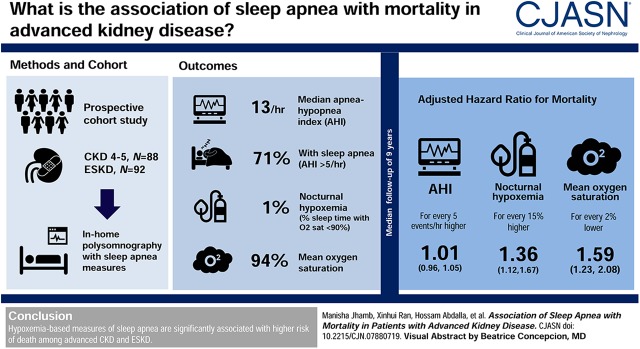
Visual Abstract
Keywords: sleep duration mortality ckd, sleep duration mortality, mortality risk, nocturnal hypoxemia, sleep apnea, female, polysomnography, proportional hazards models, body mass index, oxygen, follow-up studies, prospective studies, kidney transplantation, sleep apnea syndromes, survival analysis, kidney, chronic renal insufficiency, hypoxia, diabetes mellitus
Abstract
Background and objectives
In the general population, sleep disorders are associated with mortality. However, such evidence in patients with CKD and ESKD is limited and shows conflicting results. Our aim was to examine the association of sleep apnea with mortality among patients with CKD and ESKD.
Design, setting, participants, & measurements
In this prospective cohort study, 180 patients (88 with CKD stage 4 or 5, 92 with ESKD) underwent in-home polysomnography, and sleep apnea measures such as apnea hypopnea index (AHI) and nocturnal hypoxemia were obtained. Mortality data were obtained from the National Death Index. Cox proportional hazard models were used for survival analysis.
Results
Among the 180 patients (mean age 54 years, 37% women, 39% with diabetes, 49% CKD with mean eGFR 18±7 ml/min per 1.73 m2), 71% had sleep apnea (AHI>5) and 23% had severe sleep apnea (AHI>30). Median AHI was 13 (range, 4–29) and was not significantly different in patients with advanced CKD or ESKD. Over a median follow-up of 9 years, there were 84 (47%) deaths. AHI was not significantly associated with mortality after adjusting for age, sex, race, diabetes, body mass index, CKD/ESKD status, and kidney transplant status (AHI>30: hazard ratio [HR], 1.5; 95% confidence interval [95% CI], 0.6 to 4.0; AHI >15 to 30: HR, 2.3; 95% CI, 0.9 to 5.9; AHI >5 to 15: HR, 2.1; 95% CI, 0.8 to 5.4, compared with AHI≤5). Higher proportion of sleep time with oxygen saturation <90% and lower mean oxygen saturation were significantly associated with higher mortality in adjusted analysis (HR, 1.4; 95% CI, 1.1 to 1.7; P=0.007 for every 15% higher proportion, and HR, 1.6; 95% CI, 1.2 to 2.1; P=0.003 for every 2% lower saturation, respectively). Sleep duration, sleep efficiency, or periodic limb movement index were not associated with mortality.
Conclusions
Hypoxemia-based measures of sleep apnea are significantly associated with increased risk of death among advanced CKD and ESKD.
Introduction
Patients with CKD and ESKD have very high prevalence of sleep-disordered breathing (1–3). It is estimated that 50%–80% of these patients suffer from sleep-disordered breathing, compared with 2%–4% of the general population or 20%–30% of patients with other comorbidities such as diabetes or congestive heart failure (1,2,4–7). Moreover, poor sleep quality, altered sleep duration, fragmented sleep, excessive daytime sleepiness, restless legs syndrome, and periodic limb movement disorder are very common (2,3,8). In the general population, evidence suggests that sleep apnea and insufficient sleep may be associated with cardiovascular events and all-cause mortality (9,10). Similarly, in patients with kidney disease, poor self-reported sleep quality and sleep duration have also been associated with increased mortality (11–13). Paradoxically, a recent Medicare claims study in >180,000 older patients starting dialysis showed that sleep-disordered breathing lowered the risk of cardiovascular events and mortality (14). Given these conflicting findings, additional evidence is needed to evaluate the association of objectively measured sleep-disordered breathing, duration, and quality with mortality in patients with kidney disease.
A recent meta-analysis showed that patients with kidney disease and sleep disorders (i.e., sleep-disordered breathing, periodic limb movement, or altered sleep duration/quality) had significantly increased risk of all-cause mortality (risk ratio, 1.47; 95% confidence interval [95% CI], 1.30 to 1.66; P<0.001) (15). However, of the 12 studies included in this analysis, only five used objective polysomnography data to measure sleep and these reports demonstrated conflicting results. These five studies were limited in sample size, follow-up duration, largely included non–United States populations and were limited owing to selection biases (Supplemental Table 1). Given that self-reported sleep measures have very poor correlation with objective sleep measures in these patients (16), and the methodological limitations of the included studies, the results of this meta-analysis should be interpreted with caution. Additionally, only one of these five studies was done in patients with nondialysis CKD, and largely included patients with earlier stage of CKD (mean eGFR 49±11 ml/min per 1.73 m2) (17). Thus, additional studies are needed to evaluate the association of polysomnography-measured sleep with mortality in patients with advanced CKD and ESKD.
The aim of our study was to examine the association of sleep apnea severity, periodic limb movements, sleep duration, and sleep quality (efficiency) using polysomnography with mortality in a cohort of patients with CKD stage 4 and 5 and ESKD. Our hypothesis was that higher sleep apnea severity, short sleep duration, and reduced sleep efficiency will be associated with increased risk of mortality in these patients.
