Summary
The oral pathogen, Aggregatibacter actinomycetemcomitans, produces a number of virulence factors, including a leukotoxin (LtxA), which specifically kills human white blood cells, to provide a colonization advantage to the bacterium. Strains of A. actinomycetemcomitans that produce more LtxA have been more closely linked to disease, indicating that this toxin plays a key role in pathogenesis of the bacterium. Disruption of the activity of LtxA thus represents a promising approach to reducing the pathogenicity of the bacterium. Catechins are polyphenolic molecules derived from plants, which have shown potent antibacterial and antitoxin activities. We have previously shown that galloylated catechins are able to prevent LtxA delivery to host cells by altering the toxin’s secondary structure and preventing binding to cholesterol on the host cell membrane. Here, we have investigated how one particular galloylated catechin, epigallocatechin gallate (EGCg), affects A. actinomycetemcomitans growth and toxin secretion. Our results demonstrate that EGCg, at micromolar concentrations, inhibits A. actinomycetemcomitans growth, as has been reported for other bacterial species. At subinhibitory concentrations, EGCg promotes LtxA production, but the toxicity of the bacterial supernatant against human immune cells is reduced. The results of our biophysical studies indicate that this seemingly contradictory result is caused by an EGCg-mediated enhancement of LtxA affinity for the bacterial cell surface. Together, these results demonstrate the potential of EGCg in the treatment of virulent A. actinomycetemcomitans infections.
Keywords: Leukotoxin, Aggregatibacter actinomycetemcomitans, (-)-Epigallocatechin gallate, Toxin secretion
1. Introduction
A Gram-negative bacterium, A. actinomycetemcomitans, primarily resides in human oral cavities and is associated with localized aggressive periodontitis (LAP) (Zambon 1985). The “key” virulence factor of A. actinomycetemcomitans has been suggested to be LtxA (Zambon, Haraszthy, Hariharan, et al. 1996), which kills immune cells (Johansson 2011) thus inhibiting the host’s immune response to the infection. LtxA is a member of the repeats-in-toxin (RTX) family (Welch 1991), a family of proteins that share several common features, including secretion via the type I secretion system (TISS) from the bacterial cytosol across the inner and outer membranes in a single step (Crosby and Kachlany 2007; Kachlany, Fine and Figurski 2000). Like most other Gram-negative bacteria, A. actinomycetemcomitans also releases outer membrane vesicles (OMVs), which deliver biologically active LtxA to host cells (Kato, Kowashi and Demuth 2002; Kieselbach, Zijnge, Granström, et al. 2015; Nice, Balashova, Kachlany, et al. 2018b). OMVs are spherical vehicles derived from the outer membrane of the bacterial cell and consist of outer membrane and periplasmic lipids, proteins, and nucleic acids (Ellis and Kuehn 2010; Kuehn and Kesty 2005; Kulp and Kuehn 2010). We and others have shown that LtxA resides on the surface of A. actinomycetemcomitans OMVs (Nice, Balashova, Kachlany, et al. 2018b; Ohta, Kato, Kokeguchi, et al. 1991), suggesting that after secretion of LtxA via T1SS, some of the toxin associates with the outer membrane of the bacterial cells, enabling its release on the surface of the OMVs in addition to its release in a “free” form.
Current treatment of LAP includes mechanical debridement in combination with systemic antibiotics such as tetracycline. However, growing antibiotic resistance, including resistance to tetracycline in A. actinomycetemcomitans (Saxen and Asikainen 1993; Walker 1996), has decreased the effectiveness of this treatment (Deas and Mealey 2010; Mombelli, Gmur, Gobbi, et al. 1994). Strains of A. actinomycetemcomitans that produce more LtxA than other strains are more closely associated with LAP (Haraszathy, Hariharan, Tinoco, et al. 2000; Haubek, Ennibi, Poulsen, et al. 2008; Zambon, Slots and Genco 1983). An example of such a toxin-rich strain isJP2, which originated in North- and West Africa (Åberg, Kwamin, Claesson, et al. 2012; Haubek, Poulsen and Kilian 2007; Kwamin, Gref, Haubek, et al. 2012). The close association between LtxA and LAP suggests that this immune-modulating protein plays a significant role in disease progression. Therefore, an anti-toxin strategy that focuses on inhibiting the activity of LtxA has potential utility in the treatment of LAP.
Recently, as the need for new antibiotic options has increased, polyphenols have emerged as a promising alternative (Betts, Hornsey and La Ragione 2018; Daglia 2012). Approximately four billion people worldwide use medicinal plants, such as chewing sticks and other herbs as their primary healthcare sources; many of these plants contain naturally occurring polyphenols (Danila, Gatea and Radu 2011; Ekor 2014; Kwamin, Gref, Haubek, et al. 2012; Mukherjee 2002). Over 4,000 naturally occurring polyphenols have been reported to have biological activity (Daglia 2012), including catechins, which are extracts found in the green tea leaf, as well as in grape seeds, kiwi, strawberries, red wine, cacao, and other plant sources (Zanwar, Badole, Shende, et al. 2014). Among the catechin derivatives, epigallocatechin gallate (EGCg) has been frequently noted for its therapeutic effects (Ahn, Kawamura, Kim, et al. 2014; Chakrawarti, Agrawal, Dang, et al. 2016; Chang, Huang, Lin, et al. 2019; Mendel Friedman 2006; Osterburg, Gardner, Hyon, et al. 2009). Studies have shown that EGCg effectively lysed Acinetobacter baumannii clinical isolates that are highly antibiotic-resistant (Osterburg, Gardner, Hyon, et al. 2009) and inhibited the growth of Clostridium perfringens, a Gram-positive bacteria (Ahn, Kawamura, Kim, et al. 2014). At comparable concentrations, some galloylated catechins have been shown to be more effective than traditional antibiotics, such as tetracycline and vancomycin, against Bacillus cereus (Mendel Friedman 2006).
In addition to these bactericidal effects, some catechins have been shown to exhibit anti-virulence properties by inhibiting bacterial toxins from being secreted into the extracellular environment. For example, secretion of the α-toxin from Staphylococcus aureus was inhibited when the culture was supplemented with epicatechin gallate (ECg) (Shah, Stapleton and Taylor 2008) or licochalcone A (LicA), another natural phenolic molecule (Qiu, Jiang, Xia, et al. 2010). Although the mechanism of ECg-mediated inhibition of secretion was not specified, real-time polymerase chain reaction (RT-PCR) was used to demonstrate that LicA specifically inhibits transcription of the α-toxin gene (hla) (Qiu, Jiang, Xia, et al. 2010). Similarly, RT-PCR and Western blotting have shown that EGCg increased expression of the Shiga toxin-1 gene (stx1) while decreasing expression of the Shiga toxin-2 gene (stx2) (Yang, Tang, Xiao, et al. 2018). In addition to affecting toxin expression, catechins have been reported to deactivate bacterial toxins after secretion. Grape extract was shown to inhibit the activity of the cholera toxin (CT) produced by Vibrio cholerae (Reddy, Taylor, Zhao, et al. 2013); when the authors investigated the inhibitory activity of the specific polyphenolic compounds comprising the extract, they observed that EGCg was the most active component (Cherubin, Garcia, Curtis, et al. 2016). Similarly, we have shown that EGCg deactivates the toxic activity of the leukotoxin (LtxA) produced by A. actinomycetemcomitans by altering the secondary structure of LtxA and inhibiting the toxin’s binding to cholesterol on the host cell membrane (Chang, Huang, Lin, et al. 2019). Our finding closely reflects a previous study, in which it was shown that Psidium guajava (guava) extracts, particularly those from the leaves and twigs, neutralized the activity of LtxA by binding directly to the secreted LtxA rather than interacting with the host cell (Kwamin, Gref, Haubek, et al. 2012); guava leaves and bark have been reported to contain significant amounts of polyphenolic compounds, including catechins (Barbalho and Machado 2012).
