Supplemental Digital Content is available in the text.
Keywords: Chemical mixtures, Children, G-computation, mercury, Neurodevelopment, Perfluoroalkyl substances, Superlearner
Abstract
Background:
Exposure to mercury (Hg) is associated with adverse developmental effects. However, Hg occurs with a multitude of chemicals. We assessed the associations of developmental exposure to multiple pollutants with children’s neurodevelopment using a novel approach.
Methods:
Hg, polychlorinated biphenyls (PCBs), and perfluoroalkyl substances (PFASs) were measured in maternal and children’s blood at 5 years (n = 449 and 419). At 7 years, children were administered Boston Naming Test (BNT) and the Strengths and Difficulties Questionnaire (SDQ). We used the G-formula combined with SuperLearner to estimate independent and joint effects of chemicals at both ages. We constructed flexible exposure-response relationships and assessed interactions.
Results:
Most chemicals showed negative relationships with BNT scores. An interquartile range (IQR) increase in maternal Hg and perfluorooctanoic acid (PFOA) was associated with 0.15 standard deviation (SD) (95% confidence interval [CI] = –0.29, –0.03) and 0.14 SD (95% CI = –0.26, –0.05) lower scores in BNT, whereas a joint IQR increase in the mixture of chemicals was associated with 0.48 SD (95% CI = –0.69, –0.25) lower scores in BNT. An IQR increase in PFOA was associated with 0.11 SD (95% CI = 0.02, 0.26) higher total SDQ difficulties scores. Maternal ∑PCBs concentrations were associated with lower SDQ scores (β = –0.09 SD; 95% CI = –0.19, 0), whereas 5 years ∑PCBs showed a negative association (β = –0.09 SD; 95% CI = –0.21, 0). Finally, a joint IQR increase in the mixture was associated with 0.22 SD (95% CI = 0.04, 0.4) higher SDQ scores.
Conclusions:
Using a novel statistical approach, we confirmed associations between prenatal mercury exposure and lower cognitive function. The potential developmental effects of PFASs need additional attention.
What this study adds
Strong epidemiologic evidence suggests that early life exposure to certain chemicals impairs brain development, and therefore lead to decreased cognitive function. However, the majority of previous studies used a single pollutant-at-a-time approach, considering each chemical separately when assessing the potential effects of chemicals. In this study, we jointly investigated the potential neurodevelopmental effects of maternal and child 5-year exposure to mercury (Hg), perfluoroalkyl substances (PFASs), and polychlorinated biphenyls (PCBs) using a novel approach that combines the SuperLearner with G-computation. We found evidence that higher maternal Hg and PFOA concentrations were associated with decreases in cognitive function. The joint effect of the mixtures of chemicals was stronger, but no potential for synergistic effects was observed.
Background
Mercury (Hg) and other persistent organic pollutants (POPs) are environmental chemicals that are ubiquitous, persistent, and accumulate in marine mammals, with well-documented potential for developmental toxicity. Strong epidemiologic evidence suggests that many of these chemicals are neurotoxicants with potential to harm the developing brain, therefore resulting in long-lasting neurodevelopmental sequels.1,2 The large majority of environmental epidemiology studies typically adopt a single pollutant-at-a-time approach, considering each chemical separately when assessing the potential neurotoxic effects of chemicals; therefore, providing only limited insights on the real environment and health associations. Indeed, biomonitoring studies of environmental chemicals demonstrate that the general population experiences exposure to multiple chemicals from many different sources and at varying levels. Traditionally, chemical mixtures have been studied via. multivariable parametric regression approaches that mutually adjust for mixture components and estimate the independent effect of each component, while adjusting for the others. Recently, several statistical methods have been proposed to estimate health effects of environmental mixtures, often with an emphasis on variable selection.3 These methods include environmental-wide association studies (EWAS),4 penalized regression methods (e.g., least angle selection and shrinkage operator [LASSO]),5 dimension reduction methods, and exposure-response surface methodology such as generalized additive models (GAMs)and kernel regression methods.3,6
In this article, we investigated the potential independent and joint neurodevelopmental effects of maternal and child 5-year exposure to seven major marine pollutants in a population of Faroese children 7 years of age. We propose to use an ensemble machine learning technique called Super Learner7 that offers greater flexibility in approximating the data generating mechanism, and we combine it with G-computation,8,9 a causal inference approach that can yield valid causal effect estimates. This proposed approach can mitigate the problems of multicollinearity and model misspecification, with nonparametric prediction algorithms fitting complex exposure-response curves (Oulhote et al).32 We apply this approach to estimate valid exposure-response relationships and detect potential interactions.
Methods
Study population
The birth cohort was formed from 656 consecutive pregnancies recruited at the last antenatal examination at week 32 of pregnancy at the National Hospital in Tórshavn, Faroe Islands, during 1997–2000.10 The cohort can be considered reasonably representative of Faroese births. The Faroe Islands are located in the North Atlantic Ocean, between Norway and Iceland. The Faroese population is fairly homogeneous mainly of Scandinavian origin.11 Populations in the Faroese have depended on a traditional diet that includes fish, pilot whale, sheep, and birds. Therefore, concentrations of mercury and persistent organic pollutants in Faroese residents have been shown to be elevated compared with other populations especially due to dietary intake of pilot whale.11,12 Given the fairly homogenous population in terms of genetics and socioeconomic status (SES), and an exposure to POPs and related contaminants that covers a range of over 100-fold, this population offer a unique opportunity to study health effects of environmental contaminants with a limited potential for confounding.
Of the 656 pregnancies, 640 singleton births were included. Obstetric variables, including date of birth, birth weight, parity, and maternal age, were obtained from obstetrical and medical records. Information on prepregnancy weight and height, socioeconomic status, maternal smoking, and alcohol use during pregnancy were self-reported. The birth cohort underwent a prospective follow-up at age 7 years. A maternal interview informed questions concerning current health and past medical history, lifestyle, duration of breastfeeding, behavior, and other characteristics.
The study protocol was approved by the ethical review committee serving the Faroe Islands and by the institutional review board at the Harvard School of Public Health, and written informed consent was obtained from all mothers.
Assessment of children’s cognitive and behavioral functions at 7 years of age
For the purpose of this explorative investigation, we restricted the outcomes’ assessment to two neuropsychological tools are as follows: (1) the Boston Naming Test (BNT) that has been shown to be particularly sensitive to methylmercury exposure and 2) the Strengths and Difficulties Questionnaire (SDQ) for which we previously reported associations with environmental exposures in single pollutant approaches.13–17 A total of 567 children underwent neuropsychological assessment at age 7 years.