Materials and Methods
Study Participants
This is a prospective, observational cohort study of 180 patients with advanced CKD stage 4 and 5 (88 patients) and ESKD (72 patients on hemodialysis [HD], 20 patients on peritoneal dialysis) enrolled from outpatient nephrology clinics, transplant clinics, and local dialysis units in Western Pennsylvania from March 2004 to December 2008. These patients were enrolled as part of a larger study investigating factors contributing to sleep apnea in kidney disease patients. The inclusion exclusion criteria have been described in detail in a study examining a subset of this cohort (18). In brief, this study included patients aged >18 years and presence of advanced CKD (Modification of Diet in Renal Disease equation eGFR ≤30 ml/min per 1.73 m2) or ESKD on maintenance dialysis. Exclusion criteria included age >90 years, active medical disease (malignancy, myocardial infarction, advanced cirrhosis, advanced dementia), active alcohol abuse, or refractory psychiatric disease. In addition, patients who were using continuous positive airway pressure, oral devices, or home oxygen therapy for sleep-disordered breathing and those who had a tracheostomy were excluded. For the current analysis, all patients who had complete in-home polysomnography were included (Supplemental Figure 1). This study was approved by the University of Pittsburgh Institutional Review Board, and all participants provided written informed consent.
Sleep Assessment using Polysomnography
Sleep apnea severity was assessed using polysomnography, which remains the gold standard for diagnosis of sleep apnea. Unattended in-home polysomnography was used for objective assessment of sleep, and these were done from April 2004 to November 2008. Ambulatory Siesta monitor (Charlotte, NC) was used to record polysomnography data at habitual sleep times for one night. Such unattended in-home polysomnography has been shown to provide highly accurate and complete sleep data as compared with in-laboratory polysomnography (19). Research staff went to each patient’s home in the evening and the next morning to assist with putting on and taking off the apparatus. The apparatus included bilateral central and occipital electroencephalogram channels, bilateral electro-oculogram, and bipolar submentalis electromyogram, along with bipolar electrocardiogram for heart rate and position sensors for body position monitoring. Other monitoring included inductance plethysmography to measure abdominal and thoracic effort, pulse oximetry (Nonin, Minneapolis, MN) to measure oxyhemoglobin saturation, nasal-oral thermocouple and nasal pressure to measure airflow, and arm and leg monitors to measure periodic leg movements. Data recorded were analyzed by trained polysomnography technologists who were blinded to patient’s kidney function or dialysis status. Using the 1999 American Academy of Sleep Medicine definitions, episodes of apneas and hypopneas were scored and characterized as being obstructive, central, or mixed (20).
The polysomnography outcome variables of interest were apnea hypopnea index (AHI; number of apneas and hypopneas per hour of sleep), oxygen desaturation index (ODI; number of events per hour in which SaO2 decreased by ≥4% from the baseline for ≥5 seconds), nocturnal hypoxemia (proportion of total sleep time with oxygen saturation <90%), and mean oxygen saturation (21). Patients with AHI >5 per hour were defined as having sleep apnea. Sleep apnea severity was measured by AHI: mild (6–15 per hour), moderate (16–30 per hour), and severe (>30 per hour). Additionally, obstructive AHI and central AHI scores were calculated.
Other sleep variables examined included total sleep time (sleep time excluding periods of wakefulness during the night), sleep efficiency (percentage of total sleep time out of time in bed), and periodic leg movement index (periodic limb movement index; average number of periodic limb movements during sleep and awake periods with and without arousal per hour of sleep).
Clinical and Demographic Data
Information on participant demographics, comorbidities, kidney transplant status, and laboratory values was obtained from participant interviews and chart review. Cardiovascular disease included coronary artery disease and stroke.
Mortality Data
Mortality data including cause of death was obtained from the National Death Index until December 31, 2016. Cause of death was categorized as cardiovascular, infectious, cancer, or others.
Statistical Analyses
Categorical and continuous variables were presented using frequencies with percentages and means with SDs (or medians with interquartile range for skewed distributions), respectively. Between-group comparisons were conducted using ANOVA test and chi-squared or Fisher exact test. Kruskal–Wallis test was used for highly skewed continuous data. The extent of missing data were analyzed (Supplemental Table 2). Time to mortality and time to transplantation were calculated from date of polysomnography measurement and observations were censored by December 31, 2016 if no death was reported by the National Death Index before this date. Kaplan–Meier curves for mortality were examined across AHI groups. Unadjusted and adjusted Cox models were used to test whether each polysomnography measure was associated with mortality. Covariates used in adjusted models were age, sex, race, diabetes, body mass index (BMI), kidney function status (CKD or ESKD), and kidney transplant status. Kidney transplant was treated as a time-varying covariate as opposed to censoring at the time of transplant to adjust for it as well as accounting for potential immortal time bias. Interaction effects between polysomnography measurements with age, sex, and CKD/ESKD status were assessed. To account for multiple primary outcomes and correct for multiple comparisons, we calculated false discovery rate–corrected P values using the Benjamini–Hochberg method. In sensitivity analyses, AHI was also examined as a continuous variable that allowed for nonlinear associations with the log-hazards using restricted cubic splines. The proportional hazards assumption was assessed using Schoenfeld residuals, and if not held then interactions with log time was included in the model, and hazard ratios (HRs) at different time points (year 1, year 5, year 9, and year 12) were calculated.
To determine the relative strength of association of polysomnography measures with mortality, standardized HRs were calculated by dividing the polysomnography measures by their respective SDs before fitting the adjusted Cox model. Sensitivity analysis with censoring for kidney transplant was also done.
All statistical analyses were carried out in R (version 3.5.1) using the packages dplyr (22) for data manipulation, compare Groups for descriptive tables, survival (23) for survival analysis, and ggplot2 (24) for graphics.
