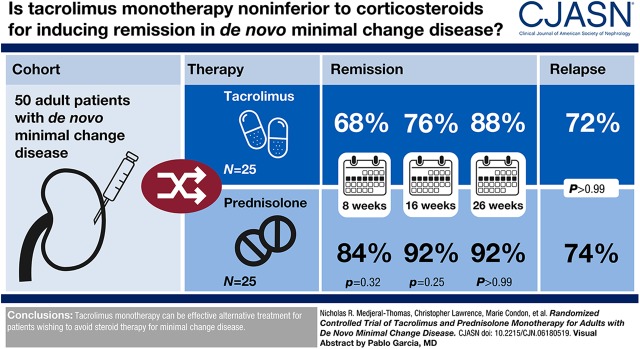
Visual Abstract
Keywords: Glomerulonephritis, nephrotic syndrome, tacrolimus, adult, humans, lipoid nephrosis, prednisolone, nephrology, prospective studies, treatment outcome, recurrence, remission induction, United Kingdom
Abstract
Background and objectives
Minimal change disease is an important cause of nephrotic syndrome in adults. Corticosteroids are first-line therapy for minimal change disease, but a prolonged course of treatment is often required and relapse rates are high. Patients with minimal change disease are therefore often exposed to high cumulative corticosteroid doses and are at risk of associated adverse effects. This study investigated whether tacrolimus monotherapy without corticosteroids would be effective for the treatment of de novo minimal change disease.
Design, setting, participants, & measurements
This was a multicenter, prospective, open-label, randomized, controlled trial involving six nephrology units across the United Kingdom. Adult patients with first presentation of minimal change disease and nephrotic syndrome were randomized to treatment with either oral tacrolimus at 0.05 mg/kg twice daily, or prednisolone at 1 mg/kg daily up to 60 mg daily. The primary outcome was complete remission of nephrotic syndrome after 8 weeks of therapy. Secondary outcomes included remission of nephrotic syndrome at 16 and 26 weeks, rates of relapse of nephrotic syndrome, and changes from baseline kidney function.
Results
There were no significant differences between the tacrolimus and prednisolone treatment cohorts in the proportion of patients in complete remission at 8 weeks (21 out of 25 [84%] for prednisolone and 17 out of 25 [68%] for tacrolimus cohorts; P=0.32; difference in remission rates was 16%; 95% confidence interval [95% CI], −11% to 40%), 16 weeks (23 out of 25 [92%] for prednisolone and 19 out of 25 [76%] for tacrolimus cohorts; P=0.25; difference in remission rates was 16%; 95% CI, −8% to 38%), or 26 weeks (23 out of 25 [92%] for prednisolone and 22 out of 25 [88%] for tacrolimus cohorts; P=0.99; difference in remission rates was 4%; 95% CI, −17% to 25%). There was no significant difference in relapse rates (17 out of 23 [74%] for prednisolone and 16 out of 22 [73%] for tacrolimus cohorts) for patients in each group who achieved complete remission (P=0.99) or in the time from complete remission to relapse.
Conclusions
Tacrolimus monotherapy can be effective alternative treatment for patients wishing to avoid steroid therapy for minimal change disease.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2020_01_16_CJN06180519.mp3
Introduction
Minimal change disease causes up to 25% of nephrotic syndrome in adults (1–3). Nephrotic syndrome is associated with significant comorbidities, including infections (4,5), thromboembolic events (6,7), and hyperlipidemia (8). Furthermore, AKI accompanies the presentation of minimal change disease in up to 25% of cases (9). Consequently, most clinicians recommend treatment for minimal change disease patients with high doses of corticosteroids. However, this is a rare disease and there is a paucity of data from randomized, controlled trials in adults on the efficacy of therapy (10–12). Many adult patients require a prolonged initial course of corticosteroids to enter remission (9,13–15). Additionally, the relapse rate in adults is high at 48%–76%, and worse in adults younger than 45 years of age (9,13). This necessitates the use of repeated courses of steroids. The resultant high-dose corticosteroid exposure is associated with adverse effects, including weight gain, diabetes mellitus, infection, osteoporosis, cosmetic changes, and gastrointestinal bleeding (14,16).
Steroid-sparing therapeutic agents have been used to treat patients with frequently relapsing or steroid-dependent minimal change disease (17). These include cyclophosphamide, ciclosporin, tacrolimus, mycophenolate, and, more recently, rituximab (18–29). Tacrolimus is widely available and generally well tolerated and therefore a relatively attractive alternative to corticosteroids for treatment of minimal change disease. Minimal change disease pathogenesis is thought to be dependent on systemic T lymphocyte changes and subsequent cytokine production that impairs glomerular function (17). Tacrolimus is a calcineurin inhibitor that suppresses IL-2 transcription and inhibits T cell activation. Tacrolimus may also reduce proteinuria by directly stabilizing podocyte structure (30). Additionally, tacrolimus may be associated with a more favorable side-effect profile than alternative calcineurin inhibitors such as ciclosporin, as well as having fewer side effects than corticosteroids (28).
Retrospective case series report successful tacrolimus treatment of frequently relapsing, steroid-dependent, and steroid-resistant minimal change disease (19,26). Likewise, ciclosporin has been used as monotherapy in prospective trials of steroid-dependent and steroid-resistant minimal change disease treatment (22,31). As significant corticosteroid toxicity can occur from the first, often prolonged, therapeutic course for minimal change disease, as well as from subsequent courses given for relapses, steroid-sparing agents such as calcineurin inhibitors and cyclophosphamide are sometimes also used for a first presentation of minimal change disease. However, there are few data on the effectiveness of steroid-free regimens for de novo disease.
In kidney transplantation, steroid avoidance or early withdrawal results in improved BP and lower rates of new-onset diabetes, dyslipidemia, weight gain, and infections (32–35). These associations appear to be dose-dependent (36). Steroid-free regimens may likewise be beneficial in minimal change disease.
This prospective, multicenter, randomized, open-label trial compared tacrolimus and prednisolone monotherapy for the treatment of adult patients with minimal change disease, and investigated the hypothesis that tacrolimus monotherapy is noninferior to corticosteroids for inducing remission and has comparable relapse rates, but is associated with fewer adverse events. This is the first prospective, multicenter trial of steroid-free treatment for de novo minimal change disease in adults.
Materials and Methods
This was a multicenter, randomized, controlled trial that recruited patients from six out of the seven units enrolled in the study. The study protocol was registered at the European Clinical Trials Database (EudraCT, number 2009–014292–52) and ClinicalTrials.gov (identifier: NCT00982072). The trial was conducted in accordance with the Declaration of Helsinki. Ethics approval was obtained from the United Kingdom (UK) National Research Ethics Service Committee (reference 09/H0711/68).