Boston Naming Test
The 60-item BNT18 is a visual confrontation naming test which measures the word retrieval or word finding performance of a subject. Stimuli are line drawings of a wide category of objects of increasing difficulty. Scores are obtained for number of correct items without cueing, and correct number of items after stimulus and phonemic cueing by the examiner.17
Strengths and Difficulties Questionnaire
The Parent’s version of the SDQ19 is comprised of 25 items scored on a 3-point Likert scale. Five behavioral subscales with a score range of 0 to 10 are calculated from the 25 SDQ items: emotional symptoms, conduct problems, hyperactivity/inattention, peer relationship problems, and prosocial behavior. A total difficulties score ranging from 0 to 40 was calculated by summing four of the subscales (emotional, conduct, hyperactivity, and peer). Higher total SDQ scores indicate higher behavioral difficulties.
Exposure assessment
Maternal exposures were assessed at the last antenatal examination at week 32 of pregnancy; 5-year exposure was assessed from the child blood at age 5.
Total mercury (Hg) concentration in whole blood was determined on a Direct Mercury Analyzer (DMA-80; Milestone Inc, Sorrisole, Italy).20 Serum polychlorinated biphenyls (PCBs) concentrations were measured after solid-phase extraction (SPE) using gas chromatography equipped with an electron capture detector (μ-ECD).21 To avoid problems with congeners not assessed and concentrations below the detection limit, a simplified ΣPCB concentration was calculated as the sum of major congeners CB-138, CB-153, and CB-180 multiplied by 2.22 The concentrations of PCBs are expressed in relation to the total lipid concentration determined using the Cypress Diagnostics kit (Langdorp, Belgium). Perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), perfluorohexane sulfonic acid (PFHxS), perfluorononanoic acid (PFNA), and perfluorodecanoic acid (PFDA) concentrations were measured using online solid-phase extraction and analyzed using high-pressure liquid chromatography with tandem mass spectrometry.23 The analysis of mercury, PCB’s, and PFAS’s were all conducted at Department of Environmental Medicine, Institute of Public Health, University of Southern Denmark.
In children with neuropsychological test scores at age 7 years, complete measurements of chemicals concentrations were available for 465 and 503 children respectively for maternal and 5-year exposures. Additionally, complete measurements for both maternal and 5-year exposures were available for 430 children with neuropsychological test scores (Supplemental Material; Figure S1; http://links.lww.com/EE/A53).
Covariates and potential confounders
We collected sociodemographic and lifestyle factors and medical history during pregnancy and at delivery via. administered questionnaires. At the 5-year follow-up visit, duration of exclusive breastfeeding was reported. We considered the following potential covariates in our models: child’s exact age (in months), sex (boy; girl), birth weight (grams), maternal age at pregnancy, prepregnancy body mass index (body mass index [BMI], kg/m2), parity (nulliparous; primipara; and multiparous), duration of exclusive breastfeeding (months), maternal intelligence (RAVEN scores), maternal socioeconomic status (SES) during pregnancy based on education (low: ≤10 years of education; intermediate: school leaving certificate and above including technical studies; and high: university studies), alcohol consumption during pregnancy (never; ever), and smoking during pregnancy (no; 1–5 cigarettes/day; more than 5 cigarettes/day). Final models for prenatal pollutant exposures included age, sex, maternal age, prepregnancy BMI, parity, maternal RAVEN scores, socioeconomic status, and alcohol and smoking during pregnancy. Models for child 5-year exposures further included prenatal pollutant exposures, duration of exclusive breastfeeding and birth weight.
Statistical analyses
Complete data on exposures, outcomes, and covariates were available for 449 and 419 children, respectively, for maternal and 5-year exposures (See supplemental material; Figure S1; http://links.lww.com/EE/A53). We did not conduct any imputations as the methods presented in this article could not be conducted on multiply imputed datasets. Mercury, PFASs, and ∑PCBs concentrations were logarithmically (base 10) transformed and centered. Initial exploratory data analyses included descriptive statistics and univariate associations between exposures and outcomes and potential covariates of interest. Neuropsychological outcomes were standardized with a mean of 0 and standard deviation (SD) of 1 in analyses investigating associations between multiple contaminants and neuropsychological functions. Estimates therefore represent the SD change in the outcome related to an interquartile range (IQR) increase in exposures.
To estimate individual and joint estimates of the associations between environmental exposures and neuropsychological scores, we first generated a valid prediction of the outcomes using the SuperLearner algorithm. SuperLearner is a data-adaptive approach that has been proposed by van der Laan et al.7,24,25 It uses cross-validated risks to find an optimal combination of predictions from a list of algorithms supplied by the user that minimizes a given loss function (e.g., squared error). We included a set of prediction algorithms in the library that can cover a large range of exposure-response relationships: the generalized linear regression model (GLM) and generalized additive models (GAM), elastic net regularization,26 multivariate adaptive polynomial spline regression,27 support vector machine,28 gradient boosting,29 random forests,30 and artificial neural networks.31,32
After obtaining a valid model for the outcome given the concurrent exposures and other covariates using SuperLearner, the resulting model was used to predict the neuropsychological test scores under specified exposure scenarios using G-computation.8,9 The marginal effect (hereafter called naïve average causal effect [NACE]) is estimated by calculating the sample average of the model predictions if exposure is set to the 75th percentile of exposure for all individuals minus the sample average of the model predictions if exposure is set to the 25th percentile of exposure for all individuals, while leaving the values of the remaining covariates at their observed values. For instance, the NACE for an IQR increase in maternal blood Hg is expressed as follows: 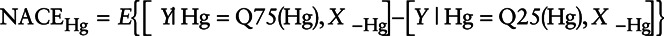 , where Q75 and Q25 are respectively the 75th and 25th percentiles of Hg distribution and
, where Q75 and Q25 are respectively the 75th and 25th percentiles of Hg distribution and 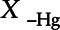 includes all the remaining covariates (including exposures and confounders) required for the identifiability of the effect estimate. The same strategy was applied to investigate the joint effect of the chemical mixture by replacing all exposures of interest first by the 75th percentile for all individuals in the cohort and then by the 25th percentile in a second time. In both cases, predictions of the outcome were calculated for all individuals, and we reported the difference between the sample averages of both sets of predictions.
includes all the remaining covariates (including exposures and confounders) required for the identifiability of the effect estimate. The same strategy was applied to investigate the joint effect of the chemical mixture by replacing all exposures of interest first by the 75th percentile for all individuals in the cohort and then by the 25th percentile in a second time. In both cases, predictions of the outcome were calculated for all individuals, and we reported the difference between the sample averages of both sets of predictions.