Results
Baseline Characteristics
Table 1 shows baseline characteristics of the participants by sleep apnea severity category. Among the 180 patients, average age was 54±14 years, 37% were women, 66% were white, 39% had diabetes, and 25% had cardiovascular disease. The patients with CKD (49%) had an average eGFR of 18±7 ml/min per 1.73 m2, and the patients with ESKD (51%) had median dialysis vintage of 18 (interquartile range, 9–44) months. The median AHI was 13 per hour (interquartile range, 4–29) and the mean was 21 (±25) per hour. About 71% of the study participants had an AHI>5, and 23% had an AHI>30. Older age and male sex were significantly associated with higher AHI (P<0.001 for both). Diabetes was more common among patients with severe sleep apnea severity (P=0.02). There was no difference among the sleep apnea severity groups with regards to race, BMI, other comorbidities, kidney function status (CKD or ESKD), antidepressant or benzodiazepine use, or any of the laboratory values. About 47% of the patients received a kidney transplant and the median time to transplant from the date of polysomnography was 0.8 (interquartile range, 0.4–1.9) years. Of the 85 transplant recipients, 47 (55%) were owing to ESKD and rest were pre-emptive. For the key variables, data were missing in <5% of the patients, and was missing completely at random, thus we performed complete-case analysis (Supplemental Table 2).
Table 1.
Baseline characteristics of participants enrolled from outpatient nephrology clinics, transplant clinics, and local dialysis units in Western Pennsylvania as part of a study investigating factors contributing to sleep apnea
| Characteristic | All, n=180 | AHI≤5, n=52 | AHI >5 to ≤15, n=45 | AHI >15 to ≤30, n=42 | AHI>30, n=41 |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, yr | 54 (14) | 48 (15) | 54 (15) | 59 (12) | 56 (12) |
| Male | 113 (63%) | 26 (50%) | 20 (46%) | 35 (81%) | 32 (78%) |
| Race | |||||
| White | 118 (66%) | 36 (69%) | 29 (66%) | 27 (63%) | 26 (63%) |
| Black | 56 (31%) | 14 (27%) | 14 (32%) | 14 (33%) | 14 (34%) |
| Asian/Pacific | 3 (2%) | 0 (0%) | 1 (2%) | 1 (2%) | 1 (2%) |
| American Indian | 1 (1%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Others | 2 (1%) | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Body mass index | 27.8 (5.6) | 26.2 (4.8) | 27.9 (5.6) | 28.7 (6.1) | 29.0 (5.8) |
| Ever smoked | 99 (58%) | 25 (50%) | 23 (58%) | 24 (57%) | 27 (68%) |
| Comorbidities | |||||
| Kidney disease | |||||
| CKD | 88 (49%) | 31 (60%) | 19 (43%) | 18 (42%) | 20 (49%) |
| ESKD | 92 (51%) | 21 (40%) | 25 (57%) | 25 (58%) | 21 (51%) |
| Dialysis vintage, moa | 18 [9; 44] | 11 [6; 26] | 19 [9; 39] | 26 [12; 52] | 18 [10; 34] |
| Diabetes | 65 (39%) | 10 (22%) | 16 (40%) | 22 (54%) | 17 (45%) |
| Hypertension | 148 (89%) | 45 (90%) | 35 (90%) | 31 (78%) | 37 (97%) |
| Cardiovascular disease | 45 (25%) | 10 (19%) | 13 (30%) | 11 (26%) | 11 (27%) |
| Medication use | |||||
| Antidepressants | 22 (13%) | 5 (10%) | 5 (12%) | 6 (15%) | 6 (16%) |
| Benzodiazepines | 13 (8%) | 3 (6%) | 5 (12%) | 4 (10%) | 1 (3%) |
| Laboratory measurements | |||||
| Albumin, g/dl | 3.9 (0.5) | 3.9 (0.6) | 3.9 (0.3) | 3.7 (0.6) | 3.9 (0.5) |
| Creatinine, mg/dl | 6.8 (3.6) | 6.2 (3.5) | 6.6 (3.7) | 6.9 (3.4) | 7.3 (4.0) |
| Bicarbonate, mEq/L | 24 (3.8) | 23 (3.7) | 24 (3.1) | 25 (4.6) | 23 (3.7) |
| Hemoglobin, mg/dl | 11.8 (1.4) | 12.0 (1.5) | 11.9 (1.4) | 11.6 (1.6) | 11.9 (1.3) |
| Dialysis adequacy, Kt/va | 1.6 (0.4) | 1.8 (0.4) | 1.8 (0.4) | 1.5 (0.4) | 1.6 (0.5) |
| Phosphate, mg/dl | 5.1 (1.6) | 4.8 (1.2) | 5.0 (1.5) | 5.2 (1.5) | 5.6 (1.9) |
| Parathyroid hormone, pg/ml | 266 (334) | 216 (180) | 188 (163) | 212 (149) | 424 (555) |
| eGFR, ml/min per 1.73 m2b | 18 (7) | 17 (5) | 20 (6) | 18 (8) | 18 (10) |
| Sleep measures | |||||
| Apnea hypopnea index, events/h | 21.1 (25.0) | 2.5 (1.5) | 8.7 (2.9) | 21.5 (4.2) | 57.6 (28.0) |
| Sleep efficiency, % | 71.7 (15.7) | 77.2 (11.5) | 71.2 (18.1) | 69.5 (15.2) | 67.5 (16.8) |
| Periodic leg movement index, events/h | 37.5 [14.6; 73] | 19.9 [7.99; 66.4] | 34.2 [20.7; 76.2] | 52.4 [33.9; 90.0] | 38.2 [15.8; 55.9] |
| Total sleep duration, h | 5.7 (1.9) | 6.3 (1.5) | 5.4 (1.9) | 5.3 (1.9) | 5.5 (2.1) |
| Nocturnal hypoxemia, % | 1.0 [0.0; 6.8] | 0.0 [0.0; 0.2] | 0.9 [0.2; 2.2] | 3.3 [0.3; 12.2] | 6.9 [2.3; 15.1] |
| Mean oxygen saturation, % | 94.2 (2.3) | 95.6 (2.0) | 94.3 (2.1) | 93.3 (2.4) | 93.5 (2.2) |
| Oxygen desaturation index, events/h | 5.6 [1.2; 15.0] | 0.3 [0.0; 1.2] | 4.4 [2.5; 6.8] | 13.4 [7.8; 16.0] | 28.2 [14.8; 38.4] |
| Obstructive apnea hypopnea index, events/h | 12.3 [4.0; 25.9] | 2.4 [1.1; 3.2] | 7.1 [6.2; 9.9] | 19.9 [17.2; 23.5] | 41.4 [33.4; 60.5] |
| Central apnea hypopnea index, events/h | 0.1 [0.0; 0.6] | 0.0 [0.0; 0.2] | 0.2 [0.0; 0.4] | 0.0 [0.0; 0.7] | 0.3 [0.0; 3.4] |
Table shows mean (SD), median [quartile 1; quartile 3], or frequency (%). API, apnea hypopnea index.
