
To study the association of the Mediterranean Diet Score on graft failure, kidney function decline and graft loss we performed Cox proportional hazard regression analyses to calculate hazard ratios (HRs) and 95% confidence intervals (95% CIs) per two-point increment of the Mediterranean Diet Score. Nonlinearity was tested by comparing a linear model to a restricted cubic spline model. We performed crude analyses with the Mediterranean Diet Score as a continuous variable and performed multivariable analyses in which we cumulatively adjusted for age, sex, and BSA (model 1); kidney function characteristics, including eGFR, urinary protein excretion, time since transplantation, primary kidney disease (model 2); and transplantation-specific characteristics, including total HLA mismatches, living or deceased kidney donor status, and pre-emptive transplantation (model 3). To prevent overfitting by including too many covariates in relation to number of events, further models were constructed additive to model 3. In these models, we adjusted for history of acute rejection, hepatitis C status, and cytomegalovirus infection (model 4); use of calcineurin inhibitors, proliferation inhibitors, and corticosteroids (model 5); diabetes, systolic BP, cardiovascular history, and use of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and thiazide diuretics (model 6); and smoking status, Short Questionnaire to Assess Health-Enhancing Physical Activity score, energy and protein intake, and weight gain in the first year after baseline (model 7). Additionally, we separately analyzed the associations of each food component as a categorical variable with study end points. The associations of the Mediterranean Diet Score on graft failure, kidney function decline, and graft loss are visualized by fitting multivariable Cox regression analyses according to model 3 with the median Mediterranean Diet Score of 5 as reference. In sensitivity analyses, we analyzed whether the Adapted Mediterranean Diet Score was associated with study end points (see Supplemental Table 2 for a detailed description of the Adapted Mediterranean Diet Score) (23). Furthermore, we analyzed the association of the Mediterranean Diet Score with mortality and performed competing risk analyses according to Fine and Gray on study end points, considering death as a competing event (24).
We evaluated potential effect modification by sex, eGFR, time since transplantation, and urinary protein excretion by fitting models containing both main effects and their crossproduct terms. To visualize effect modification, we constructed interaction plots displaying the HR and 95% CI of a two-point Mediterranean Diet Score increase on study end points according to varying values of the effect-modifying variable, on the basis of Cox regression models containing both main effects and their crossproduct terms (25).
Linear and Cox regression were performed using SPSS version 23.0 (IBM Corp., Armonk, NY). Proportionality of hazards and competing risk analyses were tested using STATA version 13 (Statacorp., College Station, TX). R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria) was used to test nonlinearity and construct interaction plots. A P value <0.1 was considered statistically significant to detect effect modification. Otherwise, a P value <0.05 was considered statistically significant.
Results
Baseline characteristics are shown in Table 1. We included 632 patients at a median of 5.7 (IQR, 1.9–12.1) years after transplantation. Mean age was 53±13 years and 354 (57%) were men. In general, patients were slightly overweight (body mass index 26.6±4.8) and 152 (24%) had diabetes. Total daily energy intake was 9102±2667 kilojoules per day and 76 (12%) patients were smokers at baseline. The Mediterranean Diet Score was associated with living kidney donor (standardized β, 0.10; 95% CI, 0.02 to 0.18; P=0.01), use of proliferation inhibitors (standardized β, 0.10; 95% CI, 0.02 to 0.17; P=0.02) and thiazide diuretics (standardized β, −0.09; 95% CI, −0.17 to −0.01; P=0.03), and diabetes (standardized β, −0.10; 95% CI, −0.18 to −0.02; P=0.01). During a median follow up of 5.4 (IQR, 4.9–6.0) years with no patients lost to follow-up, 76 (12%) developed graft failure (5-year incidence rate of 11%). Chronic allograft dysfunction was the major cause of graft failure accountable for 56 (74%) of all graft failures. Other causes for graft failure included return of primary kidney disease (9%), infection (7%), acute rejection (4%), BK nephropathy (4%), and vascular complications (3%). Decline of kidney function occurred in 119 (19%) patients (5-year incidence rate of 17%), of whom 93 experienced a doubling of serum creatinine and 26 developed death-censored graft failure. Overall, 181 (29%) patients developed graft loss (5-year incidence rate of 25%).
Table 1.
