Purpose of review
Skeletal muscle wasting during critical illness is the result of disturbed metabolism. No proven effective interventions targeting skeletal muscle mass and function during critical illness currently exist. This review summarizes recent advances regarding the complexity of metabolic factors involved and the challenge of establishing the clinical effects of metabolic interventions targeting the muscle.
Recent findings
Although the catabolic state is limited to the acute phase of critical illness, its subsequent impact on muscle mass and function persists long after ICU discharge. Immobilization, inflammation and disturbed muscle energy and nutrient metabolism are key drivers of muscle protein loss. Current research focuses on the effects of enhanced protein provision, specific substrate delivery and physical exercise. Whilst some interventions have been successful at improving muscle mass, these effects do not always carry over into muscle function or strength.
Summary
Increased understanding of metabolic derangements during critical illness provides new potential targets for treatment. The potential of dietary protein to attenuate the muscle protein catabolic state has yet to be established in clinical trials. Basic research should focus on ways to further improve the anabolic potential of nutrition by unravelling mechanisms that regulate anabolic and catabolic pathways and energy metabolism.
Keywords: dietary protein, exercise, metabolism, muscle
INTRODUCTION
Long-term disability following ICU admission is one of the major challenges of modern intensive care medicine [1]. One of the key factors contributing to the long-lasting consequences of critical illness is muscle wasting. Over the past decade, the extent of skeletal muscle wasting and its subsequent impact on both short-term and long-term outcome of patients have become increasingly clear [2,3]. Uncovering the central role of muscle wasting in the development of persistent post-ICU complaints and poor quality of life has increased the priority of research into ICU-associated muscle wasting [4]. From a metabolic perspective, muscle wasting is the result of muscle protein breakdown exceeding protein synthesis. This negative net balance is the result of a myriad of ICU-related factors including inflammation, altered energy and substrate metabolism, immobilization and drug administration (i.e. corticosteroids, sedatives and muscle relaxants) [5]. Unravelling the complex metabolic interactions involved in muscle wasting will be key to design targeted, individualized interventions with a higher chance of success.
Aim of this review is to summarize the current insights into the metabolic aspects of ICU-related muscle wasting, the impact on muscle mass and function, potential targets for treatment and the challenges for future research and clinical care.
Box 1.
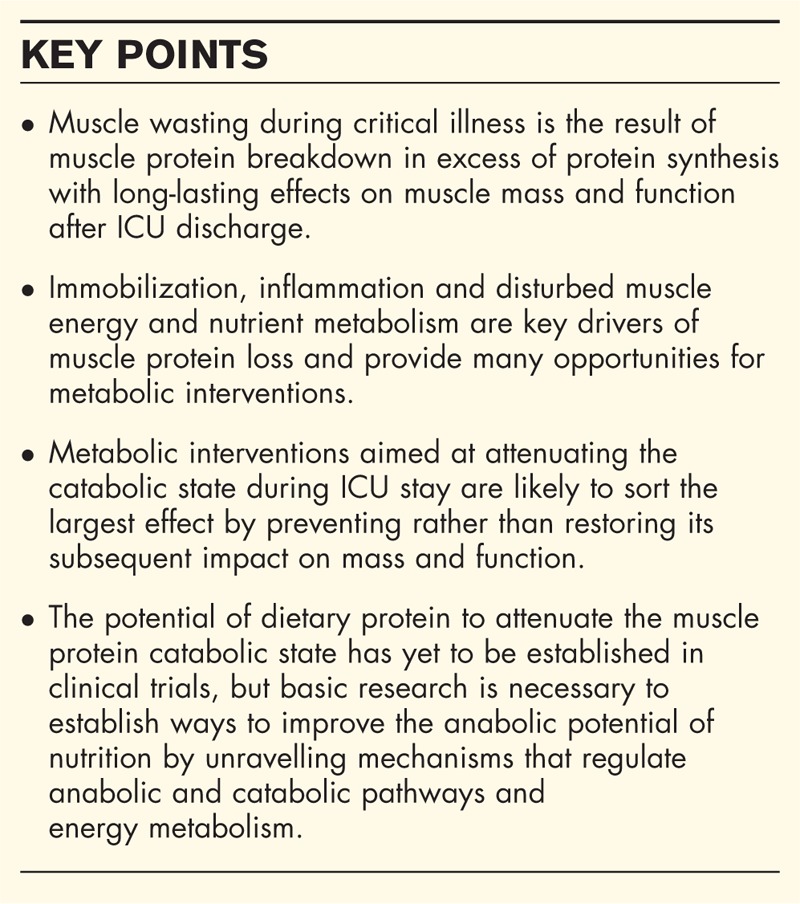
no caption available
PROTEIN METABOLISM IN CRITICAL ILLNESS: IMPACT ON MUSCLE MASS AND FUNCTION
