Abstract
Background:
Extracorporeal membrane oxygenation (ECMO) is increasingly used in acute myocardial infarction (AMI); however there are limited large-scale national data.
Methods:
Using the National Inpatient Sample database from 2000–2014, a retrospective cohort of AMI utilizing ECMO was identified. Use of percutaneous coronary intervention (PCI), intra-aortic balloon pump (IABP) and percutaneous left ventricular assist device (pLVAD) was also identified in this population. Outcomes of interest included temporal trends in utilization of ECMO alone and with concomitant procedures (PCI, IABP, and pLVAD), in-hospital mortality and resource utilization.
Results:
In ~9 million AMI admissions, ECMO was used in 2,962 (<0.01%) and implanted a median of one day after admission. ECMO was used in 0.5% and 0.3% AMI admissions complicated by cardiogenic shock and cardiac arrest respectively. ECMO was used more commonly in admissions that were younger, non-white, and with less comorbidity. ECMO use was 11-times higher in 2014 as compared to 2000 (odds ratio 11.37 [95% confidence interval 7.20–17.97]). Same-day PCI was performed in 23.1%; IABP/pLVAD was used in 57.9%, of which 30.3% were placed concomitantly. In-hospital mortality with ECMO was 59.2% overall, but decreased from 100% (2000) to 45.1% (2014). Durable LVAD and cardiac transplantation were performed in 11.7% as an exit strategy. Of the hospital survivors, 40.8% were discharged to skilled nursing facilities. Older age, male sex, non-white race, and lower socio-economic status, were independently associated with higher in-hospital mortality with ECMO use.
Conclusions:
In AMI admissions, a steady increase was noted in the utilization of ECMO alone and with concomitant procedures (PCI, IABP, pLVAD). In-hospital mortality remained high in AMI admissions treated with ECMO.
SUBJECT CODE: Heart Failure and Cardiac Disease: Myocardial Infarction
Keywords: Acute myocardial infarction, extra-corporeal membrane oxygenation, cardiogenic shock, cardiac arrest, outcomes research
Concomitant cardiac arrest and/or cardiogenic shock (CS) are seen in about 3–10% of all patients with acute myocardial infarction (AMI), and is associated with nearly 30–50% in-hospital mortality.1, 2 These patients typically present with attendant respiratory failure, hemodynamic collapse and electrical instability, all of which may result in systemic hypoperfusion and multi-organ failure.3–5 Furthermore, AMI patients with CS or cardiac arrest patients remain at high risk of clinical decompensation during coronary angiography or percutaneous coronary intervention (PCI) due to ongoing hemodynamic compromise. In contemporary practice, the advent of advanced percutaneous mechanical circulatory support (MCS) devices has made it possible to offer hemodynamic support for the management of AMI.6, 7 Though there are prior data on the intra-aortic balloon pump (IABP) and emerging data on the percutaneous left ventricular assist devices (pLVAD);8, 9 there are limited large-scale data on the use of extra-corporeal membrane oxygenation (ECMO) in AMI.10 Using a 15-year nationally-representative database we sought to assess the use, temporal trends, timing, and outcomes of ECMO in AMI. We also sought to evaluate the use of ECMO across demographic categories and the use of concomitant cardiac interventions with ECMO in AMI.
MATERIAL AND METHODS
Study Population, Variables and Outcomes
The data are publicly available with the Agency for Healthcare Research and Quality for other researchers to replicate the results of this study.11 Institutional Review Board approval was not sought due to the publicly available nature of the de-identified data. The Healthcare Quality and Utilization Project-National (Nationwide) Inpatient Sample (HCUP-NIS) is the largest all-payer database of hospital inpatient stays in the United States and contains discharge data from a 20% stratified sample of community hospitals.11 Information regarding each discharge includes patient demographics, primary payer, hospital characteristics, principal diagnosis, up to 24 secondary diagnoses, and procedural diagnoses. The HCUP-NIS does not capture individual patients, but captures all information for a given hospitalization.
Using the HCUP-NIS data from 2000–2014, a retrospective cohort study of admissions with AMI in the primary diagnosis field was identified (ST-elevation myocardial infarction – ICD-9CM 410.1x-410.6x, 410.8x, 410.9x, non ST-elevation myocardial infarction – ICD-9CM 410.70–410.79).2–5, 12, 13 CS and cardiac arrest were identified using ICD-9CM codes of 785.51, 427.5 and 427.41. CS was defined as shock resulting from diminution of cardiac output in cardiovascular disease, shock resulting from primary pump failure of the heart, as in myocardial infarction, severe cardiomyopathy, or mechanical obstruction or compression of the heart or shock resulting from the failure of the heart to maintain adequate output.14 Validation studies have shown a specificity of 99.3%, a sensitivity of 59.8%, a positive predictive value of 78.8%, and a negative predictive value of 98.1% for the ICD-9-CM code 785.51 to identify CS.14 Use and timing of ECMO (ICD-9CM 39.65), IABP (ICD-9CM 37.61) and pLVAD (ICD-9CM 37.68) were identified using procedure codes and procedure day for timing.4, 6, 7, 12 Durable LVAD (ICD-9CM 37.66) and cardiac transplant (ICD-9CM 37.5, 37.51, 33.6) were identified for all admissions.6, 7 Consistent with prior literature from the HCUP-NIS, we used the listed procedure day to define concomitant ECMO placement with PCI, IABP and pLVAD placement.15 The Deyo’s modification of Charlson Comorbidity Index was used to identify the burden of co-morbid diseases (Supplementary