Baseline characteristics and associations with the Mediterranean Diet Score
| Characteristic | Total Population (n=632) | Standardized β | 95% Confidence Interval |
|---|---|---|---|
| Mediterranean Diet Score | 4.3±1.7 | — | — |
| Demographics | |||
| Age, yr | 53±13 | 0.04 | −0.04 to 0.12 |
| Male sex, n (%) | 354 (57) | 0.04 | −0.04 to 0.12 |
| Primary kidney disease, n (%) | |||
| Primary glomerulopathy | 179 (28) | −0.02 | −0.09 to 0.06 |
| Glomerulonephritis secondary to systemic disease | 46 (7) | 0.008 | −0.07 to 0.09 |
| Tubulointerstitial nephritis | 74 (12) | 0.01 | −0.06 to 0.09 |
| Polycystic kidney disease | 135 (21) | 0.06 | −0.02 to 0.14 |
| Kidney hypoplasia and dysplasia | 23 (4) | −0.03 | −0.11 to 0.04 |
| Renovascular disease | 36 (6) | −0.03 | −0.11 to 0.05 |
| Diabetic kidney disease | 30 (5) | −0.03 | −0.11 to 0.05 |
| Other or unknown | 109 (17) | 0.01 | −0.09 to 0.07 |
| Diabetes, n (%) | 152 (24) | −0.10 | −0.18 to −0.02 |
| Cardiovascular disease, n (%) | 252 (40) | 0.03 | −0.05 to 0.11 |
| Weight, kg | 80±16 | 0.007 | −0.07 to 0.09 |
| Height, cm | 173±10 | 0.08 | −0.01 to 0.15 |
| BMI, kg/m2 | 26.6±4.8 | −0.04 | −0.12 to 0.04 |
| BSA, m2 | 1.94±0.22 | 0.03 | −0.04 to 0.11 |
| Transplant-specific characteristics | |||
| Time after transplantation, yr | 5.7 [1.9–12.1] | 0.04 | −0.04 to 0.11 |
| Pre-emptive transplantation, n (%) | 103 (16) | 0.05 | −0.03 to 0.13 |
| Living kidney donor, n (%) | 216 (34) | 0.10 | 0.02 to 0.18 |
| HLA mismatches | 2 [1–3] | 0.05 | −0.03 to 0.13 |
| Acute rejection, n (%) | 166 (26) | 0.07 | −0.01 to 0.15 |
| Cytomegalovirus infection, n (%) | 163 (26) | 0.01 | −0.06 to 0.09 |
| Hepatitis C status, n (%) | 8 (1) | −0.01 | −0.06 to 0.09 |
| Medication use, n (%) | |||
| Antihypertensive drugs | 556 (88) | −0.003 | −0.08 to 0.07 |
| ACE inhibitor | 208 (33) | −0.001 | −0.08 to 0.08 |
| Angiotensin II inhibitor | 102 (16) | −0.04 | −0.12 to 0.04 |
| β-Blocker | 401 (63) | 0.05 | −0.03 to 0.13 |
| Calcium channel antagonist | 156 (25) | −0.05 | −0.13 to 0.03 |
| Thiazide diuretic | 102 (16) | −0.09 | −0.17 to −0.01 |
| Loop diuretic | 125 (20) | −0.03 | −0.10 to 0.06 |
| Statins | 338 (54) | 0.07 | −0.01 to 0.15 |
| Calcineurin inhibitor | 362 (57) | −0.06 | −0.14 to 0.02 |
| Proliferation inhibitor | 529 (84) | 0.10 | 0.02 to 0.17 |
| Corticosteroids | 627 (99) | −0.02 | −0.09 to 0.06 |
| Hemodynamic parameters | |||
| Systolic BP, mm Hg | 136±17 | −0.05 | −0.13 to 0.03 |
| Diastolic BP, mm Hg | 83±11 | 0.01 | −0.07 to 0.09 |
| Heart rate, bpm | 69±12 | −0.04 | −0.12 to 0.04 |
| Kidney function | |||
| Serum creatinine, mg/dl | 1.4 [1.1–1.8] | −0.002 | −0.08 to 0.08 |