The muscle is in a permanent state of turnover, as muscle protein is continuously synthesized and broken down under the influence of different anabolic and catabolic stimuli. Skeletal muscle wasting in critical illness is the consequence of a prolonged catabolic state, where muscle protein breakdown exceeds the rate of protein synthesis. During critical illness, the rate of muscle protein breakdown is sharply increased, which seems to be the main driver of the catabolic state rather than a decrease in protein synthesis [6]. This catabolic state results in a rapid depletion of the body's muscle-bound protein reserves, with patients losing as much as 20% of their muscle mass in the first 10 days of ICU admission depending on the severity of disease [2]. Recent work established that this net catabolic state is attenuated over time, with protein synthesis increasing and the net release of amino acids from the muscle reduced in patients still admitted to the ICU after 10 days [7▪▪].
The breakdown of muscle protein, therefore, seems to occur mainly in the early phase of critical illness, with muscle protein turnover slowly restored after this initial phase. The impact on muscle function and quality of life can persist for years, however [3]. In recent years, an increasing number of studies have investigated muscle mass and weakness in the post-ICU period. In a recent follow-up cohort of the EPaNIC trial, muscle weakness still persisted in patients at 5 years post-ICU discharge [8▪]. In a propensity score-matched cohort, short-stay patients (length of stay <8 days) had better physical function than long-stay patients, further suggesting that the length of the catabolic insult, as well patient-specific factors, such as age, are important determinants of long-term physical function and recovery [9].
The question that arises from this observation of persistent weakness, is whether the muscle is able to recover after the initial ‘metabolic hit’ during the ICU admission. The available data suggests that recovery of muscle mass is highly variable and often incomplete as muscle atrophy persists in the majority of patients even at 6 months following ICU discharge [10]. Only a third of patients reach a muscle mass within the 95% confidence interval of expected normal values, but even they experience persistent muscle weakness [10]. Therefore, regaining muscle mass does not necessarily equals regaining muscle strength. This can be attributed to impaired neuromuscular signaling, ineffective intracellular energy metabolism or to structural alterations in the muscle like muscle fibrosis or muscle necrosis frequently identified on muscle ultrasound [11]. Further increase in lean body and muscle mass after 6 months appears limited [12].
In summary, muscle mass and function during and following critical illness is affected in three phases (Fig. 1): a catabolic phase where muscle protein catabolism subsequently drives loss of muscle mass and function, a recovery phase where protein balance is restored followed by some recovery in mass and to a lesser extent function and and enduring state where recovery of muscle mass and function stagnate and muscle function is persistently lower than prior to ICU admission.
FIGURE 1.
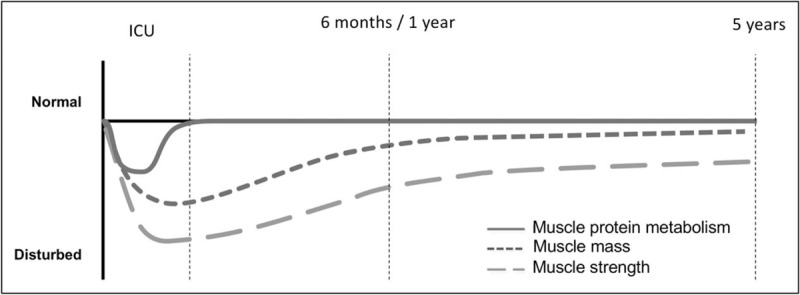
Protein metabolism in critical illness and impact on muscle mass and function. Changes over time in muscle protein metabolism, mass and function, distinguishes a short catabolic phase during ICU stay with subsequent persistent impact on muscle mass and function. Put together, this timeline further emphasizes the long-lasting impact of the relative short period of disturbed protein metabolism.
