Visual Abstract
Keywords: kidney transplantation, donor exchange, kidney donation, humans, female, United States, transplant recipients, risk factors, incidence, living donors, renal dialysis, follow-up studies, allografts, body mass index, semantic web, age factors, sex factors, hepatitis C, registries, diabetes mellitus, Hispanic Americans
Abstract
Background and objectives
In the United States, kidney paired donation networks have facilitated an increasing proportion of kidney transplants annually, but transplant outcome differences beyond 5 years between paired donation and other living donor kidney transplant recipients have not been well described.
Design, setting, participants, & measurements
Using registry-linked data, we compared National Kidney Registry (n=2363) recipients to control kidney transplant recipients (n=54,497) (February 2008 to December 2017). We estimated the risk of death-censored graft failure and mortality using inverse probability of treatment weighted Cox regression. The parsimonious model adjusted for recipient factors (age, sex, black, race, body mass index ≥30 kg/m2, diabetes, previous transplant, preemptive transplant, public insurance, hepatitis C, eGFR, antibody depleting induction therapy, year of transplant), donor factors (age, sex, Hispanic ethnicity, body mass index ≥30 kg/m2), and transplant factors (zero HLA mismatch).
Results
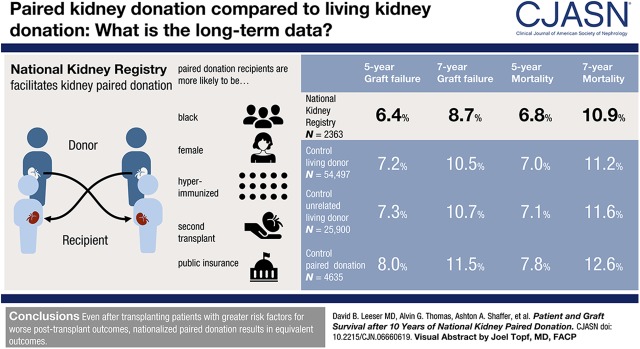
National Kidney Registry recipients were more likely to be women, black, older, on public insurance, have panel reactive antibodies >80%, spend longer on dialysis, and be previous transplant recipients. National Kidney Registry recipients were followed for a median 3.7 years (interquartile range, 2.1–5.6; maximum 10.9 years). National Kidney Registry recipients had similar graft failure (5% versus 6%; log-rank P=0.2) and mortality (9% versus 10%; log-rank P=0.4) incidence compared with controls during follow-up. After adjustment for donor, recipient, and transplant factors, there no detectable difference in graft failure (adjusted hazard ratio, 0.95; 95% confidence interval, 0.77 to 1.18; P=0.6) or mortality (adjusted hazard ratio, 0.86; 95% confidence interval, 0.70 to 1.07; P=0.2) between National Kidney Registry and control recipients.
Conclusions
Even after transplanting patients with greater risk factors for worse post-transplant outcomes, nationalized paired donation results in equivalent outcomes when compared with control living donor kidney transplant recipients.
Introduction
Kidney paired donation was first proposed in 1986 by Dr. Felix Rapaport (1). Since that time, paired donation has developed from an emerging modality of transplanting patients with incompatible donors, to one that is responsible for 12% of living donor kidney transplants in the United States each year through local and national programs (2). Prospective studies have focused on efforts to maximize the effect of nondirected donors on start chains, the effect of cold ischemia time on living donor kidneys recovered at distant centers, and characteristics of the patients transplanted through these paired donation modalities (3–5).
With the practice of paired donation still being relatively new, questions remain unanswered because lack of long-term follow-up data. Early studies of large paired donation programs have shown acceptable short-term outcomes, despite increased rates of delayed graft function in patients receiving shipped kidneys (5,6). One important unanswered question is whether nationalized kidney paired donation programs can lead to acceptable outcomes 5 years post-transplant compared with living other donor kidney transplant modalities.
The National Kidney Registry facilitated its first transplant on February 14, 2008; to date, it has facilitated over 3000 transplants, the greatest number of kidney paired donation transplants by a single network in the United States. A report of the demographics of patients transplanted through the National Kidney Registry network showed that, compared with other living donor kidney transplant recipients, the National Kidney Registry patients were more often black, hyperimmunized, previous transplant recipients, women, and on public insurance (7). Thus, National Kidney Registry recipients have greater rates of risk factors predictive of worse post-transplant outcomes relative to other living donor kidney transplant recipient populations (7,8).
Given the characteristics of the National Kidney Registry recipients, the goal of this study was to assess the short- and longer-term outcomes of National Kidney Registry recipients compared with living donor kidney transplant recipient controls. Specifically, using a person-level linkage of the National Kidney Registry database and the Scientific Registry of Transplant Recipients (SRTR), we described and compared outcomes at 3, 5, and 7 years post-transplant and assessed differences in the mortality and graft failure risk between National Kidney Registry and control living donor kidney transplant recipients.
Materials and Methods
The National Kidney Registry
This study used data from the National Kidney Registry, a nonprofit, 501(c) organization that facilitates kidney paired donations for members of its clinical network. The National Kidney Registry network comprises 85 transplant centers within the United States. The participating transplant centers carry out all transplants in concordance with National Kidney Registry and center-specific protocols. The National Kidney Registry receives quarterly updates from participating transplant centers. The clinical and research activities of this study are consistent with the Declaration of Helsinki and Declaration of Istanbul. Using the National Kidney Registry, we identified 2454 living donor kidney transplants facilitated by the National Kidney Registry between February 2008 and December 2017.