For patients with ESKD only.
For patients with CKD only.
Polysomnography-Measured Sleep Variables
On average, the sleep duration was 5.7±1.9 hours, sleep efficiency was 72%±16%, and median periodic limb movement index was 37.5 (IQR 14.6; 73) per hour of sleep (Table 1). There was no significant difference in AHI in patients with advanced CKD or ESKD (P=0.15). However, the CKD group had significantly better sleep efficiency, longer sleep duration, higher mean oxygen saturation, and lower ODI and nocturnal hypoxemia as compared with the ESKD group (Table 2).
Table 2.
Polysomnography-measured sleep variables in CKD and ESKD
| Variable | CKD, n=88 | ESKD, n=92 | P Value |
|---|---|---|---|
| Apnea hypopnea index, events/h | 8.9 [3.4; 27.0] | 14.6 [5.5; 29.5] | 0.15 |
| Total sleep, h | 6.1 (1.7) | 5.2 (2.0) | 0.001 |
| Sleep efficiency, % | 74.4 (15.0) | 69.1 (16.0) | 0.03 |
| Nocturnal hypoxemia, % | 0.5 [0.0; 5.3] | 1.9 [0.1; 9.5] | 0.01 |
| Periodic leg movement index, events/h | 34.5 [15.9; 55.8] | 39.9 [12.1; 87.2] | 0.64 |
| Mean oxygen saturation, % | 95.0 (1.9) | 93.5 (2.5) | <0.001 |
| Oxygen desaturation index, events/h | 4.2 [0.8; 13.7] | 7.6 [1.9; 16.0] | 0.05 |
Table shows mean (SD), median [quartile 1; quartile 3], or frequency (%). P values were on the basis of a two-sample t test or Wilcoxon rank-sum test for continuous variables, and chi-squared or Fisher exact test for categorical variables.
Sleep Apnea Severity (AHI) and Mortality
Over a median follow-up period of 9 years (quartiles 4, 11), there were a total of 84 (47%) deaths and 85 (47%) kidney transplants. Among transplant recipients, 20 died. The overall causes of death were cardiovascular in 31%, infectious in 4%, cancer in 14%, and other in 51%.
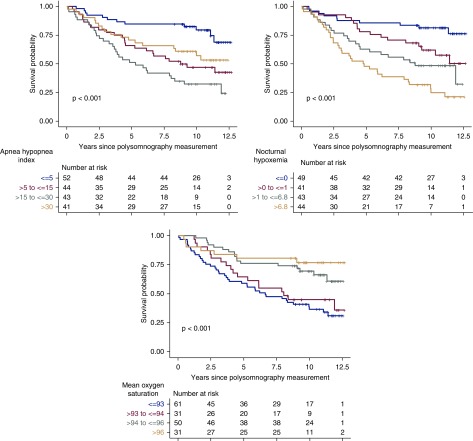
Unadjusted survival analysis showed there was significant difference in mortality between the AHI groups (log-rank P<0.001) and moderate sleep apnea had the highest hazard of death (Figure 1). However, after adjustment for age, sex, race, diabetes, BMI, CKD/ESKD status, and kidney transplant status, there was no significant association of AHI with mortality (Table 3). In sensitivity analysis using AHI as a continuous variable, there was no significant association of AHI with mortality in unadjusted or adjusted analyses (Table 4). Other covariates significantly associated with mortality were age (for every 10-year increase in age: adjusted HR, 1.4; 95% confidence interval [95% CI], 1.1 to 1.7; P<0.01), kidney function status (for ESKD: HR, 2.1; 95% CI, 1.2 to 3.7; P<0.01), and kidney transplant recipient status (adjusted HR, 0.4; 95% CI, 0.2 to 0.7; P<0.01).
Figure 1.
Moderate sleep apnea, worse nocturnal hypoxemia, and lower mean oxygen saturation were associated with higher mortality.
Table 3.
Associations of apnea hypopnea index with mortality
| Variable | Deaths, n (%) | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| Apnea hypopnea index | ||||||
| ≤5 events/h | 12 (23%) | Reference | Reference | |||
| >30 events/h | 24 (55%) | 2.23 (1.07 to 4.62) | 0.03 | 1.54 (0.59 to 4.01) | 0.38 | |
| >15 to 30 events/h | 30 (70%) | 4.41 (2.25 to 8.64) | <0.001 | 2.31 (0.91 to 5.85) | 0.08 | |
| >5 to 15 events/h | 18 (44%) | 2.88 (1.44 to 5.77) | 0.003 | 2.13 (0.84 to 5.41) | 0.11 | |
| Covariates adjusted for | ||||||
| Age, for every 10 yr higher | 1.40 (1.13 to 1.72) | 0.002 | ||||
| Male versus female | 1.82 (0.95 to 3.51) | 0.07 | ||||
| ESKD versus CKD | 2.11 (1.21 to 3.69) | 0.009 | ||||
| Kidney transplant (time varying) | 0.37 (0.20 to 0.69) | 0.002 | ||||
| Other race versus white | 0.91 (0.49 to 1.67) | 0.75 | ||||
| Diabetes | 1.17 (0.68 to 2.03) | 0.57 | ||||
| BMI, for every unit higher | 1.05 (1.00 to 1.10) | 0.05 | ||||
HR, hazard ratio; 95% CI, 95% confidence interval; BMI, body mass index.
Table 4.