Here, we investigated EGCg-mediated alterations in LtxA release by A. actinomycetemcomitans. Our results indicate that at low concentrations where EGCg does not affect A. actinomycetemcomitans growth, EGCg prevents the release of LtxA into solution, not by inhibiting LtxA expression or secretion via the T1SS machinery, but by increasing the toxin’s affinity for cell surface components.
2. Materials and Methods
2.1. Chemicals
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) was purchased from Avanti Polar Lipids (Alabaster, AL). (-)-Catechin (C), (-)-epigallocatechin gallate (EGCg), and cholesterol were purchased from Sigma-Aldrich (St. Louis, MO). N-(3-Triethylammoniumpropyl)-4-(6–4-(diethylamino) phenyl) hexatrienyl) pyridinium dibromide (FM™ 4–64), and goat anti-mouse IgG (H+L) secondary antibody-fluorescein isothiocyanate (FITC-GAM) were purchased from ThermoFisher Scientific (Waltham, MA). Goat-anti-mouse IgG, Human ads-HRP (GAM-HRP) was purchased from Southern Biotech (Birmingham, AL). Sephacryl S-1000 gel filtration resin and HiTrap SP HP cation exchange chromatography columns were purchased from GE Healthcare (Pittsburgh, PA).
2.2. Mammalian Cell Culture
THP-1 cells (ATCC), were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 0.05 mM 2-mercaptoethanol, and 1% Pen-Strep (100 U/mL penicillin and 100 μg/mL streptomycin).
2.3. Bacterial Cell Culture
An LtxA-expressing strain of A. actinomycetemcomitans, JP2 (Haubek, Poulsen and Kilian 2007), and an isogenic, non-LtxA-expressing strain AA1704 (Balashova, Crosby, Al Ghofaily, et al. 2006), were grown anaerobically in candle jar in A. actinomycetemcomitans growth medium (AAGM), following a published protocol (Kachlany, Fine and Figurski 2002).
2.4. LtxA Purification
LtxA was purified from the A. actinomycetemcomitans JP2 supernatant using a published protocol with slight modifications (Reinholdt, Poulsen, Brinkmann, et al. 2013). Briefly, an A. actinomycetemcomitans culture was grown to the late exponential phase and then centrifuged at 10,000 × g for 10 mins twice to pellet the bacteria. Any remaining bacteria were removed by filtering the supernatant through a 0.22-μm filter. The bacteria-free supernatant was run through a HiTrap SP HP cation exchange chromatography column then eluted from the column with a linear gradient of NaCl with a maximum concentration of 1 M, and 80 1 mL fractions were collected. The absorbance at 280 nm (A280) of each fraction was analyzed to quantify the amount of protein, and those fractions with the most protein were collected. The purity, identity, and activity of the purified protein was determined using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), Western blot and cytotoxicity experiments, respectively (Fig. S1).
2.5. OMV Purification
A. actinomycetemcomitans (JP2 and AA1704) OMVs were purified using ultracentrifugation. Briefly, an A. actinomycetemcomitans culture was grown to the late exponential phase and then centrifuged at 10,000 × g for 10 mins twice to pellet the bacteria. Any remaining bacteria were removed by filtering the supernatant through a 0.45-μm filter. The bacteria-free supernatant was ultracentrifuged at 105,000 × g for 30 mins, and the pellet containing the OMVs was resuspended and ultracentrifuged again under the same condition. The final OMV pellet was resuspended in 2 mL of phosphate-buffered saline (PBS), and any remaining bacteria were removed by centrifuging the sample at 5,000 × g for 2 mins. The relative concentration of OMVs was determined by measuring the amount of protein associated with OMVs using the A280 value measured on a NanoDrop spectrometer. In addition, the lipid concentration of the OMV samples was determined using a calibration curve employing the lipophilic dye, FM™ 4–64. Liposomes composed of an 80/20 molar ratio of POPC/cholesterol were produced using the lipid film technique and hydrated in PBS. The liposomes were extruded using a 100 nm filter, then diluted to concentrations of 0.1, 0.05, 0.025, 0.0125 and 0.00625 mM. The OMVs were serially diluted from the stock and both the liposome samples, with known concentrations, and OMV samples, with unknown concentrations, were incubated with the FM™ 4–64 dye for 15 sec. The fluorescence intensity at a wavelength of 640 nm of all samples was measured using an excitation wavelength of 515 nm. A calibration curve was constructed using the liposome concentrations and corresponding fluorescence intensities. The lipid concentration of the OMVs was then calculated from the best linear fit of the data.
2.6. Primary Antibody Preparation
Hybridoma cells (Lally, Golub and Kieba 1994) were gifted by Dr. Edward T. Lally, University of Pennsylvania. The cells were grown in serum-free medium, and the cells were pelleted. The cell supernatant was collected. Depending on the application, the supernatant was used directly, or the antibody was purified using a protein-G column.
2.7. Subinhibitory Dose of EGCg and C
To calculate the subinhibitory dose of EGCg and C against A. actinomycetemcomitans, the bacteria were grown for 18 hrs in AAGM, then diluted to an OD600 value of 0.15 in AAGM supplemented with different concentrations (50 μg/mL 10 μg/mL, 5 μg/mL, and 2.5 μg/mL) of EGCg, C, or a corresponding volume of PBS. The OD600 value was measured over time using a Tecan plate reader (Tecan, Männedorf, Switzerland), and each reading was normalized by dividing by the corresponding 1-hr time point. Five independent replicates were measured.
2.8. Toxicity of A. actinomycetemcomitans Supernatant
A. actinomycetemcomitans was cultured with or without EGCg, and the cells were harvested at the late exponential phase. The cells were pelleted by centrifuging 1 mL of culture at 3,000 × g for 10 mins. The supernatant was collected, and 10 μL of the supernatant from each batch or the same volume of PBS was added to 0.1 mL of 2 × 106 THP-1 cells/mL. The cells were incubated for three hrs at 37 °C under 5% CO2, before the viability was measured using a Trypan blue exclusion assay. The viability was normalized to the viability of the untreated (PBS only) control sample.
2.9. Quantification of Free LtxA and Cell-Associated LtxA
An immunoblot assay was developed to quantify the amount of LtxA in the bacterial supernatant or associated with the bacterial cells. In parallel, two A. actinomycetemcomitans cultures were grown to the late exponential phase, either with or without 5 μg/mL of EGCg in the media. One mL of each culture was centrifuged at 16,000 × g for 2 mins. Both the pellet and supernatant were collected for LtxA analysis. The pellet was dissolved in 1 mL of PBS. To 500 μL of the supernatant, 1 mL of cold ethanol was added, and the solution was incubated at −80 °C for 5 mins before centrifugation at 16,000 × g for 15 mins at 4 °C (Kachlany, Fine and Figurski 2000). The resulting pellet was collected and dissolved in 500 μL of PBS.
We have observed that EGCg treatment decreases the binding of our monoclonal anti-LtxA antibody (Fig. S2). For this reason, we used two separate nitrocellulose blots, one containing an untreated LtxA standard and the untreated LtxA samples and a second containing an EGCg-treated LtxA standard and EGCg-treated LtxA samples. This technique allowed us to more accurately quantify the mass of LtxA present under each condition by comparing the samples to the respective standards. On one nitrocellulose membrane, 2 μL of purified LtxA at a concentration of 50 μg/mL, serially diluted by a factor of four was spotted. Below this row of standards, 2 μL of the pellet and supernatant samples collected from A. actinomycetemcomitans grown in the absence of EGCg were dotted in a serial dilution by a factor of two. On a second nitrocellulose membrane, 2 μL of LtxA at a concentration of 50 μg/mL, pretreated with 2 μg/mL of EGCg, was used as the first dot of the standard, then serially diluted by a factor of two. Below this row of standards, 2 μL of the pellet and supernatant samples collected from A. actinomycetemcomitans grown in the presence of 5 μg/mL of EGCg were spotted in a serial dilution by a factor of two. Both membranes were incubated in separate chambers with an anti-LtxA antibody (mAb 107) (Lally, Golub and Kieba 1994) followed by secondary antibody (GAM-HRP). The intensities of each experimental spot were compared to that of the corresponding standard (LtxA with or without EGCg) using ImageJ.