National Registry Data Source
This study used data from the SRTR external release made available in March 2019. The SRTR data system includes data on donors, waitlist candidates, and transplant recipients in the United States, submitted by members of the Organ Procurement and Transplantation Network (OPTN) (9). The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. Using SRTR, we identified 56,860 kidney-only recipients who underwent living donor kidney transplant between February 2008 and December 2017 (including the 2363 National Kidney Registry transplants). All recipients were followed for post-transplant outcomes through December 31, 2018.
Data Linkage
Data on kidney paired donation transplants facilitated by the National Kidney Registry were linked to the SRTR using unique, encrypted person-level identifiers; they were crossvalidated using redundantly captured characteristics (transplant center, transplant date, donor blood type, donor sex, recipient blood type, and recipient sex). As a result of crossvalidation, 2363 (96%) living donor kidney transplant facilitated by the National Kidney Registry were included in the study population.
Statistical Analyses
All analyses were performed using Stata 15/MP for Linux (College Station, Texas). Differences in donor, recipient, and transplant characteristics between National Kidney Registry and control recipients were assessed using the chi-squared test (categorical variables) and Mann–Whitney rank-sum (continuous variables) tests. To assess post-transplant outcomes, death-censored graft failure, and mortality, we used Kaplan–Meier plots and compared groups using the log-rank test. We used a two-sided α of 0.05 to indicate a statistically significant difference. For Kaplan–Meier plots, we compared National Kidney Registry recipient outcomes to outcomes of (1) control related living donor kidney transplant recipients, (2) control unrelated living donor kidney transplant recipients, and (3) control kidney paired donation recipients as previously described (7). Briefly, control unrelated kidney transplant recipients report a nonbiologic relation to their living donor. Control kidney paired donation recipients report receiving a kidney through paired donation or a nondirected donor, but were not linked to the National Kidney Registry. These recipients participated through either a local/regional system or another multicenter network such as the Alliance for Paired Donation; however, we cannot systematically identify the paired donation system through the national registry.
To produce unbiased estimates of the association between National Kidney Registry participation and transplant outcomes, we used statistical models to control for potential confounders. We present multiple models to demonstrate different pathways through which the estimate could be affected by confounding. To compare post-transplant outcomes among National Kidney Registry and control transplants, we used a two-stage inverse probability of treatment weighting framework (10,11). The first stage assesses the association between transplant characteristics and receiving a National Kidney Registry facilitated transplant using logistic regression. These predicted probabilities (propensity scores) were then converted into weights to be used during the second stage. In the second stage, we used Cox regression to assess the association between transplant characteristics and post-transplant outcomes. In the second stage there is only one covariate: an indicator variable for participation in National Kidney Registry. The weighting approach often performs similarly to propensity score matching approaches, but can improve power by utilizing all observations (10). Cox models were stratified by transplant center to account for center-level differences. In sensitivity analyses, we also estimate the adjusted hazard ratio (aHR) of graft failure and mortality using unweighted Cox regression (see Supplemental Material). Using the inverse probability of treatment weighting framework, we constructed four models to assess post-transplant outcomes.
Model 1: Recipient Characteristics.
The first model (recipient only) was adjusted for recipient factors, including age, sex, black race, Hispanic ethnicity, body mass index (BMI) ≥30 kg/m2, diabetes, hypertension, college education, public insurance, hepatitis C infection, preemptive transplant, history of previous transplant, eGFR before transplant (CKD Epidemiology Collaboration 2009 equation), antibody nondepleting induction therapy, antibody depleting induction therapy, and year of transplant.
Model 2: Recipient and Donor Characteristics.
The second model (recipient, donor) was adjusted for the recipient characteristics in model 1 as well as donor factors, including age, sex, black race, Hispanic ethnicity, BMI≥30 kg/m2, and eGFR before transplant.
Model 3: Recipient, Transplant, and Donor Characteristics.
The third model (recipient, donor, and transplant) was adjusted for all of the recipient and donor characteristics in model 2 as well as ABO incompatible (ABOi) transplant and zero HLA mismatches.
Model 4: Parsimonious Model.
The parsimonious model was developed using the F-test for goodness of fit. The parsimonious model was adjusted for recipient factors (age, sex, black race, BMI≥30 kg/m2, diabetes, previous transplant, preemptive transplant, public insurance, hepatitis C, eGFR, antibody depleting induction therapy, year of transplant), donor factors (age, sex, Hispanic ethnicity, BMI≥30 kg/m2), and transplant factors (zero HLA mismatch).
Handling of Missingness
There were low levels of missingness among characteristics used in the statistical analyses. Characteristics with missingness included cold ischemia time (n=6581, 12%), panel reactive antibodies (PRA) >80% (n=5186, 9%) recipient education (n=3241, 6%), recipient hepatitis C (n=1471, 3%), recipient induction (n=686, <1%), recipient/donor HLA mismatch (n=474, <1%), recipient eGFR (n=438, <1%), donor eGFR (n=320, <1%), and recipient insurance status (n=3, <1%). Using a missing-at-random assumption, missing values were imputed using multiple imputation by chained equations to avoid potential information bias.
Results
Study Population
Among the 2363 living donor kidney transplant recipients with National Kidney Registry facilitated transplants, 46% were women, 18% were black, 12% were Hispanic, and the median age was 51 years (interquartile range [IQR], 39–60 years). These recipients spent a median 1.3 (IQR, 0.0–2.9) years on dialysis with 25% receiving a preemptive transplant. These recipients had a median BMI was 27 kg/m2 (IQR, 23–31 kg/m2), 19% had diabetes, 16% had hypertension, 1% had a HIV infection, 2% had an hepatitis C infection, and 25% had a previous transplant. A majority (65%) completed some college, and 50% were on public insurance. The median eGFR before transplant was 8 (IQR, 6–12) ml/min per 1.73 m2, and 21% of National Kidney Registry recipients had a PRA>80%. The characteristics of the National Kidney Registry and the SRTR kidney paired donation recipients are shown in Table 1. At the time of surgery, 65% received an antibody depleting induction therapy and 30% received an antibody nondepleting induction therapy (Table 2).