Table 1).16 Patient and hospital demographic characteristics, cardiac and non-cardiac diagnostic and therapeutic procedures and outcomes were identified similar to prior literature.2–7, 12, 13, 17, 18 The primary outcome was the incidence and temporal trends for ECMO use in AMI. Secondary outcomes included in-hospital mortality, resource utilization, the use of a second temporary MCS device, timing of ECMO with relation to PCI and other MCS devices, concomitant placement of ECMO and IABP/pLVAD and the use of durable LVAD or cardiac transplant as an exit strategy. Though the Agency for Healthcare Research and Quality has released the HCUP-NIS data till 2016, due to the change in coding practices from ICD-9CM to ICD-10CM in October 2015 we sought to restrict the data to 2014. The HCUP-NIS from 2015 and 2016 databases lack the Clinical Classification System for ICD-9CM codes used in the study. Furthermore, the ICD-10-CM codes lack extensive validation studies unlike the ICD-9CM codes and therefore need further evaluation prior to incorporation into temporal analyses.19
Statistical Analysis
As recommended by HCUP-NIS, survey procedures using discharge weights provided with the HCUP-NIS database were used to generate national estimates. Using the trend weights provided by the HCUP-NIS, samples from 2000–2011 were re-weighted to adjust for the 2012 HCUP-NIS re-design.20 Chi-square and t-tests were used to compare categorical and continuous variables respectively. The inherent restrictions of the HCUP-NIS database related to research design, data interpretation, and data analysis were reviewed and addressed.20 Odds ratio (OR) with 95% confidence interval (CI) were used to represent univariable and multivariable analyses. Adjusted temporal trends were assessed using multivariable logistic regression analysis incorporating age, gender, race, admission year, primary payer status, socio-economic stratum, hospital characteristics, comorbidities, acute organ failure, coronary angiography, PCI, second temporary MCS use, invasive hemodynamic monitoring, invasive ventilation and hemodialysis use for ECMO use (referent year 2000) and in-hospital mortality (referent year 2014). A multivariable logistic regression incorporating age, sex, race, primary payer status, socio-economic stratum, comorbidity, hospital location, AMI type, acute organ failure, cardiac and non-cardiac procedures was performed for predictors of in-hospital mortality in AMI admissions with the use of ECMO. For the multivariable modeling, regression analysis with purposeful selection of statistically (p<0.20) and clinically relevant variables was conducted. Two-tailed p<0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 25.0 (IBM Corp, Armonk NY).
RESULTS
There were an estimated 9,747,034 (95% CI 9,736,108–9,757,960) admissions for AMI between January 1, 2000 and December 31, 2014, of which ECMO was used in 2,962 (<0.01%). In these ~9 million AMI admissions, CS and cardiac arrest were noted in 4.6% and 4.5%, respectively. ECMO was used in 0.5% and 0.3% of AMI admissions complicated by CS and cardiac arrest respectively. The cohort receiving ECMO was on average younger, more frequently non-White, covered by private insurance, presenting with ST-elevation, on average with lower comorbidity and higher organ failure (Table 1). AMI admissions with ECMO use more often had cardiac arrest, CS, and less use of coronary angiography and PCI (Table 1). In the admissions receiving ECMO support, 86.7% and 92.2% had been admitted to large and urban teaching hospitals, respectively. The 15-year unadjusted and adjusted trends (referent year 2000) in the use of ECMO in AMI are presented in Figure 1A and 1B, respectively. There was an 11.37-times higher odds of ECMO use in 2014 as compared to 2000 after adjusting for patient and hospital characteristics (95% CI 7.20–17.97). ECMO use stratified by patient characteristics is presented in Supplementary Figure 1. Per 100,000 AMI admissions, the Northeast region had highest use of ECMO (60.3), followed by the Midwest (28.4), the West (21.7) and the Southern (18.7) regions of the United States.
Table 1.
Baseline characteristics of cohorts with and without ECMO use in acute myocardial infarction
| Characteristic | ECMO (N = 2,962) | No ECMO (N = 9,744,072) | P | |
|---|---|---|---|---|
| Age (years) | 59.0 ± 10.9 | 67.7 ± 14.3 | <0.001 | |
| Female sex | 26.1 | 40.1 | <0.001 | |
| Race | White | 70.6 | 78.0 | <0.001 |
| Black | 8.8 | 9.2 | ||
| Other | 20.6 | 12.8 | ||
| Primary payer | Medicare | 33.4 | 57.8 | <0.001 |
| Medicaid | 10.6 | 5.6 | ||
| Private | 45.2 | 28.3 | ||
| Uninsured | 6.9 | 5.2 | ||
| No charge | 1.6 | 0.5 | ||
| Quartile of median household income for zip code | 0–25th | 23.9 | 23.1 | 0.24 |
| 26th–50th | 28.3 | 27.2 | ||
| 51st–75th | 23.6 | 24.7 | ||
| 75th–100th | 24.1 | 25.0 | ||
| Charlson Comorbidity Index | 0–3 | 43.1 | 35.9 | <0.001 |
| 4–6 | 46.8 | 46.3 | ||
| ≥ 7 | 10.2 | 17.8 | ||
| AMI type | ST-segment elevation | 67.3 | 39.0 | <0.001 |
| Non-ST-segment elevation | 32.7 | 61.0 | ||
| Acute organ failure | Respiratory | 56.5 | 7.6 | <0.001 |
| Renal | 62.1 | 1.0 | <0.001 | |
| Hepatic | 30.2 | 0.8 | <0.001 | |
| Neurologic | 27.3 | 2.8 | <0.001 | |
| Out-of-hospital cardiac arrest | 47.5 | 4.5 | <0.001 | |
| Cardiogenic shock | 80.6 | 4.5 | <0.001 | |
| Coronary angiography | 50.9 | 62.0 | <0.001 | |
| Percutaneous coronary intervention | 39.3 | 40.2 | 0.31 | |
| Invasive hemodynamic monitoring | 22.2 | 4.9 | <0.001 | |
| Coronary artery bypass grafting | 37.1 | 9.3 | <0.001 | |
Represented as percentage or mean ± standard deviation
Abbreviations: ECMO: extracorporeal membrane oxygenation
Figure 1. Temporal trends of ECMO use in AMI.