In the absence of a theoretical formula for the asymptotic distributions of these parameters within the SuperLearner, we used bootstrapping (N = 200) to approximate the 95% confidence intervals (CIs).33,34
Since the NACE cannot reveal nonlinearities, we therefore constructed dose-response relationships for each exposure. We used SuperLearner to predict test scores when replacing exposure values by a specific percentile of that exposure for all subjects while keeping the values of the other exposures and covariates at their observed levels. We calculated the sample average of those predictions to obtain the predicted response at a given exposure percentile, and the process was repeated for several exposure percentiles. The resulting values were used to plot a curve of the partial relationship between Y and Xj, which we called 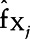 . The average partial relationship between Xj and Y can be therefore expressed as follows:
. The average partial relationship between Xj and Y can be therefore expressed as follows: 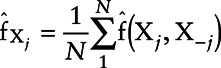 , where Xj is the exposure of interest,
, where Xj is the exposure of interest, 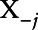 is the remaining set of exposures and covariates, N is the number of observations and
is the remaining set of exposures and covariates, N is the number of observations and 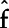 denotes predictions from the SuperLearner.
denotes predictions from the SuperLearner.
Finally, to investigate the presence of potential interactions between exposures or effect modification by other covariates, we used individual conditional expectations (ICEs) for each exposure.35 The ICE was calculated for each individual in the dataset separately for each exposure. For a given exposure, the ICE of a subject was calculated by replacing the value of that exposure by a given exposure percentile and computing the model predictions while keeping the values of the other exposures and covariates at his/her observed levels. Repeating the process for several exposure percentiles, we obtained the curve for that individual and exposure. The process was then repeated for all exposures and individuals. We therefore plotted N estimated conditional expectation curves, each reflecting the individual predicted response as a function of the exposure Xj, conditional on the observed 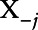 .35
.35
We did not perform any null hypothesis significance testing since we are not making any decisions. Rather, we rely on interval estimation for statistical inference and present the resulting estimates for all the exposures with their 95% CI based on nonparametric 2.5% and 97.5% percentiles.
Results
Mean age at examination was 89.9 months (IQR: 89–91), and there was a comparable number of boys and girls (49% and 51%, respectively). Most of the children had an older sibling (75%), and 32% were exclusively breastfed for 6 months or longer (Supplemental Material: Table S1; http://links.lww.com/EE/A53). Maternal age at delivery was 29.5 years (IQR: 26–33), and 8% of mothers reported a prepregnancy BMI higher than 30. Twenty-seven percent of the mothers reported ever smoking during pregnancy, and 41% reported ever consuming alcohol during pregnancy. Finally, mean maternal Raven intelligence score was 48.6 (IQR: 45–53). The 449 and 419 children included respectively in the prenatal and 5-year analyses did not significantly differ from the overall children included in the neuropsychological assessment at 7 years in terms of important characteristics (Supplemental Material; Table S1; http://links.lww.com/EE/A53).
Table 1 describes univariate associations between neuropsychological endpoints and important characteristics of the study population for the 449 included children. Mean score for BNT without cues (number of correct items without cueing) was 27.4 and mean BNT with cues (number of correct items after stimulus and phonemic cueing) was 30.3. Mean SDQ total difficulties was 6.4. BNT scores with and without cues were higher among children with no older siblings, of mothers with high SES and high Raven scores, and who reported ever drinking during pregnancy. Total SDQ difficulties scores were higher (indicating more problems) in children of younger mothers, with low SES, and who reported ever smoking during pregnancy.
Table 1.
Univariate associations between neuropsychological endpoints and important characteristics of the study population included in the prenatal analyses
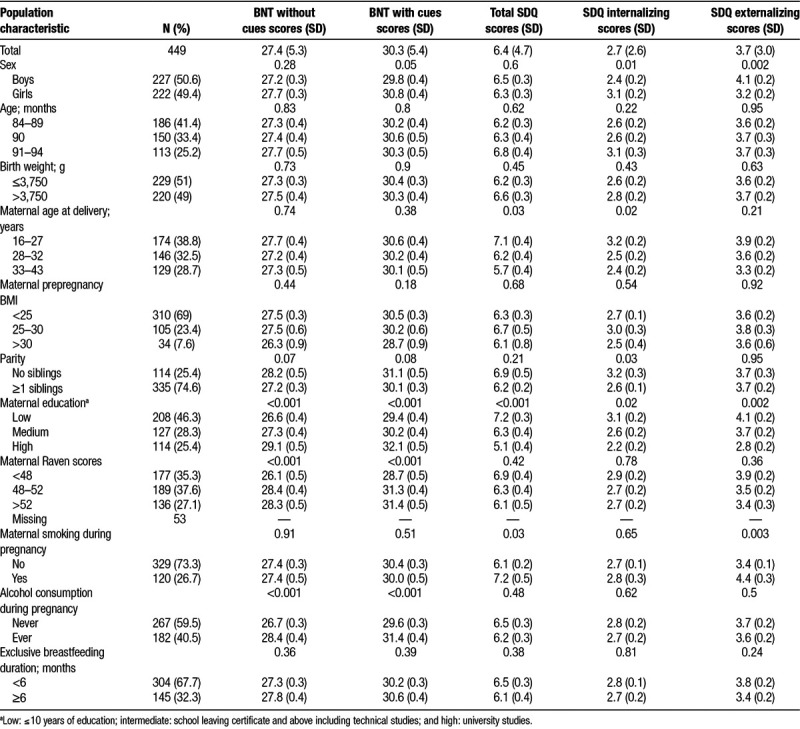
Table 2 shows distributions of maternal and 5-year environmental exposures. Among PFASs, PFOS showed the highest serum concentrations at all timepoints, followed by PFOA and PFHxS. Maternal PFOS and PFHxS concentrations were higher in comparison to 5-year child concentrations, whereas PFOA, PFNA, and PFDA concentrations were comparable between maternal and children at 5 years. Blood Hg concentrations were also higher in maternal blood compared with children’s blood, whereas ∑PCB concentrations were comparable.
Table 2.
Descriptive statistics of maternal and child’s 5-year pollutants concentrations measured in blood
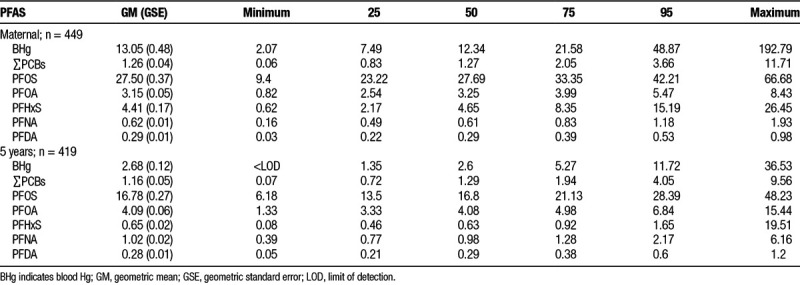
Figure 1 presents a heat map plot of within (at the same time point) and between (exposure between maternal and 5 years) exposures correlations. The highest within correlations (Spearman’s ρ) were observed between serum concentrations of PFDA and PFNA (ρ = 0.79 at both maternal and 5 years), whereas the lowest correlations were observed between serum PFOA and Hg concentrations at 5 years (ρ = –0.04). The highest between-correlation was observed between maternal and 5 years serum ∑PCB concentrations (ρ = 0.58), whereas the lowest correlation was observed between maternal and 5 years serum PFHxS concentrations (ρ = 0.09).