The inclusion criteria were age >18 years, nephrotic syndrome, and a kidney biopsy showing minimal change disease. We defined nephrotic syndrome as the combination of urine protein-to-creatinine ratio (UPCR) >100 mg/mmol and a serum albumin of <3.0 g/dl. We adopted this level of proteinuria to avoid excluding patients with hypoalbuminemia and significant but non-nephrotic range proteinuria, which may have been subsequent to severe hypoalbuminemia. Only one patient in each cohort had UPCR<300 mg/mmol, and these patients had UPCR of 249 and 261 mg/mmol, respectively. Biopsy specimens were examined and reported locally and reviewed by a single histopathologist (H.T.C.) if there was diagnostic uncertainty. Patients were excluded if they had any of the following: hepatitis B, C, or HIV infection; other untreated infections; women who were pregnant, breast feeding, or at risk of pregnancy; patients who had immunosuppression for nephrotic syndrome in the past 18 months or immunosuppression that may affect the outcome of their current episode of nephrotic syndrome; or any condition that would cause the study to be detrimental to the patient.
Local responsible clinicians directed the management of hypertension with angiotensin receptor blockers or angiotensin-converting enzyme inhibitors and hypercholesterolemia with statins. Primary venous thromboembolism prophylaxis was subcutaneous low-molecular-weight heparin while serum albumin was <2.0 g/dl, or aspirin 75 mg daily if the serum albumin was ≥2.0 g/dl but <3.0 g/dl (37). Patients considered to be at high risk of latent tuberculosis were also prescribed isoniazid 150 mg daily and pyridoxine 50 mg weekly during immunosuppression treatment.
We randomized patients at a 1:1 ratio to receive either oral tacrolimus or prednisolone. Participants were enrolled by the local principal investigators at each site. Consecutively enrolled patients were allocated a trial number by the lead site. Before the trial commenced, each trial number was randomized to a treatment arm by computer-generated random permuted blocks (with concealment of block size from the clinical team). Allocation and masking was through computer-generated sheets in opaque, tamper-evident envelopes (one for each trial number) that were stored securely at the lead site and opened by the trial team after each patient enrolment. The intervention assignment was communicated to the local principal investigator. Randomization sequence was not known by the trial investigators. The treatment was nonblinded after intervention assignment. The trial was monitored throughout by the data monitoring committee. Trial recruitment finished after the target study population had been reached and was closed when all patients had completed follow-up.
Patients allocated to tacrolimus received an initial dose of 0.05 mg/kg twice daily, with target blood trough levels of 6–8 ng/ml. In the event of inadequate clinical response at 8 weeks of treatment, the target blood trough level was increased to 9–12 ng/ml. Twelve weeks after achieving complete remission, the tacrolimus doses were gradually reduced over 8 weeks and stopped. Patients allocated to prednisolone received initial doses of 1 mg/kg per day with a maximum dose of 60 mg/d. One week after achieving complete remission, the steroid dose was halved for 4–6 weeks then gradually reduced and stopped over a further 6 weeks, ensuring patients received a minimum of 16 weeks of prednisolone. Patients receiving prednisolone were also prescribed omeprazole 20 mg and calcium carbonate/colecalciferol (1000 mg/800 IU) two tablets daily.
The primary outcome was the proportion of patients achieving complete remission at 8 weeks. We defined complete remission a priori as UPCR<50 mg/mmol. Partial remission was defined as UPCR reduction of at least 50% from enrolment and a value between 50 mg/mmol and 300 mg/mmol. The secondary outcomes were the proportion of patients achieving complete remission at 16 and 26 weeks, the proportion who relapsed after complete remission, change in serum creatinine from baseline, and the number of adverse events. Relapse was defined as UPCR>300 mg/mmol in patients who had achieved complete remission. Patients who achieved complete remission were followed up for 78±2 weeks from complete remission or until relapse. We reviewed patients weekly for 4 weeks, then monthly for 6 months, then every 3 months, or more frequently at the investigators’ discretion if clinically indicated. At each review we measured proteinuria (UPCR), and tested blood for full blood count, biochemical profile, nonfasting glucose, and trough tacrolimus levels. The per-protocol analysis evaluated patients who completed at least 8 weeks of therapy. We also compared outcomes on an intention-to-treat basis.
Statistical advice on sample size calculations was provided by the Department of Statistics, Imperial College London (London, UK). Noninferiority was assessed by estimating the two-sided 95% confidence interval (95% CI) for the difference in remission rates between the two groups using the Koopman asymptomatic score (38).We aimed to verify that the lower limit of the 95% CI was not lower than 10% (Δ value of −0.1). The expected complete remission rate for patients treated with corticosteroids at 8 weeks was estimated at 60% (23), and tacrolimus at 84% (39). Allowing for a 10% drop-out rate, we calculated we needed to enroll 52 patients. We used GraphPad Prism Version 6.00 for Windows for statistical analyses. Normally distributed continuous variables were compared using unpaired t test. Continuous variables with skewed distribution were compared using Mann–Whitney U test and confidence intervals were calculated using the Hodges–Lehmann method. We compared the proportion of patients in remission and relapse with Fisher exact test and used the Baptista–Pike method to calculate odds ratio (OR) confidence intervals and the Newcombe–Wilson method with continuity correction for confidence intervals of differences in proportions. We adjusted P values for differences in remission rates for multiple analyses with the two-stage linear step-up procedure of Benjamini et al. (40).
Results
Patients
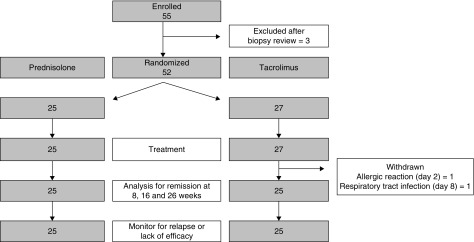
We enrolled 55 patients from six hospitals across the UK. All patients had de novo disease. Three patients were excluded after review of their biopsy specimen by electron microscopy determined they had an alternative diagnosis (features of membranous nephropathy in two patients and lupus nephritis in one patient). Randomization allocated 27 patients to tacrolimus and 25 patients to prednisolone treatment (Figure 1). Two patients in the tacrolimus group did not complete 8 weeks of treatment; one patient developed an allergic rash and diarrhea 2 days after starting tacrolimus; one patient developed lower respiratory tract infection 8 days into treatment and were withdrawn on the request of the local clinician. Ten patients in each cohort received treatment for hypertension with angiotensin receptor blockers or angiotensin-converting enzyme inhibitors (Table 1). The median total treatment time until relapse of nephrotic syndrome, or completion of withdrawal was 36 weeks (interquartile range [IQR], 26–52 weeks) for the tacrolimus cohort compared with 23 weeks (IQR, 14–29 weeks) for the corticosteroid cohort. The median follow-up across the study was 44 weeks (IQR, 22–82 weeks).