A: Unadjusted 15-year temporal trends of ECMO use in all AMI, AMI with CS and AMI with CA. B: adjusted odds ratio for temporal trends of ECMO use in AMI (referent year 2000)*, the Y-axis is presented in logarithmic scale; all p<0.001 for trend
*Adjusted for: age, sex, race, primary payer, socio-economic status, hospital location/teaching status, hospital bedsize, hospital region, comorbidity, acute organ dysfunction, cardiogenic shock, cardiac arrest, use of coronary angiography, percutaneous coronary intervention, invasive hemodynamic assessment, invasive mechanical ventilation and hemodialysis
Abbreviations: AMI: acute myocardial infarction; CA: cardiac arrest; CS: cardiogenic shock; ECMO: extra-corporeal membrane oxygenation
ECMO was implanted at a median of one day (interquartile range 0–3 days) after admission. Same-day PCI was performed in 686 (23.1%) admissions and a second temporary MCS device (IABP/pLVAD) was used in 1,716 (57.9%) admissions. Temporal trends in the use of same-day PCI, placement of concomitant IABP/pLVAD with ECMO, and use of a second temporary MCS device during the index admission are presented in Figure 2A. The timing of the second temporary MCS device relative to ECMO was available in 1,579/1,716 (92.0%) admissions (Figure 2B). Of these admissions, 30.3% received concomitant IABP/pLVAD with ECMO with an increasing trend during the study period (Figure 2A and 2B). Vascular complications, lower limb amputations, and use of blood transfusions were noted in 4.7%, 1.1% and 25.9%, respectively, of the AMI admissions with ECMO use.
Figure 2. Concomitant cardiac procedures in AMI admissions on ECMO.
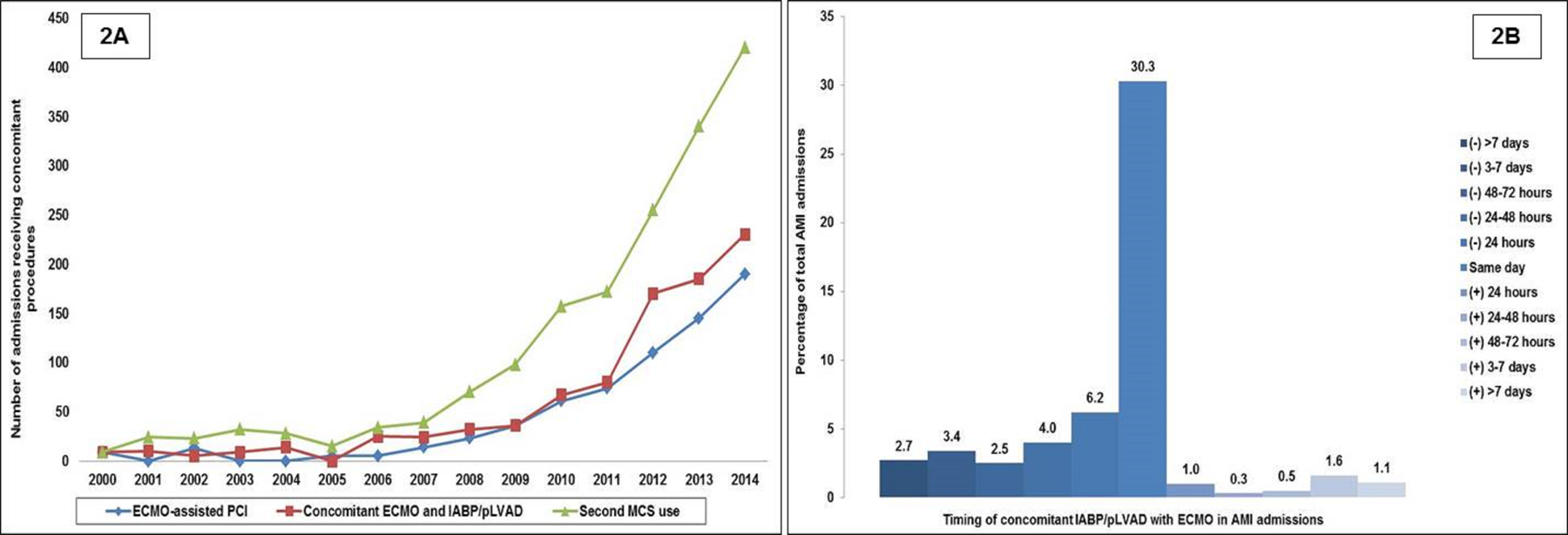
A: Fifteen-year temporal trends of same-day PCI, concomitant use of ECMO and IABP/pLVAD and use of a second MCS device (IABP/pLVAD) in AMI admissions receiving ECMO (represented as total admissions); all p<0.001 for trend. B: Timing of IABP/pLVAD in AMI admissions receiving ECMO; negative values denote IABP/pLVAD use before ECMO and positive values denote IABP/pLVAD use after ECMO (represented as percentage of total AMI admissions); all p<0.001 for trend
Abbreviations: AMI: acute myocardial infarction; ECMO: extra-corporeal membrane oxygenation; IABP: intra-aortic balloon pump; MCS: mechanical circulatory support; PCI: percutaneous coronary intervention; pLVAD: percutaneous left ventricular assist device
In-hospital mortality occurred in 1,752 (59.2%) admissions treated with ECMO during the study period. The 15-year temporal trends for mortality are presented in Figure 3A. In an adjusted analysis, with 2014 as the referent year, there was a 15-times lower odds of in-hospital mortality in this population during the study period (Figure 3B). The use of durable LVAD, cardiac transplant and discharge disposition of the AMI admissions receiving ECMO is presented in Figure 4. In a multivariable logistic regression analysis for in-hospital mortality, older age, male sex, non-white race, lower socio-economic status, acute hepatic failure and use of organ support systems were independently predictive of higher in-hospital mortality in AMI admissions receiving ECMO support (Figure 5). Age >80 years was the strongest predictor of in-hospital mortality (OR 4.14 [95% CI 1.89–9.07]; p<0.001) in the population receiving ECMO in AMI. There was a statistically significant interaction between PCI and concomitant CS (p=0.02 for interaction). Use of PCI was associated with lower in-hospital mortality in the overall cohort (OR 0.72 [95% CI 0.59–0.87); p<0.001) and when stratified by presence (OR 0.74 [95% CI 0.59–0.91]; p=0.005) or absence (OR 0.37 [95% CI 0.20–0.70]; p=0.002) of CS.