Figure 1.
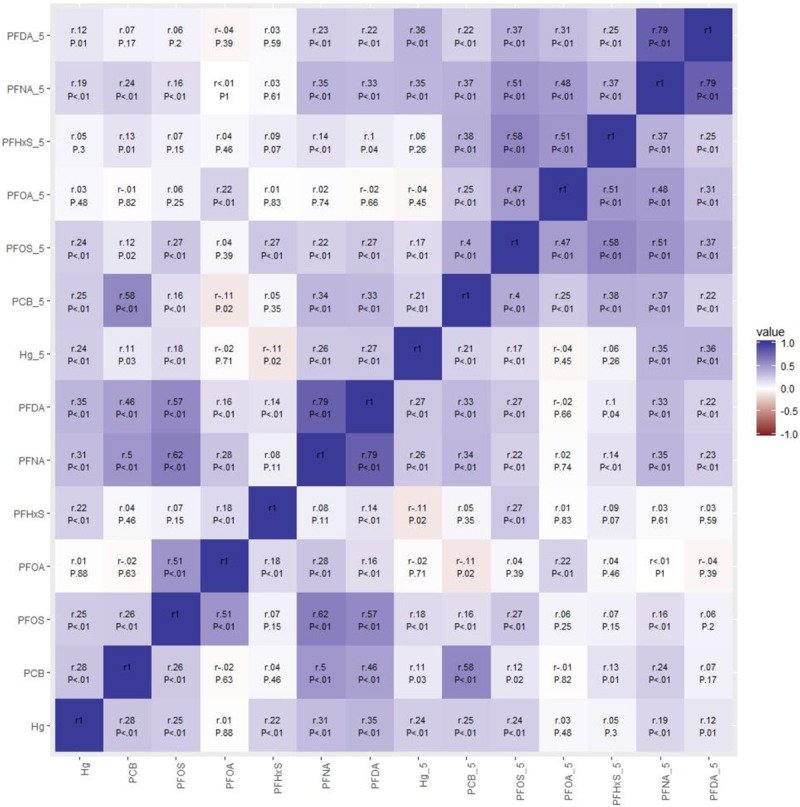
Correlation plot of prenatal and 5-year concentrations. Red indicates negative correlations, whereas blue indicates positive correlations. The intensity of the color indicates the strength of the correlation.
Univariate associations
Maternal Hg, PFOS, and 5 years PFHxS concentrations were negatively associated with BNT scores. Maternal PFOS concentrations were also associated with higher (poorer) total SDQ scores, whereas 5-year ∑PCB concentrations were associated with lower total SDQ scores (Supplemental Material: Figure S1; http://links.lww.com/EE/A53).
Insights from SuperLearner cross-validation
Figure 2 shows the distribution of the 10-fold cross-validated minimum squared error (MSE) for each included algorithm and for the SuperLearner. The figure also shows the distribution of the weighting coefficients of the convex combination applied to minimize the prediction error for each algorithm. The SuperLearner and the Elastic net algorithms yielded the lowest average MSE, although the differences were small with the other methods. However, Elastic net highly contributed to the convex combination that yielded the best predictions with a median weighting coefficient of 0.56. All the remaining algorithms included in the SuperLearner had a median weighting coefficient of 0.
Figure 2.
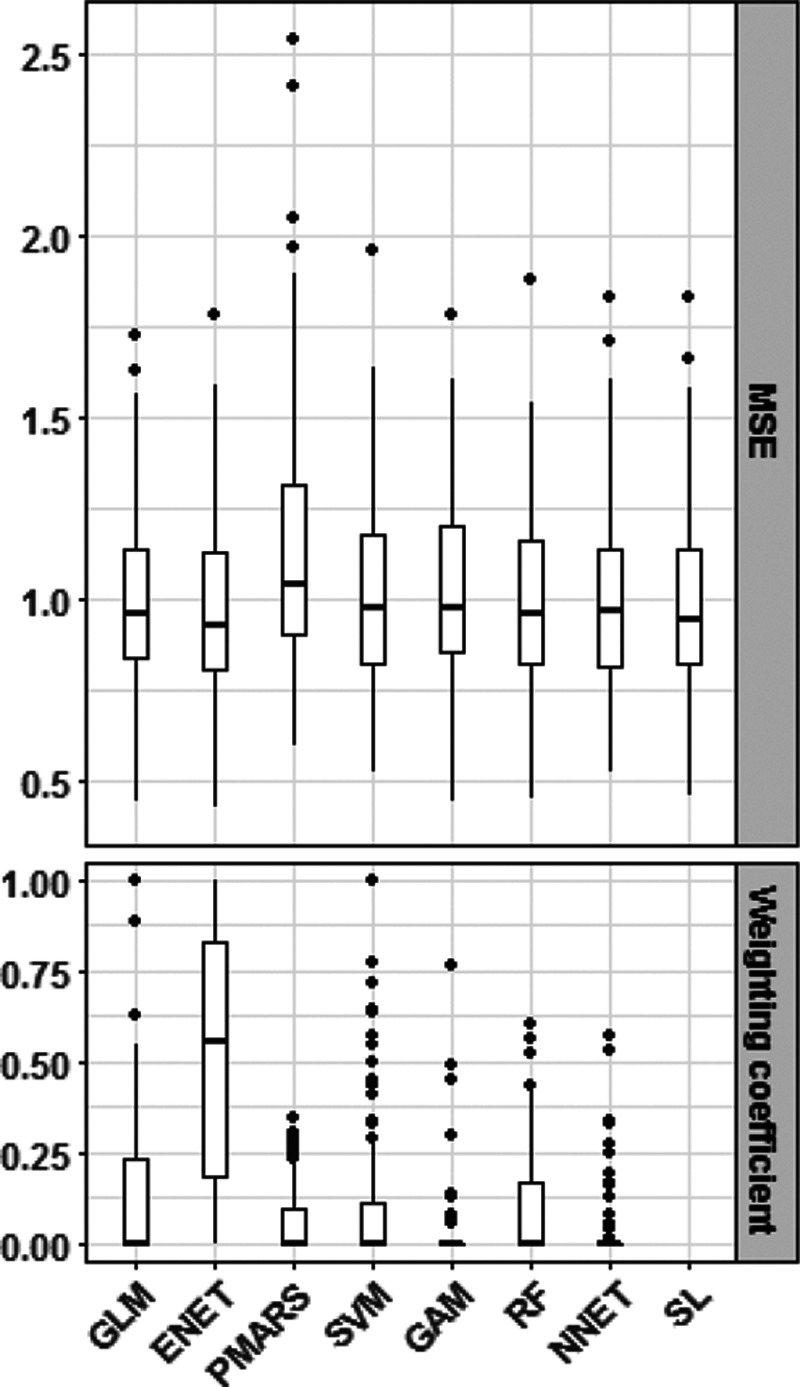
Distribution of the 10-fold cross-validated minimum squared error and weighting coefficients for each included algorithm and for the SuperLearner. ENET indicates elastic net regularization; NNET, artificial neural networks; PMARS, multivariate adaptive polynomial spline regression; RF, random forests; SVM, support vector machine and SL, Super learner.