| eGFR, ml/min per 1.73 m2 | 52±20 | −0.02 | −0.10 to 0.05 |
| Protein excretion, g/d | 0.19 [0.02–0.35] | −0.06 | −0.13 to 0.02 |
| Laboratory parameters | |||
| Glucose, mg/dl | 96 [87–108] | −0.04 | −0.12 to 0.04 |
| HbA1c, % | 5.8 [5.5–6.2] | −0.07 | −0.15 to 0.01 |
| Total cholesterol, mg/dl | 197±43 | −0.04 | −0.12 to 0.04 |
| HDL cholesterol, mg/dl | 50 [42–66] | 0.06 | −0.15 to 0.14 |
| LDL cholesterol, mg/dl | 116±35 | −0.04 | −0.12 to 0.04 |
| hs-CRP, μg/ml | 1.6 [0.7–4.5] | −0.003 | −0.08 to 0.08 |
| Albumin, g/dl | 4.3±0.3 | 0.01 | −0.07 to 0.09 |
| Lifestyle parameters | |||
| Smokers, n (%) | 76 (12) | −0.04 | −0.12 to 0.04 |
| SQUASH score, time×intensity | 5230 [2423–8029] | 0.07 | −0.01 to 0.15 |
| Total energy intake, kJ/d (kcal/d) | 9102±2667 (2172±638) | 0.08 | −0.01 to 0.15 |
| Protein intake, g/d (% of kcal/d) | 82±20 (15±3) | 0.03 | −0.05 to 0.11 |
| Fat intake, g/d (% of kcal/d) | 88±34 (36±6) | 0.03 | −0.05 to 0.11 |
| Carbohydrate intake, g/d (% of kcal/d) | 249±78 (46±6) | 0.06 | −0.01 to 0.14 |
| Weight gain first year after baseline, kg | 0.3±4.7 | −0.03 | −0.11 to 0.04 |
Data are reported as mean±SD, median [interquartile range], or numbers. BMI, body mass index; BSA, body surface area; ACE, angiotensin-converting enzyme; HbA1C, glycated hemoglobin; Hs-CRP, high-sensitivity C-reactive protein; SQUASH, Short Questionnaire to Assess Health-Enhancing Physical Activity.
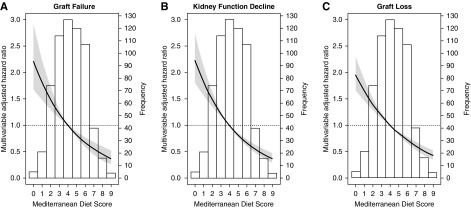
The associations of the Mediterranean Diet Score on graft failure, kidney function decline, and graft loss are depicted in Table 2. The Mediterranean Diet Score was inversely associated with graft failure (HR, 0.76; 95% CI, 0.58 to 0.98; 5-year absolute risk reduction (ARR), 3%), kidney function decline (HR, 0.76; 95% CI, 0.62 to 0.93; 5-year ARR, 4%), and graft loss (HR, 0.78; 95% CI, 0.66 to 0.92; 5-year ARR, 5%). These associations remained, independent of adjustment for age, sex, BSA, eGFR, urinary protein excretion, time since transplantation, primary kidney disease, HLA mismatches, living versus deceased donor kidney, and pre-emptive transplantation (graft failure: HR, 0.68; 95% CI, 0.50 to 0.91; kidney function decline: HR, 0.68; 95% CI, 0.55 to 0.85; and graft loss: HR, 0.74; 95% CI, 0.63 to 0.88). Further adjustment for immunosuppressive treatment (model 5), cardiovascular risk factors (model 6), and lifestyle parameters (model 7) did not materially alter these associations. We found no indication of nonlinear associations of the Mediterranean Diet Score on study end points. The associations of the Mediterranean Diet Score on study end points using multivariable Cox regression analyses are presented in Figure 1.
Table 2.
