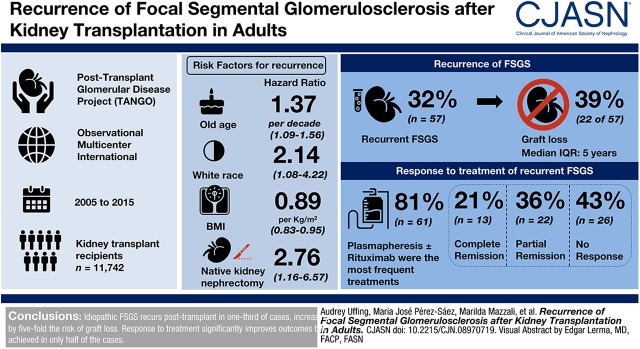
Visual Abstract
Keywords: focal segmental glomerulosclerosis, transplant outcomes, recurrence, renal transplantation, risk factors, humans, adult, kidney transplantation, graft survival, rituximab, incidence, cohort studies, plasmapheresis, sample size, body mass index, transplant recipients, kidney diseases, kidney, Nephrectomy, treatment outcome, registries
Abstract
Background and objectives
FSGS recurrence after kidney transplantation is a major risk factor for graft loss. However, the natural history, clinical predictors, and response to treatment remain unclear because of small sample sizes and poor generalizability of single-center studies, and disease misclassification in registry-based studies. We therefore aimed to determine the incidence, predictors, and treatment response of recurrent FSGS in a large cohort of kidney transplant recipients.
Design, setting, participants, & measurements
The Post-Transplant Glomerular Disease (TANGO) project is an observational, multicenter, international cohort study that aims to investigate glomerular disease recurrence post-transplantation. Transplant recipients were screened for the diagnosis of idiopathic FSGS between 2005 and 2015 and details were recorded about the transplant, clinical outcomes, treatments, and other risk factors.
Results
Among 11,742 kidney transplant recipients screened for FSGS, 176 had a diagnosis of idiopathic FSGS and were included. FSGS recurred in 57 patients (32%; 95% confidence interval [95% CI], 25% to 39%) and 39% of them lost their graft over a median of 5 (interquartile range, 3.0–8.1) years. Multivariable Cox regression revealed a higher risk for recurrence with older age at native kidney disease onset (hazard ratio [HR], 1.37 per decade; 95% CI, 1.09 to 1.56). Other predictors were white race (HR, 2.14; 95% CI, 1.08 to 4.22), body mass index at transplant (HR, 0.89 per kg/m2; 95% CI, 0.83 to 0.95), and native kidney nephrectomies (HR, 2.76; 95% CI, 1.16 to 6.57). Plasmapheresis and rituximab were the most frequent treatments (81%). Partial or complete remission occurred in 57% of patients and was associated with better graft survival.
Conclusions
Idiopathic FSGS recurs post-transplant in one third of cases and is associated with a five-fold higher risk of graft loss. Response to treatment is associated with significantly better outcomes but is achieved in only half of the cases.
Introduction
FSGS is one of the leading glomerular causes of kidney failure in adults (1). When no secondary cause (genetic, viral, drug-associated, or adaptive [2]) can be identified and the patient presents with a clinical history of nephrotic syndrome, FSGS is termed primary or idiopathic. The accurate distinction between idiopathic and secondary causes, however, remains challenging, particularly if ultrastructural evaluation of the kidney biopsy or genetic testing are missing (3).
Distinguishing between idiopathic and secondary FSGS is especially important in patients being considered for kidney transplantation because idiopathic forms frequently recur in the graft with a substantial rate of subsequent graft loss. Reported rates of recurrence are wide, ranging from 17% to 55% (4–23) in smaller studies and from 9% to 15% (24–27) in registry-based studies. Because FSGS is rare, most published studies are limited by small sample size and thus insufficient power to precisely determine incidence, predictors, and outcomes of FSGS recurrence. Administrative registries with large sample sizes, such as the US Renal Data System, are limited by missing data, misclassification of glomerular disease diagnoses (28), and failure to capture cases of FSGS recurrence that do not lead to graft loss, rendering data unreliable for drawing robust conclusions (29). The same limitations are present in prior studies that aimed to identify predictors of recurrence, in which most proposed predictors were identified by univariable analysis without adjustment for relevant confounding factors (Supplemental Table 1).
To combine the strengths of a large registry, with the detailed and accurate nature of single-center studies, we established the Post-Transplant Glomerular Disease (TANGO) study (30). This international network of centers, located in three different continents, studies the recurrence of glomerular disease after kidney transplantation, using a standardized approach to data and biosample collection. Herein, we examine retrospectively collected data of patients with biopsy-proven native kidney FSGS from 15 centers and report FSGS recurrence rates, risk factors for recurrence, and responses to therapy. To our knowledge, this is the largest nonregistry-based cohort ever created to study this patient population.
Materials and Methods
Study Design, Objectives, and Predictors
We performed a multicenter, retrospective study in patients from 15 kidney transplant centers participating in the TANGO study in Europe, Brazil, and the United States. Our primary objective was to determine the incidence of FSGS recurrence after kidney transplantation in patients with idiopathic FSGS. We also aimed to identify risk factors for FSGS recurrence, to compare clinical outcomes of patients with and without recurrent FSGS, and to evaluate the effect of different treatments on recurrent FSGS outcomes. A more detailed description of used methods can be found in Supplemental Appendix 1.
Patient Selection and Data Collection
All adults (aged ≥16 years) who received a kidney transplant between January 2005 and December 2015 were reviewed by TANGO investigators in the 15 collaborating centers. Patients with a biopsy-proven native kidney diagnosis of idiopathic FSGS were included. Patients with secondary causes of FSGS, as determined by kidney biopsy (e.g., only mild effacement of foot processes, glomerular hypertrophy) and/or by clinical features (e.g., absent nephrotic syndrome at disease onset or a clear secondary cause, including infections, drugs, reduced kidney mass, long-standing diabetes, or morbid obesity) were excluded. Electron microscopy evaluation of the kidney biopsy was performed and available for 36 patients. From all patients who were included in the study, data were extracted from their medical records. Two patients with less than half a year of follow-up were excluded from analysis. Ten other patients who were lost to follow-up (median time to lost to follow-up, 5.7 years; interquartile range [IQR], 1.84–7.36) were censored at time of loss to follow-up in the Cox proportional hazards analysis.