Table 1.
Characteristics of kidney paired donation recipients by participation in the National Kidney Registry (February 2008 to December 2017)
| Characteristic | National Kidney Registry Recipients | SRTR Kidney Paired Donation Recipients |
|---|---|---|
| N | 2363 | 4635 |
| Recipient characteristicsa | ||
| Women, % | 46 | 43 |
| Black, % | 18 | 14 |
| Hispanic, % | 12 | 14 |
| Age, yr | 51 (39–60) | 50 (39–59) |
| Preemptive transplant, % | 25 | 29 |
| Years on dialysis | 1.3 (0.0–2.9) | 0.9 (0.0–2.3) |
| BMI, kg/m2 | 26.5 (23.2–30.9) | 27.4 (23.7–31.6) |
| College educated, % | 65 | 64 |
| Public insurance, % | 50 | 46 |
| Diabetes, % | 19 | 20 |
| Hypertension, % | 16 | 16 |
| HIV, % | 1 | 0 |
| Hepatitis C, % | 2 | 2 |
| Previous transplant, % | 25 | 17 |
| PRA>80 at transplant, % | 21 | 10 |
| Antibody depleting induction, % | 65 | 73 |
| Antibody nondepleting induction, % | 30 | 21 |
| eGFR pretransplant, ml/min per 1.73 m2 | 7.9 (5.6–11.5) | 8.4 (5.7–12.0) |
| Delayed graft function, % | 5.2 | 3.8 |
| Donor characteristics | ||
| Women, % | 62 | 64 |
| Black, % | 10 | 9 |
| Hispanic, % | 10 | 12 |
| Age, yr | 45 (35–53) | 44 (34–52) |
| BMI, kg/m2 | 26.2 (23.3–28.9) | 26.5 (23.8–29.6) |
| eGFR pretransplant, ml/min per 1.73 m2 | 98 (85–109) | 97 (85–109) |
| Transplant characteristics | ||
| ABO incompatible, % | 2 | 2 |
| Zero HLA mismatch, % | 1 | 1 |
| Cold ischemia time, hr | 8.8 (5.5–12.0) | 1.5 (0.9–3.0) |
BMI, body mass index; PRA, panel reactive antibodies; SRTR, Scientific Registry of Transplant Recipients.
Continuous factors are reported as the median (25th percentile, 75th percentile).
Table 2.
Characteristics of living donor kidney transplant recipients by participation in the National Kidney Registry (February 2008 to December 2017)
| Characteristic | Control Living Donor Kidney Transplant Recipients | National Kidney Registry Recipients |
|---|---|---|
| N | 54,497 (96%) | 2363 (4%) |
| Recipient characteristicsa | ||
| Women, % | 38 | 46 |
| Black, % | 13 | 18 |
| Hispanic, % | 15 | 12 |
| Age, yr | 49 (36–59) | 51 (39–60) |
| Preemptive transplant, % | 36 | 25 |
| Years on dialysis | 0.5 (0.0–1.6) | 1.3 (0.0–2.9) |
| BMI, kg/m2 | 27 (24–31) | 27 (23–31) |
| College educated, % | 60 | 65 |
| Public insurance, % | 42 | 50 |
| Diabetes, % | 21 | 19 |
| Hypertension, % | 16 | 16 |
| HIV, % | 0.4 | 0.8 |
| Hepatitis C, % | 2 | 2 |
| Previous transplant, % | 12 | 25 |
| PRA>80 at transplant | 4 | 21 |
| Antibody depleting induction, % | 62 | 65 |
| Antibody nondepleting induction, % | 29 | 30 |
| eGFR pretransplant, ml/min per 1.73 m2 | 9 (6–13) | 8 (6–12) |
| Delayed graft function, % | 3 | 5 |
| Donor characteristics | ||
| Women, % | 62 | 62 |
| Black, % | 11 | 10 |
| Hispanic, % | 14 | 10 |
| Age, yr | 42 (33–51) | 45 (35–53) |
| BMI, kg/m2 | 27 (24–30) | 26 (23–29) |
| eGFR pretransplant, ml/min per 1.73 m2 | 99 (86–112) | 98 (86–109) |
| Transplant characteristics | ||
| ABO incompatible, % | 2 | 2 |
| Zero HLA mismatch, % | 7 | 1 |
| Cold ischemia time | 1 (1–2) | 9 (6–12) |
BMI, body mass index; PRA, panel reactive antibodies.
Continuous factors are reported as the median (25th percentile, 75th percentile).
Among National Kidney Registry donors, 62% were women, 10% were black, 10% were Hispanic, and the median age was 45 (IQR, 35–53) years. Their median BMI was 26 kg/m2 (IQR, 23–29 kg/m2) and median eGFR before transplant was 98 (IQR, 86–109) ml/min per 1.73 m2. Among National Kidney Registry transplants, 2% were ABOi, 1% had 0 HLA mismatches, and the median cold ischemia time was 9 (IQR, 6–12) hours (Table 2). Controls with 0 HLA mismatches were mostly siblings (83%) (Supplemental Table 1).