Prospective analyses of the association of Mediterranean Diet Score on risk of graft failure, kidney function decline, and graft loss per two-point increment
| Primary End Point | Secondary End Points | |||||
|---|---|---|---|---|---|---|
| Graft Failure | Kidney Function Decline | Graft Loss | ||||
| No. of Events | 76 | 119 | 181 | |||
| Model | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value |
| Crude | 0.76 (0.58 to 0.98) | 0.04 | 0.76 (0.62 to 0.93) | 0.009 | 0.78 (0.66 to 0.92) | 0.004 |
| Model 1 | 0.76 (0.58 to 0.99) | 0.04 | 0.76 (0.61 to 0.93) | 0.008 | 0.78 (0.66 to 0.92) | 0.003 |
| Model 2 | 0.68 (0.50 to 0.91) | 0.01 | 0.67 (0.54 to 0.83) | <0.001 | 0.72 (0.61 to 0.86) | <0.001 |
| Model 3 | 0.68 (0.50 to 0.91) | 0.01 | 0.68 (0.55 to 0.85) | 0.001 | 0.74 (0.63 to 0.88) | 0.001 |
| Model 4 | 0.66 (0.49 to 0.90) | 0.008 | 0.67 (0.54 to 0.84) | 0.001 | 0.73 (0.61 to 0.87) | <0.001 |
| Model 5 | 0.67 (0.50 to 0.91) | 0.009 | 0.68 (0.55 to 0.86) | 0.001 | 0.74 (0.62 to 0.88) | 0.001 |
| Model 6 | 0.64 (0.47 to 0.88) | 0.005 | 0.68 (0.54 to 0.85) | 0.001 | 0.72 (0.60 to 0.86) | <0.001 |
| Model 7 | 0.68 (0.50 to 0.92) | 0.01 | 0.70 (0.56 to 0.88) | 0.003 | 0.73 (0.61 to 0.87) | 0.001 |
Model 1: Mediterranean Diet Score, age, sex, and body surface area. Model 2: model 1 plus primary kidney disease, eGFR, 24-hour protein excretion, and time since transplantation. Model 3: model 2 plus total HLA mismatches, living donor kidney status, and pre-emptive transplantation. Model 4: model 3 plus history of acute rejection, cytomegalovirus infection, and hepatitis C status. Model 5: model 3 plus calcineurin inhibitor use, proliferation inhibitor use, and prednisolone use. Model 6: model 3 plus diabetes, systolic BP, cardiovascular history, ACE inhibitor, angiotensin II antagonist, and thiazide diuretic. Model 7: model 3 plus smoking status, physical activity (SQUASH score), energy intake, protein intake, and weight gain 1 year after baseline. 95% CI, 95% confidence interval; ACE, angiotensin-converting enzyme; SQUASH, Short Questionnaire to Assess Health-Enhancing Physical Activity.
Figure 1.
Associations of the Mediterranean Diet Score with graft failure, kidney function decline, and graft loss in stable kidney transplant recipients. Data were fitted by Cox proportional-hazards regression using a Mediterranean Diet Score of 5 as reference value. The black line represents the hazard ratio and the gray area represents the 95% confidence interval.
In subgroup analyses of the individual food components, we did not find a consistent association of a specific food component across all study end points. However, higher than median intake of legumes and nuts was inversely associated with graft failure (HR, 0.57; 95% CI, 0.36 to 0.91), moderate alcohol consumption was inversely associated with kidney function decline (HR, 0.61; 95% CI, 0.40 to 0.95), and higher than median intake of cereals (HR, 0.66; 95% CI, 0.49 to 0.89) and moderate alcohol intake (HR, 0.63; 95% CI, 0.44 to 0.89) were both inversely associated with graft loss (Supplemental Table 3).
Effect Modifications
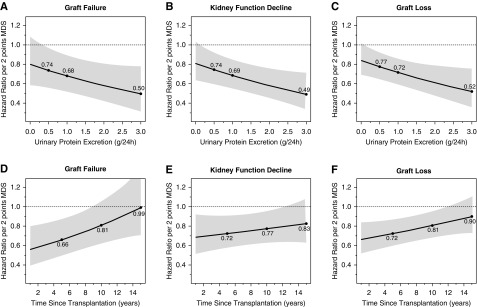
We observed effect modification by urinary protein excretion and time since transplantation on the association of the Mediterranean Diet Score on risk for graft failure (interaction term: β, −0.19; 95% CI, −0.39 to 0.01; P=0.05 and interaction term: β, 0.04; 95% CI, 0.01 to 0.08; P=0.03, respectively), independent of age, sex, and eGFR. As depicted in Figure 2 the magnitude of the association of the Mediterranean Diet Score on study end points was greater in patients with higher urinary protein excretion (Figure 2, A–C) and more recently transplanted patients (Figure 2, D–F). We found no indication for effect modification of the Mediterranean Diet Score with sex or eGFR (P≥0.05).