Figure 1.
Study design and study cohort flowchart. Of 55 patients enrolled, 52 were randomized to treatment, and 25 patients in each cohort completed treatment. Two patients in the tacrolimus group did not complete treatment; one patient developed diarrhea and rash 2 days after starting tacrolimus; one patient developed lower respiratory tract infection 8 days into treatment; both were withdrawn from the study to receive nonprotocol treatment. These patients are included in the intention-to-treat analysis and excluded from the per-protocol analysis.
Table 1.
Patient characteristics at enrollment
| Characteristic | Overall (n=50) | Prednisolone (n=25) | Tacrolimus (n=25) | |
|---|---|---|---|---|
| Male | n (%) | 27 (54%) | 15 (60%) | 12 (48%) |
| Age | Median (range) | 43 (18–74) | 39 (18–73) | 43 (18–74) |
| White | n (%) | 33 (66%) | 17 (68%) | 16 (64%) |
| Serum creatinine, mg/dl | Median (range) | 0.82 (0.53–2.96) | 0.81 (0.59–2.33) | 0.82 (0.53–2.96) |
| eGFR, ml/min per 1.73 m2 | Median (range) | 98 (27–120) | 94 (29–120) | 99 (27–120) |
| Urine protein-to-creatinine ratio, mg/g | Median (range) | 7310 (2204–15,708) | 6504 (2310–15,708) | 7717 (2204–15,619) |
| Urine protein-to-creatinine ratio, mg/mmol | Median (range) | 826 (249–1775) | 735 (261–1775) | 872 (249–1765) |
| Serum albumin, g/dl | Median (range) | 1.6 (0.7–2.9) | 1.7 (0.7–2.9) | 1.5 (0.7–2.4) |
| Systolic BP, mm Hg | Median (range) | 127 (101–145) | 128 (101–145) | 126 (108–140) |
| Diastolic BP, mm Hg | Median (range) | 80 (50–90) | 80 (67–89) | 73 (50–90) |
| Antihypertensive treatment | n (%) | 20 (40%) | 10 (40%) | 10 (40%) |
Primary Outcome
There were no significant differences between the treatment arms in the proportion of patients in complete remission at 8 weeks, although the results did not meet the a priori definition of noninferiority.
Intention-to-Treat Analysis.
A total of 21 out of 25 (84%) patients in the prednisolone cohort and 17 out of 27 (63%) patients in the tacrolimus cohort were in remission at 8 weeks (P=0.12). The difference in remission rates was 21% (95% CI, −6% to 44%).
Per-Protocol Analysis.
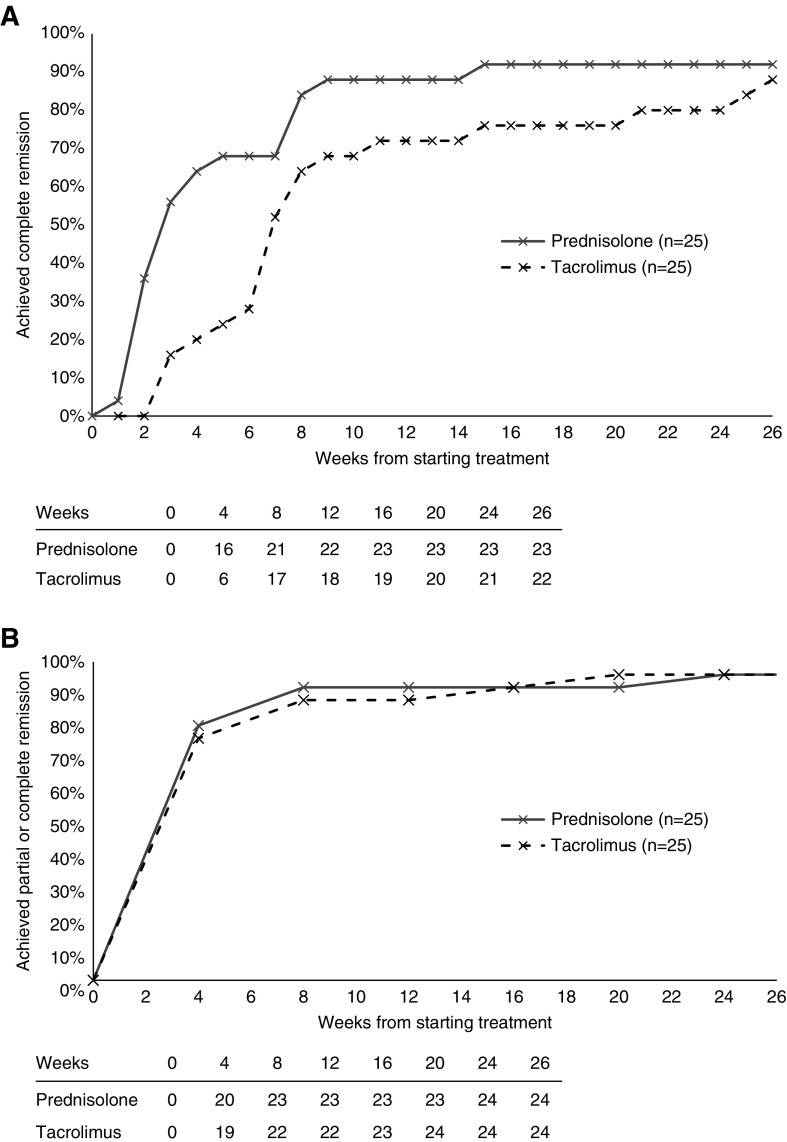
Of patients who completed 8 weeks of treatment, 21 out of 25 (84%) in the prednisolone cohort and 17 out of 25 (68%) in the tacrolimus cohort were in remission at 8 weeks (P=0.32). The differences in remission rates were 16% (95% CI, −11% to 40%) (Figure 2, Table 2).
Figure 2.
The proportion of patients achieving complete remission. (A) Complete remission and (B) all remission (complete or partial remission) against weeks from the start of treatment in the prednisolone (gray, solid line) and tacrolimus (black, dashed line) cohorts. The number of patients achieving remission is shown in the table below each graph.
Table 2.