2.10. Labeling of LtxA on the Surface of A. actinomycetemcomitans
To quantify the amount of LtxA on the surface of A. actinomycetemcomitans, an antibody staining technique was used. A. actinomycetemcomitans, strain JP2, was grown in the presence or absence of EGCg at a concentration of 5 μg/mL. The cells were harvested at the late exponential phase, and 1 mL of the culture was centrifuged at 3,000 × g for 10 mins. The pellet was washed with PBS and resuspended in 200 μL of PBS. Then 100 μL of an anti-LtxA antibody (mAb28) (Lally, Golub and Kieba 1994), at a concentration of 78 μg/mL was added to label LtxA, and the cells were incubated for 15 mins at 37 °C. The labeled cells were then washed twice with PBS to remove excess antibody, and the final pellet was resuspended in 200 μL of PBS. Finally, 5 μL of FITC-GAM (Molecular Probes, Eugene, OR) was added to label the primary antibody, and the cells were washed with PBS. Additionally, controls lacking either the primary or secondary antibody were prepared to exclude the possibility of non-specific binding. The fluorescence emission of each sample was measured between 500 nm and 550 nm, with an excitation wavelength of 490 nm. The emission intensities at 525 nm of the controls were subtracted from the related sample intensity at 525 nm, and the resulting peak was normalized to the maximum fluorescence intensity of both samples.
2.11. Association of LtxA with Bacterial Outer Membrane
To measure binding of LtxA to the outer membrane of A. actinomycetemcomitans, size exclusion chromatography (SEC) using a sephacryl-1000 resin (SEC-1000) column was performed. AA1704 OMVs were used as an LtxA-free model of the JP2 surface and were incubated with EGCg-treated or untreated LtxA. First, 375 μL of LtxA (0.4 μg/μL) was incubated with either 12 μL of EGCg (1 μg/μL) or PBS for 15 mins at room temperature. Then, the mixture was incubated with 600 μL of AA1704 OMVs at a lipid concentration of 1.8 mM for 15 mins at room temperature. The whole mixture was added to the SEC column, and after 10 mL flowed through, 80 1-mL fractions were collected. To quantify the amount of OMVs in each fraction, 50 μL of each fraction was collected and incubated with 2.5 μL of FM™ 4–64 dye for 15 s in a Corning™ 96-well solid plate; the fluorescence was measured using a Tecan plate reader with excitation and emission wavelengths set at 515 nm and 640 nm, respectively. To quantify the amount of LtxA in each fraction, an immunoblot assay was employed. Because the concentration of LtxA in each fraction was small, 400 μL of each fraction was collected, and every four fractions were combined to make 1.6 mL. This combined fraction was then lyophilized and dissolved in 100 μL of DI water. To quantify the amount of LtxA in each combined fraction, an LtxA standard of known concentration was used. We have observed that our anti-LtxA antibodies bind with different affinity to EGCg-treated LtxA; thus, two sets of standards were created, one untreated LtxA and one EGCg-treated LtxA. For the untreated LtxA standard, 2 μL of untreated LtxA (5 μg/mL, serially diluted by a factor of two) was spotted on the top row of one blot. For the EGCg-treated standard, 2 μL of EGCg-treated LtxA at a mass ratio of 0.08 (5 μg/mL, serially diluted by a factor of two) of LtxA was spotted on the top row of a second blot. On each blot, below the standards, the combined, concentrated fractions collected from the SEC-1000 were spotted. The blots were incubated in blotto (2.5 mg of powdered milk in 50 mL of Tris-buffered saline, 0.1% Tween 20) for 1 hr, followed by overnight incubation with the mAb 107 antibody at 4 °C. The blot was washed with Tris-buffered saline buffer for 5 mins, three times, then incubated in GAM-HRP for 1 hr before imaging. The dot intensities of the standards were quantified using ImageJ, and a normalized concentration of LtxA was calculated from the standard. The amount of LtxA was normalized to the total amount of LtxA added to the column.
2.12. Statistical Analysis
All data are presented as the mean ± standard deviation. Statistical analysis was performed using an unpaired two-tailed student’s t-test or two-way analysis of variance (ANOVA), as noted, where p values less than 0.01 were considered to be statistically significant.
3. Results and Discussion
3.1. Inhibition of A. actinomycetemcomitans Growth by EGCg
Because catechins, particularly EGCg, have reported antibacterial properties (Aron and Kennedy 2008; Kawashima 2011; Osterburg, Gardner, Hyon, et al. 2009; Saito, Tsuzukibashi and Takada 2012), we investigated the EGCg-mediated inhibition of A. actinomycetemcomitans growth by measuring the OD600 of the culture at several time points. Each curve was normalized by dividing by the corresponding 1-hr time point of each growth curve to facilitate comparison between the curves. For a negative control, the same concentrations of C, a non-galloylated catechin was used; non-galloylated catechins, such as C, have been shown to have limited antibacterial properties (Ikigai, Nakae, Hara, et al. 1993), and we have previously shown that C has limited anti-toxin activity, in contrast to the galloylated catechins (Chang, Huang, Lin, et al. 2019). Fig. 1A shows that micromolar concentrations of EGCg inhibit the growth of A. actinomycetemcomitans. At an EGCg concentration of 5 μg/mL, A. actinomycetemcomitans growth was not significantly affected; however, 10 μg/mL of EGCg decreased the growth of A. actinomycetemcomitans, and this inhibition was further increased with a higher concentration (50 μg/mL) of EGCg. On the other hand, equivalent concentrations of C did not have any effect on the growth of A. actinomycetemcomitans (Fig. S3), consistent with published reports that this molecule is not as strong of an antibacterial agent as EGCg (Ikigai, Nakae, Hara, et al. 1993). To confirm that low concentrations of EGCg do not affect A. actinomycetemcomitans growth, an additional growth inhibition experiment was conducted using 2.5, 5 and 10 μg/mL of EGCg (Fig. 1B). The lowest concentration of EGCg where we observed inhibition of growth was 10 μg/mL; we defined that concentation to be the minimum inhibitory concentration (MIC), and in the remainder of this work, a subinhibitory concentration of 5 μg/mL was used.
Figure 1. EGCg-Mediated Inhibition of A. actinomycetemcomitans Growth.