Figure 3. Temporal trends of in-hospital mortality in AMI admissions receiving ECMO therapy.

A: Unadjusted 15-year temporal trends of in-hospital mortality in AMI admissions receiving ECMO therapy represented as percentage. B: Adjusted odds ratio for temporal trends of in-hospital mortality of ECMO use in AMI (referent year 2014)*, the Y-axis is presented in logarithmic scale; all p<0.001 for trend
*Adjusted for: age, sex, race, primary payer, socio-economic status, hospital location/teaching status, hospital bedsize, hospital region, comorbidity, acute organ dysfunction, cardiogenic shock, cardiac arrest, use of a second temporary mechanical circulatory support device, coronary angiography, percutaneous coronary intervention, invasive hemodynamic assessment, invasive mechanical ventilation and hemodialysis
The odds ratio for year 2000 in Figure 3B could not be calculated due to 100% mortality (infinite odds ratio)
Abbreviations: AMI: acute myocardial infarction; ECMO: extra-corporeal membrane oxygenation
Figure 4. In-hospital mortality, disposition and use of permanent exit-strategies in AMI admissions receiving ECMO therapy.
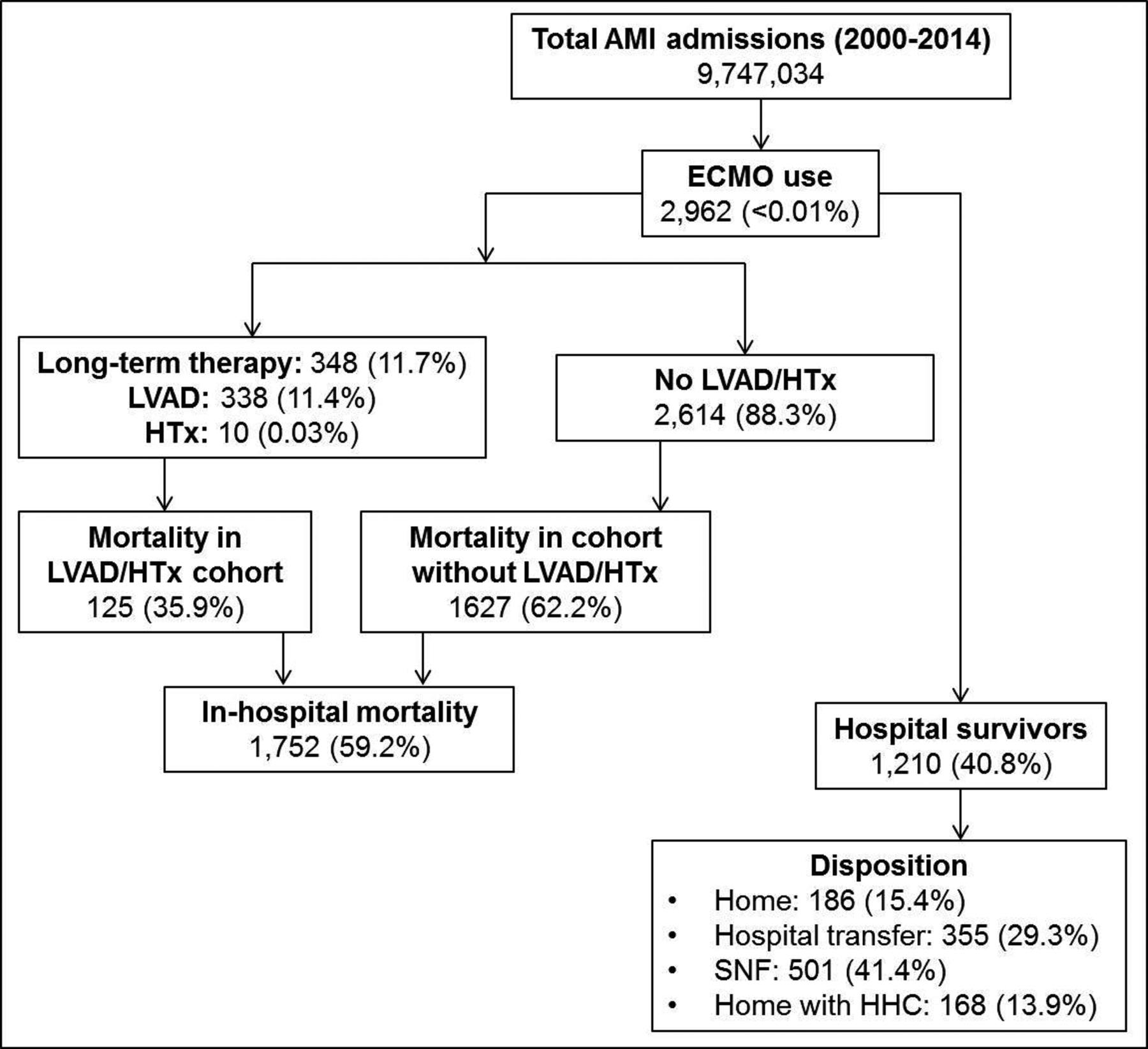
Abbreviations: AMI: acute myocardial infarction; ECMO: extra-corporeal membrane oxygenation; HHC: home health care; HTx: heart transplantation; LVAD: left ventricular assist device; SNF: skilled nursing facilities
Figure 5. Adjusted odds ratio for in-hospital mortality in AMI admissions receiving ECMO therapy.