The Elastic net algorithm outperformed all the other included algorithms, which points to two main insights: (1) the exposure-response relationships are likely linear and (2) the absence of potential interactive effects between exposures and between exposures and potential confounders.
Associations between chemical exposures and neuropsychological outcomes
Table 3 shows the estimates resulting from the G-computation method combined with SuperLearner predictions. An IQR increase in maternal Hg concentrations was associated with 0.08 SD (95% CI = –0.18, 0) and 0.15 SD (95% CI = –0.29, –0.03) lower scores in the BNT without and with cues, respectively. Maternal PFOA concentrations were also associated with lower BNT scores (β = 0.07 SD; 95% CI = –0.16, 0 and 0.14 SD; 95% CI = –0.26, –0.05 for BNT without and with cues, respectively). Finally, an IQR increase in maternal PFOS concentrations was associated with 0.11 SD (95% CI = –0.27, 0.01) lower BNT with cues scores (Table 3). A joint IQR increase in the mixture concentrations was associated with 0.15 SD (95% CI, –0.41, 0.13) and 0.48 SD (95% CI = –0.69, –0.25) lower scores in the BNT without and with cues. Regarding SDQ scores, an IQR increase in maternal PFOS and PFOA concentrations were associated with 0.15 SD (95% CI = 0.08, 0.23) and 0.11 SD (95% CI = 0.02, 0.26) higher total SDQ scores, whereas an IQR increase in maternal ∑PCBs concentrations was associated with 0.09 SD (95% CI = –0.19, 0) lower SDQ scores. A joint IQR increase in the mixture concentrations was associated with 0.19 SD (95% CI = –0.11, 0.42) higher total SDQ scores. No other pattern of associations was observed.
Table 3.
Associations between prenatal and 5 years exposures and neuropsychological test scores at 7 years using G-computation and SuperLearner predictions
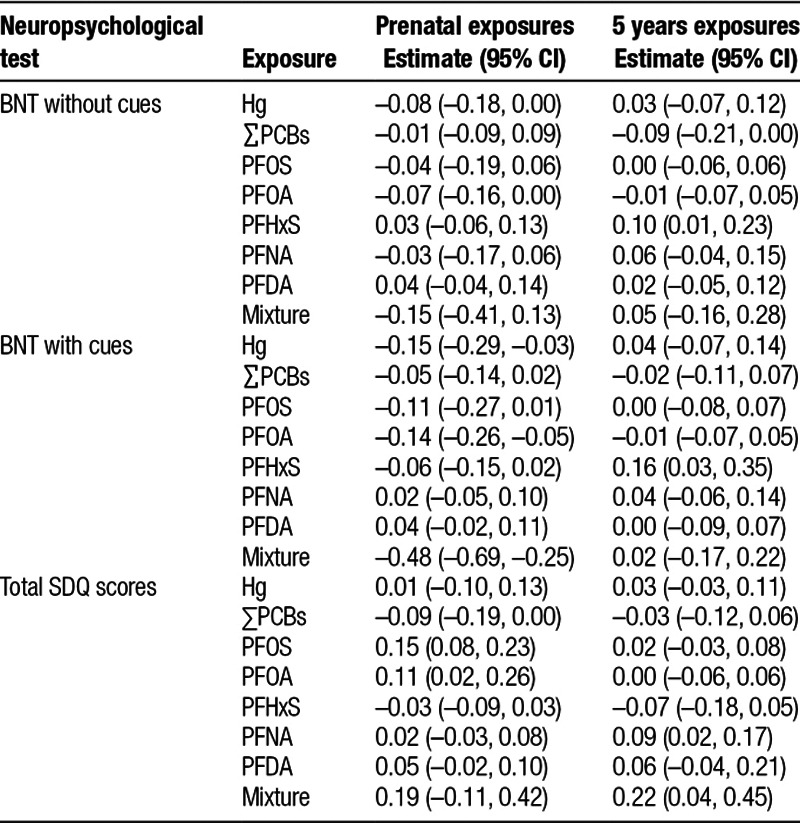
Regarding child 5-year exposures, we observed both positive and negative associations with BNT scores. An IQR increase in 5 years ∑PCB concentrations was associated with 0.09 SD (95% CI = –0.21, 0) lower BNT with cues scores, whereas an IQR increase in 5 years PFHxS concentrations was associated with 0.16 SD (95% CI = 0.03, 0.35) higher BNT with cues scores (Table 3). No association was observed for a joint increase in the chemical mixture. Regarding SDQ scores, an IQR increase in 5-year PFNA concentrations was associated with 0.09 SD (95% CI = 0.02, 0.17) higher total SDQ scores. Finally, a joint IQR increase in the mixture of chemicals was associated with 0.22 SD (95% CI = 0.04, 0.45) higher total SDQ scores.
Figure 3 shows the individual conditional expectations and exposure-response relationships for maternal exposure to individual chemicals and the mixture of chemicals. Most observed associations showed a linear pattern, however, some associations showed patterns of nonlinear relationships. The association between ∑PCBs and BNT with cues showed a slight increase after the 80th percentile, whereas the association for the joint effect of chemicals with BNT scores showed a steeper decrease after the 30th percentile for BNT with cues and after the 60th percentile for BNT without cues. Predictions for individuals showed mainly parallel patterns, which points to a lack of potential interactions with other exposures or covariates in the models. At age 5 years, exposure-response relationships exhibited a linear pattern (data not shown).
Figure 3.