Primary, secondary, and exploratory outcomes
| Outcome | Overall | Prednisolone | Tacrolimus | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Cohort | Percentage | n | Cohort | Percentage | n | Cohort | Percentage | ||
| Primary outcome | ||||||||||
| Complete remission by 8 wk | 38 | 50 | 76 | 21 | 25 | 84 | 17 | 25 | 68 | 0.32 (0.54) |
| Secondary outcomes | ||||||||||
| Complete remission by 16 wk | 42 | 50 | 84 | 23 | 25 | 92 | 19 | 25 | 76 | 0.25 (0.54) |
| Complete remission by 26 wk | 45 | 50 | 90 | 23 | 25 | 92 | 22 | 25 | 88 | 0.99 (1.00) |
| Any relapse after complete remission | 33 | 45 | 73 | 17 | 23 | 74 | 16 | 22 | 73 | 0.99 |
| Change in serum creatinine at 12 mo, mg/dl | 50 | Median = 0.02 Range = −1.25 to 0.64 |
25 | Median = 0.01 Range = −1.25 to 0.20 |
25 | Median = 0.02 Range =−0.47 to 0.64 |
0.16 | |||
| Exploratory outcomes | ||||||||||
| Complete remission by 4 wk | 22 | 50 | 44 | 16 | 25 | 64 | 6 | 25 | 24 | 0.01 (0.05) |
| Complete or partial remission by 4 wk | 39 | 50 | 78 | 20 | 25 | 80 | 19 | 25 | 76 | 0.99 (1.00) |
| Failed to achieve complete remission | 5 | 50 | 10 | 2 | 25 | 8 | 3 | 25 | 12 | 0.99 |
Adjusted P values for multiple analyses are shown in parentheses.
Secondary Outcomes
Remission Rates.
There were no significant differences between the treatment arms in the proportion of patients in complete remission at 16 or 26 weeks.
Intention-to-Treat Analysis.
At 16 weeks, 23 out of 25 (92%) patients in the prednisolone cohort and 19 out of 27 (70%) patients in the tacrolimus cohort were in complete remission (P=0.08; difference in remission rates, 22%; 95% CI, −3% to 43%). At 26 weeks, the proportions were 23 out of 25 (92%) in the prednisolone cohort and 22 out of 27 (81%) in the tacrolimus cohort (P=0.71; difference in rates 11%; 95% CI, −12% to 32%).
Per-Protocol Analysis.
Analysis of patients completing 8 weeks of treatment showed 23 out of 25 (92%) patients in the prednisolone cohort and 19 out of 25 (76%) patients in the tacrolimus cohort were in complete remission at 16 weeks (P=0.25; difference in remission rates, 16%; 95% CI, −8% to 38%). At 26 weeks, the proportions were 23 out of 25 (92%) for the prednisolone cohort and 22 out of 25 (88%) for the tacrolimus cohort (P=0.99; difference in remission rates, 4%; 95% CI, −17% to 25%) (Figure 2, Table 2).
Relapse Rates
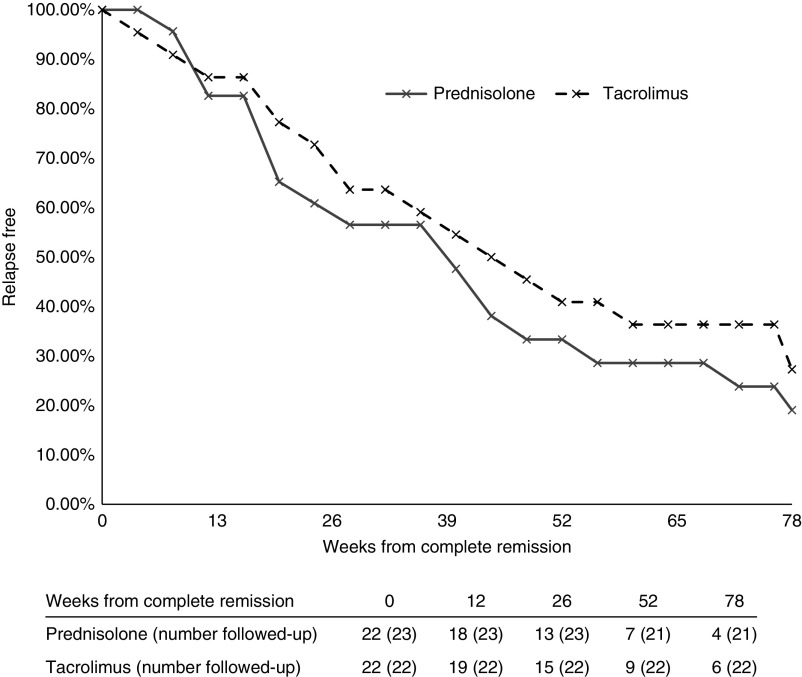
There was no significant difference between the tacrolimus and prednisolone cohorts in the proportion of patients who relapsed after achieving complete remission (Table 2). Relapse after complete remission was recorded in 17 out of 23 in the prednisolone cohort, and 16 out of 22 in the tacrolimus cohort (P=0.99; difference in relapse rates, 11%; 95% CI, −22% to 26%). The timings of relapses were similar between the two treatment groups (Figure 3). At 26 weeks from complete remission, 13 out of 23 (57%) in the prednisolone cohort and 15 out of 22 (68%) in the tacrolimus cohort were relapse free (P=0.54; difference in relapse rates, 11%; 95% CI, −16% to 42%). Two of the patients in remission in the prednisolone group subsequently left the United Kingdom and were lost to follow-up; one at 36 weeks and one at 40 weeks. At 52 weeks, seven out of 21 (33%) in the prednisolone cohort and nine out of 22 (41%) in the tacrolimus cohort were relapse free (P=0.75; difference in relapse rates, 8%; 95% CI, −21% to 38%). At 78 weeks, four out of 21 (19%) in the prednisolone cohort and six out of 22 (27%) in the tacrolimus cohort were relapse free (P=0.72; difference in relapse rates, 8%; 95% CI, −18% to 37%). Most relapses occurred after immunosuppression treatment had been stopped (nine out of 17 [53%] relapses in the prednisolone cohort and nine out of 16 [56%] in the tacrolimus cohort). The duration of remission was not statistically different between the prednisolone and tacrolimus cohorts (median time complete remission to relapse 22.0 weeks in the prednisolone cohort and 32.7 weeks in tacrolimus cohort; P=0.72).
Figure 3.
The proportion of patients who suffered a relapse after complete remission in the prednisolone (gray, solid line) and tacrolimus (black, dashed line) cohorts. The number of patients with relapse after complete remission at 26-week intervals is shown. Two of the patients in remission in the prednisolone group were lost to follow-up at 36 weeks and 40 weeks.