Statistical Analyses
Data are shown as frequencies (percentages) for categorical variables, and as medians (IQR) or means±SD for continuous variables. Statistical analysis of Table 1 was done by complete case analysis. Continuous variables were analyzed by t test, and binary and categorical variables by chi-squared or Fisher exact test, as appropriate. Confidence intervals around proportions were calculated by Wald testing.
Table 1.
Baseline recipient and donor characteristics in all patients and according to FSGS recurrence
| Baseline Characteristic | Overall Cohort (n=176) | No Recurrence (n=119) | Recurrence (n=57) |
|---|---|---|---|
| Follow-up, yr | 5.2 [3.0–8.1] | 6.2 [3.9–8.4] | 3.3 [1.6–5.9] |
| Age at transplantation, yr | 38 [29–47] | 38 [30–46] | 39 [28–49] |
| Age at diagnosis, yr | 27 [17–40] | 27 [17–36] | 29 [17–43] |
| Male sex | 106 (60) | 72 (61) | 34 (60) |
| Race/ethnicity | |||
| White | 98 (56) | 63 (53) | 35 (61) |
| Black | 19 (11) | 13 (11) | 6 (11) |
| Hispanic | 8 (5) | 6 (5) | 2 (4) |
| Asian | 8 (5) | 8 (7) | 0 (0) |
| Mixed | 18 (10) | 14 (12) | 4 (7) |
| Other/unknown | 25 (14) | 15 (13) | 10 (18) |
| BMI at transplantation | 25±5 | 26±5 | 24±5 |
| Manifestation of FSGS in native kidney | |||
| Nephrotic syndrome | 122 (69) | 78 (66) | 44 (77) |
| Unknown | 54 (31) | 41 (34) | 13 (23) |
| Time from diagnosis to ESKD, mo | 38 [14–75] | 40 [12–83] | 32 [15–62] |
| Time on dialysis, mo | 29 [11–57] | 29 [11–60] | 32 [12–51] |
| Type of dialysis | |||
| Hemodialysis | 127 (73) | 84 (71) | 43 (77) |
| Peritoneal dialysis | 21 (12) | 13 (11) | 8 (14) |
| Both | 15 (9) | 12 (10) | 3 (5) |
| Pre-emptive transplant | 12 (7) | 10 (8) | 2 (4) |
| Nephrectomy of native kidneys | 12 (7) | 4 (3) | 8 (14) |
| Prior transplant loss due to FSGS | 16 (9) | 6 (5) | 10 (18) |
| Number of prior transplants | |||
| None | 132 (75) | 89 (75) | 44 (75) |
| 1 | 39 (22) | 30 (25) | 9 (16) |
| 2 | 5 (3) | 0 (0) | 5 (9) |
| PRA>50% | 28 (18) | 20 (18) | 8 (17) |
| DSA at transplantation | 14 (9) | 10 (9) | 4 (8) |
| Deceased donor | 118 (67) | 82 (69) | 36 (63) |
| Extended criteria donor (KDPI>85%) | 16 (14) | 12 (15) | 4 (12) |
| Cold ischemia time, h | 19±7.0 | 18.6±6.9 | 20.1±7.2 |
| Living donor | 58 (33) | 37 (31) | 21 (37) |
| Living related donor | 39 (67) | 22 (59) | 17 (81) |
| Donor age, yr | 40±14 | 40±15 | 40±14 |
| HLA-A/B/DR mismatch | 3.2±1.6 | 3.3±1.6 | 2.9±1.6 |
| Induction therapy | |||
| None | 22 (13) | 15 (13) | 7 (12) |
| Anti-thymocyte globulin | 72 (42) | 46 (40) | 26 (46) |
| Basiliximab | 73 (42) | 51 (44) | 22 (39) |
| Daclizumab | 6 (3) | 4 (3) | 2 (4) |
| Immunosuppressive regimen | |||
| Tacrolimus + MMF + steroids | 126 (72) | 85 (72) | 41 (72) |
| Cyclosporine + MMF + steroids | 29 (17) | 21 (18) | 8 (14) |
| Tacrolimus + MMF | 9 (5) | 5 (4) | 4 (7) |
| Other | 11 (6) | 7 (6) | 4 (7) |
| Prophylactic plasmapheresis | 22 (13) | 13 (11) | 9 (16) |
Values represent frequency (percentage), mean±SD, or median [interquartile range]. BMI, body mass index; PRA, panel reactive antibody; DSA, donor-specific antibody; KDPI, kidney donor profile index; MMF, mycophenolate mofetil.
Cumulative incidence, Kaplan–Meier curves, and 95% confidence intervals (95% CIs) around curves were graphed by Prism 5.0b software (GraphPad). Predictors were selected informed by prior literature and clinical practice (4–22,24–27,31). Univariable Cox proportional hazards regression was performed by complete case analysis. Missing data per predictor is shown in Supplemental Table 2. Because of the large amount of missing data in the predictor “time to ESKD” (40 missing values, 23%), this predictor was not imputed and was removed from the multivariable model. STATA’s multiple imputation by chained equations (MICE) procedure was used to impute missing data (described in more detail in Supplemental Appendix 1). Cox proportional hazards regression was performed, with categorical variables entered as binary variables, analyzed as largest group versus other groups (e.g., white race versus other races). Backward selection was used to remove collinear variables and select a final model from previously defined predictors with both stay and entry criteria of P=0.05. Schoenfeld residuals were evaluated to assess for violation of the proportional-hazard assumption. Deviance residuals were used to examine model accuracy and outliers, after which time on dialysis was log-transformed to improve random scatter of residuals. Longitudinal data were collected yearly after transplantation and means of groups were graphed over time. For eGFR, in case an in between year of follow-up was missing, the value was imputed using interpolation by STATA. An eGFR value of 5 was imputed for patients after experiencing graft loss. Other longitudinal data were not imputed. A two-sided P value of <0.05 was deemed significant in all used tests. Statistical analyses were performed using Prism 5.0b software (GraphPad) and STATA version 15.1 (StataCorp.).