Associations of sleep measures with mortality
| Unadjusted | Adjusteda | ||
|---|---|---|---|
| Variable | HR (95% CI) | HR (95% CI) | P Value (FDR-Correctedb) |
| Hypoxemia-based measures | |||
| Apnea hypopnea index, for every 5 events/h higherc | 1.02 (0.99 to 1.06)d | 1.01 (0.96 to 1.05)d | 0.99 |
| Nocturnal hypoxemia (% of sleep time with oxygen saturation <90%), for every 15% highere,f | 1.53 (1.30 to 1.78) | 1.36 (1.12 to 1.67) | 0.007 |
| Mean oxygen saturation, for every 2% lowerf | 1.76 (1.47 to 2.14) | 1.59 (1.23 to 2.08) | 0.003 |
| Oxygen desaturation index, for every 17 events/h highere,f | 1.19 (1.03 to 1.38) | 1.02 (0.84 to 1.25) | 0.99 |
| Other polysomnography measures | |||
| Total sleep, for every h lowerg,c | 1.22 (1.08 to 1.36)h | 1.06 (0.93 to 1.22)h | 0.88 |
| Sleep efficiency, for every 15% lowerf | 1.40 (1.15 to 1.69) | 1.08 (0.83 to 1.40) | 0.99 |
| Periodic leg movement index, for every 57 events/h higherf | 1.32 (1.06 to 1.63) | 1.00 (0.79 to 1.32) | 0.99 (0.99) |
HR, hazard ratio; 95% CI, 95% confidence interval; FDR, false discovery rate.
Adjusted model is adjusted for age, sex, race, diabetes, body mass index, kidney function status (CKD or ESKD), and time-varying kidney transplant status. Within each model, HR estimates and the corresponding P value are given.
FDR-corrected P values used Benjamini–Hochberg method.
On the basis of clinically meaningful increment.
For apnea hypopnea index, the HR for every 25 events/h (1 SD) higher were as follows: unadjusted HR, 1.10 (95% CI, 0.95 to 1.34) and adjusted HR, 1.05 (95% CI, 0.82 to 1.28).
There was a significant interaction between nocturnal hypoxemia and CKD/ESKD (P=0.01) and between oxygen desaturation index and sex (P=0.02), so HRs within these subgroups were also calculated from the adjusted model. The adjusted HR for every 15% higher nocturnal hypoxemia for CKD and ESKD, respectively, were HR, 2.39 (95% CI, 1.55 to 3.65; P<0.001) and HR, 1.15 (95% CI, 1.00 to 1.55; P=0.07). Because of the small sample size for women, the HR estimates for oxygen desaturation index by sex were not shown.
On the basis of 1 SD increment.
For adjusted analysis, the proportional hazard assumption did not hold for total sleep time. Thus, an interaction term with time was included and HRs at different time points (year 1, year 5, year 9, and year 12) were calculated but revealed estimates in the same direction as the overall HR (data not shown).
For total sleep, the HR for every 1.86 hour (1 SD) lower were as follows: unadjusted HR, 1.44 (95% CI, 1.15 to 1.77) and adjusted HR, 1.11 (95% CI, 0.87 to 1.45).
In subgroup analysis, there was a significant interaction of AHI category with sex (P=0.05), but the stratified analysis was not done owing to very small number of women with severe sleep apnea. There was no evidence of a significant interaction of AHI with age or kidney function status (CKD or ESKD), thus the survival analysis results are not different in these subgroups.
Hypoxemia-Based Polysomnography Measures and Mortality
In unadjusted analyses, worse nocturnal hypoxemia (proportion of total sleep time with oxygen saturation <90%), lower mean oxygen saturation, and higher ODI (number of events per hour in which SaO2 decreased by ≥4% from the baseline for ≥5 seconds) were associated with higher mortality (Figure 1, Table 4). In adjusted analysis, worse nocturnal hypoxemia was associated with higher mortality (for every 15% higher: HR, 1.36; 95% CI, 1.12 to 1.67; P=0.007). There was a significant interaction of nocturnal hypoxemia with kidney function status (CKD or ESKD) (P=0.01) and in both of these groups, the hazards for mortality were significant and in the same direction (Table 4, footnote e). Association of lower mean oxygen saturation with higher mortality also remained significant in adjusted analysis (for every 2% lower: HR, 1.59; 95% CI, 1.23 to 2.08; P=0.003). There was a significant interaction between ODI and sex (P=0.02), but because of the limited number of women, estimates stratified by sex were not calculated.
To determine the relative strength of association of different polysomnography measures with mortality in adjusted analysis, standardized covariate HRs were calculated. Lower mean oxygen saturation had the highest hazard of mortality in adjusted analysis (HR, 1.7; 95% CI, 1.3 to 2.3; P<0.001), followed by nocturnal hypoxemia (HR, 1.4; 95% CI, 1.1 to 1.7; P=0.002).
Other Polysomnography Measures and Mortality
Lower sleep efficiency was associated with increased mortality in unadjusted analysis, but this association became insignificant in adjusted models (Table 4). Sleep duration and periodic limb movement index did not have a significant association with mortality in unadjusted or adjusted analyses. There was no significant interaction of age, sex, or kidney function status (CKD or ESKD) with any of these polysomnography variables.
Sensitivity Analysis
Censoring for kidney transplant has been a prevalent practice in prior literature, but we believe that it may not be the best approach because transplant is associated with the primary outcome of mortality and hence violates the assumption of noninformative censoring. Nevertheless, we performed the analysis and found that the results were similar to our original analyses, except nocturnal hypoxemia is now not significant (Supplemental Table 3).
Discussion
In this study of patients with advanced CKD or ESKD, prevalence of sleep apnea was high, with 71% diagnosed with sleep apnea and 23% with severe sleep apnea. There were no significant differences in sleep apnea severity (measured by AHI) among patients with advanced CKD or ESKD. Over a median follow-up period of 9 years (quartiles 4, 11), there was no significant association of AHI with mortality after adjusting for age, sex, race, diabetes, BMI, CKD/ESKD status, and kidney transplant status. However, nocturnal hypoxemia, mean oxygen saturation, and ODI (in women only) were significantly associated with mortality in adjusted analysis. There was no association of sleep duration, sleep efficiency, or periodic limb movement index with mortality in these patients.