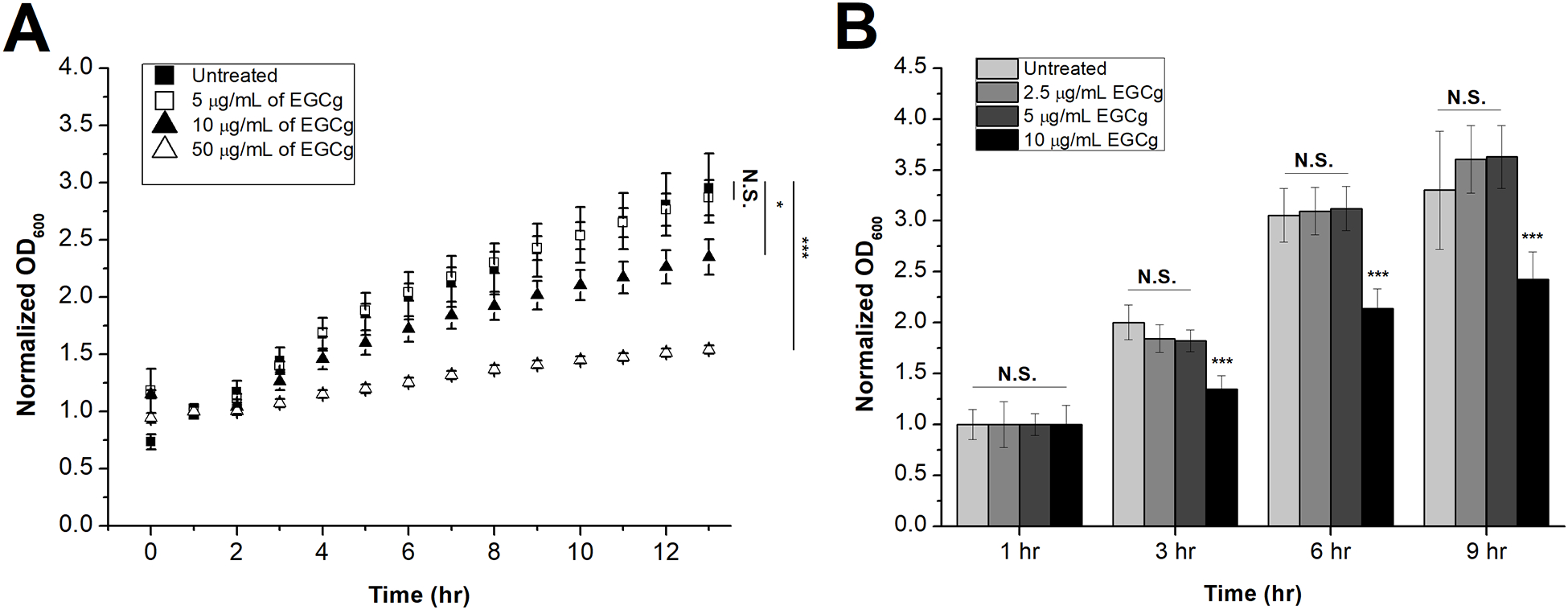
An overnight culture of A. actinomycetemcomitans was diluted to OD600 = 0.15 and treated with varying concentrations of EGCg. (A) Growth curve of A. actinomycetemcomitans treated with PBS (black squares) or EGCg at concentrations of 5 μg/mL (white squares), 10 μg/mL (black triangles), or 50 μg/mL (white triangles). The optical densities of five independent samples and five independent baselines for each concentration were observed for a 13-hr period, and the average of each baseline was subtracted from its corresponding samples and averaged. The resultant data points were normalized to the corresponding 1-hr time point. Data are presented as the mean (N=5) ± standard deviation. The level of significance, relative to untreated control was determined using ANOVA. N.S., not significant; *, p < 0.01; ***, p < 0.001. (B) Growth of A. actinomycetemcomitans treated with PBS or EGCg at concentrations of 2.5 μg/mL, 5 μg/mL, and 10 μg/mL of EGCg. The optical densities of five independent samples and five independent baselines for each concentration were observed for 1, 3, 6, 9-hr time points, and the average of each baseline line was subtracted from its corresponding sample and averaged. The resultant data points were normalized to the 1-hr time point of each corresponding curve. Data are presented as the mean (N=5) ± standard deviation. The level of significance, relative to untreated control was determined using a two-tailed student’s t-test. N.S., not significant; ***, p < 0.001.
EGCg has been shown to inhibit the growth of other bacteria, including Porphyromonas gingivalis and Prevotella melaninogenica, with MICs ranging from 250 to 2000 μg/mL (Sakanaka, Aizawa, Kim, et al. 1996). Here, we have observed that EGCg inhibits A. actinomycetemcomitans growth at a much lower concentration of 10 μg/mL, suggesting that the molecule has potential antibiotic utility for the treatment of A. actinomycetemcomitans infections. Importantly, the inhibitory concentration of 10 μg/mL that we observe here is comparable to the inhibitory concentrations of several antibiotics against A. actinomycetemcomitans, including tetracycline (1 μg/mL), metronidazole (32 μg/mL), azithromycin (2 μg/mL), and clindamycin (4 μg/mL) (Veloo, Seme, Raangs, et al. 2012).
3.2. Effect of Subinhibitory Concentration of EGCg on LtxA Secretion
Because of our observation that EGCg inhibits A. actinomycetemcomitans growth, we hypothesized that this molecule might also inhibit LtxA secretion, as secretion is growth-phase dependent. To study this, we grew A. actinomycetemcomitans in the presence or absence of 5 μg/mL EGCg and collected the supernatant from both cultures at a time of 6 hrs. (We have previously observed that this time is when A. actinomycetemcomitans strain JP2 begins to secrete signficant amounts of LtxA, under our culture conditions). The cell-free bacterial supernatants were incubated with THP-1 cells, and the viability of each cell culture after 3 hr was determined using a Trypan blue cytotoxicity assay. The viability of each sample was normalized to that of the negative control (PBS-treated cells). As shown in Fig. 2A, the untreated A. actinomycetemcomitans supernatant killed approximately 50% of the cells in this time period, while the supernatant from EGCg-treated A. actinomycetemcomitans had no significant effect on THP-1 cell viability.
Figure 2. Release of LtxA into A. actinomycetemcomitans Supernatant.
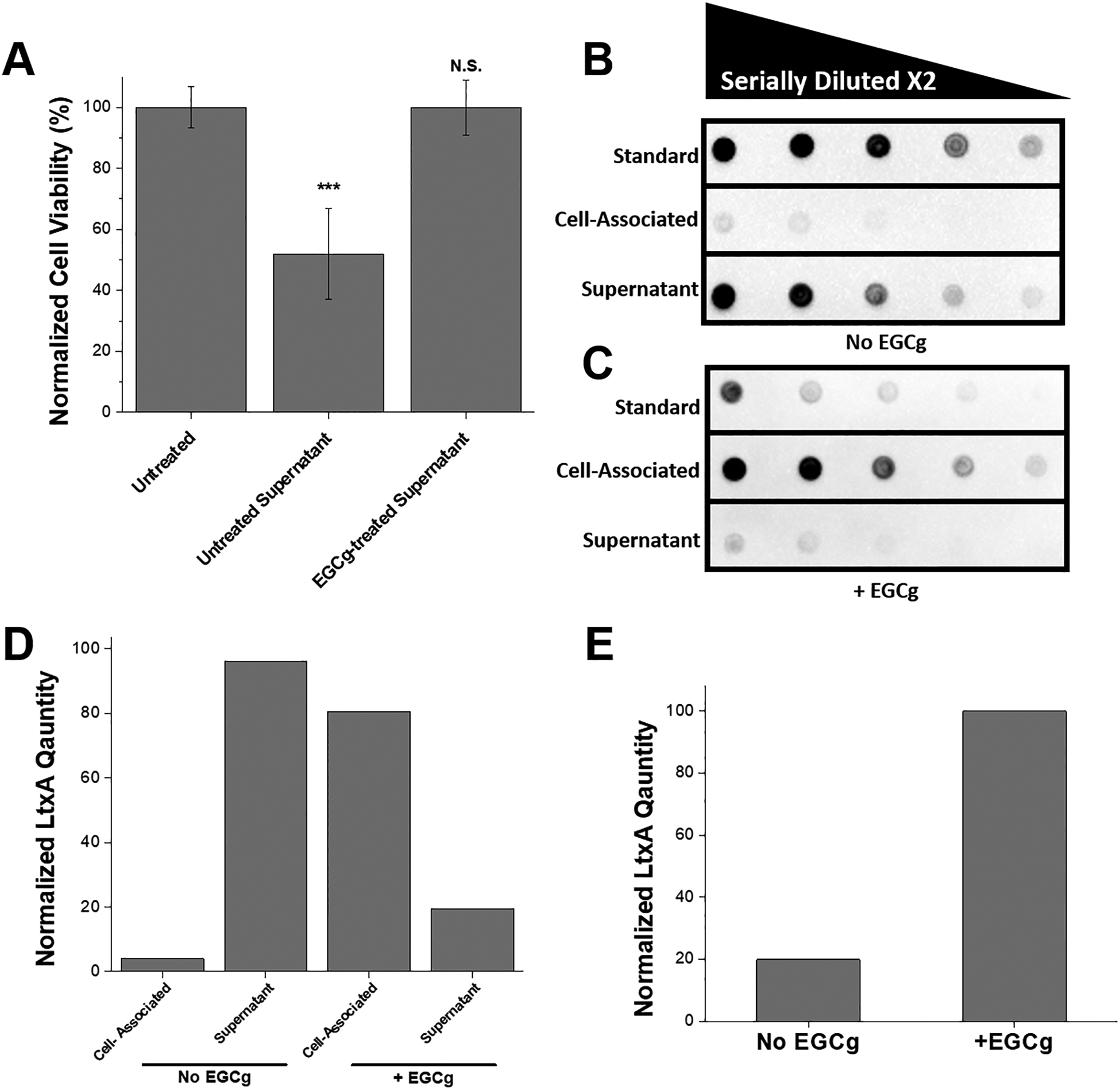
(A) Cytotoxicity of supernatant collected from untreated A. actinomycetemcomitans and EGCg-treated A. actinomycetemcomitans. The bacteria were pelleted from 1 mL culture, and 10 μL from the supernatant was collected and added to 100 μL of THP-1 cells at a concentration of 2 × 106 cells/mL. The cell viability was measured using Trypan blue after 3 hrs of incubation at 37 °C under 5% CO2, and the viability was normalized to that of untreated THP-1 cells. Data are presented as the mean (N=3) ± standard deviation. The level of significance, relative to untreated control was determined using a two-tailed student’s t-test. N.S., not significant; ***, p < 0.001. (B) Immunoblot for LtxA associated with A. actinomycetemcomitans cells or released into the supernatant. The first row represents the LtxA standard, where the first dot consists of 50 μg/mL of free LtxA, then serially diluted by a factor of two. The second row represents the amount of LtxA associated with untreated A. actinomycetemcomitans cells, where the first dot is the bacterial cell pellet resuspended in PBS. The third row represents the LtxA in the untreated A. actinomycetemcomitans supernatant, after cold ethanol precipitation, and resuspended in PBS. Rows two and three are also serially diluted by a factor of two. (C) Immunoblot for LtxA associated with EGCg-treated A. actinomycetemcomitans cells or released into the supernatant. The first row represents the EGCg-treated LtxA standard, where the first dot consists of 50 μg/mL of free LtxA that has been pretreated with 4 μg/mL of EGCg, then serially diluted by a factor of two. The second row represents the amount of LtxA associated with EGCg-treated A. actinomycetemcomitans cells, where the first dot is the bacterial cell pellet resuspended in PBS. The third row represents the LtxA in the EGCg-treated A. actinomycetemcomitans supernatant, after cold ethanol precipitation, and resuspended in PBS. Lanes two and three are also serially diluted by a factor of two. (D) Normalized amounts of LtxA associated with the A. actinomycetemcomitans cells or released into the supernatant in the absence and presence of 5 μg/mL EGCg. The mass of LtxA was calculated from the standard, and the percentage of the total is shown. (E) Quantification of the total amount of LtxA produced by EGCg-treated and untreated A. actinomycetemcomitans.