METABOLIC DRIVERS OF MUSCLE ATROPHY
Based on the above, it becomes clear that metabolic derangements during the first 1--2 weeks of ICU admission are able to induce muscle injury that can take years to recover from. Therefore, interventions able to successfully attenuate the muscle catabolic state during ICU stay will likely sort the largest effect by preventing the subsequent cascade of muscle mass and functional loss. Understanding the metabolic drivers of the muscle catabolic state during critical illness are, therefore, essential to identify potential targets for treatment.
Nutrient and substrate availability
Availability of nutrients, in particular amino acids, is an important prerequisite for muscle maintenance as they serve as the precursors required to build muscle protein. In addition, nutrients serve as regulators of muscle protein synthesis on the cellular level, as intracellular abundance of amino acids activates the mammalian target of rapamycin (mTOR), which is the main pathway driving protein synthesis [13]. During health, the intake of dietary protein is a potent stimulator of muscle protein synthesis by increasing amino acid delivery to the muscle, with approximately 11% of diet-derived protein being directly incorporated into muscle myofibrillar protein [14]. During critical illness, however, it seems that endogenous nutrients are diverted away from the muscle as muscle-bound amino acids are released into the circulation, which has been hypothesized to be an adaptive response in an effort to meet the increased metabolic demands of the body [15]. However, the actual metabolic fate of muscle-derived amino acids during critical illness is still unclear. Whether exogenous nutrients in form of dietary protein are also diverted away from the muscle during critical illness or if they are able to reach the muscle and stimulate muscle protein synthesis and inhibit proteolysis during critical illness remains unknown and is a major unresolved question with potential for intervention [16].
Mitochondrial function and bioenergetic failure
In addition to nutrient availability as precursor for muscle protein, muscle protein synthesis and turnover is an energy-dependent process. Contrasting the anabolic mTOR pathway, autophagy and the Ubiquitin Proteasome System (UPS) are the two main pathways of the cell for protein breakdown [17]. They are activated when intracellular AMP levels rise as a result of decreased nutrient and energy levels, resulting in protein degradation in order to make substrates available for ATP production and cell survival [13]. Both mitochondrial and ATP content of muscle cells are known to be reduced in the early phase of critical illness, indicating that normal energy production is compromised [18]. A recent network analysis investigating the metabolic phenotype in muscle biopsy samples collected from patients in the early phase of ICU admission indeed showed that decreased muscle ATP, creatinine and phosphocreatine content was closely related to muscle protein loss, a phenomenon referred to as bioenergetic failure of the muscle [19▪▪]. Accordingly, during this catabolic phase, the muscle may lack the energy required for maintenance and turnover and targeting mitochondrial function might aid in protecting the muscle from energy stress [20].
Modulators of muscle protein signalling
Finally, the balance of muscle protein synthesis and breakdown is coordinated by the input of various anabolic and catabolic signals. Inflammation is not only a hallmark of critical illness but also a powerful driver of muscle catabolism and atrophy via cytokine mediated activation of the ubiquitin proteasome system (UPS) required for proteolysis [21]. Intramuscular inflammation, evidenced by inflammatory infiltrates of leukocytes in muscle biopsies of ICU patients, further exacerbates the catabolic impact of inflammation [10]. Intramuscular inflammation is often concurrent with hypoxia and both are closely related to decreased protein synthesis and anabolic resistance witnessed in the early phase of critical illness [19▪▪].
Exercise is an important anabolic stimulus and requirement for muscle protein gain under normal conditions, in part via direct activation of the mTOR pathway [22]. Immobilization, which occurs in virtually all ICU patients, is therefore, a catabolic stimulus able to induce severe muscle atrophy even in healthy volunteers [23]. Muscle relaxants and use of intravenous sedation further exacerbate the immobilization component and is a strong predictor of post-ICU functional outcome [24].