Figure 2.
Urinary protein excretion and time since transplantation significantly modify the associations between the Mediterranean Diet Score and kidney endpoints. The black line represents the hazard ratio and the gray area represents the 95% confidence interval estimated using Cox regression analyses. The dots refer to the hazard ratios at cut-off values of urinary protein excretion set at 0.5, 1.0, and 3.0 g/d, respectively, and cut-off values of time since transplantation set at 5, 10, and 15 years, respectively.
Sensitivity Analyses
The Adapted Mediterranean Diet Score was inversely associated with graft failure (HR, 0.76; 95% CI, 0.59 to 0.99; P=0.04), kidney function decline (HR, 0.73; 95% CI, 0.60 to 0.90; P=0.003), and graft loss (HR, 0.79; 95% CI, 0.67 to 0.94; P=0.006) in univariable analyses. In multivariable analyses, the associations of the Adapted Mediterranean Diet Score on graft failure showed similar HRs but did not remain statistically significant, but the associations of the Adapted Mediterranean Diet Score on kidney function decline and graft loss remained significantly associated (Supplemental Table 4).
Additionally, we performed competing risk analyses with death as a competing event for graft failure and kidney function decline. The association of the Mediterranean Diet Score and mortality is shown in Supplemental Table 5. In competing risk analyses we observed that the Mediterranean Diet Score remained inversely associated with graft failure (HR, 0.76; 95% CI, 0.59 to 0.99; P=0.04) and kidney function decline (HR, 0.77; 95% CI, 0.63 to 0.93; P=0.007). Adjustment for potential confounders did not materially affect these associations (Supplemental Table 6).
Discussion
In this study we showed that adherence to the Mediterranean diet was associated with better kidney graft outcomes in a large cohort of stable kidney transplant recipients. In addition, this study shows that the association of adherence to the Mediterranean diet with graft survival was more pronounced in kidney transplant recipients with more proteinuria and who are transplanted more recently. Chrysohoou et al. were the first to demonstrate that higher adherence to the Mediterranean diet was associated with better kidney function in a cross-sectional study of 1212 participants in the ATTICA study (10) and similar results were reported by Huang et al. (11) in 1110 Swedish males. Furthermore, prospective studies have demonstrated that adherence to the Mediterranean diet is also protective for the development of CKD and kidney function decline and is associated with lower albuminuria (12,13,26). Moreover, in sensitivity analyses, we showed that the Adapted Mediterranean Diet Score, which recently has been associated with incident CKD in the general population (27), is also associated with better kidney outcomes in kidney transplant recipients.
Multiple mechanisms might explain the beneficial effect of the Mediterranean diet because high adherence to this prudent diet could modify several factors, including oxidative stress, inflammation, endothelial dysfunction, dietary acid load, lipid profile, protein intake, and glycemic index, and thereby mediate the association of the Mediterranean diet on kidney function outcomes. Several characteristics of the Mediterranean diet, including fruit and vegetable intake, which leads to a higher intake of antioxidants and fibers, and higher intake of ω-3 fatty acids, have been associated with reducing oxidative stress and inflammation (23,28,29). Furthermore, low meat intake and high fruit and vegetables intake is associated with improving the acid-base balance (30) and low dietary acid load has been associated with lower incidence of CKD (31). Additionally, proteins predominantly from plant-based foods have favorable effects on lipid profile and are associated with lower risk of incident CKD and ESKD (32–34). The protective effect on the Mediterranean diet on cardiovascular risk factors such as hypertension, diabetes, and obesity may also contribute to a beneficial effect on kidney outcomes (10–13).
In our study, the Mediterranean Diet Score was inversely associated with diabetes, a known risk factor for graft failure in kidney transplant recipients (35–37). We found that the magnitude of the inverse association of the Mediterranean Diet Score on kidney graft survival was stronger in participants with higher urinary protein excretion and in patients transplanted more recently. Considering protein excretion is a well established risk factor for kidney function decline and ESKD (38–40), it can be expected that a healthier diet particularly benefits kidney transplant recipients with higher urinary protein excretion. It is known that risks for graft failure are time-dependent and the mechanisms for early and late graft failure differ, which may explain why kidney transplant recipients with a longer time since transplantation benefit less from a Mediterranean diet (41).