This result suggested that, compared to untreated A. actinomycetemcomitans, EGCg-treated A. actinomycetemcomitans releases into the supernatant either fewer LtxA molecules or LtxA molecules that are less active. To quantify the amount of LtxA released into the supernatant in cultures grown with or without EGCg, an immunoblot was used. Each culture was centrifuged, and a sample of the supernatant and a sample of the pelleted cells were collected and spotted on a nitrocellulose membrane. The results from the untreated culture are shown in Fig. 2B, and those from the EGCg-treated culture are shown in Fig. 2C. Purified LtxA was used as a standard in row one of both blots. In row two of the blots, the cell pellet was spotted, and in row three the supernatant was spotted. In the untreated A. actinomycetemcomitans culture (Fig. 2B), most of the LtxA was observed in the supernatant fractions, with very little residing on the cell surface. In contrast, in the EGCg-treated A. actinomycetemcomitans culture (Fig. 2C), very little LtxA was observed in the supernatant. Most of the toxin in this culture was observed in association with the bacterial cells instead.
The total amount of LtxA produced from A. actinomycetemcomitans treated with or without EGCg was quantified from the dot intensities using a calibration curve. The dot intensities of the standards, with known concentrations, were measured with ImageJ, and the concentration versus the intensity was plotted. The LtxA concentration of each experimental sample could then be calculated from the intensity of the spot. As shown in Fig. 2D, the presence of EGCg in the bacterial culture altered the location of the LtxA from primarily being in the supernatant to primarily being associated with the bacterial cell. EGCg treatment increased the total amount of LtxA produced (Fig 2E) by approximately five-fold.
We have previously shown that EGCg inhibits the activity of LtxA against THP-1 cells (Chang, Huang, Lin, et al. 2019). However, the results shown here suggest that the decreased toxicity of the supernatant of the EGCg-treated A. actinomycetemcomitans cultures (Fig. 2A) is due to increased association of the toxin with the bacterial cells, not decreased toxin expression or reduced activity of the secreted toxin. LtxA is secreted via a Type I secretion system in which the synthesized toxin is transferred across both the inner and outer membranes of the bacterium in a single-step (Kachlany 2010). Upon secretion, most of the toxin is released into the supernatant, but some reports have shown that LtxA has some affinity for the bacterial membrane, resulting in an association of the toxin with the outer surface of the bacterial membrane (Ohta, Hara, Fukui, et al. 1993; Ohta, Kato, Kokeguchi, et al. 1991).
Because our results demonstrated that EGCg treatment increases the amount of toxin produced by the cells, but decreases the amount of toxin in the supernatant, we next wondered if EGCg might be inhibiting the proper function of the T1SS in some way to prevent LtxA release from the bacterial cytosol. To determine if LtxA is located within or on the surface of the A. actinomycetemcomitans cells, we specifically labeled cell-surface-associated LtxA using a primary anti-LtxA antibody followed by a fluorescently labeled secondary antibody. A. actinomycetemcomitans, grown in the presence or absence of 5 μg/mL EGCg, was collected and labeled with mAb 28 antibody (Lally, Golub and Kieba 1994), followed by a FITC-labeled secondary antibody. The cells were washed, and the fluorescence, corresponding to the amount of surface-associated LtxA, was recorded. As shown in Fig. 3, untreated A. actinomycetemcomitans exhibited a slight fluorescence, indicating association of only a small amount of LtxA on the surface. This result is consistent with our immunoblot, where we detected minor amounts of LtxA associated with the bacterial cells. On the other hand, EGCg-treated A. actinomycetemcomitans exhibited strong fluorescence, indicating the presence of a large amount of surface-bound LtxA in these cells. This result suggests that in the presence of EGCg, LtxA is secreted via the T1SS, but most of the secreted LtxA then associates with the outer surface of the bacterial cell membrane.
Figure 3. Quantification of A. actinomycetemcomitans Cell Surface-Associated LtxA.