Our study builds on prior literature, and extends the findings to a larger, more diverse CKD/ESKD population, followed over a longer time period. Two studies in patients on HD using nocturnal pulse oximetry reported increased mortality with sleep-disordered breathing/nocturnal hypoxemia (25,26). Using polysomnography, Jung et al. (27) reported significant association of nocturnal hypoxemia with mortality in 30 patients on HD. However, given the limited sample size, this study was not able to account for multiple confounders in adjusted analysis. Our study provides further robust evidence that hypoxemia-based measures remain significantly associated with mortality in both advanced CKD and ESKD, even after adjusting for a number of confounders, including kidney transplant status. The strongest association was for mean oxygen saturation for which every 2% lower saturation was associated with 1.6 times increased risk of mortality. The lack of any significant association of AHI with mortality in our study is consistent with prior studies in patients on HD and patients with CKD done using polysomnography (17,27–29). In contrast, Tang et al. (30) reported increased mortality in those with moderate-to-severe sleep apnea, but was only limited to patients on peritoneal dialysis.
Overall, our results are consistent with findings from the general population, which support the role of hypoxemic burden as a predictor of mortality. Azarbarzin et al. (31) combined data from two very large sleep study cohorts and demonstrated that hypoxic burden of sleep apnea, rather than AHI, more significantly and consistently associated with cardiovascular mortality in over 7700 diverse patients. It may be that AHI, a frequency-based measure of arousal, does not adequately characterize the physiologic disturbances. Hypoxemic burden may be the driving force leading to elevated oxidative stress, coronary calcification, sympathetic hyperactivity, systemic inflammation, and left ventricular hypertrophy, all potential mechanisms that are hypothesized to cause increased mortality with sleep-disordered breathing in the general and ESKD population (32–35). Moreover, the high prevalence of sleep apnea (measured by AHI) in CKD/ESKD is thought to be owing to rostral fluid shifts, fluid overload, uncontrolled hypertension, metabolic acidosis, and uremia (36). It is plausible that these unique mechanisms that lead to increased apneic or hypopneic arousal events may not cause significant changes in blood gas, thus not mediating risk for mortality the same way as hypoxemic-based measures. Lastly, AHI may be subject to measurement challenges (interscorer and intrascorer variability), but hypoxia-based measures are accurate and reproducible, and thus more representative of underlying physiologic changes.
Our findings have clinical and research implications in patients with CKD/ESKD and sleep-disordered breathing. Hypoxemic burden could be derived from basic and commonly used tools in clinical practice, such as pulse oximetry, without the need for extensive sleep study, and could help characterize mortality risk in these patients. Moreover, patients with sleep disorder–related nocturnal hypoxemia may be more prone to hypoxemia during HD, which is associated with morbidity and mortality and hypothesized to contribute to intradialytic complications such as hypotension and cramps (37,38). Thus, research into screening all patients with CKD/ESKD with a simple overnight home pulse oximetry device is warranted. Whether improvement in mean oxygen saturation with supplemental oxygen improves mortality remains to be seen. Given the modest beneficial effect of positive airway therapy on cardiovascular disease and mortality in the general population or patients with comorbidities such as diabetes or cardiovascular disease (39–42), future research could study the effect of supplemental oxygen alone versus positive airway therapy on survival in this population. Also, whether these treatment options improve other symptoms that may be associated with AHI severity (such as daytime sleepiness and mood disorders) remains to be studied.
Our findings in patients with advanced CKD stages 4 and 5 are novel and we are unaware of any other study that has evaluated the association of polysomnography-obtained sleep measures with mortality in this patient population. Xu et al. (17) reported association of central sleep apnea with mortality in 103 patients with CKD; however, these patients had much earlier stage of CKD as compared with our cohort (mean eGFR 49±11 versus 18±7 ml/min per 1.73 m2). Additionally, this study was conducted in patients referred to a sleep clinic and did not adjust for kidney transplant. In our study, we did not find any significant differences in AHI among patients with CKD or ESKD, although patients with ESKD had worse nocturnal hypoxemia (P=0.01). The results of our survival analysis did not differ among CKD or ESKD. This suggests that many of the underlying mechanisms for increased mortality with nocturnal hypoxemia in this group are similar to ESKD.
We did not find association of sleep duration, sleep efficiency, or periodic limb movement index with mortality. In the general population, both short and long sleep have been associated with increased mortality and cardiovascular events (43). Similar results have been reported in CKD, but are on the basis of patient self-report, which often poorly correlates with objective sleep measures (12,16). Although periodic limb movement index has been associated with increased mortality in two studies of patients on HD, the scores in our cohort were much lower, and could explain the negative findings (27,28).
Strengths of our study include a diverse, representative population; large sample size; long follow-up duration; and lack of selection bias. Our cohort had similar age, sex, and race distribution as the prevalent United States patients on dialysis, thus our results are generalizable (44). Additionally, although the rate of kidney transplant in our study was high at 47%, we had accurate information on timing of kidney transplantation. Furthermore, we used time-varying model to account for presence and timing of kidney transplantation, thus limiting survivorship bias.
Limitations of our study included a lower median AHI as compared with prior studies using polysomnography in this population, which may have attenuated the association of sleep apnea with mortality. This is because our cohort was recruited from nephrology, transplant, and dialysis clinics as opposed to sleep clinics. However, 46% of patients in our study still had moderate-to-severe sleep apnea. Also, our results remained unchanged using AHI as continuous variable. Secondly, the 1999 American Academy of Sleep Medicine definitions for AHI were used in our study. There have been subsequent changes in AHI definitions, and this may limit translation of our results to the currently clinically reported AHI. Thirdly, we did not have information on certain confounders such as underlying lung disease, congestive heart failure, or hypoxemia measurement during wakefulness, which may have affected our results and interpretation. Fourthly, we do not have information whether patients with sleep-disordered breathing received any subsequent treatment. However, given the low rates of continuous positive airway pressure use in the general population, we do not expect this to significantly affect our results. Lastly, our study may have been underpowered to detect an association of AHI with mortality, and a larger sample size may be needed to detect a smaller HR.