Odds ratio with 95% confidence interval using multivariable hierarchical regression analysis for prediction of in-hospital mortality; for cohorts with multiple categories (i.e. age, sex, race, primary payer, CCI, SES, hospital region, AMI type) the first category was used as reference category for calculating odds ratios. The X-axis is presented in logarithmic scale.
Abbreviations: AMI: acute myocardial infarction; CCI: Charlson comorbidity index; ECMO: extracorporeal membrane oxygenation; IHDM: invasive hemodynamic monitoring; IMV: invasive mechanical ventilation; MCS: mechanical circulatory support; NSTEMI: non-ST-elevation myocardial infarction; OHCA: out-of-hospital cardiac arrest; PCI: percutaneous coronary intervention; SES: socio-economic status; STEMI: ST-elevation myocardial infarction
DISCUSSION
In this nationally-representative study of ~9 million AMI admissions, ECMO use was noted in 2,962 (<0.01%). There was an 11-times higher odds of use of ECMO for AMI in 2014 compared to 2000. There was a temporal increase in same-day PCI, use of concomitant IABP/pLVAD with ECMO and the use of multiple MCS devices during a single admission. During this 15-year period, the overall in-hospital mortality for AMI admissions receiving ECMO remained high at 59.2%, although there was a significant decrease from 100% mortality in 2000 to 45.1% mortality in 2014. Older age, male sex, non-white race, lower socio-economic status, and acute non-cardiac organ failure were independently associated with in-hospital mortality. Of the hospital survivors, 41.4% of those receiving ECMO were discharged to skilled nursing facilities suggestive of high post-hospitalization resource utilization.
In patients with AMI complicated by CS and/or cardiac arrest, ECMO offers significant advantages over other MCS devices by virtue of respiratory and biventricular support, higher flow rates, ease of deployment and relatively low costs.21–23 Prior work from large databases has either focused on unselected ECMO use or use in unselected CS admissions.10, 24 Our data are consistent with prior studies that demonstrate an increased adoption of ECMO for AMI-CS.12, 22, 25, 26 In addition to CS, in AMI ECMO is often used to facilitate cardiopulmonary resuscitation (eCPR).27 Consistent with the Extracorporeal Life Support Organization registry that reported a 10-times increase in the adoption of eCPR between 2003 and 2014, we note a steady increase in ECMO adoption in AMI with cardiac arrest.28 In AMI with refractory cardiac arrest, ECMO can be used to facilitate PCI despite not attaining return of spontaneous circulation.27, 29 However, given the limitations of this administrative database, we are unable to discern the timing of ECMO placement in cardiac arrest and if it was used per an eCPR protocol.
The improvements of ECMO technology have resulted in its increasing adoption to facilitate PCI in AMI with CS or cardiac arrest.25–27 Consistent with these data, we demonstrate a rapid increase in ECMO-facilitated PCI in this nationally representative population of AMI. The lower rates of angiography and PCI in this study are consistent with prior real-world literature that reflects reluctance to perform angiography in higher risk cohorts despite robust guideline recommendations.30 Furthermore, use of MCS in AMI needs careful assessment of clinical and hemodynamic profiles prior to selection of MCS device. Despite strong guideline recommendations, angiography and PCI may frequently be deferred in patients with non-ST-elevation AMI placed on ECMO to evaluate for organ recovery or neurological function.31 The higher rates of coronary artery bypass grafting in this population may have resulted in consequent lower rates of PCI. The heterogeneity of this population and associated complex decision-making often requires multi-disciplinary care teams to achieve optimal outcomes.5, 32
In this study we noted that nearly 57.9% of admissions had a second temporary MCS device besides ECMO which is increasingly being used in modern practice.21, 22 In a prior meta-analysis, we noted the ECMO+IABP strategy to have lower mortality compared to ECMO alone only in AMI-CS.22 Preliminary data with unloading the left ventricle with a pLVAD has shown greater myocardial recovery and faster weaning of ECMO and is worthy of further study.21 Despite well-established randomized trial data on the use of IABP in cardiogenic shock, there are limited adequately powered studies evaluating the VA-ECMO with equal rigor.33 Furthermore, all studies on combination therapy (ECMO+IABP/pLVAD) are observational in nature, and therefore are prone to confounding by indication and heterogeneity.21, 22 Though hemodynamically, the combination therapy of ECMO with pLVAD has a strong physiological rationale, further studies are needed prior to adoption in routine clinical practice. Our study reflects the contemporary practice in the United States. Despite the lack of strong evidence from randomized trials, 57.9% of our population received combination therapy.