Post-Transplant Outcomes
National Kidney Registry recipients had a median 3.7 (IQR, 2.1–5.6; maximum 10.9) years of follow-up compared with controls, who had a median 5.3 (IQR, 3.0–7.9; maximum 10.9) years. Unrelated kidney transplant recipients had a median 5.0 (IQR, 2.8–7.7) years and other paired donation recipients had a median 4.9 (IQR, 2.8–7.3) years of follow-up. National Kidney Registry participants had similar graft failure and mortality cumulative incidence compared with control recipients (Table 3). Results were similar in comparisons of National Kidney Registry facilitated living donor kidney transplant recipients compared with control unrelated living donor kidney transplant recipients and control kidney paired donation recipients.
Table 3.
Unadjusted 1-, 3-, 5-, and 7-yr death-censored graft failure and mortality by participation in the National Kidney Registry
| Outcome | National Kidney Registry | Control Living Donor Kidney Transplant | Control Unrelated Living Donor Kidney Transplant | Control Kidney Paired Donation |
|---|---|---|---|---|
| N | 2363 | 54,497 | 25,900 | 4635 |
| 1-yr graft failure risk (events)a | 1.6 (38) | 1.7 (899) | 1.7 (448) | 1.7 (77) |
| 3-yr graft failure risk (events) | 3.4 (72) | 4.0 (2026) | 4.1 (977) | 4.5 (191) |
| 5-yr graft failure risk (events) | 6.4 (106) | 7.2 (3181) | 7.3 (1516) | 8.0 (295) |
| 7-yr graft failure risk (events) | 8.7 (120) | 10.5 (4034) | 10.7 (1901) | 11.5 (361) |
| 1-yr mortality risk (events) | 1.0 (23) | 1.3 (712) | 1.3 (338) | 1.3 (61) |
| 3-yr mortality risk (events) | 3.0 (62) | 3.7 (1874) | 3.7 (888) | 4.2 (180) |
| 5-yr mortality risk (events) | 6.8 (107) | 7.0 (3129) | 7.1 (1480) | 7.8 (290) |