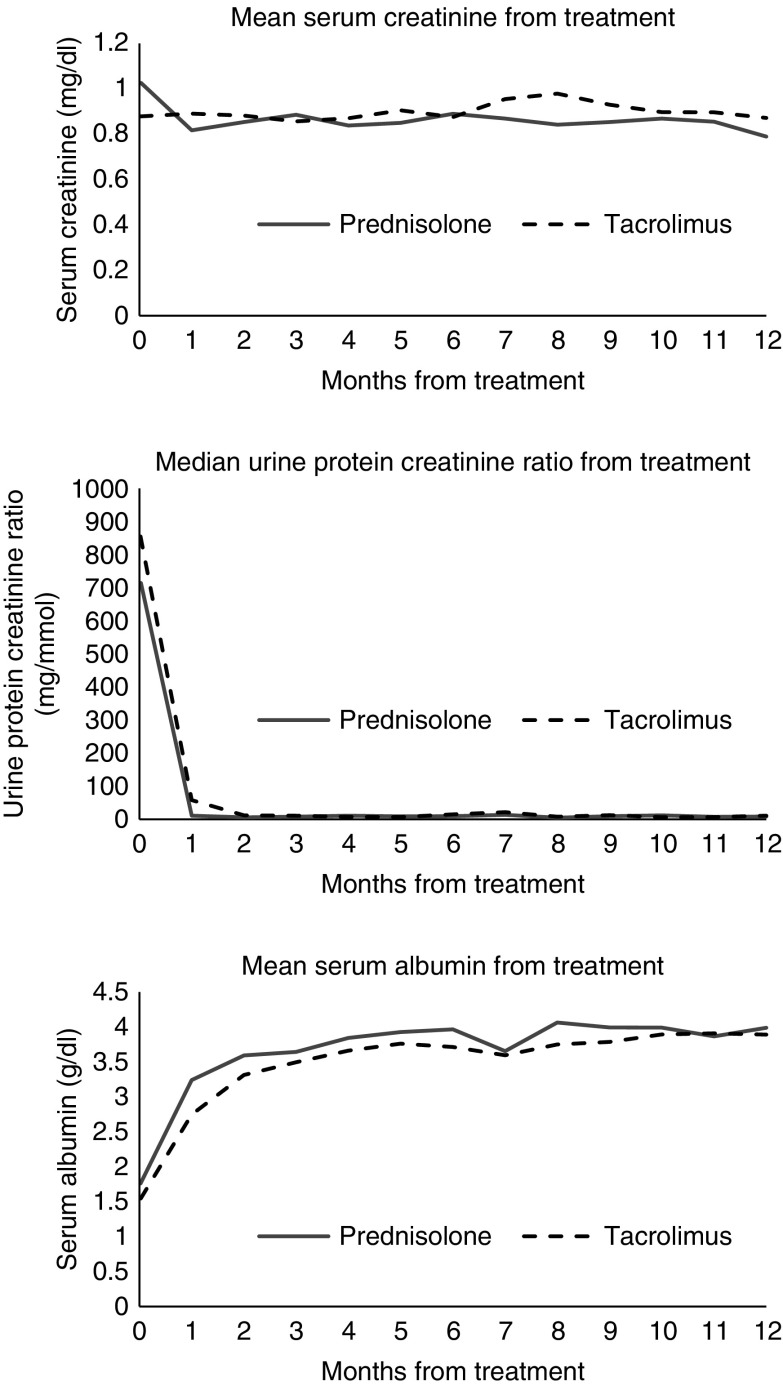
There were no significant differences in mean serum creatinine between the treatment groups during the 12-month follow-up period (Figure 4).
Figure 4.
Changes in mean serum creatinine, median urine protein-to-creatinine ratio, and mean serum albumin from the start of treatment and during follow-up in the prednisolone and tacrolimus treated cohorts. The urine protein-to-creatinine ratio data were nonparametrically distributed.
Other Outcomes
Post hoc analysis demonstrated that a significantly greater proportion of patients treated with prednisolone were in complete remission at 4 weeks. By intention-to-treat analysis, complete remission rates were 16 out of 25 (64%) in the prednisolone cohort and six out of 27 (22%) in the tacrolimus cohort (P=0.005; difference in remission rates, 42%; 95% CI, 12% to 63%). The per-protocol analysis showed 16 out of 25 (64%) patients in the prednisolone cohort and six out of 25 (24%) in the tacrolimus cohort were in complete remission at 4 weeks (P=0.01; difference in remission rates, 40%; 95% CI, 13% to 60%). However, post hoc analysis of total remission rates (complete or partial remission) at 4 weeks showed no significant difference between treatment arms (Figure 2B). By intention-to-treat analysis, 20 out of 25 patients (80%) in the prednisolone cohort and 19 out of 27 patients (70%) in the tacrolimus cohort achieved complete or partial remission at 4 weeks (P=0.53; difference in remission rates, 10%; 95% CI, −17% to 34%). The per-protocol analysis showed 20 out of 25 patients (80%) in the prednisolone cohort and 19 out of 25 patients (76%) in the tacrolimus cohort achieved complete or partial remission at 4 weeks (P=0.99; difference in remission rates, 4%; 95% CI, −22% to 29%).
Post hoc analysis of the per-protocol cohorts showed the mean serum albumin was significantly higher in the prednisolone than the tacrolimus cohort at 4 weeks follow-up (3.24 g/dl in the prednisolone and 2.75 g/dl in the tacrolimus cohorts; P=0.03; difference in means, 0.49 g/dl; 95% CI, 0.05 to 0.93 g/dl). No significant difference in albumin was detected at the other time points. Differences in UPCR did not reach statistical significance at 4-week follow-up (median prednisolone cohort UPCR, 11 mg/g [IQR, 6–109 mg/g]; median tacrolimus cohort UPCR, 58 mg/g [IQR, 6–216 mg/g]; P=0.36) or at subsequent time points.
Adverse Events
Adverse events were recorded from the intention-to-treat cohorts. Eighteen adverse events were recorded from 25 patients in the prednisolone cohort (0.72 events per patient), and 20 adverse events were recorded in the 27 patients in the tacrolimus cohort (0.74 events per patient; P=0.99 compared with the prednisolone cohort) (Table 3). There were four serious adverse events that required admission in the prednisolone and three in the tacrolimus cohorts (P=0.99 for difference in serious adverse event rates between the treatment cohorts). Specifically, there were admissions for perforated diverticular disease, severe headache, traumatic fractured radius, and lower respiratory tract infection in the prednisolone cohort (Table 3). In the tacrolimus cohort, there were admissions for severe hypertension, diarrhea and rash 2 days into treatment with subsequent withdrawal from the study, and lower respiratory tract infection 8 days into treatment with subsequent withdrawal from the study. There were no deaths during follow-up in either cohort. We recorded a similar number of infective episodes in each cohort (Table 3). We recorded one episode of hyperglycemia in each cohort, but both were treated with diet alone; we recorded no new cases of diabetes mellitus in either cohort (Table 3).