Results
Cohort Demographics
A total of 11,742 patients who received a kidney transplant between 2005 and 2015 were screened for FSGS in four European centers, five Brazilian centers, and five United States centers; 253 patients with idiopathic FSGS were included in our online database. Fifty nine patients did not meet inclusion criteria and were excluded from analysis (Supplemental Figure 1). Twenty-two of these patients were excluded because their primary kidney disease did not manifest with nephrotic syndrome (none of these patients experienced recurrence of FSGS). Finally, 176 patients with biopsy-proven primary FSGS were included for analysis. Patient and donor characteristics of all patients, as well as by recurrence status (recurrent/nonrecurrent FSGS), are shown in Table 1.
Recurrence of FSGS
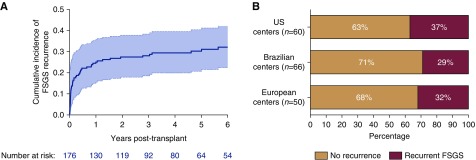
Fifty-seven patients experienced a recurrence of FSGS post-transplant (32%; 95% CI, 25% to 39%). Most recurrences occurred early after transplant (Figure 1A), with a median time to recurrence of 1.5 months (IQR, 0–11 months). The incidence of recurrent FSGS differed across geographical regions (Figure 1B). A higher percentage of patients in the recurrence group (9%) had received two prior transplants compared with nonrecurrent patients (P=0.003). Body mass index (BMI) of patients with a recurrence was lower than of patients without a recurrence (24±5 kg/m2 versus 26±5 kg/m2, respectively; P=0.02). More patients with a recurrence had a prior nephrectomy of native kidneys (14% versus 3%, respectively; P=0.02). Other parameters did not differ between patients with and without recurrent FSGS (Table 1).
Figure 1.
FSGS recurs in one third of kidney transplant recipients. (A) Cumulative incidence curve of FSGS recurrence in kidney transplant recipients with biopsy-proven idiopathic FSGS. Overall recurrence of FSGS was 32%, with median time to recurrence of 1.5 months. Shaded area around the curve represents the 95% confidence interval. (B) Recurrence rates per geographical location of the centers.
FSGS Recurrence and Graft Survival
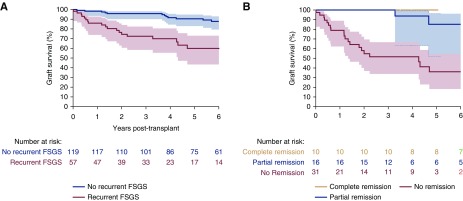
Graft failure occurred in 18 patients (15%) without recurrence and in 22 patients with recurrence (39%) (Figure 2A). After adjusting for important confounders such as HLA mismatch, pretransplant donor-specific antibody, donor type and age, and post-transplant rejection, Cox regression revealed a hazard ratio (HR) for graft loss of 4.80 (95% CI, 2.85 to 12.16; P<0.001) in patients with versus without recurrence. Graft loss was mainly confined to patients who failed to enter remission (20 out of 31 patients, 65%), with a median time from recurrence to graft loss of 7 months (IQR, 2–18 months) (Figure 2B). Two out of 16 patients (13%) with partial remission experienced graft loss after 3.2 and 6.4 years after recurrence, whereas ten patients with complete remission did not experience graft loss. Overall, FSGS recurrence was associated with a five-fold higher risk of graft loss, mainly because of the high rate of graft loss in the patients without response to treatment of recurrent FSGS. Ten patients died during the follow-up time: seven patients in the nonrecurrence group (6%) versus three patients (5%) who experienced FSGS recurrence.
Figure 2.
Recurrence of FSGS is associated with reduced graft survival, especially in patients with no response to treatment. (A) Kaplan–Meier graft survival curve comparing patients with and without recurrent FSGS after kidney transplantation. (B) Kaplan–Meier graft survival curve comparing only patients with recurrent FSGS stratified by their treatment response. Areas around the curve represent the 95% confidence intervals.
Predictors of FSGS Recurrence
Univariable Cox regression revealed associations between FSGS recurrence and BMI at transplant as well as history of native kidney nephrectomies (Table 2). After multivariable analysis and backward selection of variables in the model, age at diagnosis (HR, 1.31; 95% CI, 1.09 to 1.56 per decade; P=0.003), white race (HR, 2.14; 95% CI, 1.08 to 4.22; P=0.03), BMI (HR, 0.89; 95% CI, 0.83 to 0.95 per 1 kg/m2; P=0.001), and native nephrectomy (HR, 2.76; 95% CI, 1.16 to 6.57; P=0.02) remained significant variables. The geographical region was also of importance in multivariable analysis, with centers in Brazil and Europe having lower recurrence rates than in the United States (HR, 0.46; 95% CI, 0.24 to 0.90; P=0.02 and HR, 0.39; 95% CI, 0.19 to 0.79; P=0.009, respectively). Time from native kidney FSGS onset to ESKD was not included in the multivariable model because of 23% of missing data. A univariable complete case analysis did not reveal an association between time to ESKD and recurrent FSGS (HR, 1.00; 95% CI, 0.99 to 1.00; P=0.26).
Table 2.