It is important to note that the overall survival in these observational studies was low at <50%. This is consistent with the cumulative in-hospital mortality in this study was 59.2%, with 45.1% mortality in 2014. The decrease in mortality could potentially be due to improvements in the delivery of AMI care in patients with concomitant cardiac arrest and CS.2 Our data are consistent with unselected ECMO patients from a population database in Taiwan that demonstrated 30% hospital survival.34 Chang et al. noted only 24% of the total population to be alive at one-year emphasizing the high post-hospitalization mortality and morbidity as noted indirectly in this study.34 This study identifies significant health care disparities in in-hospital mortality in AMI admissions with ECMO use. Admissions of non-white race, without insurance, and lower socio-economic status were independently associated with higher in-hospital mortality, similar to disparities in patients with other acute cardiovascular conditions.35 Sex-based disparities were also noted in this study given the high male preponderance in ECMO use that is consistent with prior literature.36 We noted a lower mean age in ECMO recipients compared to the general AMI population. This could potentially be explained by the higher comorbidity burden and the lack of a well-defined exit strategy in the older population. Importantly, older patients are often not candidates for cardiac transplantation or durable LVAD which was used in 11.7% of this population.6 The reasons for these patient-specific disparities are incompletely understood and are likely due to differences in cultural and religious beliefs, treatment preferences and incomplete knowledge.5 Lastly, it is important to note that the use of ECMO is associated with significant financial incentives.37 The insertion and maintenance of ECMO is has high hospitalization costs and resource utilization.38, 39 Recent policy changes from the Center for Medicare and Medicaid Services that treat peripheral and central ECMO differently were met with strong resistance needing reversal of the Diagnosis Relation Groups policy. In contemporary practice, peripheral ECMO is treated on par with central ECMO for reimbursement, which is significantly higher in costs than other percutaneous MCS devices.40 In light of these issues, careful assessment of the indications, continued need and management of ECMO is needed in AMI patients.
Limitations
This study has several limitations, some of which are inherent to the analysis of a large administrative database. Given the administrative nature of this database, we cannot accurately distinguish type-1 from type-2 AMIs. However, this study included admissions with a primary diagnosis of AMI (i.e. the reason most likely for the admission), and therefore is less likely to include type-2 AMIs which often have an alternate primary diagnosis. Nonetheless, this could be an important issue where additional research is necessary. Concomitant use of other MCS or PCI with ECMO was defined as those performed on the same procedure day. However since further granularity in timing is unavailable and AMI often evolves dynamically, it is possible this study included admissions that received ECMO for cardiopulmonary resuscitation, worsening CS or post-PCI complications. However, since the HCUP-NIS does not record duration of support or explantation of organ support, this study did not evaluate duration of ECMO support alone or in-combination with another MCS device. The lack of angiographic data, such PCI location, lesion classification, presence of multi-vessel disease, and revascularization failure, that may significantly influence outcomes, were not available in this database. Though in patients with AMI with or without CS, veno-arterial ECMO is the most commonly used configuration of ECMO support, it is possible that the ICD-9CM code used in this study may refer to the veno-venous ECMO, used for respiratory support. Due to the limitations of the administrative coding, this database is unable to distinguish veno-arterial and veno-venous configurations. Despite these limitations, this study addresses an important knowledge gap highlighting the contemporary use of ECMO in AMI.
CONCLUSIONS
In this study, we observed a rapid increase in the use of ECMO in AMI over a 15-year study period. There was a temporal increase in same day PCI, use of concomitant IABP and pLVAD with ECMO and the use of multiple MCS devices in a single admission. Fifty nine percent of the admissions that received ECMO for AMI died during the hospitalization and those discharged had high post-hospitalization resource utilization. The use of ECMO in AMI needs further careful study in randomized studies prior to widespread adoption given the high costs and mortality in this sick population.
Supplementary Material
SHORT COMMENTARY.
What is new?
In the largest study of extracorporeal membrane oxygenation use in acute myocardial infarction, we note an 11-times higher odds increase in extracorporeal membrane oxygenation use between 2000 and 2014.
The in-hospital mortality (59.2%) continues to be high in this population.
What are the clinical implications?
The use of extracorporeal membrane oxygenation in acute myocardial infarction needs further careful study in randomized studies prior to widespread adoption given the high costs and mortality in this sick population.
SOURCES OF FUNDING
Dr. Saraschandra Vallabhajosyula is supported by the Clinical and Translational Science Award (CTSA) Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
ABBREVIATIONS
- AMI
acute myocardial infarction
- CS
cardiogenic shock
- ECMO
extracorporeal membrane oxygenation
- eCPR
extracorporeal membrane oxygenation-facilitated cardiopulmonary resuscitation
- HCUP
Healthcare Cost and Utilization Project
- IABP
intra-aortic balloon pump
- ICD-9CM
International Classification of Diseases-9 Clinical Modification
- MCS
mechanical circulatory support