Finally, anabolic and catabolic signaling also occurs on the whole-body level via the endocrine system. For the muscle, hormones evolved around feeding and fasting are especially relevant as these coordinate the flux of nutrients either towards or away from the muscle. The anabolic and catabolic effects of insulin and glucagon, respectively, are well established, and critical illness is associated with elevated levels of glucagon, overpowering the effects of insulin and inducing protein catabolism [25]. Whilst insulin and glucagon react early to feeding and fasting, recent discoveries have unearthed their late-acting counterparts in the form of FGF19 and FGF21, respectively. FGF19 is a bile acid-induced hormone released by the gut following a meal, and is an insulin-independent modulator of macronutrient metabolism and regulator of muscle mass [26,27]. In ICU patients, the postprandial FGF19 response is absent, indicating impaired postprandial anabolic signaling with potential for intervention [28]. Its counterpart FGF21 is a regulator of muscle mass in conditions of nutrient stress and starvation, and plasma FGF21 levels are elevated during critical illness similarly to glucagon [29,30]. Whilst these novel modulators of protein and muscle anabolism and catabolism are gaining increased attention and provide novel opportunities for intervention, their exact role and relevance during critical illness requires further investigation.
TOWARDS EFFECTIVE METABOLIC CARE FOR THE MUSCLE
It is clear that a complex network of various metabolic factors contributes to muscle atrophy during critical illness, providing many avenues for potential therapeutic interventions. However, establishing the clinical effectiveness of all these potential therapeutic interventions also brings along a major challenge for researchers, and the true clinical effectiveness of interventions targeting the muscle is still uncertain [4].
Nutrition and exercise: effective or not?
Whilst enhancing nutritional support improves outcome in hospitalized patients [31], numerous large RCTs did not show an effect of increasing energy delivery in critically ill patients on clinical outcome [32]. It is important to realize that all high-impact nutrition studies in critical care have been aimed at ‘hard clinical endpoints’, such as mortality or length of ICU stay that are presumably affected by much more factors than nutrition alone. Unfortunately, studies evaluating the effects of nutritional interventions on muscle mass or function are rare [33▪]. Only two studies prospectively investigated the impact of nutrition on muscle-related function. In the EAT-ICU trial, increased nutrition intake based on indirect calorimetry was not able to significantly improve muscle function measured by the physical component score (PCS), a sub-score of the SF-36 questionnaire [34]. However, Ferrie et al.[35] were able to show improvements of both handgrip strength and muscle mass measured by ultrasound on day 7 of admission by increasing parenteral amino acid supply. Currently, enteral feeds are becoming available on the market with a high protein content that may facilitate the achievement of higher protein targets than hitherto common [36]. The question remains whether increased dietary protein provision during ICU stay can improve muscle function and adequately powered, randomized controlled trials with muscle-related outcomes highly warranted [37].
Several studies have investigated the effect of mobilization of patients in the early phase of critical illness in order to attenuate muscle disuse atrophy with promising effects on maintenance of muscle fiber cross sectional area [38]. However, even when muscle-activating strategies result in the preservation of myofiber size, no significant effect on measures of muscle strength were detected [39▪]. Two larger randomized controlled trials looking at either intensive physical therapy or in-bed cycling combined with neuromuscular electrical stimulation (NMES) also found no effect on muscle strength or function [40,41].
These experiences in both nutrition and exercise interventions show a common challenge in metabolic research, as effects of interventions on muscle mass do not necessarily carry over into effects on muscle function of clinical outcomes. When moving farther away from the initial target of a metabolic intervention (i.e. from muscle metabolism to mass and subsequently from muscle mass to function), more factors start to play a role and the original signal can be lost in the increasing noise [42▪]. The challenge for future metabolic interventions is, therefore, to either increase the signal (i.e. anabolic potential) or aim to cancel out the noise (i.e. metabolic heterogeneity).
Optimizing the anabolic potential: 1 + 1 = 3?
Plasma amino acid levels have to exceed a certain ‘anabolic threshold’ to exert an anabolic effect, which can be affected both positively and negatively. Inflammation and hypoxia are known to supress the anabolic signals in the muscle [19▪▪], whereas exercise is able to increase the anabolic potential in health [43]. Systemic availability of enteral protein-derived amino acids is impaired in critically ill patients, making it more difficult to reach the ‘anabolic threshold’ [44]. Efforts should be put in increasing the impaired systemic availability of dietary amino acids and in improving anabolic potential of the muscle. Increasing protein dose, providing hydrolysed protein that is more readily absorbed than whole protein or bolus rather than continuous feeding could all improve systemic availability of amino acids [45]. Certain amino acids or substrates, such as leucine, its metabolite HMB (beta-hydroxy-beta-methylbutyrate) or ketone bodies such as 3OHB (3-hydroxybutyrate) have direct anabolic signalling effects on the muscle and could further increase the effect of dietary protein and exercise on the muscle [46–48]. Using smaller metabolic studies could help identify the most promising interventions creating the strongest ‘signal’ on muscle metabolism or mass, before a combination of these is validated in a larger clinical study.