Univariable and multivariable Cox-hazard analysis in patients without FSGS recurrence compared with patients with FSGS recurrence
| Predictors | Missing Values, n (%) | Univariable Analysis | Univariable Analysis | Multivariable Analysis | Multivariable Analysis | Model Selection |
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI), P Value | ||
| Geographic location of center | ||||||
| United States | 0 (0) | Ref | Ref | Ref | ||
| Brazil | 0 (0) | 1.42 (0.77–2.62) | 0.27 | 0.45 (0.21 to 0.98) | 0.05 | 0.46 (0.24 to 0.90), 0.02 |
| Europe | 0 (0) | 1.11 (0.57 to 2.17) | 0.74 | 0.39 (0.18 to 0.87) | 0.02 | 0.39 (0.19 to 0.79), 0.009 |
| Age at diagnosis FSGS, per 10 yr | 4 (2) | 1.14 (0.96 to 1.37) | 0.12 | 1.37 (1.12 to 1.68) | 0.002 | 1.31 (1.09 to 1.56), 0.003 |
| White race | 19 (11)a | 1.80 (0.95 to 3.41) | 0.07 | 2.39 (1.16 to 4.96) | 0.02 | 2.14 (1.08 to 4.22), 0.03 |
| BMI, per 1 kg/m2 | 1 (1) | 0.94 (0.89 to 0.99) | 0.03 | 0.89 (0.83 to 0.96) | 0.002 | 0.89 (0.83 to 0.95), 0.001 |
| Time on dialysis, per mo (log) | 2 (1) | 1.04 (0.86 to 1.27) | 0.69 | 1.10 (0.85 to 1.42) | 0.49 | NS |
| Native nephrectomy | 1 (1) | 2.56 (1.21 to 5.41) | 0.01 | 2.89 (1.09 to 7.66) | 0.03 | 2.76 (1.16 to 6.57), 0.02 |
| Living donor | 0 (0) | 1.23 (0.72 to 2.11) | 0.45 | 1.55 (0.76 to 3.16) | 0.23 | NS |
| Age donor, per 10 yr | 14 (8) | 0.99 (0.82 to 1.19) | 0.90 | 0.84 (0.66 to 1.06) | 0.14 | NS |
| HLA mismatch >3 | 10 (6) | 0.65 (0.37 to 1.14) | 0.14 | 0.71 (0.39 to 1.31) | 0.27 | NS |
| Presence of DSA | 15 (9) | 0.98 (0.35 to 2.72) | 0.97 | 0.83 (0.25 to 2.79) | 0.76 | NS |
| Use of induction | 3 (2) | 1.12 (0.51 to 2.47) | 0.79 | 1.23 (0.53 to 2.89) | 0.63 | NS |
| Plasmapheresis pretransplant | 0 (0) | 1.28 (0.63 to 2.61) | 0.50 | 1.27 (0.54 to 2.97) | 0.58 | NS |
| Immunosuppression with tacrolimus + MMF + steroids | 1 (1) | 1.01 (0.57 to 1.81) | 0.96 | 0.88 (0.46 to 1.66) | 0.69 | NS |
| Time to ESKD, per mo | 40 (23) | 1.00 (0.99 to 1.00) | 0.26 |
HR, hazard ratio; 95% CI, 95% confidence interval; Ref, reference; BMI, body mass index; NS, not significant; DSA, donor-specific antibody; MMF, mycophenolate mofetil.
Most patients with missing race come from France, where race/ethnicity is not allowed to be reported.
Previous Allografts and Recurrent FSGS
Previous allograft loss owing to FSGS has been described as a risk factor for recurrent FSGS in subsequent allografts. In our cohort, 45% and 100% of patients who had lost one or two prior allografts, respectively, because of recurrent FSGS experienced another recurrence (Supplemental Figure 2). Patients who lost a previous graft because of reasons other than recurrent FSGS were associated with a lower incidence of FSGS recurrence in the new allograft (14%).
Response to Treatment of Recurrent FSGS
We evaluated treatment response in 75 patients with FSGS recurrence: 57 patients from the primary cohort and an additional 18 from two centers that did not have native biopsy results available (see Supplemental Appendix 1). Immunosuppressive treatments varied across the cohort and included steroids, cyclosporine, plasmapheresis, rituximab, cyclophosphamide, and immunoadsorption (Table 3). Five patients did not receive any further immunosuppressive treatment because of active infection or advanced scarring on kidney biopsy. Plasmapheresis with or without rituximab was the most frequent treatment (61 patients, 81%) and the only one inducing complete remission. Frequency of plasmapheresis varied from one to three sessions a week, with duration ranging from 2 weeks to a year. The median number of rituximab doses was two (IQR, 1–3).
Table 3.
Immunosuppressive treatment modalities for recurrent FSGS and corresponding outcomes
| Treatment | No Remission | Partial Remission | Complete Remission | Total |
|---|---|---|---|---|
| Plasmapheresis | 7 (28) | 11 (44) | 7 (28) | 25 |
| Plasmapheresis + rituximab | 16 (53) | 9 (30) | 5 (17) | 30 |
| Immunoadsorptiona | 2 (67) | 1 (33) | 3 | |
| Rituximab only | 1 (50) | 1 (50) | 2 | |
| Plasmapheresis + cyclophosphamide | 1 (100) | 1 | ||
| Steroids only | 5 (83) | 1 (17) | 6 | |
| Cyclosporineb | 2 (67) | 1 (33) | 3 | |
| No treatment | 5 (100) | 5 | ||
| Total | 38 (51) | 24 (32) | 13 (17) | 75 |
Two patients dependent on immunoadsorption had received plasmapheresis and rituximab treatment without remission; one patient had received only rituximab and immunoadsorption.
Patients were switched from oral tacrolimus to oral cyclosporine. Treatment was not given intravenously.
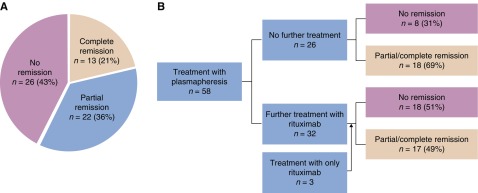
Plasmapheresis with or without rituximab was associated with complete remission in 13 patients (21%), partial remission in 22 patients (36%), and no response in 26 patients (43%) (Figure 3A). Thirty-two patients received both plasma exchange and rituximab, 25 received only plasmapheresis, and three only received rituximab (Figure 3B). Two patients with a complete remission experienced a relapse that responded to repeated treatments.
Figure 3.
Treatment of recurrent FSGS with plasmapheresis with or without rituximab leads to complete remission in only a minority of patients. (A) Overall effect of treatment in patients treated with plasmapheresis and/or rituximab. (B) Flow chart of treatment outcome per treatment modality. One patient treated with plasmapheresis was also treated with cyclophosphamide, without remission.
Univariable logistic regression analysis in patients treated with plasmapheresis with or without rituximab did not show an association between early FSGS recurrence (within 6 months) and response to treatment (odds ratio [OR], 0.83; 95% CI, 0.24 to 2.85; P=0.77) (Supplemental Table 3). Younger age at transplantation (OR, 0.97; 95% CI, 0.93 to 1.01; P=0.10) and female sex (OR, 2.38; 95% CI, 0.82 to 6.91; P=0.11) showed a trend for a higher chance of responding to treatment, but we could not assess this in a multivariable model because of the small group sizes.