- NIS
National/Nationwide Inpatient Sample
- PCI
percutaneous coronary intervention
- pLVAD
percutaneous left ventricular assist device
Footnotes
DISCLOSURES
Dr. Jaffe has been a consultant for Beckman, Abbott, Siemens, Roche, ET Healthcare, Sphingotoec, Quidel, Brava, Blade, and Novartis. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Scholz KH, Maier SKG, Maier LS, Lengenfelder B, Jacobshagen C, Jung J, Fleischmann C, Werner GS, Olbrich HG, Ott R, Mudra H, Seidl K, Schulze PC, Weiss C, Haimerl J, Friede T and Meyer T. Impact of treatment delay on mortality in ST-segment elevation myocardial infarction (STEMI) patients presenting with and without haemodynamic instability: results from the German prospective, multicentre FITT-STEMI trial. Eur Heart J. 2018;39:1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vallabhajosyula S, Dunlay SM, Prasad A, Kashani K, Sakhuja A, Gersh BJ, Jaffe AS, Holmes DR Jr. and Barsness GW. Acute noncardiac organ failure in acute myocardial infarction with cardiogenic shock. J Am Coll Cardiol. 2019;73:1781–1791. [DOI] [PubMed] [Google Scholar]
- 3.Vallabhajosyula S, Dunlay SM, Kashani K, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Jaffe AS and Barsness GW. Temporal trends and outcomes of prolonged invasive mechanical ventilation and tracheostomy use in acute myocardial infarction with cardiogenic shock in the United States. Int J Cardiol. 2019;285:6–10. [DOI] [PubMed] [Google Scholar]
- 4.Vallabhajosyula S, Dunlay SM, Murphree DH, Barsness GW, Sandhu GS, Lerman A and Prasad A. Cardiogenic shock in takotsubo cardiomyopathy versus acute myocardial infarction: An 8-year national perspective on clinical characteristics, management, and outcomes. JACC Heart Fail. 2019;7:469–476. [DOI] [PubMed] [Google Scholar]
- 5.Vallabhajosyula S, Prasad A, Dunlay SM, Murphree DH Jr., Ingram C, Mueller PS, Gersh BJ, Holmes DR Jr. and Barsness GW. Utilization of palliative care for cardiogenic shock complicating acute myocardial infarction: A 15-year national perspective on trends, disparities, predictors, and outcomes. J Am Heart Assoc. 2019;8:e011954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallabhajosyula S, Arora S, Lahewala S, Kumar V, Shantha GPS, Jentzer JC, Stulak JM, Gersh BJ, Gulati R, Rihal CS, Prasad A and Deshmukh AJ. Temporary mechanical circulatory support for refractory cardiogenic shock before left ventricular assist device surgery. J Am Heart Assoc. 2018;7:e010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallabhajosyula S, Arora S, Sakhuja A, Lahewala S, Kumar V, Shantha GPS, Egbe AC, Stulak JM, Gersh BJ, Gulati R, Rihal CS, Prasad A and Deshmukh AJ. Trends, predictors, and outcomes of temporary mechanical circulatory support for postcardiac surgery cardiogenic shock. Am J Cardiol. 2019;123:489–497. [DOI] [PubMed] [Google Scholar]
- 8.O’Neill WW, Grines C, Schreiber T, Moses J, Maini B, Dixon SR and Ohman EM. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am Heart J. 2018;202:33–38. [DOI] [PubMed] [Google Scholar]
- 9.Schrage B, Ibrahim K, Loehn T, Werner N, Sinning JM, Pappalardo F, Pieri M, Skurk C, Lauten A, Landmesser U, Westenfeld R, Horn P, Pauschinger M, Eckner D, Twerenbold R, Nordbeck P, Salinger T, Abel P, Empen K, Busch MC, Felix SB, Sieweke JT, Moller JE, Pareek N, Hill J, MacCarthy P, Bergmann MW, Henriques JPS, Mobius-Winkler S, Schulze PC, Ouarrak T, Zeymer U, Schneider S, Blankenberg S, Thiele H, Schafer A and Westermann D. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation. 2019;139:1249–1258. [DOI] [PubMed] [Google Scholar]
- 10.Strom JB, Zhao Y, Shen C, Chung M, Pinto DS, Popma JJ and Yeh RW. National trends, predictors of use, and in-hospital outcomes in mechanical circulatory support for cardiogenic shock. EuroIntervention. 2018;13:e2152–e2159. [DOI] [PubMed] [Google Scholar]
- 11.Introduction to the HCUP Nationwide Inpatient Sample 2009. http://www.hcup-us.ahrq.gov/db/nation/nis/NIS_2009_INTRODUCTION.pdf. Accessed Jan 18, 2015.
- 12.Vallabhajosyula S, Dunlay SM, Barsness GW, Rihal CS, Holmes DR Jr. and Prasad A. Hospital-level disparities in the outcomes of acute myocardial infarction with cardiogenic shock. Am J Cardiol. 2019;124:491–498. [DOI] [PubMed] [Google Scholar]
- 13.Vallabhajosyula S, Ya’Qoub L, Dunlay SM, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Jaffe AS, Gersh BJ and Kashani K. Sex disparities in acute kidney injury complicating acute myocardial infarction with cardiogenic shock. ESC Heart Fail. 2019;6:874–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambert L, Blais C, Hamel D, Brown K, Rinfret S, Cartier R, Giguere M, Carroll C, Beauchamp C and Bogaty P. Evaluation of care and surveillance of cardiovascular disease: can we trust medico-administrative hospital data? Can J Cardiol. 2012;28:162–8. [DOI] [PubMed] [Google Scholar]
- 15.Khera R, Cram P, Vaughan-Sarrazin M, Horwitz PA and Girotra S. Use of mechanical circulatory support in percutaneous coronary intervention in the United States. Am J Cardiol. 2016;117:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC and Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- 17.Vallabhajosyula S, Dunlay SM, Barsness GW, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Gersh BJ, Jaffe AS and Kashani K. Temporal trends, predictors, and outcomes of acute kidney injury and hemodialysis use in acute myocardial infarction-related cardiogenic shock. PLoS One. 2019;14:e0222894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vallabhajosyula S, Prasad A, Gulati R and Barsness GW. Contemporary prevalence, trends, and outcomes of coronary chronic total occlusions in acute myocardial infarction with cardiogenic shock. Int J Cardiol Heart Vasc. 2019;24:100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauridsen MD, Gammelager H, Schmidt M, Nielsen H and Christiansen CF. Positive predictive value of International Classification of Diseases, 10th revision, diagnosis codes for cardiogenic, hypovolemic, and septic shock in the Danish National Patient Registry. BMC Med Res Methodol. 