To our knowledge, this is the first study to evaluate the association between adherence to the Mediterranean diet and kidney function outcomes in kidney transplant recipients. Strengths of this study include the prospective design of the study, the relatively long median follow-up of 5.4 years, and clinically relevant end points without participants lost for follow-up. On the other hand, we acknowledge that this study has several limitations. Dietary intake was assessed using a self-reporting FFQ, which could lead to possible misclassification and over- or under-reporting of dietary intake. Previously, a slight underreporting of protein intake by this FFQ was demonstrated (42). Another limitation of our study is that dietary intake was assessed once at baseline and therefore possible changes in dietary intake and adherence to diet over time could not be accounted for. Nevertheless, most epidemiologic studies use single baseline measurements to study associations with long-term outcome, which is likely to adversely affect strengths of these associations with outcomes and might lead to underestimation of associations between baseline measurements and long-term outcome, rather than overestimation (43,44). Furthermore, adherence to the Mediterranean Diet Score was assessed in a Dutch population and consumption of food items may differ from populations in Mediterranean regions, which warrants careful consideration when attempting to extrapolate these results to other populations. In addition, sicker individuals could have received more frequent follow-up visits, which could have led to a greater chance of detecting doubling of serum creatinine. However, if such an effect occurred, it is likely to have been very small, because all participants were seen at 3-month intervals. As with any observational study, residual confounding may exist despite having adjusted for several potential confounders in our analyses. Finally, the observational nature of the study precludes us from drawing conclusions of causality.
In conclusion, in this single-center cohort of kidney transplant recipients we showed that adherence to the Mediterranean diet is associated with better kidney function outcomes in kidney transplant recipients, independent of potential confounders. These findings suggest that adopting a Mediterranean diet may benefit kidney graft survival after kidney transplantation, particularly in individuals with higher protein excretion and patients transplanted more recently. Randomized trials with dietary interventions, including guidance and education of a dietician, aimed to improve adherence to the Mediterranean diet in kidney transplant recipients are warranted to further substantiate these findings.
Disclosures
Dr. Bakker, Dr. Berger, Dr. Gans, Dr. Geleijnse, Dr. Gomes-Neto, Dr. Navis, Dr. Osté, Dr. Sotomayor, and Dr. van den Berg have nothing to disclose.
Funding
The generation of the Transplant Lines Food and Nutrition Biobank and Cohort Study (ClinicalTrials.gov; #NCT02811835) was funded by the Top Institute Food and Nutrition (grant A-1003).
Supplementary Material
Acknowledgments
Dr. Gomes-Neto and Dr. Osté performed data analyses and wrote the manuscript. Dr. van den Berg was responsible for data acquisition and contributed to manuscript revisions. Dr. Berger, Dr. Gans, Dr. Geleijnse, and Dr. Sotomayor contributed to data interpretation and manuscript revisions. Dr. Bakker and Dr. Navis were responsible for the study design and contributed to data interpretation and manuscript revisions.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06710619/-/DCSupplemental.
Supplemental Table 1. Overview of food items used to determine the Mediterranean Diet Score.
Supplemental Table 2. Overview of food items used to determine the adapted Mediterranean Diet score by Fung et al. (23).
Supplemental Table 3. Association per component of the Mediterranean Diet score on graft failure, kidney function decline, and graft loss.
Supplemental Table 4. Association of the adapted Mediterranean Diet score by Fung et al. (23) on graft failure, kidney function decline, and graft loss.
Supplemental Table 5. Association of the Mediterranean Diet score on all-cause mortality.
Supplemental Table 6. Competing risk analyses of the Mediterranean Diet Score on graft failure and kidney function decline, considering death as a competing event.