In conclusion, we found that patients with advanced CKD and ESKD have similar sleep apnea severity (measured by AHI). Hypoxemia-based measures of sleep apnea are significantly associated with mortality in advanced CKD and ESKD. Given the strong association of nocturnal mean oxygen saturation with mortality, further studies are needed to evaluate whether screening all patients with CKD or ESKD with a simple overnight home pulse oximetry device is warranted. There is no association of AHI, sleep duration, sleep efficiency, or periodic limb movement index with mortality in these patients. Future research is needed to elucidate underlying mechanisms and potential treatments such as supplemental oxygen for minimizing hypoxemic burden and perhaps improving survival in patients with advanced kidney disease.
Disclosures
Dr. Patel reports receiving grants from Bayer and Philips Respironics and personal fees from the American Academy of Sleep Medicine outside of the submitted work. Dr. Abdalla, Dr. Davis, Dr. Hou, Dr. Jhamb, Dr. Ran, Dr. Roumelioti, Dr. Unruh, and Dr. Yabes have nothing to disclose.
Funding
Dr. Jhamb was supported by National Institute of Diabetes and Kidney Diseases (NIDDK) grants P30DK079307 and R01DK114085 and American Heart Association grant 11FTF7520014. Dr. Unruh was supported by NIDDK grants K23DK66006 and R01DK77785.
Supplementary Material
Acknowledgments
These findings were presented in a poster at the National Kidney Foundation Spring Clinical meetings from April 10–14, 2018 in Austin, Texas.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07880719/-/DCSupplemental.
Supplemental Table 1. Summary of studies reporting association of polysomnography-measured sleep apnea severity with mortality in patients with CKD and/or ESKD.
Supplemental Table 2. Missing data for key variables.
Supplemental Table 3. Sensitivity analysis of association of sleep measures with mortality by censoring at the time of kidney transplant.
Supplemental Figure 1. Participant enrollment in the study.
References
- 1.Hanly P: Sleep disorders and end-stage renal disease. Curr Opin Pulm Med 14: 543–550, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Huang Z, Tang X, Zhang T, Qiu S, Xia Z, Fu P: Prevalence of sleep apnoea in non-dialysis chronic kidney disease patients: A systematic review and meta-analysis. Nephrology (Carlton) 24: 1041–1049, 2019. [DOI] [PubMed] [Google Scholar]
- 3.Merlino G, Piani A, Dolso P, Adorati M, Cancelli I, Valente M, Gigli GL: Sleep disorders in patients with end-stage renal disease undergoing dialysis therapy. Nephrol Dial Transplant 21: 184–190, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S: The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235, 1993. [DOI] [PubMed] [Google Scholar]
- 5.West SD, Nicoll DJ, Stradling JR: Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax 61: 945–950, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansfield D, Naughton MT: Obstructive sleep apnoea, congestive heart failure and cardiovascular disease. Heart Lung Circ 14[Suppl 2]: S2–S7, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Tasali E, Mokhlesi B, Van Cauter E: Obstructive sleep apnea and type 2 diabetes: Interacting epidemics. Chest 133: 496–506, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Unruh ML, Sanders MH, Redline S, Piraino BM, Umans JG, Hammond TC, Sharief I, Punjabi NM, Newman AB: Sleep apnea in patients on conventional thrice-weekly hemodialysis: Comparison with matched controls from the Sleep Heart Health Study. J Am Soc Nephrol 17: 3503–3509, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Liu TZ, Xu C, Rota M, Cai H, Zhang C, Shi MJ, Yuan RX, Weng H, Meng XY, Kwong JS, Sun X: Sleep duration and risk of all-cause mortality: A flexible, non-linear, meta-regression of 40 prospective cohort studies. Sleep Med Rev 32: 28–36, 2017. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Ouyang Y, Wang Z, Zhao G, Liu L, Bi Y: Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: A meta-analysis of prospective cohort studies. Int J Cardiol 169: 207–214, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Elder SJ, Pisoni RL, Akizawa T, Fissell R, Andreucci VE, Fukuhara S, Kurokawa K, Rayner HC, Furniss AL, Port FK, Saran R: Sleep quality predicts quality of life and mortality risk in haemodialysis patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 23: 998–1004, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Ricardo AC, Knutson K, Chen J, Appel LJ, Bazzano L, Carmona-Powell E, Cohan J, Kurella Tamura M, Steigerwalt S, Thornton JD, Weir M, Turek NF, Rahman M, Van Cauter E, Lash JP; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The association of sleep duration and quality with CKD progression. J Am Soc Nephrol 28: 3708–3715, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kutner N, Zhang R, Johansen K, Bliwise D: Associations among nocturnal sleep, daytime intradialytic sleep, and mortality risk in patients on daytime conventional hemodialysis: US Renal Data System special study data. Hemodial Int 17: 223–229, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Tuohy CV, Montez-Rath ME, Turakhia M, Chang TI, Winkelman JW, Winkelmayer WC: Sleep disordered breathing and cardiovascular risk in older patients initiating dialysis in the United States: A retrospective observational study using medicare data. BMC Nephrol 17: 16, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang XH, Zhang BL, Gu YH, Zhan XL, Guo LL, Jin HM: Association of sleep disorders, chronic pain, and fatigue with survival in patients with chronic kidney disease: A meta-analysis of clinical trials. Sleep Med 51: 59–65, 2018. [DOI] [PubMed] [Google Scholar]
- 16.Unruh ML, Sanders MH, Redline S, Piraino BM, Umans JG, Chami H, Budhiraja R, Punjabi NM, Buysse D, Newman AB: Subjective and objective sleep quality in patients on conventional thrice-weekly hemodialysis: Comparison with matched controls from the sleep heart health study. Am J Kidney Dis 52: 305–313, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Yoon IY, Chin HJ: The effect of sleep apnea on all-cause mortality in nondialyzed chronic kidney disease patients. Sleep Med 27–28: 32–38, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Unruh ML, Hartunian MG, Chapman MM, Jaber BL: Sleep quality and clinical correlates in patients on maintenance dialysis. Clin Nephrol 59: 280–288, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Bruyneel M, Ninane V: Unattended home-based polysomnography for sleep disordered breathing: Current concepts and perspectives. Sleep Med Rev 18: 341–347, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of sleep medicine task force. Sleep 22: 667–689, 1999. [PubMed] [Google Scholar]
- 21.American Academy of Sleep Medicine : The AASM Manual for the Scoring of Sleep and Associated Events, 2019. Available at: https://aasm.org/clinical-resources/scoring-manual/. Accessed June 15, 2019
- 22.Wickham H, François R, Henry L, Müller K: dplyr: A Grammar of Data Manipulation. R Package Version 0.7.6. Available at: https://cran.r-project.org/web/packages/dplyr/index.html. Accessed January 10, 2019
- 23.Therneau T: A Package for Survival Analysis in S. version 2.38, 2015. Available at: https://CRAN.R-project.org/package=survival. Accessed January 13, 2019
- 24. Wickham H: ggplot2: Elegant Graphics for Data Analysis, New York, Springer-Verlag, 2016.