2015;15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khera R and Krumholz HM. With great power comes great responsibility: big data research from the National Inpatient Sample. Circ Cardiovasc Qual Outcomes. 2017;10:e003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallabhajosyula S, O’Horo JC, Antharam P, Ananthaneni S, Vallabhajosyula S, Stulak JM, Dunlay SM, Holmes DR Jr. and Barsness GW. Venoarterial extracorporeal membrane oxygenation with concomitant Impella versus venoarterial extracorporeal membrane oxygenation for cardiogenic shock. ASAIO J. 2019; doi: 10.1097/MAT.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 22.Vallabhajosyula S, O’Horo JC, Antharam P, Ananthaneni S, Vallabhajosyula S, Stulak JM, Eleid MF, Dunlay SM, Gersh BJ, Rihal CS and Barsness GW. Concomitant intra-aortic balloon pump use in cardiogenic shock requiring veno-arterial extracorporeal membrane oxygenation. Circ Cardiovasc Interv. 2018;11:e006930. [DOI] [PubMed] [Google Scholar]
- 23.Vallabhajosyula S, Patlolla SH, Sandhyavenu H, Vallabhajosyula S, Barsness GW, Dunlay SM, Greason KL, Holmes DR Jr. and Eleid MF. Periprocedural Cardiopulmonary Bypass or Venoarterial Extracorporeal Membrane Oxygenation During Transcatheter Aortic Valve Replacement: A Systematic Review. J Am Heart Assoc. 2018;7: e009608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stretch R, Sauer CM, Yuh DD and Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64:1407–15. [DOI] [PubMed] [Google Scholar]
- 25.Chung ES, Lim C, Lee HY, Choi JH, Lee JS and Park KH. Results of extracorporeal membrane oxygenation (ECMO) support before coronary reperfusion in cardiogenic shock with acute myocardial infarction. Korean J Thorac Cardiovasc Surg. 2011;44:273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung SY, Tong MS, Sheu JJ, Lee FY, Sung PH, Chen CJ, Yang CH, Wu CJ and Yip HK. Short-term and long-term prognostic outcomes of patients with ST-segment elevation myocardial infarction complicated by profound cardiogenic shock undergoing early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention. Int J Cardiol. 2016;223:412–417. [DOI] [PubMed] [Google Scholar]
- 27.Yannopoulos D, Bartos JA, Raveendran G, Conterato M, Frascone RJ, Trembley A, John R, Connett J, Benditt DG, Lurie KG, Wilson RF and Aufderheide TP. Coronary artery disease in patients with out-of-hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol. 2017;70:1109–1117. [DOI] [PubMed] [Google Scholar]
- 28.Richardson AS, Schmidt M, Bailey M, Pellegrino VA, Rycus PT and Pilcher DV. ECMO Cardio-Pulmonary Resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12-years. Resuscitation. 2017;112:34–40. [DOI] [PubMed] [Google Scholar]
- 29.Yannopoulos D, Bartos JA, Martin C, Raveendran G, Missov E, Conterato M, Frascone RJ, Trembley A, Sipprell K, John R, George S, Carlson K, Brunsvold ME, Garcia S and Aufderheide TP. Minnesota Resuscitation Consortium’s advanced perfusion and reperfusion cardiac life support strategy for out-of-hospital refractory ventricular fibrillation. J Am Heart Assoc. 2016;5: e003732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko DT, Wang Y, Alter DA, Curtis JP, Rathore SS, Stukel TA, Masoudi FA, Ross JS, Foody JM and Krumholz HM. Regional variation in cardiac catheterization appropriateness and baseline risk after acute myocardial infarction. J Am Coll Cardiol. 2008;51:716–23. [DOI] [PubMed] [Google Scholar]
- 31.Bougouin W, Dumas F, Karam N, Maupain C, Marijon E, Lamhaut L, Jost D, Geri G, Beganton F, Varenne O, Spaulding C, Jouven X and Cariou A. Should we perform an immediate coronary angiogram in all patients after cardiac arrest?: insights from a large French registry. JACC Cardiovasc Interv. 2018;11:249–256. [DOI] [PubMed] [Google Scholar]
- 32.Vallabhajosyula S, Barsness GW and Vallabhajosyula S. Multidisciplinary teams for cardiogenic shock. Aging (Albany NY). 2019;11:4774–4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Bohm M, Ebelt H, Schneider S, Schuler G and Werdan K. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–96. [DOI] [PubMed] [Google Scholar]
- 34.Chang CH, Chen HC, Caffrey JL, Hsu J, Lin JW, Lai MS and Chen YS. Survival analysis after extracorporeal membrane oxygenation in critically ill adults: A nationwide cohort study. Circulation. 2016;133:2423–33. [DOI] [PubMed] [Google Scholar]
- 35.Ziaeian B, Kominski GF, Ong MK, Mays VM, Brook RH and Fonarow GC. National Differences in Trends for Heart Failure Hospitalizations by Sex and Race/Ethnicity. Circ Cardiovasc Qual Outcomes. 2017;10:e003552. doi: 10.1161/CIRCOUTCOMES.116.003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bermudez CA, Rocha RV, Toyoda Y, Zaldonis D, Sappington PL, Mulukutla S, Marroquin OC, Toma C, Bhama JK and Kormos RL. Extracorporeal membrane oxygenation for advanced refractory shock in acute and chronic cardiomyopathy. Ann Thorac Surg. 2011;92:2125–31. [DOI] [PubMed] [Google Scholar]
- 37.Crow S, Fischer AC and Schears RM. Extracorporeal life support: utilization, cost, controversy, and ethics of trying to save lives. Semin Cardiothorac Vasc Anesth. 2009;13:183–91. [DOI] [PubMed] [Google Scholar]
- 38.Blum JM, Lynch WR and Coopersmith CM. Clinical and billing review of extracorporeal membrane oxygenation. Chest. 2015;147:1697–1703. [DOI] [PubMed] [Google Scholar]
- 39.Subramaniam AV, Barsness GW, Vallabhajosyula S and Vallabhajosyula S. Complications of temporary percutaneous mechanical circulatory support for cardiogenic shock: an appraisal of contemporary literature. Cardiol Ther. 2019; doi: 10.1007/s40119-019-00152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maini B, Gregory D, Scotti DJ and Buyantseva L. Percutaneous cardiac assist devices compared with surgical hemodynamic support alternatives: cost-effectiveness in the emergent setting. Catheter Cardiovasc Interv. 2014;83:E183–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