FSGS Recurrence and Post-Transplant Complications
Increased intensity of immunosuppression in patients with recurrent FSGS may lead to a higher risk of infectious complications such as BK virus and cytomegalovirus reactivation. Using Cox regression and adjusting for confounders, we did not find differences in incidence of BK or cytomegalovirus viremia, acute rejection rates, or cancer between patients with or without a recurrence (Table 4), nor did we find differences in delayed graft function or diabetes using logistic regression.
Table 4.
Complications post-transplantation in all patients and according to FSGS recurrence
| Variable | Overall Cohort (n=176) | No Recurrence (n=119) | Recurrence (n=57) | Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|---|
| Acute rejection | 29 (16) | 23 (19) | 6 (11) | 0.63 (0.25 to 1.56)a | 0.31 |
| Cellular mediated | 18 (62) | 15 (65) | 3 (50) | ||
| Antibody mediated | 11 (38) | 8 (35) | 3 (50) | ||
| CMV | 20 (11) | 18 (14) | 3 (5) | 0.34 (0.09 to 1.15)b | 0.08 |
| BK viremia | 10 (6) | 5 (4) | 5 (9) | 2.69 (0.77 to 9.36) | 0.12 |
| Cancer | 9 (5) | 7 (6) | 2 (4) | 1.07 (0.22 to 5.17) | 0.91 |
| New-onset DM | 21 (12) | 13 (11) | 8 (14) | NA | 0.55 |
| Delayed graft function | 64 (38) | 42 (36) | 22 (41) | NA | 0.26c |
Acute rejection, CMV, BK viremia, and cancer were assessed by Cox hazard regression. New-onset DM and delayed graft function were analyzed by logistic regression. 95% CI, 95% confidence interval; CMV, cytomegalovirus; DM, diabetes mellitus; NA, not applicable.
Hazard ratio and P value are corrected for pretransplant HLA mismatch and pretransplant donor-specific antibody, by adding those variables to the regression model.
Hazard ratio and P value are corrected for pretransplant recipient/donor CMV IgG status.
P value is corrected for donor type and age.
Graft Function
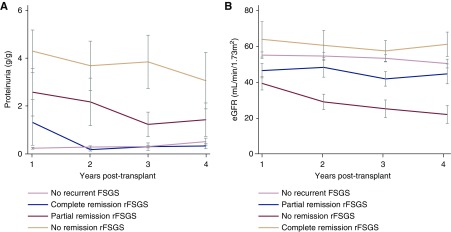
As predicted, proteinuria in patients with partial or no remission was higher than in patients with complete remission (Figure 4A). eGFRs of patients with complete remission were comparable to those of patients who did not experience recurrent FSGS (Figure 4B). For patients with a partial or no remission, eGFR was lower and progressively declined, especially in patients without remission. Other clinical parameters, such as BP and serum calcium, phosphate, and total cholesterol, are shown in Supplemental Figure 3, illustrating that patients with partial or complete remission, in most parameters, cluster with patients who did not experience a recurrence, whereas the mean values of patients with recurrence and no response were often abnormal.
Figure 4.
Clinical outcomes of patients who achieve complete remission of rFSGS are comparable with patients who did not experience recurrence. Comparison of levels of (A) proteinuria and (B) eGFR calculated by Modification of Diet in Renal Disease Study (MDRD) in patients without recurrence with patients with recurrent FSGS (rFSGS) stratified by their response to treatment. eGFR after graft loss was imputed with a value of 5 ml/min per 1.73 m2. Error bars represent SEMs.
Discussion
In this large, multicenter, international cohort of adults with idiopathic FSGS, we observed that FSGS recurred in 32% of patients, with subsequent graft loss in 39%. Multivariable analysis revealed older age at primary disease onset, native kidney nephrectomies, white race, and lower BMI at transplant to be associated with a higher risk for recurrence. Recurrent FSGS was most often treated with plasmapheresis with or without rituximab, and this regimen resulted in partial or complete remission in 57% of patients.
Previous studies on recurrent FSGS have reported a wide range of recurrence rates and potential predictors (Supplemental Table 1) (4–22). However, most of these studies have major limitations, including small sample size in single-center studies and poor data quality in registry-based analyses. A small sample size lowers the precision of incidence estimates and results in broad confidence intervals (Supplemental Table 1). Furthermore, studies with modest sample sizes are not only underpowered to detect predictors (32), but also inflate effect sizes because of wider sample distributions (33,34), thus reducing the likelihood that a statistically significant result reflects a true effect. Lastly, small studies are limited in multivariable analysis in which adjustment of confounders can be performed. Registry studies have larger sample sizes, but suffer from potential FSGS misclassification and failure to capture cases of FSGS recurrence that do not lead to allograft loss (28), as suggested by the generally lower reported recurrence rates (24–27,31). Another source of bias is the inclusion of multiple transplants per patient (Supplemental Table 1) because it creates correlation within the data set and makes use of standard statistical tests and related P values invalid.
The analyses of our large data set showed that only a few of the previously described predictors of recurrence were independently associated with risk for FSGS recurrence. Contrary to the previously reported association between younger age at disease onset and higher risk of recurrence (4,6,14,15,17,22,26,27), patients in our cohort with older age at disease onset had a higher HR of recurrence. This conflicting finding could be due to our different study population (adults with mixed race/ethnicities), our stringent exclusion of secondary FSGS that is more common at older age, or our ability to perform multivariable analyses. On the other hand, only few of the patients in our cohort were tested for genetic causes of FSGS. The fact that older age at onset was a predictor of recurrence may capture the fact that some cases diagnosed at younger age were, in fact, genetic and less likely to recur (35–37). Therefore, our result regarding age at diagnosis should be considered with caution.
A higher rate of FSGS recurrence in white recipients has previously been reported in United States studies (11,27,38), whereas data from New-Zealand and Australia showed a higher risk in nonwhite recipients (26). There is no consensus on how ethnicity affects recurrent FSGS. Black (or mixed race) patients, the second-largest race group in our cohort, have a higher prevalence of APOL1 high-risk alleles that are associated primarily with FSGS (39). Because APOL1-related FSGS is not associated with greater risk of recurrence post-transplantation (due to the effect of APOL1 alleles in the kidney itself [36,37,40]), this could contribute to our observed difference.