References
- 1.Jofré R, López-Gómez J, Moreno F, Sanz-Guajardo D, Valderrábano F: Changes in quality of life after renal transplantation. Am J Kidney Dis 32: 93–100, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Junchotikul P, Charoenthanakit C, Saiyud A, Parapiboon W, Ingsathit A, Jirasiritham S, Sumethkul V: Assessment of the changes in health-related quality of life after kidney transplantation in a cohort of 232 Thai patients. Transplant Proc 47: 1732–1735, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Oniscu GC, Brown H, Forsythe JL: Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol 16: 1859–1865, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, Klarenbach S, Gill J: Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 11: 2093–2109, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Schippers HM, Kalff MW: Cost comparison haemodialysis and renal transplantation. Tissue Antigens 7: 86–90, 1976 [DOI] [PubMed] [Google Scholar]
- 6.Gondos A, Döhler B, Brenner H, Opelz G: Kidney graft survival in Europe and the United States: Strikingly different long-term outcomes. Transplantation 95: 267–274, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group : KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9[Suppl 3]: S1–S155, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Sofi F, Abbate R, Gensini GF, Casini A: Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am J Clin Nutr 92: 1189–1196, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Serra-Majem L, Roman B, Estruch R: Scientific evidence of interventions using the Mediterranean diet: A systematic review. Nutr Rev 64: S27–S47, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Chrysohoou C, Panagiotakos DB, Pitsavos C, Skoumas J, Zeimbekis A, Kastorini CM, Stefanadis C: Adherence to the Mediterranean diet is associated with renal function among healthy adults: The ATTICA study. J Ren Nutr 20: 176–184, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Jiménez-Molén JJ, Lindholm B, Cederholm T, Arnlöv J, Risérus U, Sjögren P, Carrero JJ: Mediterranean diet, kidney function, and mortality in men with CKD. Clin J Am Soc Nephrol 8: 1548–1555, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khatri M, Moon YP, Scarmeas N, Gu Y, Gardener H, Cheung K, Wright CB, Sacco RL, Nickolas TL, Elkind MS: The association between a Mediterranean-style diet and kidney function in the Northern Manhattan study cohort. Clin J Am Soc Nephrol 9: 1868–1875, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asghari G, Farhadnejad H, Mirmiran P, Dizavi A, Yuzbashian E, Azizi F: Adherence to the Mediterranean diet is associated with reduced risk of incident chronic kidney diseases among Tehranian adults. Hypertens Res 40: 96–102, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Osté MC, Corpeleijn E, Navis GJ, Keyzer CA, Soedamah-Muthu SS, van den Berg E, Postmus D, de Borst MH, Kromhout D, Bakker SJ: Mediterranean style diet is associated with low risk of new-onset diabetes after renal transplantation. BMJ Open Diabetes Res Care 5: e000283, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Berg E, Pasch A, Westendorp WH, Navis G, Brink EJ, Gans RO, van Goor H, Bakker SJ: Urinary sulfur metabolites associate with a favorable cardiovascular risk profile and survival benefit in renal transplant recipients. J Am Soc Nephrol 25: 1303–1312, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feunekes GI, Van Staveren WA, De Vries JH, Burema J, Hautvast JG: Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am J Clin Nutr 58: 489–496, 1993 [DOI] [PubMed] [Google Scholar]
- 17.van den Berg E, Engberink MF, Brink EJ, van Baak MA, Joosten MM, Gans RO, Navis G, Bakker SJ: Dietary acid load and metabolic acidosis in renal transplant recipients. Clin J Am Soc Nephrol 7: 1811–1818, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutch Food Composition Table 2006 NEVO-tabel: Nederlands Voedingsstoffenbestand. Netherlands Nutr Cent, 2006 [Google Scholar]
- 19.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D: Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 348: 2599–2608, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Du BOIS D, Du Bois EF: Clinical calorimetry. Arch Intern Med (Chic) XVII: 863–871, 1916 [Google Scholar]