Table 3.
Adverse events recorded in the prednisolone and tacrolimus cohorts
| Adverse Events | Prednisolone (n=25) | Tacrolimus (n=27) |
|---|---|---|
| Total | 18 (4 SAEa) | 20 (3 SAEb) |
| Infections requiring antibiotics | 6 | 5 |
| URTI requiring antibiotics | 2 | 3 |
| LRTI requiring antibiotics | 3 (1 SAE) | 1 SAE |
| Urinary tract infections | 1 | |
| Infective diarrhea | 1 | |
| Vaginal candidiasis | 1 | |
| Musculoskeletal pain | 4 | |
| Gastrointestinal symptoms | 1 SAE | 5 (1 SAE) |
| Headache | 2 (1 SAE) | 1 |
| Gum pain | 2 | |
| Weight gain | 1 | |
| Low mood | 2 | |
| Acneiform rash | 2 | |
| Hyperglycemia | 1 | 1 |
| Uncontrolled hypertension | 1 SAE | |
| Deranged LFTs | 1 | |
| Traumatic limb fracture | 2 (1 SAE) |
SAE, serious adverse event; URTI, upper respiratory tract infection; LRTI, lower respiratory tract infection; LFT, liver function test.
SAEs recorded in the prednisolone cohort were one admission with perforated diverticular ulcer, one episode of severe headache requiring investigation (no organic cause identified), one patient with traumatic fractured radius, and one patient with lower respiratory tract infection requiring intravenous antibiotics and supplemental oxygen.
The SAE recorded in the tacrolimus cohort were one admission with uncontrolled hypertension, one admission with diarrhea and rash 2 days into treatment, and one admission with lower respiratory tract infection.
Discussion
Minimal change disease is a rare disease in adults, and there is a paucity of clinical trials. Corticosteroids are effective in minimal change disease, but the side effects are significant, hence many patients and their physicians would prefer to consider alternative treatments. However, there are few data in the literature on the effectiveness of steroid-free regimens in minimal change disease to inform clinicians and allow appropriate counseling of patients on the likely success of alternative regimens. This is the first prospective, multicenter, randomized trial of a corticosteroid-free treatment regimen for de novo minimal change disease in adults. Although the results did not meet our a priori definition of noninferiority, the lack of significant differences in primary and secondary outcomes between the treatment cohorts demonstrate tacrolimus monotherapy is an effective alternative to corticosteroids for the treatment of minimal change disease. To counter any ambiguity, we have provided intention-to-treat analysis as well as the per-protocol analysis of patient who completed at least 8 weeks of therapy. Post hoc analysis demonstrated more patients treated with prednisolone achieved complete remission at 4 weeks than in the tacrolimus arm. However, the clinical significance of this is not clear, as most patients in both groups had achieved significant reductions in proteinuria (UPCR<300 mg/mmol) by 4 weeks, and analysis of total remission rates (complete or partial remission) at 4 weeks showed no significant difference between treatment arms. This warrants further study, as it may be relevant in some patients, especially perhaps those presenting with severe complications of nephrotic syndrome. For patients treated with steroids initially, concomitant tacrolimus treatment, may allow early steroid withdrawal and limitation of steroid-associated toxicity, as in the study by Li et al. (39).
Rates of complete remission in this trial are comparable with those documented in previous minimal change disease patient series for both steroids and calcineurin inhibitors. Retrospective studies of prednisolone treatment regimens (1 mg/kg daily for 4–16 weeks, and with 24 weeks total treatment) similar to our study, documented remission rates of 70%–90% by 16 weeks of treatment (8,9). In general, calcineurin inhibitor use has predominantly been reported in steroid-resistant, steroid-dependent, and frequently relapsing minimal change disease cases and involved 9–12 months of treatment, making direct comparison difficult. However, remission rates from these studies were similar at between 60% and 80% with an average time to remission of about 5 weeks (8). With regards to de novo minimal change disease, a single case series of 12 minimal change disease patients with relative contraindications to corticosteroids treated with cyclosporine alone for 12 months documented response rates of 75% and minimal adverse effects (24). Data from patients with minimal change disease treated with tacrolimus without prednisolone are limited. However, a number of studies suggest tacrolimus is effective in combination with prednisolone (16) and is better tolerated than cyclosporine as a steroid-sparing agent (19,26,28). The most comprehensive clinical trial of de novo minimal change disease in adults comes from China. In this, Li et al. compared the use of tacrolimus and prednisolone monotherapy in adults with minimal change disease (39) after treatment of all patients with high-dose daily intravenous methylprednisolone (0.8 mg/kg) for 10 days (39). Notably high remission rates were achieved in both cohorts, more than 90% of both treatment cohorts were in remission by 4 weeks (39). This may be because of the initial use of high-dose intravenous corticosteroids in all their patients, but there may also be a difference in response rates between Chinese and UK patients (13,23).
Relapse rates were similar in our tacrolimus and prednisolone treatment groups and comparable to the 48%–76% relapse rates reported in other case series (9,13,14,42). Our relapse rate is higher than either of the cohorts in the Li et al. study (39). This may also be explained by the use of intravenous methylprednisolone in the Li et al. study or to racial differences in relapse rates. Other studies have documented relapse rates of 73%–76% in predominantly white cohorts compared with 48% in a large cohort from China (9,13,23). The relapse rates may also reflect treatment regimen durations and doses. At trial design, the sparsity of available data on tacrolimus treatment for minimal change disease led us to adopt a regimen of modest treatment exposure. We also aimed to use a corticosteroid regimen of similar duration for comparison. This resulted in regimens of relatively modest duration and dosing. Regimens of longer duration and higher dosing may improve relapse rates and should be investigated in future trials.
This study was motivated by the significant adverse effect burden associated with the use of corticosteroids in patients with minimal change disease (11,41). Obesity and metabolic syndromes consistently associate with increased all-cause mortality (43). There are also significant unpleasant cosmetic side effects associated with steroids such as striae and “moon facies,” which can be especially debilitating for this group of patients, particularly the young adults, and also have an effect on adherence with treatment. Although our study was not powered to demonstrate differences in side effects, it was reasoned that noninferiority in terms of remission from nephrotic syndrome in patients with minimal change disease would be likely to portend side-effect benefits for patients treated with tacrolimus compared with corticosteroids. We recorded equal and relatively few adverse events in both treatment regimens. The study by Li et al. recorded a higher rate of total adverse events (209 adverse events in 119 study patients), and did show an increased adverse event frequency in the prednisolone cohort, particularly osteoporosis and new-onset hypertension (39). After intravenous methylprednisolone for 10 days, the prednisolone treatment regimen used by Li et al. provided 1 mg/kg oral prednisolone (up to a maximum of 80 mg/d) and 22–24 weeks total prednisolone treatment. In comparison our regimen involved no intravenous methylprednisolone, prednisolone doses up to a maximum of 60 mg/d, and a minimum of 16 weeks of treatment. The duration and target plasma levels of tacrolimus were similar in the two studies. The lower prednisolone doses and the steroid-free study arm in our study may explain the differences in adverse event burden between the studies. Additional adverse events may be recorded with longer duration of follow-up, especially in patients with relapsing minimal change disease who require repeated courses of treatment to achieve and maintain remission.