| 7-yr mortality risk (events) | 10.9 (131) | 11.2 (4306) | 11.6 (2042) | 12.6 (392) |
We estimated risk using the Kaplan–Meier method. Graft failure and mortality risks were compared between National Kidney Registry recipients and (1) control living donor kidney transplant recipients (graft failure log-rank P=0.2; mortality log-rank P=0.4) (2), control unrelated kidney transplant recipients (graft failure log-rank P=0.1; mortality log-rank P=0.3), and (3) control kidney paired donation recipients (graft failure log-rank P=0.03; mortality log-rank P=0.1).
We present risk (cumulative incidence) as a percentage.
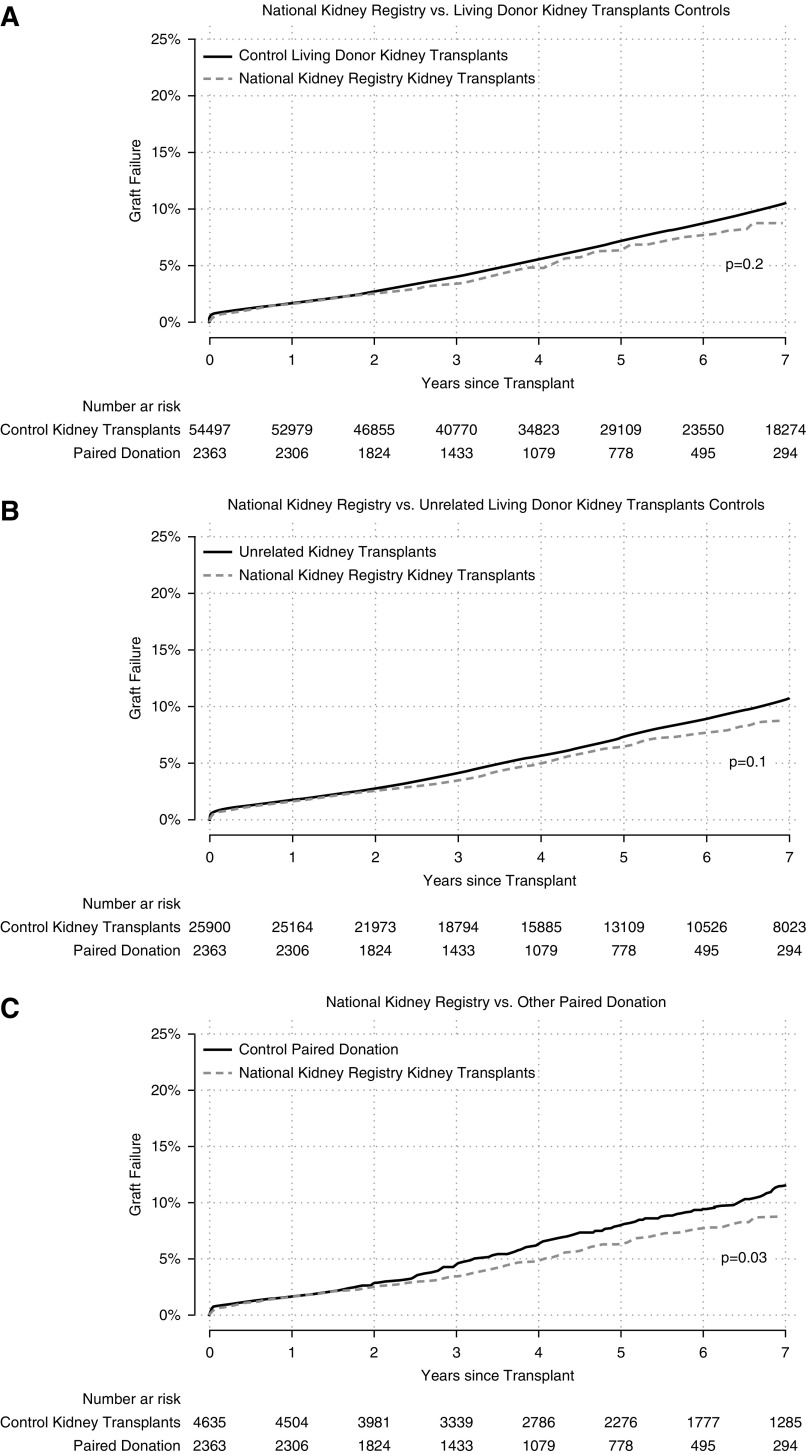
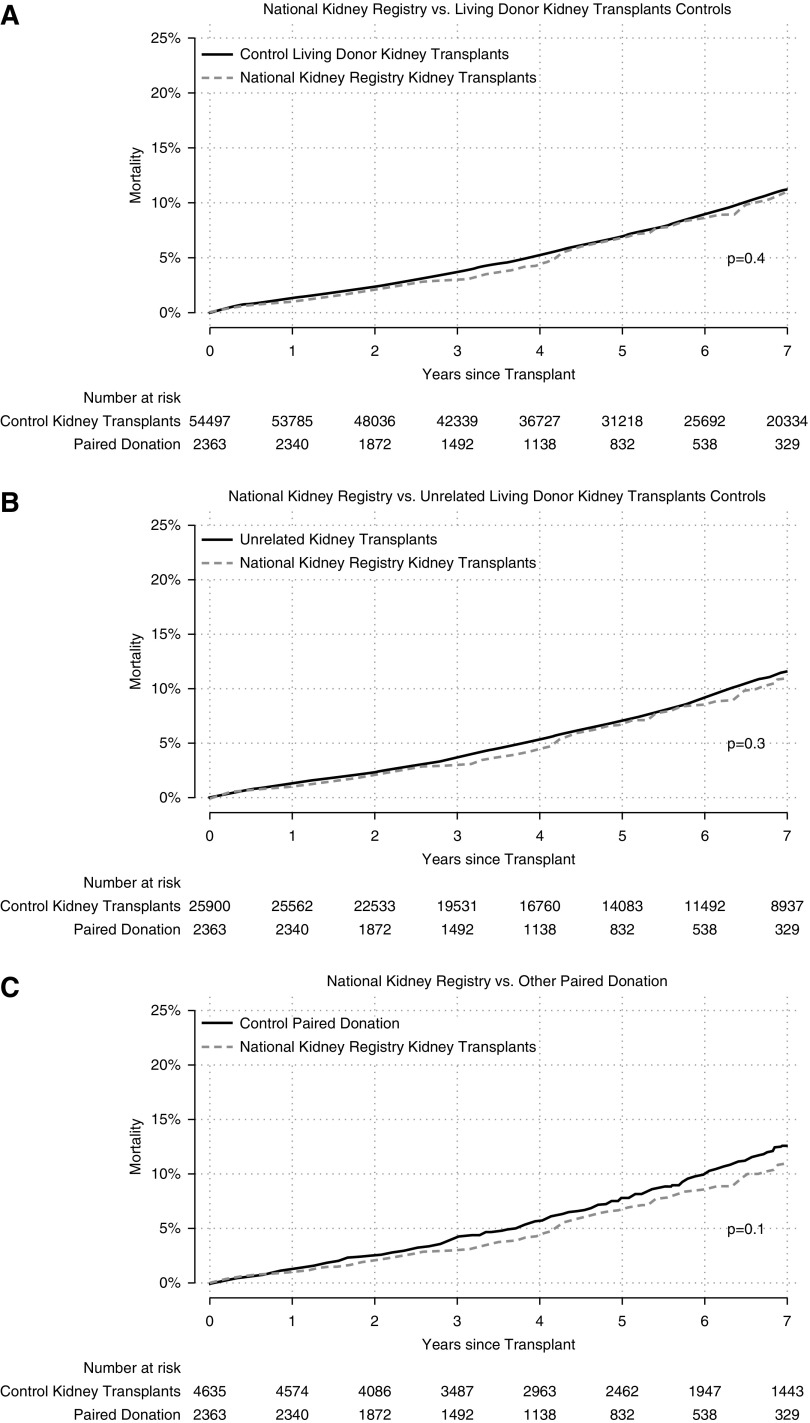
In the time-to-event framework, which properly accounts for censoring, there was no evidence of a difference in survival between National Kidney Registry living donor kidney transplant recipients and control living donor kidney transplant recipients (graft failure log-rank P=0.2; mortality log-rank P=0.4) and control unrelated living donor kidney transplant recipients (graft failure log-rank P=0.1; mortality log-rank P=0.3). Interestingly, National Kidney Registry recipients compared with control kidney paired donation recipients had a statistically lower graft failure but not mortality (graft failure log-rank P=0.03; mortality log-rank P=0.1) (Table 4). Kaplan–Meier curves show similar cumulative incidence of graft failure (Figure 1, A–C) and mortality (Figure 2, A–C) between National Kidney Registry and control living donor kidney transplant recipients during the first 7 years of the study period.
Table 4.