- 21.Wendel-Vos GC, Schuit AJ, Saris WH, Kromhout D: Reproducibility and relative validity of the Short Questionnaire to Assess Health-Enhancing Physical Activity. J Clin Epidemiol 56: 1163–1169, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fung TT, McCullough ML, Newby PK, Manson JA, Meigs JB, Rifai N, Willett WC, Hu FB: Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 82: 163–173, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 25.Lamina C, Sturm G, Kollerits B, Kronenberg F: Visualizing interaction effects: A proposal for presentation and interpretation. J Clin Epidemiol 65: 855–862, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Mazaraki A, Tsioufis C, Dimitriadis K, Tsiachris D, Stefanadi E, Zampelas A, Richter D, Mariolis A, Panagiotakos D, Tousoulis D, Stefanadis C: Adherence to the Mediterranean diet and albuminuria levels in Greek adolescents: Data from the Leontio Lyceum Albuminuria (3L study). Eur J Clin Nutr 65: 219–225, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Hu EA, Steffen LM, Grams ME, Crews DC, Coresh J, Appel LJ, Rebholz CM: Dietary patterns and risk of incident chronic kidney disease: The Atherosclerosis Risk in Communities study. Am J Clin Nutr 110: 713–721, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smidowicz A, Regula J: Effect of nutritional status and dietary patterns on human serum C-reactive protein and interleukin-6 concentrations. Adv Nutr 6: 738–747, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin J, Fung TT, Hu FB, Curhan GC: Association of dietary patterns with albuminuria and kidney function decline in older white women: A subgroup analysis from the nurses’ health study. Am J Kidney Dis 57: 245–254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siener R: Dietary treatment of metabolic acidosis in chronic kidney disease. Nutrients 10: E512, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rebholz CM, Coresh J, Grams ME, Steffen LM, Anderson CAM, Appel LJ, Crews DC: Dietary acid load and incident chronic kidney disease: Results from the ARIC Study. Am J Nephrol 42: 427–435, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins DJ, Kendall CW, Vidgen E, Augustin LS, Van Erk M, Geelen A, Parker T, Faulkner D, Vuksan V, Josse RG, Leiter LA, Connelly PW: High-protein diets in hyperlipidemia: Effect of wheat gluten on serum lipids, uric acid, and renal function. Am J Clin Nutr 74: 57–63, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Haring B, Selvin E, Liang M, Coresh J, Grams ME, Petruski-Ivleva N, Steffen LM, Rebholz CM: Dietary protein sources and risk for incident chronic kidney disease: Results from the Atherosclerosis Risk in Communities (ARIC) study. J Ren Nutr 27: 233–242, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lew QJ, Jafar TH, Koh HW, Jin A, Chow KY, Yuan JM, Koh WP: Red meat intake and risk of ESRD. J Am Soc Nephrol 28: 304–312, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morales JM, Marcén R, del Castillo D, Andres A, Gonzalez-Molina M, Oppenheimer F, Serón D, Gil-Vernet S, Lampreave I, Gainza FJ, Valdés F, Cabello M, Anaya F, Escuin F, Arias M, Pallardó L, Bustamante J: Risk factors for graft loss and mortality after renal transplantation according to recipient age: A prospective multicentre study. Nephrol Dial Transplant 27[Suppl 4]: iv39–iv46, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matas AJ, Gillingham KJ, Humar A, Ibrahim HN, Payne WD, Gruessner RW, Dunn TB, Sutherland DE, Najarian JS, Kandaswamy R: Posttransplant diabetes mellitus and acute rejection: Impact on kidney transplant outcome. Transplantation 85: 338–343, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ: Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 3: 178–185, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Ruggenenti P, Perna A, Mosconi L, Pisoni R, Remuzzi G: Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. "Gruppo Italiano di Studi Epidemiologici in Nefrologia" (GISEN). Kidney Int 53: 1209–1216, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Ishani A, Grandits GA, Grimm RH, Svendsen KH, Collins AJ, Prineas RJ, Neaton JD: Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol 17: 1444–1452, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M; Alberta Kidney Disease Network : Relation between kidney function, proteinuria, and adverse outcomes. JAMA 303: 423–429, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Prommool S, Jhangri GS, Cockfield SM, Halloran PF: Time dependency of factors affecting renal allograft survival. J Am Soc Nephrol 11: 565–573, 2000 [DOI] [PubMed] [Google Scholar]
- 42.van den Berg E, Engberink MF, Brink EJ, van Baak MA, Gans RO, Navis G, Bakker SJ: Dietary protein, blood pressure and renal function in renal transplant recipients. Br J Nutr 109: 1463–1470, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R: Underestimation of risk associations due to regression dilution in long- term follow-up of prospective studies. Am J Epidemiol 150: 341–353, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Koenig W, Sund M, Fröhlich M, Löwel H, Hutchinson WL, Pepys MB: Refinement of the association of serum C-reactive protein concentration and coronary heart disease risk by correction for within-subject variation over time: The MONICA Augsburg studies, 1984 and 1987. Am J Epidemiol 158: 357–364, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.