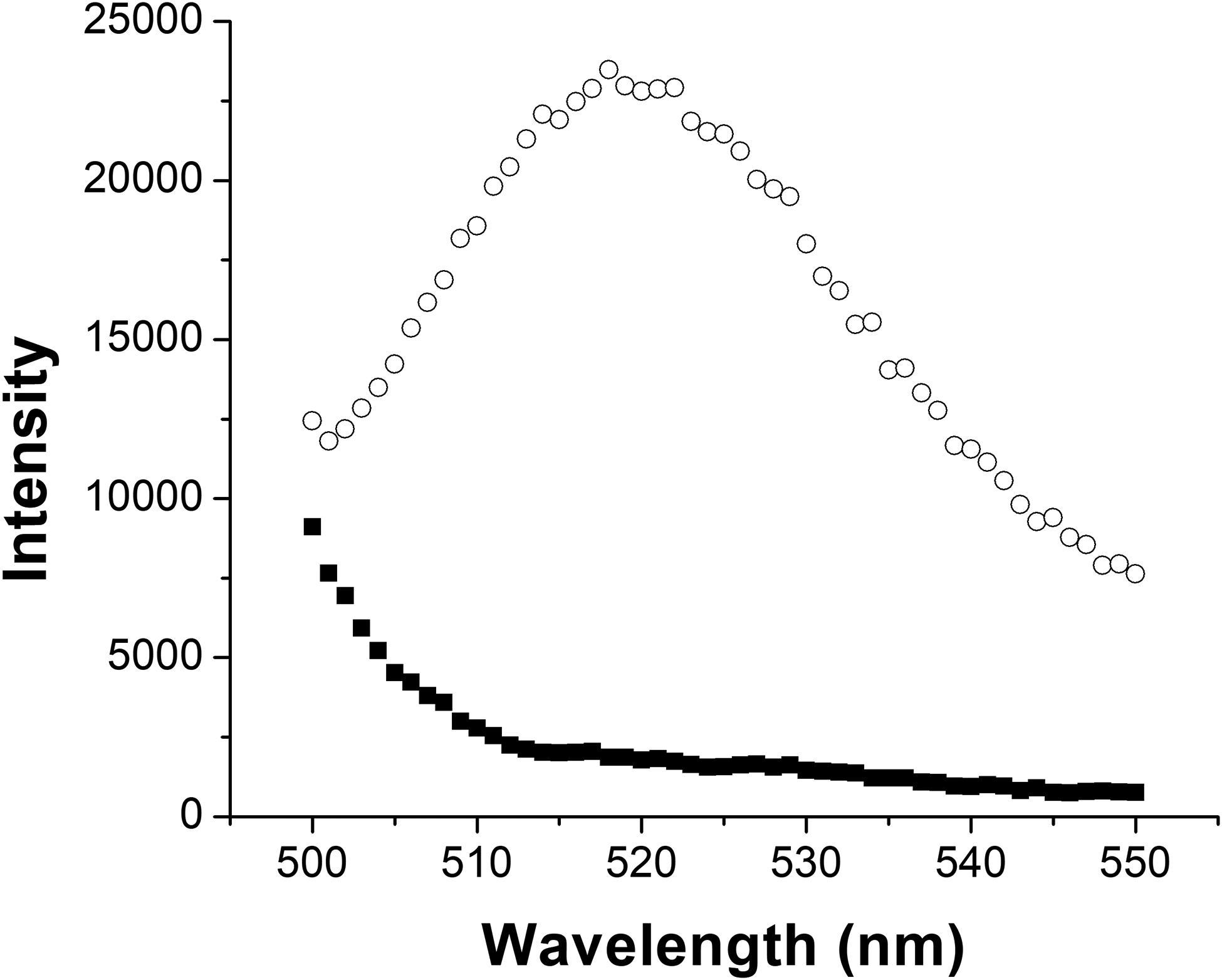
Untreated A. actinomycetemcomitans (black squares) and EGCg-treated A. actinomycetemcomitans cells (white circles) were treated with an anti-LtxA antibody, followed by FITC-GAM to fluorescently label cell surface-associated LtxA. The fluorescence intensity represents the amount of LtxA present on the surface of untreated or EGCg-treated A. actinomycetemcomitans cells.
3.3. Effect of EGCg on Association of LtxA with Bacterial Cell Membrane
We have previously demonstrated that EGCg induces a major change in the secondary structure of LtxA, resulting in a decreased affinity to cholesterol on host cell membranes (Chang, Huang, Lin, et al. 2019). We hypothesized that this large-scale conformational change might increase the affinity of the toxin to bacterial cell surface components. To investigate whether EGCg affects the binding affinity of LtxA for the outer membrane of A. actinomycetemcomitans, LtxA that has been treated with EGCg or untreated was incubated with AA1704 OMVs. AA1704 is an isogenic, LtxA-deficient mutant of JP2 (Balashova, Crosby, Al Ghofaily, et al. 2006); we therefore used the AA1704 OMVs as an LtxA-free model of the outer membrane of A. actinomycetemcomitans strain JP2. Bound and unbound LtxA was separated from the OMVs using SEC. The lipid content of each 1-mL fraction was analyzed using the FM™ 4–64 dye. The emission intensity of the dye in each fraction was normalized to the sum of all the emission intensities to obtain the percentage of OMVs in each fraction. As shown in Fig. 4, OMVs began to elute from the column near fraction number 20. A second population of OMVs eluted in later fractions (beginning in fraction 35). We have previously reported that A. actinomycetemcomitans produces two OMV populations, one with larger diameters (~350 nm) and one with smaller diameters (~100 nm) (Nice, Balashova, Kachlany, et al. 2018b). The OMV population eluting in fractions 20–23 corresponds to the larger OMVs and that in fractions 35–38 corresponds to smaller OMVs.
Figure 4. Effect of EGCg on LtxA Association with AA1704 OMVs.
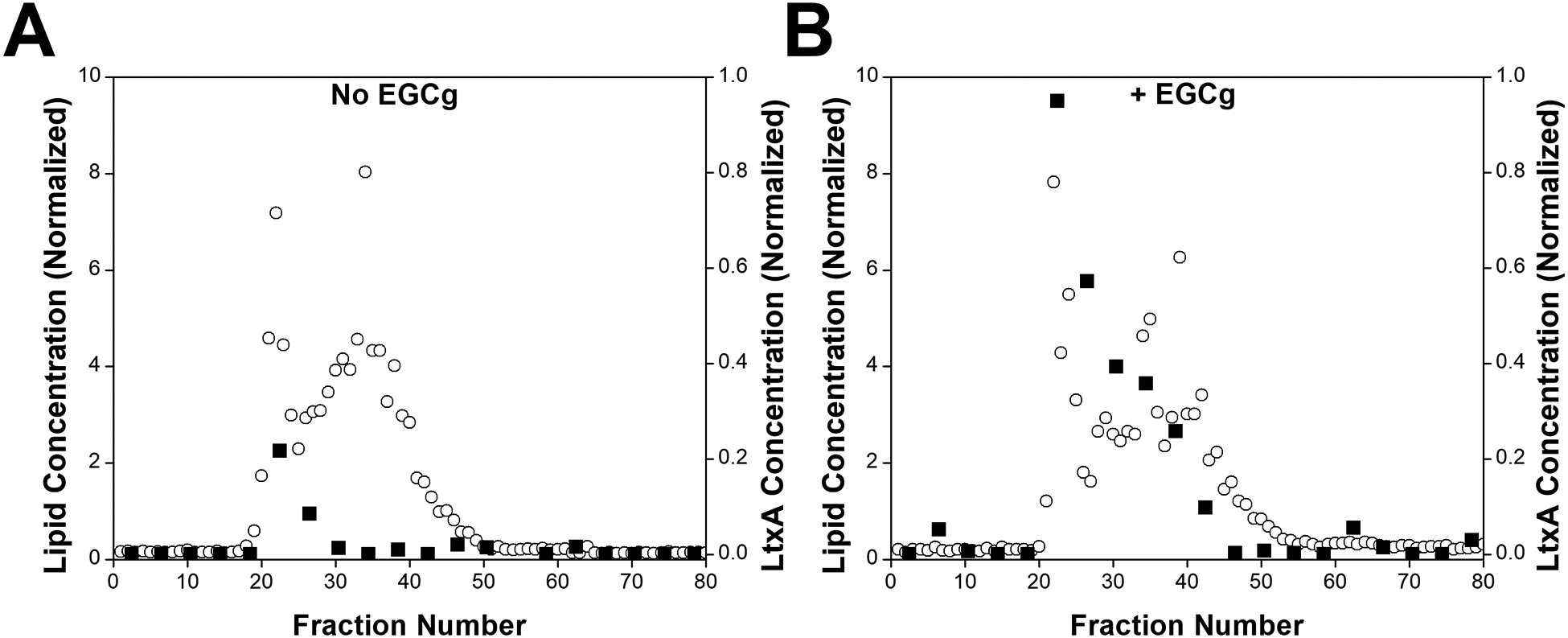
AA1704 OMVs were incubated with purified LtxA, untreated or pretreated with EGCg, for 15 mins at room temperature. OMV-associated and unbound LtxA were separated by SEC. One-mL fractions were collected, and the lipid concentration (white circles, primary y-axis) in each fraction was quantified using the FM 4–64 dye, and the LtxA concentration (black squares, secondary y-axis) was quantified using an immunoblot. (A) AA1704 OMVs incubated with untreated LtxA. (B) AA1704 OMVs incubated with EGCg-treated LtxA.