Expected and observed number of death-censored graft failure and mortality events
| Population | Graft Failure Observed | Graft Failure Expected | P Value | Mortality Observed | Mortality Expected | P Value |
|---|---|---|---|---|---|---|
| Living donor transplant | ||||||
| National Kidney Registry kidney transplant | 126 | 143 | 0.2 | 142 | 152 | 0.4 |
| Control | 4623 | 2606 | 5545 | 5535 | ||
| Unrelated living donor transplant | ||||||
| National Kidney Registry kidney transplant | 126 | 145 | 0.1 | 142 | 155 | 0.3 |
| Control | 2157 | 2138 | 2570 | 2557 | ||
| Kidney paired donation | ||||||
| National Kidney Registry kidney transplant | 126 | 149 | 0.03 | 142 | 160 | 0.1 |
| Control | 404 | 381 | 463 | 445 |
Observed and expected number of events as determined by the log-rank test of the survivor function. Recipients of National Kidney Registry–facilitated living donor kidney transplants were compared with three control groups: (1) control living donor kidney transplant recipients, (2) unrelated living donor kidney transplant recipients, and (3) recipients who participated in a kidney paired donation program that was not the National Kidney Registry.
Figure 1.
Death-censored graft failure cumulative incidence by participation in the National Kidney Registry. (A) Cumulative death-censored graft failure comparing national kidney registry recipients (dashed line) to control living donor recipient during the study period (solid line). (B) Cumulative death-censored graft failure comparing national kidney registry recipients (dashed line) to control unrelated living donor recipient during the study period (solid line). Unrelated recipients were identified using the donor/recipient relationship as captured in the national registry. (C) Cumulative death-censored graft failure comparing national kidney registry recipients (dashed line) to control paired donation living donor recipient during the study period (solid line). Paired donation recipients outside the national kidney registry were identified using the donor/recipient relationship as captured in the national registry.
Figure 2.
Mortality cumulative incidence by participation in the National Kidney Registry. (A) Cumulative mortality comparing national kidney registry recipients (dashed line) to control living donor recipient during the study period (solid line). (B) Cumulative mortality comparing national kidney registry recipients (dashed line) to control unrelated living donor recipient during the study period (solid line). Unrelated recipients were identified using the donor/recipient relationship as captured in the national registry. (C) Cumulative mortality comparing national kidney registry recipients (dashed line) to control paired donation living donor recipient during the study period (solid line). Paired donation recipients outside the national kidney registry were identified using the donor/recipient relationship as captured in the national registry.
After adjustment, there was no evidence of a difference in graft failure and mortality risk comparing National Kidney Registry living donor kidney transplant recipients to control living donor kidney transplant recipients (Table 5). In the parsimonious models, National Kidney Registry recipients’ risk of graft failure (aHR, 0.95; 95% confidence interval [95% CI], 0.77 to 1.18; P=0.6) and mortality (aHR, 0.86; 95% CI, 0.70 to 1.07; P=0.2) was similar to control living donor kidney transplant recipients. The various confounding assumptions of Models 1–3 also failed to detect a difference in graft failure and mortality between National Kidney Registry and control living donor kidney transplant recipients. We also saw few differences between kidney paired donation recipients in NKR and outside of NKR (Table 1). In sensitivity analyses, Cox regression did not show any difference between National Kidney Registry and control transplants (Supplemental Table 2).
Table 5.
Association between receiving a National Kidney Registry–facilitated transplant and post-transplant outcomes (n=56,860)
| Model | Graft Failure, aHR (95% CI) | P Value | Mortality, aHR (95% CI) | P Value |
|---|---|---|---|---|
| Model 1: recipienta | 1.00 (0.81 to 1.25) | >0.9 | 0.90 (0.74 to 1.11) | 0.3 |
| Model 2: recipient, transplantb | 0.92 (0.75 to 1.16) | 0.5 | 0.90 (0.72 to 1.12) | 0.3 |
| Model 3, recipient, transplant, donorc | 0.94 (0.75 to 1.17) | 0.6 | 0.89 (0.71 to 1.11) | 0.3 |
| Model 4: parismoniousd | 0.95 (0.77 to 1.18) | 0.6 | 0.86 (0.70 to 1.07) | 0.2 |
We estimate the aHR of graft failure and mortality comparing National Kidney Registry recipients (index population) to living donor kidney transplant recipients (reference population) using inverse probability of treatment weighted Cox regression. There were 126 graft failure events in the National Kidney Registry and 2237 events in the reference population. There were 142 deaths in the National Kidney Registry and 2221 events in the reference population. aHR, adjusted hazard ratio; 95% CI, 95% confidence interval.
Model adjusted for recipient age, sex, black race, Hispanic ethnicity, body mass index (BMI) ≥30 kg/m2, diabetes, hypertension, college education, public insurance, hepatitis C infection, preemptive transplant, history of previous transplant, eGFR before transplant (CKD Epidemiology Collaboration 2009 equation), antibody nondepleting induction therapy, antibody depleting induction therapy, and year of transplant.
Model adjusted for model 1 factors in addition to donor age, sex, black race, Hispanic ethnicity, BMI≥30 kg/m2, and eGFR before transplant.
Model adjusted for model 2 factors in addition to ABO incompatible transplant and zero HLA mismatches.
Model adjusted for recipient factors (age, sex, black race, BMI≥30 kg/m2, diabetes, previous transplant, preemptive transplant, public insurance, hepatitis C, eGFR, antibody depleting induction therapy, year of transplant), donor factors (age, sex, Hispanic ethnicity, BMI≥30 kg/m2), and transplant factors (zero HLA mismatch).
In terms of delayed graft function, there was a higher proportion of recipients with delayed graft function in National Kidney Registry compared with control living donor kidney transplant recipients (5% versus 3%; P<0.001) (Table 2). Because the prevalence of delayed graft function was low, the adjusted odds ratio approximates the adjusted risk ratio. After adjustment in the parsimonious model, National Kidney Registry recipients had 1.36 times the risk of delayed graft function compared with control living donor kidney transplant recipients (aOR, 1.36; 95% CI, 1.05 to 1.75; P=0.02) (Supplemental Table 3). This association was consistent across all models.