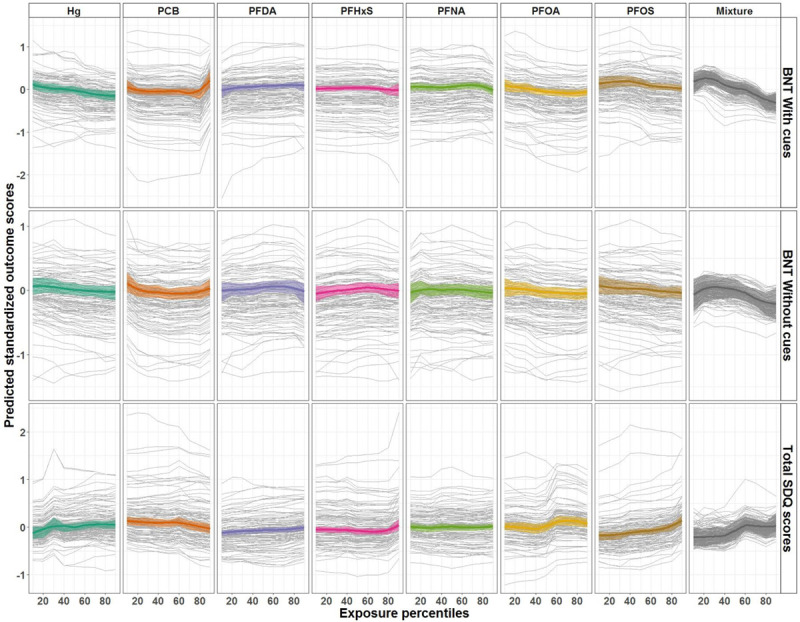
Exposure-response relationships and individual conditional expectations for prenatal exposures and neuropsychological test scores at 7 years using G-computation and SuperLearner predictions. The lines represent locally weighted scatterplot smoothing for mean predictions at different percentiles. For the sake of clarity, only 100 individuals were randomly sampled to represent the individual conditional expectations.
Discussion
In this study, we introduce a novel approach combining SuperLearner and G-computation to estimate the associations between both prenatal and child 5-year environmental exposures and child cognitive and behavioral functions at 7 years of age. By investigating all chemicals jointly with no a priori assumptions on the model specification, we were able to account for three main challenges that hamper investigations using single pollutant approaches: (1) multiple comparisons, (2) potential model misspecifications (i.e., nonlinear terms and interactions), and (3) confounding by omitted correlated exposures. Overall, our findings suggest that maternal Hg exposure may be a risk factor of poorer cognitive function, as assessed by the BNT scores. Additionally, PFOA and PFOS also showed negative associations with BNT scores independently of Hg. We also found indications of positive associations between maternal PFOS and PFOA and behavioral problems as assessed by the SDQ. Exposures at age 5 years did not appear to negatively impact child cognitive function at 7 years, but we rather report a positive association between 5 years PFHxS concentrations and BNT scores. Only 5 years PFNA concentrations appeared to be associated with higher SDQ behavioral difficulties scores. The cumulative impact of all the exposures appeared stronger and pointed to a cumulative effect on both cognitive and behavioral functions. We found no evidence supporting potential interactive effects between chemical exposures and no strong indications of nonlinear exposure-response relationships, except for the model for cumulative effects.
Generally, the results from this investigation corroborate our previous findings from individual chemical analyses.15,16,36 The chemicals associated with a decrease in cognitive function and those associated with an increase in behavioral problems by individual chemical analyses were also selected as potential risk factors by this novel approach. These include maternal Hg and 5-year PFNA exposures as potential developmental neurotoxicants. Unlike our previous investigation, which also pointed to a positive association between PFOA and PFDA concentrations and higher SDQ behavioral difficulties scores, this joint analysis did not provide evidence of such associations and pointed only to PFNA as a potential culprit, although PFDA also exhibited a pattern of detrimental impact. Instead, maternal PFOA and PFOS concentrations appeared to be associated with lower cognitive function. Although some results suggest a negative slope for ∑PCBs with SDQ behavioral difficulties scores, it appears unlikely that these chemicals may have any positive effects on behavioral function, and this finding may be driven by a correlation between ∑PCBs and unmeasured omega-3 polyunsaturated fatty acids that have been shown to exert a positive effect on cognitive function.37,38 Such correlation was not found for mercury and PFASs in a subset of children from this cohort (data not shown). The positive association between 5 years PFHxS concentrations and cognitive function warrants further investigation to elucidate whether this might be a false positive or indeed a true beneficial effect. Some previous investigations reported positive associations between some PFASs and neurodevelopment (Stein et al)39 and adult memory functions (Gallo et al).40 Although unlikely, these favorable associations were hypothesized to be mediated by the activation of the peroxisome proliferator-activated receptor (PPAR) γ receptor that has been shown to prevent the expression of inflammatory cytokines and other inflammatory mediators in brains of Alzheimer disease animal models.41 Also, some PPAR agonist drugs have been proposed as preventive drugs for neurodegenerative conditions, including Alzheimer dementia.42 However, the question of why would only PFHxS have these favorable effects while other PFASs showed negative associations with cognitive function remains to be settled. It is difficult to compare doses between the two contexts of an environmental exposure such as PFAS and a therapeutic drug. However, drugs impacting PPAR have shown decreased levels of amyloid-β peptide and the number of activated microglia and astrocytes only in the context of high doses as lower doses showed only modest effects on plaque burden or microglia activation. Thus, our interpretation of this potential positive effect remains speculative.42
The findings from the present study point to weak associations for independent exposures, with the strongest estimate being a change of 0.2 SD for an IQR increase. It is therefore worth mentioning that the observed effect sizes in this study are relatively modest, failing to reach the level of clinical significance. However, the estimates regarding the joint effect of the mixture of exposures point to moderate effects that can have a big influence on the prevalence of neurodevelopmental disorders at the population level since subtle effects of chemical exposures may shift the distribution of cognitive and behavioral traits to increase the risk of clinical neurodevelopmental disorders.12,43 The impact of a factor at the population level depends not only on the magnitude of its impact on health, or its effect size but also on the distribution of the factor. Given the widespread and ubiquitous exposure to PFAS, these small effect sizes may have a considerable impact at the population level.44
A strength of our statistical approach is that it brings together the strong and unparalleled predictive performance the SuperLearner and the G-computation method to open the black box of machine learning techniques. It therefore allows to investigate both the overall potential effect of a mixture and to provide marginal estimates for each exposure, and estimation of dose-response relationships. Under assumptions of conditional exchangeability, consistency, and positivity, these estimates may be interpreted causally. The literature on the use of ensemble learning methods for estimating causal effects is limited,45 especially in the field of environmental epidemiology. To our knowledge, this approach has not been explored in settings involving multi-pollutant exposures. A comparable approach is the Bayesian Kernel Machine Regression3,6 that uses a kernel regression to estimate the joint exposure-response function of a chemical mixture. One major difference between the two methods is that our approach does not require fixing the levels of other chemicals in the mixture to a specific value when estimating their individual contributions. This allows inferring marginal estimates, and to assess potential interactions visually when the exposure-response relationship varies across individuals.