To measure the amount of LtxA associated with each fraction, an immunoblot was used. Because the concentration of LtxA in each fraction was low, every four fractions were first combined and concentrated. The dot intensity from the blot was converted into a concentration of LtxA using a calibration curve. The blot is shown in Fig. S4, and the fraction of toxin in each fraction is quantified in Fig. 4. The amount of LtxA in each fraction was normalized to the total amount of LtxA to obtain a percentage of LtxA in each fraction. Most of the untreated LtxA eluted in fractions 21 to 28. These are the fractions where the larger OMVs eluted, indicating that most LtxA is associated with larger OMVs. However, when LtxA was treated with EGCg, a much greater amount of LtxA was observed in the OMV-containing fractions and across more OMV-containing fractions, indicating that EGCg promotes binding of LtxA to bacterial surface components.
It has been shown through a variety of techniques, including Western blotting and proteomics that OMVs from several A. actinomycetemcomitans strains contain LtxA (Kato, Kowashi and Demuth 2002; Kieselbach, Zijnge, Granström, et al. 2015; Nice, Balashova, Kachlany, et al. 2018b). As a result, it has been proposed that A. actinomycetemcomitans OMVs play important roles in both offensive and defensive strategies of the bacteria (Kieselbach, Zijnge, Granström, et al. 2015). Our goal in this experiment was simply to use the OMVs as a model of the bacterial membrane; however, our results suggest that EGCg treatment might enhance association of LtxA to OMVs, thereby altering the offensive function of these vesicles, a possibility that we are currently examining.
In early reports about LtxA, there existed significant controversy about its mechanism of secretion. The RTX family of toxins was known to be secreted via the T1SS; however, LtxA was often found in large quantities in association with the bacterial membrane (Berthold, Forti, Kieba, et al. 1992; Ohta, Kato, Kokeguchi, et al. 1991; Tsai, Shenker, DiRienzo, et al. 1984). Subsequent work showed that A. actinomycetemcomitans expresses functional T1SS machinery (Crosby and Kachlany 2007; Guthmiller, Kolodrubetz, Cagle, et al. 1990; Guthmiller, Kolodrubetz and Kraig 1995; Lally, Golub, Kieba, et al. 1991), and smooth strains of A. actinomycetemcomitans do release significant amounts of LtxA into the supernatant, but this release is pH-, time-, and composition-dependent (Johansson, Claesson, Hänström, et al. 2003; Kachlany, Fine and Figurski 2000). Our results presented here further support the importance of growth medium composition on the release or cell-association of LtxA.
To our knowledge, this effect, in which EGCg promotes association of a secreted toxin with the bacterial cell, has not been reported. However, our previous results indicated that EGCg has a significant effect on the secondary structure of the toxin, resulting in a decreased affinity for cholesterol on the host cell membrane (Chang, Huang, Lin, et al. 2019). It is therefore conceivable, that this alteration of secondary structure, which inhibits cholesterol binding, additionally promotes binding of the toxin to the bacterial cell. In other words, in the presence of EGCg, the structure of LtxA is altered, resulting in a decreased affinity to cholesterol on the host cell membrane, and an increased affinity to cell surface components of the bacterial cell membrane, resulting in the observed decrease in toxicity of the bacterial supernatant.
4. Conclusions
Here, we have observed that growth in the presence of subinhibitory concentrations of EGCg greatly reduces the toxicity of the A. actinomycetemcomitans JP2 supernatant by promoting association of the secreted toxin with the bacterial cell surface. We propose that galloylated catechins are promising candidates for the treatment of periodontitis due to their unique anti-toxin activity.
Supplementary Material
Acknowledgments:
This work was supported by the National Institutes of Health grants DE025275 (ACB) and DE009517 (E.T. Lally and NVB) and the National Science Foundation grant 1554417 (ACB).
References
- Åberg CH, Kwamin F, Claesson R, et al. (2012) Presence of JP2 and Non-JP2 Genotypes of Aggregatibacter actinomycetemcomitans and Attachment Loss in Adolescents in Ghana. Journal of Periodontology 83: 1520–1528. [DOI] [PubMed] [Google Scholar]
- Ahn Y-J, Kawamura T, Kim M, et al. (2014) Tea Polyphenols: Selective Growth Inhibitors of Clostridiumspp. Agricultural and Biological Chemistry 55: 1425–1426. [Google Scholar]
- Aron PM and Kennedy JA (2008) Flavan-3-ols: Nature, occurrence and biological activity. Molecular Nutrition & Food Research 52: 79–104. [DOI] [PubMed] [Google Scholar]
- Balashova NV, Crosby JA, Al Ghofaily L, et al. (2006) Leukotoxin Confers Beta-Hemolytic Activity to Actinobacillus actinomycetemcomitans. Infection and Immunity 74: 2015–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbalho S and Machado F (2012) Psidium Guajava (Guava): A Plant of Multipurpose Medicinal Applications. Medicinal & Aromatic Plants 01. [Google Scholar]
- Berthold P, Forti D, Kieba IR, et al. (1992) Electron immunocytochemical localization of Actinobacillus actinomycetemcomitans leukotoxin. Oral Microbiology and Immunology 7: 24–27. [DOI] [PubMed] [Google Scholar]
- Betts JW, Hornsey M, and La Ragione RM (2018) Chapter Four - Novel Antibacterials: Alternatives to Traditional Antibiotics. Advances in Microbial Physiology 73: 123–169. [DOI] [PubMed] [Google Scholar]
- Chakrawarti L, Agrawal R, Dang S, et al. (2016) Therapeutic effects of EGCG: a patent review. Expert Opinion on Therapeutic Patents 26: 907–916. [DOI] [PubMed] [Google Scholar]
- Chang EH, Huang J, Lin Z, et al. (2019) Catechin-mediated restructuring of a bacterial toxin inhibits activity. Biochimica et Biophysica Acta (BBA) - General Subjects 1863: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubin P, Garcia MC, Curtis D, et al. (2016) Inhibition of Cholera Toxin and Other AB Toxins by Polyphenolic Compounds. PloS one 11: e0166477–e0166477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby JA and Kachlany SC (2007) TdeA, a TolC-like protein required for toxin and drug export in Aggregatibacter (Actinobacillus) actinomycetemcomitans. Gene 388: 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daglia M (2012) Polyphenols as antimicrobial agents. Current Opinion in Biotechnology 23: 174–181. [DOI] [PubMed] [Google Scholar]
- Danila AO, Gatea F, and Radu GL (2011) Polyphenol composition and antioxidant activity of selected medicinal herbs. Chemistry of Natural Compounds 47: 22–26. [Google Scholar]
- Deas DE and Mealey BL (2010) Response of chronic and aggressive periodontitis to treatment. Periodontology 2000 53: 154–166. [DOI] [PubMed] [Google Scholar]
- Ekor M (2014) The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in pharmacology 4: 177–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis TN and Kuehn MJ (2010) Virulence and Immunomodulatory Roles of Bacterial Outer Membrane Vesicles. Microbiology and Molecular Biology Reviews 74: 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthmiller JM, Kolodrubetz D, Cagle MP, et al. (1990) Sequence of the lktB gene from Actinobacillus actinomycetemcomitans. Nucleic acids research 18: 5291–5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthmiller JM, Kolodrubetz D, and Kraig E (1995) Mutational analysis of the putative leukotoxin transport genes in Actinobacillus actinomycetemcomitans. Microbial Pathogenesis 18: 307–321. [DOI] [PubMed] [Google Scholar]
- Haraszathy VI, Hariharan G, Tinoco EMB, et al. (2000) Evidence for the role of highly leukotoxic Actinobacillus actinomycetemcomitans in the pathogenesis of localized juvenile and other forms of early-onset periodontitis. Journal of Periodontology 71: 912–922. [DOI] [PubMed] [Google Scholar]
- Haubek D, Ennibi O-K, Poulsen K, et al. (2008) Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. The Lancet 371: 237–242. [DOI] [PubMed] [Google Scholar]