Our study is relatively small but is none the less important, as most other published data in adults with de novo minimal change disease relates to retrospective studies and case series, particularly in Western populations, reflecting the difficulty of conducting studies in this group of patient with a rare disease.
Corticosteroids are standard therapy for de novo minimal change disease. This study is the first multicenter, randomized, controlled trial of a steroid-free regimen in adults. On the basis of remission, relapse and adverse event rates, it provides evidence that tacrolimus monotherapy is an effective alternative to corticosteroids for de novo minimal change disease. This is highly significant for the treatment of patients with this disease.
Disclosures
Dr. Barratt reports receiving consultant fees from Aduro Biotech, Alnylam, Calliditas, EMD Serono, Novartis, Omeros, Retrophin, and Visterra outside the submitted work. Dr. Cook reports receiving consultant fees from Achillion Pharmaceuticals and PsiOxus Therapeutics outside the submitted work. Dr. Galliford reports receiving consultant fees as a scientific advisory board member from Alexion Pharmaceuticals and Astellas Pharmaceuticals outside of the submitted work. Dr. Griffith reports receiving consultant fees as a scientific advisory board member from Vifor Pharma UK outside the submitted work. Dr. Levy reports receiving consultant fees as an educational advisor from Gilead Sciences outside the submitted work. Dr. Lightstone reports receiving consultant fees from Achillion, AstraZeneca, Aurinia, Genentech, GSK, Medimmune, Pfizer, Roche, and UCB, and research support from Roche outside the submitted work. Dr. Sood reports receiving travel support from Alexion Pharmaceuticals to attend a sponsored educational event, and from Vifor Pharma UK outside the submitted work. Dr. Turner reports receiving consultant fees as an advisory board member from Otsuka, Sanofi Genzyme, and Shire (Takeda). Dr. Warwicker reports receiving lecture fees from Alexion Pharmaceuticals outside of the submitted work. All other authors have nothing to disclose.
Funding
We acknowledge the National Institute for Health Research Imperial Biomedical Research Centre for support of this study.
Data Sharing Statement
We are happy to share deidentified, collected cohort data and results for comparative and replication research. Additional related documents, such as the study protocol, will be available on request. The planned data use must not breach the ethical approval obtained for our study. There is no prespecified time limit to data availability. Please contact the corresponding author to request access.
Acknowledgments
We thank clinical research nurse Tom Walters for his support and assistance, Bernard North (Department of Statistics, Imperial College London) for advice on calculation of samples size, and Dr. Adam Mclean for generating the random allocation sequence.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Haas M, Meehan SM, Karrison TG, Spargo BH: Changing etiologies of unexplained adult nephrotic syndrome: A comparison of renal biopsy findings from 1976-1979 and 1995-1997. Am J Kidney Dis 30: 621–631, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Gesualdo L, Di Palma AM, Morrone LF, Strippoli GF, Schena FP; Italian Immunopathology Group, Italian Society of Nephrology : The Italian experience of the national registry of renal biopsies. Kidney Int 66: 890–894, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Tune BM, Mendoza SA: Treatment of the idiopathic nephrotic syndrome: Regimens and outcomes in children and adults. J Am Soc Nephrol 8: 824–832, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Huang JJ, Hsu SC, Chen FF, Sung JM, Tseng CC, Wang MC: Adult-onset minimal change disease among Taiwanese: Clinical features, therapeutic response, and prognosis. Am J Nephrol 21: 28–34, 2001. [DOI] [PubMed] [Google Scholar]
- 5.McIntyre P, Craig JC: Prevention of serious bacterial infection in children with nephrotic syndrome. J Paediatr Child Health 34: 314–317, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Kerlin BA, Ayoob R, Smoyer WE: Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol 7: 513–520, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahmoodi BK, ten Kate MK, Waanders F, Veeger NJ, Brouwer JL, Vogt L, Navis G, van der Meer J: High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: Results from a large retrospective cohort study. Circulation 117: 224–230, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Radhakrishnan J, Appel AS, Valeri A, Appel GB: The nephrotic syndrome, lipids, and risk factors for cardiovascular disease. Am J Kidney Dis 22: 135–142, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Waldman M, Crew RJ, Valeri A, Busch J, Stokes B, Markowitz G, D’Agati V, Appel G: Adult minimal-change disease: Clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol 2: 445–453, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Black DA, Rose G, Brewer DB: Controlled trial of prednisone in adult patients with the nephrotic syndrome. BMJ 3: 421–426, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coggins CH: Adult minimal change nephropathy: Experience of the collaborative study of glomerular disease. Trans Am Clin Climatol Assoc 97: 18–26, 1986. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L, Cheng J, Zhou J, Wu C, Chen J: Enhanced steroid therapy in adult minimal change nephrotic syndrome: A systematic review and meta-analysis. Intern Med 54: 2101–2108, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Szeto CC, Lai FM, Chow KM, Kwan BC, Kwong VW, Leung CB, Li PK: Long-term outcome of biopsy-proven minimal change nephropathy in Chinese adults. Am J Kidney Dis 65: 710–718, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Shinzawa M, Yamamoto R, Nagasawa Y, Oseto S, Mori D, Tomida K, Hayashi T, Izumi M, Fukunaga M, Yamauchi A, Tsubakihara Y, Isaka Y: Comparison of methylprednisolone plus prednisolone with prednisolone alone as initial treatment in adult-onset minimal change disease: A retrospective cohort study. Clin J Am Soc Nephrol 9: 1040–1048, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mak SK, Short CD, Mallick NP: Long-term outcome of adult-onset minimal-change nephropathy. Nephrol Dial Transplant 11: 2192–2201, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Eguchi A, Takei T, Yoshida T, Tsuchiya K, Nitta K: Combined cyclosporine and prednisolone therapy in adult patients with the first relapse of minimal-change nephrotic syndrome. Nephrol Dial Transplant 25: 124–129, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Hogan J, Radhakrishnan J: The treatment of minimal change disease in adults. J Am Soc Nephrol 24: 702–711, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Al-Khader AA, Lien JW, Aber GM: Cyclophosphamide alone in the treatment of adult patients with minimal change glomerulonephritis. Clin Nephrol 11: 26–30, 1979. [PubMed] [Google Scholar]