In agreement with our results, nephrectomy of native kidneys has been associated with a higher risk for recurrence (10,17). One of the hypotheses is that the native kidneys left in situ may act as a “sponge” to absorb the potential pathogenic circulating factor, leading to reduction of the free circulating factor that may injure the transplanted kidney (41). Nonetheless, the incidence of nephrectomy of native kidneys is low (7% in our cohort) and it is usually only performed in severe disease, which could both bias outcomes.
We found that patients with a recurrence had, on average, a lower BMI at time of transplant. Despite our careful selection of our cohort, it is possible that some patients with obesity-induced FSGS were misdiagnosed as primary FSGS, explaining this finding. Obesity is known to cause secondary FSGS through increased mechanical and metabolic stress on glomeruli, although some of these patients are difficult to differentiate from primary FSGS because obesity-induced FSGS can manifest with nephrotic-range or even massive proteinuria (42,43). Regardless, patients with high BMI are not exempted from recurrence as six patients in our cohort with BMI >30 kg/m2 at transplantation experienced recurrent FSGS.
Our data reflect the lack of official guidelines on the treatment of recurrent FSGS. Although most centers used plasmapheresis with or without rituximab, treatment intensity and duration varied considerably. This nonstandardized treatment approach could explain the lower rate of treatment response (57%) compared with previous literature, especially regarding the achievement of complete remission (44–46). An alternative hypothesis is that prior reports have a publication bias, which highlights the importance of systematic, unbiased, multicenter approaches to data collection, such as TANGO. Interestingly, although prior literature suggested the use of high-dose intravenous cyclosporine to treat FSGS recurrence (44,47,48), none of our included patients received this treatment, possibly because of concern for nephrotoxicity and/or logistical challenges with continuous intravenous infusion (49). Irrespective of the regimen, response to treatment was associated with graft survival, as graft loss mainly occurred in patients who failed to enter remission.
Our study has some limitations, including its retrospective design, which could have resulted in selection bias. Further, adjustment for all potential confounders was not possible. Selection of patients with FSGS by history and pathology was performed by clinicians in the participating centers and was not centralized. Although detailed history on secondary causes and biopsy reports were obtained to minimize variation, differences across center may still be present. We were not able to correct our analyses for center variation because the number of patients per center was low and we had a high number of participating centers. Instead, we adjusted for the continent of residence. Another limitation is the lack of genetic testing in most patients, which might create bias as genetic FSGS usually does not recur after transplantation (35–37).
Herein, we demonstrate that international, multicenter collaborations as in the TANGO study are logistically possible and scientifically pivotal to better understand the natural history of rare diseases such as FSGS recurrence. Besides clinical information, a large number of biologic samples from patients with FSGS are currently being collected as part of the TANGO study, which will allow further investigation into the pathophysiologic mechanisms underlying recurrent FSGS, including validation of prior biomarkers and expansion of the biologic understanding of this challenging disease.
Disclosures
Dr. Cheng reports grants from American Heart Association outside the submitted work. Dr. Riella received an investigator-initiated grant from Bristol-Myers Squibb and consulting fees from Mallinckrodt Pharmaceuticals outside the submitted work. Dr. Tedesco-Silva, Jr. received grants and personal fees from Novartis and grants and personal fees from Pfizer outside the submitted work. Dr. Agena, Dr. Akalin, Dr. Alani, Dr. Arias-Cabrales, Dr. Berger, Dr. Bini, Dr. Bugnazet, Dr. Buxeda, Dr. Chin, Dr. Comai, Dr. Cravedi, Dr. Elias David-Neto, Dr. de Sottomaior Drumond, Dr. Farouk, Dr. Haverly, Dr. Hokazono, Dr. Jouve, Dr. La Manna, Dr. Malvezzi, Dr. Mansur Siliano, Dr. Kirsztajn, Dr. Mazzali, Dr. Muhsin, Dr. O'Shaughnessy, Dr. Pérez-Sáez, Dr. Mothi, Dr. Uffing, Dr. Ventura, and Dr. Gilberto M.V. Neto have nothing to disclose.
Funding
This work was conducted with support from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health award UL1 TR001102), and financial contributions from Harvard University and its affiliated academic health care centers. This work was supported in part by the Safra Foundation and Nephcure Foundation. No other funding was received.
Supplementary Material
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic health care centers, or the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Facing the Vexing Problem of Recurrent FSGS after Kidney Transplantation,” on pages 171–173.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08970719/-/DCSupplemental.
Supplemental Table 1. Published studies on incidence of recurrent FSGS since 1990, including >20 participants.
Supplemental Table 2. Missing data in predictors for patients without and with recurrent FSGS.
Supplemental Table 3. Univariable logistic regression for any response to treatment.
Supplemental Figure 1. Flow chart of the study population.
Supplemental Figure 2. Prior graft loss owing to FSGS recurrence and risk of recurrence in subsequent graft.
Supplemental Figure 3. Clinical outcomes post-transplantation in patients with a functioning allograft.
Supplemental Appendix 1. Supplemental methods.