Other approaches that have been suggested to address the mixtures issue include LASSO,46 EWAS,4,47 weighted quantile sum regression,48,49 and Elastic Net.50,51 A major disadvantage of such approaches is that they typically assume specific and often restrictive parametric functional forms for the exposure-response relationship, often resulting in a model that does not accurately capture the complexity of the relationships among high dimensional covariates and health outcomes. Some of these methods, such as penalized regressions result in highly biased estimates as they rely on a procedure that reduces the variance of estimators by introducing substantial bias.5
The present work has several limitations. First, the ability of the SuperLearner approach depends on the choice of candidate learners that should be guided by theoretical and practical considerations. We believe that we have included a diverse set of algorithms that can capture a variety of potential exposure-response relationships in addition to interactive effects, if present. Second, we used the bootstrap to estimate valid confidence intervals in the absence of a theoretical formula for the asymptotic distributions of the parameters of interest. This gave rise to a heavy computational burden, especially that the method is based on cross-validation. Further efforts to incorporate the method within a parallel computing framework will substantially reduce the running time. Third, our approach, at this point, does not handle exposure misclassification that can arise from measurement errors, one of the most important issues in environmental epidemiology studies. Finally, and although this was out of the scope of this investigation, our estimates are still based on the overall performance of the predictive model, and require a targeting step to infer doubly robust estimates, which will be incorporated in additional developments.
Regarding the neuropsychological instruments used in this study, we relied on parent-reported SDQ and the BNT for measurement of the outcomes rather than clinical diagnoses. Although there are no studies that validated these tests in the Faroese population, the two tests are part of a battery of tests that was designed to allow assessment of mercury and other potential neurotoxicants associations with deficits in a wide range of abilities and have been previously used in this population.13,15,16,36 The SDQ has excellent psychometric properties and was used as a screening and/or assessment tool by psychologists and clinicians.52–55 The SDQ has been used to assess children’s behavior across age and culture and is commonly used in longitudinal birth cohorts and national surveys.56–58 A recent study confirmed the usefulness of the SDQ as a screening tool for boys and girls across age groups and raters in the general Danish population.59
Conclusions
Our findings from this study point to the neurodevelopmental effect of mercury and corroborate previous results from our Faroese cohort studies using a mixtures approach. Additionally, some PFASs showed a detrimental impact on both cognitive and behavioral functions and deserve more attention in future investigations.
Conflicts of interest statement
P.G. served as a health expert for the State of Minnesota in a recent lawsuit against a PFAS-producing company. The other authors have no conflicts to report.
Supported by the National Science Foundation-National Institutes of Health’ Oceans and Human Health Program (OCE-1321612).
Supplementary Material
Footnotes
Published online 23 September 2019
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
Sponsorships or competing interests that may be relevant to content are disclosed at the end of the article.
Data access: Faroese birth cohorts data can be made available to interested researchers upon request. Requests can be directed to the PIs P.G. (pgrand@hsph.harvard.edu) or P.W. (pal@health.fo). We are not allowed to place data in a public repository due to legal and ethical restraints. Sharing of individual participant data was not included in the informed consent of the study, and there is potential risk of revealing participants’ identities.
References
- 1.Lanphear BP. The impact of toxins on the developing brain. Annu Rev Public Health 201536211–230 [DOI] [PubMed] [Google Scholar]
- 2.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol 201413330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valeri L, Mazumdar MM, Bobb JF, et al. The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20-40 months of age: evidence from rural Bangladesh. Environ Health Perspect 2017125067015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel CJ, Bhattacharya J, Butte AJ. An Environment-Wide Association Study (EWAS) on type 2 diabetes mellitus. PLoS One 20105e10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tibshirani R. Regression shrinkage and selection via the Lasso. J R Stat Soc B Methodol 199658267–288 [Google Scholar]
- 6.Bobb JF, Valeri L, Claus Henn B, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 201516493–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Laan MJ, Polley EC, Hubbard AE. Super learner. Stat Appl Genet Mol Biol 20076Article25. [DOI] [PubMed] [Google Scholar]
- 8.Robins J. A new approach to causal inference in mortality studies with a sustained exposure period—application to control of the healthy worker survivor effect. Math Model 198671393–1512 [Google Scholar]
- 9.Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health 200660578–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grandjean P, Andersen EW, Budtz-Jørgensen E, et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 2012307391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weihe P, Kato K, Calafat AM, et al. Serum concentrations of polyfluoroalkyl compounds in Faroese whale meat consumers. Environ Sci Technol 2008426291–6295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barr DB, Weihe P, Davis MD, Needham LL, Grandjean P. Serum polychlorinated biphenyl and organochlorine insecticide concentrations in a Faroese birth cohort. Chemosphere 2006621167–1182 [DOI] [PubMed] [Google Scholar]
- 13.Oulhote Y, Debes F, Vestergaard S, Weihe P, Grandjean P. Aerobic fitness and neurocognitive function scores in young faroese adults and potential modification by prenatal methylmercury exposure. Environ Health Perspect 2017125677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oulhote Y, Bouchard MF. Urinary metabolites of organophosphate and pyrethroid pesticides and behavioral problems in Canadian children. Environ Health Perspect 20131211378–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oulhote Y, Steuerwald U, Debes F, Weihe P, Grandjean P. Behavioral difficulties in 7-year old children in relation to developmental exposure to perfluorinated alkyl substances. Environ Int 201697237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grandjean P, Weihe P, White RF, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 199719417–428 [DOI] [PubMed] [Google Scholar]
- 17.Debes F, Weihe P, Grandjean P. Cognitive deficits at age 22 years associated with prenatal exposure to methylmercury. Cortex 201674358–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan E, Goodglass H, Weintraub S, Goodglass H. Boston Naming Test 1983Philadelphia, PA: Lea & Febiger [Google Scholar]
- 19.Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry 199738581–586 [DOI] [PubMed] [Google Scholar]
- 20.Grandjean P, Weihe P, Jørgensen PJ, Clarkson T, Cernichiari E, Viderø T. Impact of maternal seafood diet on fetal exposure to mercury, selenium, and lead. Arch Environ Health 199247185–195 [DOI] [PubMed] [Google Scholar]
- 21.Petersen MS, Halling J, Damkier P, et al. Caffeine N3-demethylation (CYP1A2) in a population with an increased exposure to polychlorinated biphenyls. Eur J Clin Pharmacol 2006621041–1048 [DOI] [PubMed] [Google Scholar]
- 22.Grandjean P, Weihe P, Needham LL, et al. Relation of a seafood diet to mercury, selenium, arsenic, and polychlorinated biphenyl and other organochlorine concentrations in human milk. Environ Res 19957129–38 [DOI] [PubMed] [Google Scholar]
- 23.Haug LS, Thomsen C, Becher G. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J Chromatogr A 20091216385–393 [DOI] [PubMed] [Google Scholar]
- 24.Dudoit S, van der Laan MJ. Asymptotics of cross-validated risk estimation in estimator selection and performance assessment. Stat Meth 20052131–154 [Google Scholar]
- 25.Polley EC, van der Laan MJ. Super learner in prediction. UC Berkeley Division of Biostatistics Working Paper Series 2010; Working Paper 266. 2010Available at: https://biostats.bepress.com/ucbbiostat/paper266/. Accessed August 3, 2018.