Discussion
The data presented in this study confirms that kidney paired donation has evolved from a computational exercise to daily clinical practice. The underlying goal of kidney paired donation is the facilitation of new kidney transplants with comparable outcomes from a pool of willing donors and recipients that were unable to proceed with transplantation at their designated centers. Using the largest prospective cohort study of kidney paired donation transplants with 3-, 5-, and 7-year outcomes reported to date, this study found that patients transplanted within the first 10 years of the National Kidney Registry had similar outcomes as control living donor kidney transplant recipients despite a higher burden of risk factors, as shown in Table 1. This is demonstrated by both unadjusted and adjusted analytic approaches. In adjusted analyses, the incidence of graft failure (aHR, 0.95; 95% CI, 0.77 to 1.18; P=0.6) and mortality (aHR, 0.86; 95% CI, 0.70 to 1.07; P=0.2) were similar between National Kidney Registry and control recipients during the study period with a maximum follow-up time of 11 years. National Kidney Registry recipients did have 1.4 times the risk of delayed graft function compared with control recipients (aOR, 1.36; 95% CI, 1.05 to 1.75; P=0.02), but this did not appear to affect outcomes up to 7 years post-transplant. Further follow-up beyond 10 years may establish whether National Kidney Registry transplants, with focused HLA matching, can also deliver improved outcomes.
Previous studies of national and single-center kidney paired donation programs have introduced early successes and concerns in kidney paired donation (3,5,12). Work that is more recent demonstrated that longer transit and cold ischemia times were not associated with short-term outcomes, but were associated with increased risk of delayed graft function (4,6). Our findings suggest that this increased risk of delayed graft function may not affect long-term outcomes.
Compared with control recipients, National Kidney Registry recipients were more likely to be black (18% versus 13%), had a PRA>80% (21% versus 4%), spent longer on dialysis (median 1.3 versus 0.5 years), on public insurance at the time of transplant (50% versus 42%), had a higher rate of delayed graft function (5% versus 3%), had a longer cold ischemia time (median 8.8 versus 1.0 hours), and had a previous transplant (25% versus 12%). Despite all of these characteristics of the National Kidney Registry cohort, the unadjusted analyses suggest lower risk of graft loss and mortality up to 7 years post-transplant for National Kidney Registry recipients compared with control recipients. These trends again are demonstrated in the Kaplan–Meier survival curves, with statistical significance being demonstrated only when comparing National Kidney Registry transplants to other kidney paired donation transplants (Figure 1). Finally, the trend to lower graft failure and mortality is demonstrated in the observed-to-expected event analysis (Table 4). In this analysis, the graft failure observed-to-expected ratio between the National Kidney Registry recipients and all other kidney paired donation recipients was statistically significant. These differences did not stand after adjustment for confounders, but these results warrant future analyses to see if outcomes differ as the median follow-up time increases.
Despite a rigorous treatment of the data, we were unable to connect any specific characteristics of the National Kidney Registry cohort with the outcomes that were found. We hypothesize that the programmatic HLA matching, the low rates of desensitization, and the low number of ABOi transplants may contribute to the trends toward better outcomes over increasingly long follow-up periods, but we were unable to ascertain these associations with the present cohort. If these trends persist and can be attributed to increased HLA matching, avoidance of ABOi, and desensitization before transplant, the data will further support increasing the use of national kidney paired donation programs to improve long-term outcomes in living donor kidney transplant. Although it appears the data trends in that direction, a more mature cohort is required to show this conclusively.
In the past decade, three major hurdles have been overcome that can assure sustainability and the continued growth of kidney paired donation, which currently represents about 12% of live donor transplants in the United States. First, the National Kidney Registry has resolved most of the technical aspects of sharing kidneys over wide distances including shipping with longer cold ischemia times, anatomic, physiologic, and clinical practice differences that exist between transplant centers. Participating centers retain their independence and have final say in their degree of comfort with acceptance of potential donor and recipient combinations. Second, by assuring the inclusion of many transplant centers with a diverse patient mix the entry of pairs is fair and equitable, quite similar to the demographics and characteristics found in national live donor registries. In fact, the National Kidney Registry patient pool tends to over represent more difficult to match pairs that included more retransplants, hyperimmunized recipients, black recipients, longer dialysis time, and those on public insurance (8,13). Finally, by incorporating widespread geographic (transcontinental) sharing within the National Kidney Registry, it has become possible to identify donors that unlocked hard to match pairs (hyperimmunized and/or with common HLA phenotypes). The third leg of growth and sustainability should be to speed up exchanges and encourage greater participation around the country to further facilitate the ability to find donors for hard to match pairs. In particular, the use of blood group O and A2 donors within kidney paired donation networks should be emphasized. Future growth incorporating compatible pairs, modest degrees of HLA sensitization, planned future exchanges via vouchers, and novel sharing algorithms exploring deceased donor chain initiation should also be encouraged.
Through a comprehensive analysis of this cohort, we conclude that nationalized kidney paired donation results in equivalent outcomes when compared with all other living donor kidney transplant recipients, unrelated living donor kidney transplant recipients, and control kidney paired donation recipients. We see trends in the data that indicate that there may be advantages to the large size of the National Kidney Registry program, and the matching algorithms used by the National Kidney Registry to maximize HLA matching, avoid desensitization and/or ABOi. Further, our results show that the National Kidney Registry, which comprises both larger >200 and smaller <50 per year transplant programs representing all regions of the country, has achieved equivalent results when transplanting patients with risk factors associated with poorer expected outcomes. These results should reassure the larger transplant community that a national kidney paired donation program is a safe and effective way to treat patients with incompatible living donors.