References
- 1.Saran R, Robinson B, Abbott KC, Agodoa LY, Albertus P, Ayanian J, Balkrishnan R, Bragg-Gresham J, Cao J, Chen JL, Cope E, Dharmarajan S, Dietrich X, Eckard A, Eggers PW, Gaber C, Gillen D, Gipson D, Gu H, Hailpern SM, Hall YN, Han Y, He K, Hebert H, Helmuth M, Herman W, Heung M, Hutton D, Jacobsen SJ, Ji N, Jin Y, Kalantar-Zadeh K, Kapke A, Katz R, Kovesdy CP, Kurtz V, Lavalee D, Li Y, Lu Y, McCullough K, Molnar MZ, Montez-Rath M, Morgenstern H, Mu Q, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Pearson J, Pisoni R, Plattner B, Port FK, Potukuchi P, Rao P, Ratkowiak K, Ravel V, Ray D, Rhee CM, Schaubel DE, Selewski DT, Shaw S, Shi J, Shieu M, Sim JJ, Song P, Soohoo M, Steffick D, Streja E, Tamura MK, Tentori F, Tilea A, Tong L, Turf M, Wang D, Wang M, Woodside K, Wyncott A, Xin X, Zang W, Zepel L, Zhang S, Zho H, Hirth RA, Shahinian V: US renal data system 2016 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 69[Suppl 1]: A7–A8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Sprangers B, Meijers B, Appel G: FSGS: Diagnosis and diagnostic work-up. BioMed Res Int 2016: 4632768, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senggutuvan P, Cameron JS, Hartley RB, Rigden S, Chantler C, Haycock G, Williams DG, Ogg C, Koffman G: Recurrence of focal segmental glomerulosclerosis in transplanted kidneys: Analysis of incidence and risk factors in 59 allografts. Pediatr Nephrol 4: 21–28, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Banfi G, Colturi C, Montagnino G, Ponticelli C: The recurrence of focal segmental glomerulosclerosis in kidney transplant patients treated with cyclosporine. Transplantation 50: 594–596, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Ingulli E, Tejani A: Incidence, treatment, and outcome of recurrent focal segmental glomerulosclerosis posttransplantation in 42 allografts in children--a single-center experience. Transplantation 51: 401–405, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Dantal J, Baatard R, Hourmant M, Cantarovich D, Buzelin F, Soulillou JP: Recurrent nephrotic syndrome following renal transplantation in patients with focal glomerulosclerosis. A one-center study of plasma exchange effects. Transplantation 52: 827–831, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Dall’Amico R, Ghiggeri G, Carraro M, Artero M, Ghio L, Zamorani E, Zennaro C, Basile G, Montini G, Rivabella L, Cardillo M, Scalamogna M, Ginevri F: Prediction and treatment of recurrent focal segmental glomerulosclerosis after renal transplantation in children. Am J Kidney Dis 34: 1048–1055, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Tejani A, Stablein DH: Recurrence of focal segmental glomerulosclerosis posttransplantation: A special report of the North American Pediatric Renal Transplant Cooperative Study. J Am Soc Nephrol 2[Suppl]: S258–S263, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Odorico JS, Knechtle SJ, Rayhill SC, Pirsch JD, D’Alessandro AM, Belzer FO, Sollinger HW: The influence of native nephrectomy on the incidence of recurrent disease following renal transplantation for primary glomerulonephritis. Transplantation 61: 228–234, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Artero M, Biava C, Amend W, Tomlanovich S, Vincenti F: Recurrent focal glomerulosclerosis: Natural history and response to therapy. Am J Med 92: 375–383, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Choi KH, Kim SI, Yoon SY, Kim JH, Kang SW, Ha SK, Lee HY, Han DS, Kim YS, Park K, Jeong HJ, Kim DK: Long-term outcome of kidney transplantation in adult recipients with focal segmental glomerulosclerosis. Yonsei Med J 42: 209–214, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Pardon A, Audard V, Caillard S, Moulin B, Desvaux D, Bentaarit B, Remy P, Sahali D, Roudot-Thoraval F, Lang P, Grimbert P: Risk factors and outcome of focal and segmental glomerulosclerosis recurrence in adult renal transplant recipients. Nephrol Dial Transplant 21: 1053–1059, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Hickson LJ, Gera M, Amer H, Iqbal CW, Moore TB, Milliner DS, Cosio FG, Larson TS, Stegall MD, Ishitani MB, Gloor JM, Griffin MD: Kidney transplantation for primary focal segmental glomerulosclerosis: Outcomes and response to therapy for recurrence. Transplantation 87: 1232–1239, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Moroni G, Gallelli B, Quaglini S, Banfi G, Montagnino G, Messa P: Long-term outcome of renal transplantation in adults with focal segmental glomerulosclerosis. Transpl Int 23: 208–216, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Mahesh S, Del Rio M, Feuerstein D, Greenstein S, Schechner R, Tellis V, Kaskel F: Demographics and response to therapeutic plasma exchange in pediatric renal transplantation for focal glomerulosclerosis: A single center experience. Pediatr Transplant 12: 682–688, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Sener A, Bella AJ, Nguan C, Luke PP, House AA: Focal segmental glomerular sclerosis in renal transplant recipients: Predicting early disease recurrence may prolong allograft function. Clin Transplant 23: 96–100, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Schachter ME, Monahan M, Radhakrishnan J, Crew J, Pollak M, Ratner L, Valeri AM, Stokes MB, Appel GB: Recurrent focal segmental glomerulosclerosis in the renal allograft: Single center experience in the era of modern immunosuppression. Clin Nephrol 74: 173–181, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Canaud G, Dion D, Zuber J, Gubler MC, Sberro R, Thervet E, Snanoudj R, Charbit M, Salomon R, Martinez F, Legendre C, Noel LH, Niaudet P: Recurrence of nephrotic syndrome after transplantation in a mixed population of children and adults: Course of glomerular lesions and value of the Columbia classification of histological variants of focal and segmental glomerulosclerosis (FSGS). Nephrol Dial Transplant 25: 1321–1328, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Maas RJ, Deegens JK, van den Brand JA, Cornelissen EA, Wetzels JF: A retrospective study of focal segmental glomerulosclerosis: Clinical criteria can identify patients at high risk for recurrent disease after first renal transplantation. BMC Nephrol 14: 47, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelletier JH, Kumar KR, Engen R, Bensimhon A, Varner JD, Rheault MN, Srivastava T, Straatmann C, Silva C, Davis TK, Wenderfer SE, Gibson K, Selewski D, Barcia J, Weng P, Licht C, Jawa N, Kallash M, Foreman JW, Wigfall DR, Chua AN, Chambers E, Hornik CP, Brewer ED, Nagaraj SK, Greenbaum LA, Gbadegesin RA: Recurrence of nephrotic syndrome following kidney transplantation is associated with initial native kidney biopsy findings. Pediatr Nephrol 33: 1773–1780, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharief S, Mahesh S, Del Rio M, Telis V, Woroniecki RP: Recurrent focal segmental glomerulosclerosis in renal