Addressing metabolic heterogeneity
Critical care research is heavily affected by patient heterogeneity as large inter-individual differences in admission diagnosis and disease severity exist. New tools using machine learning and clustering analysis by integrating clinical and biological data aim to address this heterogeneity by identifying relevant subphenotypes that are not distinguished by traditional classification scores [49]. The aim is to filter out the excess noise of heterogeneity in larger trials by identifying subphenotypes of patients that will either benefit, not responded or be harmed by an intervention [50]. Advances in proteomics and metabolomics analysis could provide the metabolic field with new tools to address metabolic heterogeneity in critical care trials, by integrating clinical data, muscle-related outcomes and data derived from biomaterials. Puthucheary et al.[19▪▪] recently applied this principle on a smaller scale in a network analysis, but larger trials with muscle-related and clinical outcomes should consider this in their design to further increase our understanding of metabolic interventions [51].
CONCLUSION
Muscle wasting during critical illness is the consequence of muscle protein breakdown exceeding protein synthesis, with persistent impact on muscle mass and function after ICU discharge. Increased understanding of the drivers of muscle protein catabolism is essential in designing effective interventions protecting the muscle. A combination of larger clinical studies with relevant muscle-related outcomes as well as smaller basic studies focussing on improving the anabolic potential are needed to select and establish effective metabolic care for the muscle.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
The authors have received an unrestricted research grant from Fresenius-kabi and received competitive research awards funded by Nestlé and Nutricia.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Iwashyna TJ. Survivorship will be the defining challenge of critical care in the 21st century. Ann Intern Med 2010; 153:204–205. [DOI] [PubMed] [Google Scholar]
- 2.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013; 310:1591–1600. [DOI] [PubMed] [Google Scholar]
- 3.Herridge MS, Tansey CM, Matté A, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. New Engl J Med 2011; 364:1293–1304. [DOI] [PubMed] [Google Scholar]
- 4.Latronico N, Herridge M, Hopkins RO, et al. The ICM research agenda on intensive care unit-acquired weakness. Intensive Care Med 2017; 43:1270–1281. [DOI] [PubMed] [Google Scholar]
- 5.Batt J, Herridge M, Dos Santos C. Mechanism of ICU-acquired weakness: skeletal muscle loss in critical illness. Intensive Care Med 2017; 43:1844–1846. [DOI] [PubMed] [Google Scholar]
- 6.Klaude M, Mori M, Tjäder I, et al. Protein metabolism and gene expression in skeletal muscle of critically ill patients with sepsis. Clin Sci (Lond) 2012; 122:133–142. [DOI] [PubMed] [Google Scholar]
- 7▪▪.Gamrin-Gripenberg L, Sundstrom-Rehal M, Olsson D, et al. An attenuated rate of leg muscle protein depletion and leg free amino acid efflux over time is seen in ICU long-stayers. Crit Care 2018; 22:13. [DOI] [PMC free article] [PubMed] [Google Scholar]; First study performing repeated measure of leg muscle protein turnover in ICU patients using stable isotope methodology. This study established that muscle protein catabolism occurs mainly in the early phase of critical illness and is amended over time.
- 8▪.Hermans G, Van Aerde N, Meersseman P, et al. Five-year mortality and morbidity impact of prolonged versus brief ICU stay: a propensity score matched cohort study. Thorax 2019; 74:1037–1045. [DOI] [PubMed] [Google Scholar]; Long-term follow-up of the EPANIC trial, confirming the presence of persistent functional disability after 5 years and the importance of ICU-length of stay as a determinant in functional disability.