Disclosures
Dr. Leeser, Dr. Kapur, and Dr. Veale report that they are members of the Medical Board of the National Kidney Registry (NKR). Dr. Leeser reports a position on the Data Safety Monitoring Board at Laminate Technologies. Dr. Segev reports receiving honoraria from CSL Behring, Novartis, and Sanofi, and that Johns Hopkins University receives institutional support from NKR to provide analytical support for general research activities. Dr. Cooper, Dr. Flechner, Dr. Massie, Dr. Shaffer, Mr. Thomas, Dr. Turgeon, and Dr. Waterman have nothing to disclose.
Funding
Dr. Leeser is a member of the National Kidney Registry Medical Board. Dr. Massie is supported by National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) grant K01DK101677. Dr. Segev is supported by NIDDK grant K24DK101828. Dr. Shaffer is supported by NIDDK grant F30DK116658. Mr. Thomas is supported by National Heart, Lung, and Blood Institute grant T32HL007055.
Supplementary Material
Acknowledgments
Dr. Leeser participated in research design, writing, and data analysis. Mr. Thomas participated in research design, writing, and data analysis. Dr. Shaffer participated in research design and data analysis. Dr. Veale participated in research design and writing. Dr. Massie participated in research design and data analysis. Dr. Cooper participated in research design and writing. Dr. Kapur participated in research design and writing. Dr. Turgeon participated in research design and writing. Dr. Segev participated in research design, writing, and data analysis. Dr. Waterman participated in research design, writing, and data analysis. Dr. Flechner participated in research design, writing, and data analysis.
The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR, Organ Procurement and Transplantation Network, or the US Government.
The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “The National Kidney Registry: Time to Buy In?” on pages 168–170.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06660619/-/DCSupplemental.
Supplemental Table 1. Detailed HLA mismatches by participation in the National Kidney Registry (N=56,860).
Supplemental Table 2. Association between receiving a National Kidney Registry–facilitated transplant and post-transplant outcomes using unweighted cox regression (N=56,860).
Supplemental Table 3. Association between receiving a National Kidney Registry–facilitated transplant and delayed graft function (N=56,860).
References
- 1.Rapaport FT: The case for a living emotionally related international kidney donor exchange registry. Transplant Proc 18[Suppl 2]: 5–9, 1986 [PubMed] [Google Scholar]
- 2.Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Robinson A, Wainright JL, Haynes CR, Snyder JJ, Kasiske BL, Israni AK: OPTN/SRTR 2016 annual data report: Kidney. Am J Transplant 18[Suppl 1]: 18–113, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melcher ML, Leeser DB, Gritsch HA, Milner J, Kapur S, Busque S, Roberts JP, Katznelson S, Bry W, Yang H, Lu A, Mulgaonkar S, Danovitch GM, Hil G, Veale JL: Chain transplantation: Initial experience of a large multicenter program. Am J Transplant 12: 2429–2436, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Segev DL, Veale JL, Berger JC, Hiller JM, Hanto RL, Leeser DB, Geffner SR, Shenoy S, Bry WI, Katznelson S, Melcher ML, Rees MA, Samara EN, Israni AK, Cooper M, Montgomery RJ, Malinzak L, Whiting J, Baran D, Tchervenkov JI, Roberts JP, Rogers J, Axelrod DA, Simpkins CE, Montgomery RA: Transporting live donor kidneys for kidney paired donation: Initial national results. Am J Transplant 11: 356–360, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Bingaman AW, Wright FH Jr., Kapturczak M, Shen L, Vick S, Murphey CL: Single-center kidney paired donation: The Methodist San Antonio experience. Am J Transplant 12: 2125–2132, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Treat E, Chow EKH, Peipert JD, Waterman A, Kwan L, Massie AB, Thomas AG, Bowring MG, Leeser D, Flechner S, Melcher ML, Kapur S, Segev DL, Veale J: Shipping living donor kidneys and transplant recipient outcomes. Am J Transplant 18: 632–641, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flechner SM, Thomas AG, Ronin M, Veale JL, Leeser DB, Kapur S, Peipert JD, Segev DL, Henderson ML, Shaffer AA, Cooper M, Hil G, Waterman AD: The first 9 years of kidney paired donation through the National Kidney Registry: Characteristics of donors and recipients compared with National Live Donor Transplant Registries. Am J Transplant 18: 2730–2738, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holscher CM, Jackson K, Chow EKH, Thomas AG, Haugen CE, DiBrito SR, Purcell C, Ronin M, Waterman AD, Garonzik Wang J, Massie AB, Gentry SE, Segev DL: Kidney exchange match rates in a large multicenter clearinghouse. Am J Transplant 18: 1510–1517, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massie AB, Kucirka LM, Segev DL: Big data in organ transplantation: Registries and administrative claims. Am J Transplant 14: 1723–1730, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Austin PC, Stuart EA: Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 34: 3661–3679, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durand CM, Bowring MG, Thomas AG, Kucirka LM, Massie AB, Cameron A, Desai NM, Sulkowski M, Segev DL: The drug overdose epidemic and deceased-donor transplantation in the United States: A National Registry Study. Ann Intern Med 168: 702–711, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reese PP, Boudville N, Garg AX: Living kidney donation: Outcomes, ethics, and uncertainty. Lancet 385: 2003–2013, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Holscher CM, Jackson K, Thomas AG, Haugen CE, DiBrito SR, Covarrubias K, Gentry SE, Ronin M, Waterman AD, Massie AB, Garonzik Wang J, Segev DL: Temporal changes in the composition of a large multicenter kidney exchange clearinghouse: Do the hard-to-match accumulate? Am J Transplant 18: 2791–2797, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.