- Haubek D, Poulsen K, and Kilian M (2007) Microevolution and Patterns of Dissemination of the JP2 Clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans. Infection and Immunity 75: 3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikigai H, Nakae T, Hara Y, et al. (1993) Bactericidal catechins damage the lipid bilayer. Biochimica et Biophysica Acta (BBA) - Biomembranes 1147: 132–136. [DOI] [PubMed] [Google Scholar]
- Johansson A (2011) Aggregatibacter actinomycetemcomitans Leukotoxin: A Powerful Tool with Capacity to Cause Imbalance in the Host Inflammatory Response. Toxins 3: 242–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson A, Claesson R, Hänström L, et al. (2003) Serum-mediated release of leukotoxin from the cell surface of the periodontal pathogen Actinobacillus actinomycetemcomitans. European Journal of Oral Sciences 111: 209–215. [DOI] [PubMed] [Google Scholar]
- Kachlany SC (2010) Aggregatibacter actinomycetemcomitans Leukotoxin: from Threat to Therapy. Journal of Dental Research 89: 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachlany SC, Fine DH, and Figurski DH (2000) Secretion of RTX leukotoxin by Actinobacillus actinomycetemcomitans. Infection and immunity 68: 6094–6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachlany SC, Fine DH, and Figurski DH (2002) Purification of secreted leukotoxin (LtxA) from Actinobacillus actinomycetemcomitans. Protein Expression and Purification 25: 465–471. [DOI] [PubMed] [Google Scholar]
- Kato S, Kowashi Y, and Demuth DR (2002) Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microbial Pathogenesis 32: 1–13. [DOI] [PubMed] [Google Scholar]
- Kawashima Y (2011) Effects of Catechin Gallate on Bactericidal Action and Leukotoxic Activity of Aggregatibacter actinomycetemcomitans. International Journal of Oral-Medical Sciences 10: 20–24. [Google Scholar]
- Kieselbach T, Zijnge V, Granström E, et al. (2015) Proteomics of Aggregatibacter actinomycetemcomitans Outer Membrane Vesicles. PLOS ONE 10: e0138591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn MJ and Kesty NC (2005) Bacterial outer membrane vesicles and the host–pathogen interaction. Genes & Development 19: 2645–2655. [DOI] [PubMed] [Google Scholar]
- Kulp A and Kuehn MJ (2010) Biological Functions and Biogenesis of Secreted Bacterial Outer Membrane Vesicles. Annual Review of Microbiology 64: 163–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwamin F, Gref R, Haubek D, et al. (2012) Interactions of extracts from selected chewing stick sources with Aggregatibacter actinomycetemcomitans. BMC research notes 5: 203–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally ET, Golub EE, and Kieba IR (1994) Identification and immunological characterization of the domain of Actinobacillus actinomycetemcomitans leukotoxin that determines its specificity for human target cells. Journal of Biological Chemistry 269: 31289–95. [PubMed] [Google Scholar]
- Lally ET, Golub EE, Kieba IR, et al. (1991) Structure and function of the B and D genes of the Actinobacillus actinomycetemcomitans leukotoxin complex. Microbial Pathogenesis 11: 111–121. [DOI] [PubMed] [Google Scholar]
- Friedman Mendel, H. PR, Levin Carol E., Mandrell Robert E., Kozukue Nobuyuki. (2006) Antimicrobial Activities of Tea Catechins and Theaflavins and Tea Extracts against Bacillus cereus. Journal of Food Protection 69: 354–361. [DOI] [PubMed] [Google Scholar]
- Mombelli A, Gmur R, Gobbi C, et al. (1994) Actinobacillus actinomycetemcomitans in Adult Periodontitis. II. Characterization of Isolated Strains and Effect of Mechanical Periodontal Treatment. Journal of Periodontology 65: 827–834. [DOI] [PubMed] [Google Scholar]
- Mukherjee P (2002) Quality Control of Herbal Drugs: An Approach to Evaluation of Botanicals. Business Horizons. [Google Scholar]
- Nice JB, Balashova NV, Kachlany SC, et al. (2018) Aggregatibacter actinomycetemcomitans Leukotoxin Is Delivered to Host Cells in an LFA-1-Independent Manner When Associated with Outer Membrane Vesicles. Toxins 10: 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Hara H, Fukui K, et al. (1993) Association of Actinobacillus actinomycetemcomitans leukotoxin with nucleic acids on the bacterial cell surface. Infection and Immunity 61: 4878–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Kato K, Kokeguchi S, et al. (1991) Nuclease-sensitive binding of an Actinobacillus actinomycetemcomitans leukotoxin to the bacterial cell surface. Infection and Immunity 59: 4599–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterburg A, Gardner J, Hyon SH, et al. (2009) Highly antibiotic-resistant Acinetobacter baumannii clinical isolates are killed by the green tea polyphenol (–)-epigallocatechin-3-gallate (EGCG). Clinical Microbiology and Infection 15: 341–346. [DOI] [PubMed] [Google Scholar]
- Qiu J, Jiang Y, Xia L, et al. (2010) Subinhibitory concentrations of licochalcone A decrease alpha-toxin production in both methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Letters in Applied Microbiology 50: 223–229. [DOI] [PubMed] [Google Scholar]
- Reddy S, Taylor M, Zhao M, et al. (2013) Grape Extracts Inhibit Multiple Events in the Cell Biology of Cholera Intoxication. PLOS ONE 8: e73390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholdt J, Poulsen K, Brinkmann CR, et al. (2013) Monodisperse and LPS-free Aggregatibacter actinomycetemcomitans leukotoxin: Interactions with human β2 integrins and erythrocytes. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 1834: 546–558. [DOI] [PubMed] [Google Scholar]
- Saito M, Tsuzukibashi O, and Takada K (2012) Anticytotoxic Effect of Green Tea Catechin on Aggregatibacter actinomycetemcomitans Vesicles. International Journal of Oral-Medical Sciences 11: 101–105. [Google Scholar]
- Sakanaka S, Aizawa M, Kim M, et al. (1996) Inhibitory Effects of Green Tea Polyphenols on Growth and Cellular Adherence of an Oral Bacterium, Porphyromonas gingivalis. Bioscience, Biotechnology, and Biochemistry 60: 745–749. [DOI] [PubMed] [Google Scholar]
- Saxen L and Asikainen S (1993) Metronidazole in the treatment of localized juvenile periodontitis. Journal of Clinical Periodontology 20: 166–171. [DOI] [PubMed] [Google Scholar]
- Shah S, Stapleton PD, and Taylor PW (2008) The polyphenol (-)-epicatechin gallate disrupts the secretion of virulence-related proteins by Staphylococcus aureus. Letters in Applied Microbiology 46: 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CC, Shenker BJ, DiRienzo JM, et al. (1984) Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infection and Immunity 43: 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloo ACM, Seme K, Raangs E, et al. (2012) Antibiotic susceptibility profiles of oral pathogens. International Journal of Antimicrobial Agents 40: 450–454. [DOI] [PubMed] [Google Scholar]
- Walker CB (1996) The acquisition of antibiotic resistance in the periodontal microflora. Periodontology 2000 10: 79–88. [DOI] [PubMed] [Google Scholar]
- Welch RA (1991) Pore-forming cytolysins of gram-negative bacteria. Molecular Microbiology 5: 521–528. [DOI] [PubMed] [Google Scholar]
- Yang J, Tang CB, Xiao J, et al. (2018) Influences of epigallocatechin gallate and citric acid on Escherichia coli O157:H7 toxin gene expression and virulence-associated stress response. Letters in Applied Microbiology 67: 435–441. [DOI] [PubMed] [Google Scholar]
- Zambon JJ (1985) Actinobacillus actinomycetemcomitans in human periodontal disease. Journal of Clinical Periodontology 12: 1–20. [DOI] [PubMed] [Google Scholar]
- Zambon JJ, Haraszthy VI, Hariharan G, et al. (1996) The Microbiology of Early-Onset Periodontitis: Association of Highly Toxic Actinobacillus actinomycetemcomitans Strains with Localized Juvenile Periodontitis. Journal of Periodontology 67: 282–290. [DOI] [PubMed] [Google Scholar]
- Zambon JJ, Slots J, and Genco RJ (1983) Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infection and immunity 41: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanwar AA, Badole SL, Shende PS, et al. (2014) Chapter 21 - Antioxidant Role of Catechin in Health and Disease In: Polyphenols in Human Health and Disease, Watson RR, Preedy VR, and Zibadi S (eds). San Diego: Academic Press; pp. 267–271. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