- 26.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Ser B Methodol 200567301–320 [Google Scholar]
- 27.Kooperberg C, Bose S, Stone CJ. Polychotomous regression. J Am Stat Assoc 199792117–127 [Google Scholar]
- 28.Cortes C, Vapnik V. Support-vector networks. Mach Learn 199520273–297 [Google Scholar]
- 29.Breiman L. Classification and Regression Trees 1984Monterey: Wadsworth International Group; Brooks/Cole Publishing [Google Scholar]
- 30.Ho TK. Random decision forests. Proceedings of the Third International Conference on Document Analysis and Recognition (Volume 1)14–16 August 1995IEEE Computer Society; 278–228 [Google Scholar]
- 31.Haykin S. Neural Networks: A Comprehensive Foundation 1994New Jersey: Prentice Hall PTR [Google Scholar]
- 32.Oulhote Y, Bind MA, Coull B, Patel CJ, Grandjean P. Combining ensemble learning techniques and G-computation to investigate chemical mixtures in environmental epidemiology studies. BioRxiv 2017 10.1101/147413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreif N, Grieve R, Díaz I, Harrison D. Evaluation of the effect of a continuous treatment: a machine learning approach with an application to treatment for traumatic brain injury. Health Econ 2015241213–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidson AC, Hinkley DV. Bootstrap Methods and their Application 1997New York, NY: Cambridge University Press [Google Scholar]
- 35.Goldstein A, Kapelner A, Bleich J, Pitkin E. Peeking inside the black box: visualizing statistical learning with plots of individual conditional expectation. J Comput Graph Stat 20152422 [Google Scholar]
- 36.Grandjean P, Weihe P, Debes F, Choi AL, Budtz-Jørgensen E. Neurotoxicity from prenatal and postnatal exposure to methylmercury. Neurotoxicol Teratol 20144339–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr 2007137855–859 [DOI] [PubMed] [Google Scholar]
- 38.Strain JJ, Davidson PW, Bonham MP, et al. Associations of maternal long-chain polyunsaturated fatty acids, methyl mercury, and infant development in the Seychelles Child Development Nutrition Study. Neurotoxicology 200829776–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein CR, Savitz DA, Bellinger DC. Perfluorooctanoate and neuropsychological outcomes in children. Epidemiology 201324590–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallo V, Leonardi G, Brayne C, Armstrong B, Fletcher T. Serum perfluoroalkyl acids concentrations and memory impairment in a large cross-sectional study. BMJ Open 20133e002414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Serum perfluoroalkyl acids concentrations and memory impairment in a large cross-sectional study. BMJ Open 201320558–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lleo A, Galea E, Sastre M. Molecular targets of non-steroidal anti-inflammatory drugs in neurodegenerative diseases. Cell Mol Life Sci 2007641403–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korrick SA, Bellinger DC. Invited commentary: persistent organic pollutants and childhood learning and behavioural disorders. J Epidemiol Community Health 200761564–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellinger DC. Interpretation of small effect sizes in occupational and environmental neurotoxicology: individual versus population risk. Neurotoxicology 200728245–251 [DOI] [PubMed] [Google Scholar]
- 45.Austin PC. Using ensemble-based methods for directly estimating causal effects: an investigation of tree-based G-computation. Multivariate Behav Res 201247115–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park SK, Tao Y, Meeker JD, Harlow SD, Mukherjee B. Environmental risk score as a new tool to examine multi-pollutants in epidemiologic research: an example from the NHANES study using serum lipid levels. PLoS One 20149e98632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel CJ, Ioannidis JP. Placing epidemiological results in the context of multiplicity and typical correlations of exposures. J Epidemiol Community Health 2014681096–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czarnota J, Gennings C, Colt JS, et al. Analysis of environmental chemical mixtures and non-hodgkin lymphoma risk in the NCI-SEER NHL Study. Environ Health Perspect 2015123965–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gennings C, Carrico C, Factor-Litvak P, Krigbaum N, Cirillo PM, Cohn BA. A cohort study evaluation of maternal PCB exposure related to time to pregnancy in daughters. Environ Health 20131266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Govarts E, Remy S, Bruckers L, et al. Combined effects of prenatal exposures to environmental chemicals on birth weight. Int J Environ Res Public Health 201613495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lenters V, Portengen L, Rignell-Hydbom A, et al. Prenatal phthalate, perfluoroalkyl acid, and organochlorine exposures and term birth weight in three birth cohorts: multi-pollutant models based on elastic net regression. Environ Health Perspect 2016124365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woerner W, Fleitlich-Bilyk B, Martinussen R, et al. The strengths and difficulties questionnaire overseas: evaluations and applications of the SDQ beyond Europe. Eur Child Adolesc Psychiatry 200413suppl 2II47–II54 [DOI] [PubMed] [Google Scholar]
- 53.Goodman R. The extended version of the strengths and difficulties questionnaire as a guide to child psychiatric caseness and consequent burden. J Child Psychol Psychiatry 199940791–799 [PubMed] [Google Scholar]
- 54.Borg AM, Kaukonen P, Salmelin R, Joukamaa M, Tamminen T. Reliability of the strengths and difficulties questionnaire among Finnish 4-9-year-old children. Nord J Psychiatry 201266403–413 [DOI] [PubMed] [Google Scholar]
- 55.Yao S, Zhang C, Zhu X, Jing X, McWhinnie CM, Abela JR. Measuring adolescent psychopathology: psychometric properties of the self-report strengths and difficulties questionnaire in a sample of Chinese adolescents. J Adolesc Health 20094555–62 [DOI] [PubMed] [Google Scholar]
- 56.Russell G, Rodgers LR, Ford T. The strengths and difficulties questionnaire as a predictor of parent-reported diagnosis of autism spectrum disorder and attention deficit hyperactivity disorder. PLoS One 20138e80247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Griffiths LJ, Dezateux C, Hill A. Is obesity associated with emotional and behavioural problems in children? Findings from the Millennium Cohort Study. Int J Pediatr Obes 20116e423–e432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chittleborough CR, Lawlor DA, Lynch JW. Young maternal age and poor child development: predictive validity from a birth cohort. Pediatrics 2011127e1436–e1444 [DOI] [PubMed] [Google Scholar]
- 59.Niclasen J, Teasdale TW, Andersen AM, Skovgaard AM, Elberling H, Obel C. Psychometric properties of the Danish strength and difficulties questionnaire: the SDQ assessed for more than 70,000 raters in four different cohorts. PLoS One 20127e32025. [DOI] [PMC free article] [PubMed] [Google Scholar]