- 19.Li H, Shi X, Shen H, Li X, Wang H, Li H, Xu G, Chen J: Tacrolimus versus intravenous pulse cyclophosphamide therapy in Chinese adults with steroid-resistant idiopathic minimal change nephropathy: A multicenter, open-label, nonrandomized cohort trial. Clin Ther 34: 1112–1120, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Mak SK, Lo KY, Wong CY, Tong GM, Wong PN, Wong AK: Treatment with cyclophosphamide in elderly-onset nephrotic syndrome. Nephron Clin Pract 101: c25–c32, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Uldall PR, Feest TG, Morley AR, Tomlinson BE, Kerr DN: Cyclophosphamide therapy in adults with minimal-change nephrotic syndrome. Lancet 1: 1250–1253, 1972. [DOI] [PubMed] [Google Scholar]
- 22.Ponticelli C, Edefonti A, Ghio L, Rizzoni G, Rinaldi S, Gusmano R, Lama G, Zacchello G, Confalonieri R, Altieri P: Cyclosporin versus cyclophosphamide for patients with steroid-dependent and frequently relapsing idiopathic nephrotic syndrome: A multicentre randomized controlled trial. Nephrol Dial Transplant 8: 1326–1332, 1993. [PubMed] [Google Scholar]
- 23.Nolasco F, Cameron JS, Heywood EF, Hicks J, Ogg C, Williams DG: Adult-onset minimal change nephrotic syndrome: A long-term follow-up. Kidney Int 29: 1215–1223, 1986. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto H, Nakao T, Okada T, Nagaoka Y, Takeguchi F, Tomaru R, Iwasawa H: Favorable outcome of low-dose cyclosporine after pulse methylprednisolone in Japanese adult minimal-change nephrotic syndrome. Intern Med 43: 668–673, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Bruchfeld A, Benedek S, Hilderman M, Medin C, Snaedal-Jonsdottir S, Korkeila M: Rituximab for minimal change disease in adults: Long-term follow-up. Nephrol Dial Transplant 29: 851–856, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Li H, Chen J, He Q, Lv R, Lin W, Li Q, He X, Qu L, Suya W: Tacrolimus as a steroid-sparing agent for adults with steroid-dependent minimal change nephrotic syndrome. Nephrol Dial Transplant 23: 1919–1925, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Li H, Ye H, Li Q, He X, Zhang X, Chen Y, Han F, He Q, Wang H, Chen J: Tacrolimus therapy in adults with steroid- and cyclophosphamide-resistant nephrotic syndrome and normal or mildly reduced GFR. Am J Kidney Dis 54: 51–58, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Choudhry S, Bagga A, Hari P, Sharma S, Kalaivani M, Dinda A: Efficacy and safety of tacrolimus versus cyclosporine in children with steroid-resistant nephrotic syndrome: A randomized controlled trial. Am J Kidney Dis 53: 760–769, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Xu N, Li H, Han F, Wang R, He Q, He X, Chen J: Tacrolimus as rescue therapy for adult-onset refractory minimal change nephrotic syndrome with reversible acute renal failure. Nephrol Dial Transplant 28: 2306–2312, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Liao R, Liu Q, Zheng Z, Fan J, Peng W, Kong Q, He H, Yang S, Chen W, Tang X, Yu X: Tacrolimus protects podocytes from injury in lupus nephritis partly by stabilizing the cytoskeleton and inhibiting podocyte apoptosis. PLoS One 10: e0132724, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyrier A, Condamin MC, Broneer D: Treatment of adult idiopathic nephrotic syndrome with cyclosporin A: Minimal-change disease and focal-segmental glomerulosclerosis. Collaborative Group of the French Society of Nephrology. Clin Nephrol 35[Suppl 1]: S37–S42, 1991. [PubMed] [Google Scholar]
- 32.Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, Grinyo J; FREEDOM Study Group : A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplant 8: 307–316, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Vanrenterghem Y, Lebranchu Y, Hené R, Oppenheimer F, Ekberg H: Double-blind comparison of two corticosteroid regimens plus mycophenolate mofetil and cyclosporine for prevention of acute renal allograft rejection. Transplantation 70: 1352–1359, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Van Veldhuisen P; Astellas Corticosteroid Withdrawal Study Group : A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg 248: 564–577, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Nematalla AH, Bakr MA, Gheith OA, Elagroudy AE, Elshahawy M, Aghoneim M: Steroid-avoidance immunosuppression regimen in live-donor renal allotransplant recipients: A prospective, randomized, controlled study. Exp Clin Transplant 5: 673–679, 2007. [PubMed] [Google Scholar]
- 36.Knight SR, Morris PJ: Interaction between maintenance steroid dose and the risk/benefit of steroid avoidance and withdrawal regimens following renal transplantation. Transplantation 92: e63–e64, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Medjeral-Thomas N, Ziaj S, Condon M, Galliford J, Levy J, Cairns T, Griffith M: Retrospective analysis of a novel regimen for the prevention of venous thromboembolism in nephrotic syndrome. Clin J Am Soc Nephrol 9: 478–483, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gart JJ, Nam JM: Approximate interval estimation of the difference in binomial parameters: Correction for skewness and extension to multiple tables. Biometrics 46: 637–643, 1990. [PubMed] [Google Scholar]
- 39.Li X, Liu Z, Wang L, Wang R, Ding G, Shi W, Fu P, He Y, Cheng G, Wu S, Chen B, Du J, Ye Z, Tao Y, Huo B, Li H, Chen J: Tacrolimus monotherapy after intravenous methylprednisolone in adults with minimal change nephrotic syndrome. J Am Soc Nephrol 28: 1286–1295, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benjamini Y, Krieger AM, Yekutieli D: Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93: 491–507, 2006 [Google Scholar]
- 41.Imbasciati E, Gusmano R, Edefonti A, Zucchelli P, Pozzi C, Grassi C, Della Volpe M, Perfumo F, Petrone P, Picca M: Controlled trial of methylprednisolone pulses and low dose oral prednisone for the minimal change nephrotic syndrome. Br Med J (Clin Res Ed) 291: 1305–1308, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szeto CC, Lai FM, To KF, Wong TY, Chow KM, Choi PC, Lui SF, Li PK: The natural history of immunoglobulin a nephropathy among patients with hematuria and minimal proteinuria. Am J Med 110: 434–437, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Flegal KM, Kit BK, Orpana H, Graubard BI: Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 309: 71–82, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]