- 9.Herridge MS, Chu LM, Matte A, et al. The RECOVER program: disability risk groups and 1-year outcome after 7 or more days of mechanical ventilation. Am J Respir Crit Care Med 2016; 194:831–844. [DOI] [PubMed] [Google Scholar]
- 10.Dos Santos C, Hussain SN, Mathur S, et al. Mechanisms of chronic muscle wasting and dysfunction after an intensive care unit stay. A pilot study. Am J Respir Crit Care Med 2016; 194:821–830. [DOI] [PubMed] [Google Scholar]
- 11.Parry SM, El-Ansary D, Cartwright MS, et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care 2015; 30:1151.e9–1151.e14. [DOI] [PubMed] [Google Scholar]
- 12.Chan KS, Mourtzakis M, Aronson Friedman L, et al. National Institutes of Health National Heart, Lung, and Blood Institute (NHLBI) Acute Respiratory Distress Syndrome (ARDS) Network. Evaluating muscle mass in survivors of acute respiratory distress syndrome: a 1-year multicenter longitudinal study. Crit Care Med 2018; 46:1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palm W, Thompson CB. Nutrient acquisition strategies of mammalian cells. Nature 2017; 546:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groen BBL, Horstman AM, Hamer HM, et al. Post-prandial protein handling: you are what you just ate. PLos One 2015; 10:e0141582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preiser JC, Ichai C, Orban JC. Metabolic response to the stress of critical illness. Br J Anaesth 2014; 113:945–954. [DOI] [PubMed] [Google Scholar]
- 16.Sundstrom-Rehal M, Tardif N, Rooyackers O. Can exercise and nutrition stimulate muscle protein gain in the ICU patient? Curr Opin Clin Nutr Metab Care 2019; 22:146–151. [DOI] [PubMed] [Google Scholar]
- 17.Zhao J, Zhai B, Gygi SP, et al. mTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proc Natl Acad Sci U S A 2015; 112:15790–15797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredriksson K, Hammarqvist F, Strigard K, et al. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol Endocrinol Metab 2006; 291:E1044–E1050. [DOI] [PubMed] [Google Scholar]
- 19▪▪.Puthucheary ZA, Astin R, McPhail MJW, et al. Metabolic phenotype of skeletal muscle in early critical illness. Thorax 2018; 73:926–935. [DOI] [PubMed] [Google Scholar]; Excellent study intergrating clinical and metabolic data from plasma and muscle biopsies in a network analysis in an effort to identify the metabolic factors that have the strongest relationship to muscle atrophy.
- 20.Wesselink E, Koekkoek WAC, Grefte S, et al. Feeding mitochondria: potential role of nutritional components to improve critical illness convalescence. Clin Nutr 2019; 38:982–995. [DOI] [PubMed] [Google Scholar]
- 21.Schiaffino S, Dyar KA, Ciciliot S, et al. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 2013; 280:4294–4314. [DOI] [PubMed] [Google Scholar]
- 22.Ogasawara R, Jensen TE, Goodman CA, et al. Resistance exercise-induced hypertrophy: a potential role for rapamycin-insensitive mTOR. Exerc Sport Sci Rev 2019; 47:188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wall BT, Dirks ML, Snijders T, et al. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol (Oxf) 2014; 210:600–611. [DOI] [PubMed] [Google Scholar]
- 24.Gandotra S, Lovato J, Case D, et al. Physical function trajectories in survivors of acute respiratory failure. Ann Am Thorac Soc 2019; 16:471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thiessen SE, Derde S, Derese I, et al. Role of glucagon in catabolism and muscle wasting of critical illness and modulation by nutrition. Am J Respir Crit Care Med 2017; 196:1131–1143. [DOI] [PubMed] [Google Scholar]
- 26.Kir S, Beddow SA, Samuel VT, et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science (New York, N Y ) 2011; 331:1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benoit B, Meugnier E, Castelli M, et al. Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat Med 2017; 23:990–996. [DOI] [PubMed] [Google Scholar]
- 28.Koelfat KVK, Plummer MP, Schaap FG, et al. Gallbladder dyskinesia is associated with an impaired postprandial fibroblast growth factor 19 response in critically ill patients. Hepatology 2019; 70:308–318. [DOI] [PubMed] [Google Scholar]
- 29.Thiessen SE, Vanhorebeek I, Derese I, et al. FGF21 response to critical illness: effect of blood glucose control and relation with cellular stress and survival. J Clin Endocrinol Metab 2015; 100:E1319–E1327. [DOI] [PubMed] [Google Scholar]
- 30.Oost LJ, Kustermann M, Armani A, et al. Fibroblast growth factor 21 controls mitophagy and muscle mass. J Cachexia Sarcopenia Muscle 2019; 10:630–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuetz P, Fehr R, Baechli V, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet 2019; 393:2312–2321. [DOI] [PubMed] [Google Scholar]
- 32.Peake SL, Chapman MJ. TARGET Investigators. Energy-dense versus routine enteral nutrition in the critically ill. N Engl J Med 2019; 380:499–500. [DOI] [PubMed] [Google Scholar]
- 33▪.Taverny G, Lescot T, Pardo E, et al. Outcomes used in randomised controlled trials of nutrition in the critically ill: a systematic review. Crit Care 2019; 23:12. [DOI] [PMC free article] [PubMed] [Google Scholar]; Structural overview of endpoints used in nutritional trial, clearly indicating the lack, and therefore, need of more muscle-related outcome measures in clinical trials.