allograft recipients: Role of human leukocyte antigen mismatching and other clinical variables. Int J Nephrol 2011: 506805, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang JH, Han SS, Huh W, Park SK, Joo DJ, Kim MS, Kim YS, Min SI, Ha J, Kim SJ, Kim S, Kim YS: Outcome of kidney allograft in patients with adulthood-onset focal segmental glomerulosclerosis: Comparison with childhood-onset FSGS. Nephrol Dial Transplant 27: 2559–2565, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Allen PJ, Chadban SJ, Craig JC, Lim WH, Allen RDM, Clayton PA, Teixeira-Pinto A, Wong G: Recurrent glomerulonephritis after kidney transplantation: Risk factors and allograft outcomes. Kidney Int 92: 461–469, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Jiang SH, Kennard AL, Walters GD: Recurrent glomerulonephritis following renal transplantation and impact on graft survival. BMC Nephrol 19: 344, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis A, Trnka P, McTaggart SJ: Long-Term outcome of kidney transplantation in recipients with focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 11: 2041–2046, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nehus EJ, Goebel JW, Succop PS, Abraham EC: Focal segmental glomerulosclerosis in children: Multivariate analysis indicates that donor type does not alter recurrence risk. Transplantation 96: 550–554, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Layton JB, Hogan SL, Jennette CE, Kenderes B, Krisher J, Jennette JC, McClellan WM: Discrepancy between Medical Evidence Form 2728 and renal biopsy for glomerular diseases. Clin J Am Soc Nephrol 5: 2046–2052, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan RM, Chambers DA, Glasgow RE: Big data and large sample size: A cautionary note on the potential for bias. Clin Transl Sci 7: 342–346, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uffing A, Pérez-Sáez MJ, La Manna G, Comai G, Fischman C, Farouk S, Manfro RC, Bauer AC, Lichtenfels B, Mansur JB, Tedesco-Silva H, Kirsztajn GM, Manonelles A, Bestard O, Riella MC, Hokazono SR, Arias-Cabrales C, David-Neto E, Ventura CG, Akalin E, Mohammed O, Khankin EV, Safa K, Malvezzi P, O’Shaughnessy MM, Cheng XS, Cravedi P, Riella LV: A large, international study on post-transplant glomerular diseases: The TANGO project. BMC Nephrol 19: 229, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guan I, Singer P, Frank R, Chorny N, Infante L, Sethna CB: Role of race in kidney transplant outcomes in children with focal segmental glomerulosclerosis. Pediatr Transplant 20: 790–797, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafò MR: Power failure: Why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14: 365–376, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Xu X, Ni H: Small studies may overestimate the effect sizes in critical care meta-analyses: A meta-epidemiological study. Crit Care 17: R2, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjaergard LL, Villumsen J, Gluud C: Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 135: 982–989, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Gribouval O, Boyer O, Hummel A, Dantal J, Martinez F, Sberro-Soussan R, Etienne I, Chauveau D, Delahousse M, Lionet A, Allard J, Pouteil Noble C, Tête M-J, Heidet L, Antignac C, Servais A: Identification of genetic causes for sporadic steroid-resistant nephrotic syndrome in adults. Kidney Int 94: 1013–1022, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Conlon PJ, Lynn K, Winn MP, Quarles LD, Bembe ML, Pericak-Vance M, Speer M, Howell DN: Spectrum of disease in familial focal and segmental glomerulosclerosis. Kidney Int 56: 1863–1871, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Machuca E, Hummel A, Nevo F, Dantal J, Martinez F, Al-Sabban E, Baudouin V, Abel L, Grünfeld J-P, Antignac C: Clinical and epidemiological assessment of steroid-resistant nephrotic syndrome associated with the NPHS2 R229Q variant. Kidney Int 75: 727–735, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Abbott KC, Sawyers ES, Oliver JD 3rd, Ko CW, Kirk AD, Welch PG, Peters TG, Agodoa LY: Graft loss due to recurrent focal segmental glomerulosclerosis in renal transplant recipients in the United States. Am J Kidney Dis 37: 366–373, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jungraithmayr TC, Hofer K, Cochat P, Chernin G, Cortina G, Fargue S, Grimm P, Knueppel T, Kowarsch A, Neuhaus T, Pagel P, Pfeiffer KP, Schäfer F, Schönermarck U, Seeman T, Toenshoff B, Weber S, Winn MP, Zschocke J, Zimmerhackl LB: Screening for NPHS2 mutations may help predict FSGS recurrence after transplantation. J Am Soc Nephrol 22: 579–585, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fine RN: Recurrence of nephrotic syndrome/focal segmental glomerulosclerosis following renal transplantation in children. Pediatr Nephrol 22: 496–502, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sethi S, Glassock RJ, Fervenza FC: Focal segmental glomerulosclerosis: Towards a better understanding for the practicing nephrologist. Nephrol Dial Transplant 30: 375–384, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Darouich S, Goucha R, Jaafoura MH, Zekri S, Ben Maiz H, Kheder A: Clinicopathological characteristics of obesity-associated focal segmental glomerulosclerosis. Ultrastruct Pathol 35: 176–182, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Canaud G, Zuber J, Sberro R, Royale V, Anglicheau D, Snanoudj R, Gaha K, Thervet E, Lefrère F, Cavazzana-Calvo M, Noël L-H, Méjean A, Legendre C, Martinez F: Intensive and prolonged treatment of focal and segmental glomerulosclerosis recurrence in adult kidney transplant recipients: A pilot study. Am J Transplant 9: 1081–1086, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Garrouste C, Canaud G, Büchler M, Rivalan J, Colosio C, Martinez F, Aniort J, Dudreuilh C, Pereira B, Caillard S, Philipponnet C, Anglicheau D, Heng AE: Rituximab for recurrence of primary focal segmental glomerulosclerosis after kidney transplantation: Clinical outcomes. Transplantation 101: 649–656, 2017 [DOI] [PubMed] [Google Scholar]
- 46.Alasfar S, Matar D, Montgomery RA, Desai N, Lonze B, Vujjini V, Estrella MM, Manllo Dieck J, Khneizer G, Sever S, Reiser J, Alachkar N: Rituximab and therapeutic plasma exchange in recurrent focal segmental glomerulosclerosis postkidney transplantation. Transplantation 102: e115–e120, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cravedi P, Kopp JB, Remuzzi G: Recent progress in the pathophysiology and treatment of FSGS recurrence. Am J Transplant 13: 266–274, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rudnicki M: FSGS recurrence in adults after renal transplantation. BioMed Res Int 2016: 3295618, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naesens M, Kuypers DR, Sarwal M: Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 4: 481–508, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.