- 25.Masuda T, Murata M, Honma S, Iwazu Y, Sasaki N, Ogura M, Onishi A, Ando Y, Muto S, Shimada K, Kario K, Kusano E, Asano Y: Sleep-disordered breathing predicts cardiovascular events and mortality in hemodialysis patients. Nephrol Dial Transplant 26: 2289–2295, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Zoccali C, Mallamaci F, Tripepi G: Nocturnal hypoxemia predicts incident cardiovascular complications in dialysis patients. J Am Soc Nephrol 13: 729–733, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Jung HH, Lee JH, Baek HJ, Kim SJ, Lee JJ: Nocturnal hypoxemia and periodic limb movement predict mortality in patients on maintenance hemodialysis. Clin J Am Soc Nephrol 5: 1607–1613, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benz RL, Pressman MR, Hovick ET, Peterson DD: Potential novel predictors of mortality in end-stage renal disease patients with sleep disorders. Am J Kidney Dis 35: 1052–1060, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Sivalingam M, Chakravorty I, Mouatt S, Farrington K: Obstructive sleep apnea in incremental hemodialysis: Determinants, consequences, and impact on survival. Hemodial Int 17: 230–239, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Tang SC, Lam B, Yao TJ, Leung WS, Chu CM, Ho YW, Ip MS, Lai KN: Sleep apnea is a novel risk predictor of cardiovascular morbidity and death in patients receiving peritoneal dialysis. Kidney Int 77: 1031–1038, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, Ancoli-Israel S, Ensrud K, Purcell S, White DP, Redline S, Wellman A: The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: The Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J 40: 1149–1157, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudenbostel T, Calhoun DA: Resistant hypertension, obstructivesleep apnoea and aldosterone. J Hum Hypertens 26: 281–287, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung HH, Han H, Lee JH: Sleep apnea, coronary artery disease, and antioxidant status in hemodialysis patients. Am J Kidney Dis 45: 875–882, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Yamauchi M, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Suzuki T, Kimura H: Oxidative stress in obstructive sleep apnea. Chest 127: 1674–1679, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Zoccali C, Benedetto FA, Tripepi G, Cambareri F, Panuccio V, Candela V, Mallamaci F, Enia G, Labate C, Tassone F: Nocturnal hypoxemia, night-day arterial pressure changes and left ventricular geometry in dialysis patients. Kidney Int 53: 1078–1084, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Chu G, Choi P, McDonald VM: Sleep disturbance and sleep-disordered breathing in hemodialysis patients. Semin Dial 31: 48–58, 2018. [DOI] [PubMed] [Google Scholar]
- 37.Meyring-Wösten A, Zhang H, Ye X, Fuertinger DH, Chan L, Kappel F, Artemyev M, Ginsberg N, Wang Y, Thijssen S, Kotanko P: Intradialytic hypoxemia and clinical outcomes in patients on hemodialysis. Clin J Am Soc Nephrol 11: 616–625, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campos I, Chan L, Zhang H, Deziel S, Vaughn C, Meyring-Wösten A, Kotanko P: Intradialytic hypoxemia in chronic hemodialysis patients. Blood Purif 41: 177–187, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mokhlesi B, Ayas NT: Cardiovascular events in obstructive sleep apnea - can CPAP therapy SAVE lives? N Engl J Med 375: 994–996, 2016. [DOI] [PubMed] [Google Scholar]
- 40. Guo J, Sun Y, Xue LJ, Huang ZY, Wang YS, Zhang L, Zhou GH, Yuan LX: Effect of CPAP therapy on cardiovascular events and mortality in patients with obstructive sleep apnea: A meta-analysis. Sleep Breath 20: 965–974, 2016. [DOI] [PubMed]
- 41.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi-Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS; SAVE Investigators and Coordinators : CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 375: 919–931, 2016. [DOI] [PubMed] [Google Scholar]
- 42.Quan W, Zheng D, Douglas McEvoy R, Barbe F, Chen R, Liu Z, Loffler K, Lorenzi-Filho G, Luo Y, Mukherjee S, Tripathi M, Woodman R, Li Q, Wang X, Arima H, Xiao Y, Zhang X, Anderson CS; SAVE Investigators : High risk characteristics for recurrent cardiovascular events among patients with obstructive sleep apnoea in the SAVE study. EClinicalMedicine 2–3: 59–65, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin J, Jin X, Shan Z, Li S, Huang H, Li P, Peng X, Peng Z, Yu K, Bao W, Yang W, Chen X, Liu L: Relationship of sleep duration with all-cause mortality and cardiovascular events: A systematic review and dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc 6: e005947, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.United States Renal Data System : USRDS Annual Data Report, 2007. Available at: https://www.usrds.org/atlas07.aspx. Accessed October 7, 2019
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.