- 34.Allingstrup MJ, Kondrup J, Wiis J, et al. Early goal-directed nutrition versus standard of care in adult intensive care patients: the single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med 2017; 43:1637–1647. [DOI] [PubMed] [Google Scholar]
- 35.Ferrie S, Allman-Farinelli M, Daley M, Smith K. Protein requirements in the critically ill: a randomized controlled trial using parenteral nutrition. JPEN J Parenter Enteral Nutr 2016; 40:795–805. [DOI] [PubMed] [Google Scholar]
- 36.van Zanten ARH, Petit L, De Waele J, et al. Very high intact-protein formula successfully provides protein intake according to nutritional recommendations in overweight critically ill patients: a double-blind randomized trial. Crit Care 2018; 22:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arabi YM, Casaer MP, Chapman M, et al. The intensive care medicine research agenda in nutrition and metabolism. Intensive Care Med 2017; 43:1239–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hickmann CE, Castanares-Zapatero D, Deldicque L, et al. Impact of very early physical therapy during septic shock on skeletal muscle: a randomized controlled trial. Crit Care Med 2018; 46:1436–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39▪.Wollersheim T, Grunow JJ, Carbon NM, et al. Muscle wasting and function after muscle activation and early protocol-based physiotherapy: an explorative trial. J Cachexia Sarcopenia Muscle 2019; 10:734–747. [DOI] [PMC free article] [PubMed] [Google Scholar]; Clinical trial including both functional outcomes and musclebiopsies, showing the disconnect between effects on muscle fiber size and muscle strength.
- 40.Wright SE, Thomas K, Watson G, et al. Intensive versus standard physical rehabilitation therapy in the critically ill (EPICC): a multicentre, parallel-group, randomised controlled trial. Thorax 2018; 73:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fossat G, Baudin F, Courtes L, et al. Effect of in-bed leg cycling and electrical stimulation of the quadriceps on global muscle strength in critically ill adults: a randomized clinical trial. JAMA 2018; 320:368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42▪.Bear DE, Griffith D, Puthucheary ZA. Emerging outcome measures for nutrition trials in the critically ill. Curr Opin Clin Nutr Metab Care 2018; 21:417–422. [DOI] [PubMed] [Google Scholar]; Great overview detailing the potential and different possibilities of muscle-related outcome measures that are increasingly incorporated in critical care research.
- 43.Trommelen J, Betz MW, van Loon LJC. The muscle protein synthetic response to meal ingestion following resistance-type exercise. Sports Med 2019; 49:185–197. [DOI] [PubMed] [Google Scholar]
- 44.Liebau F, Wernerman J, van Loon LJ, Rooyackers O. Effect of initiating enteral protein feeding on whole-body protein turnover in critically ill patients. Am J Clin Nutr 2015; 101:549–557. [DOI] [PubMed] [Google Scholar]
- 45.Bear DE, Hart N, Puthucheary Z. Continuous or intermittent feeding: pros and cons. Curr Opin Crit Care 2018; 24:256–261. [DOI] [PubMed] [Google Scholar]
- 46.Holwerda AM, Paulussen KJM, Overkamp M, et al. Leucine co-ingestion augments the muscle protein synthetic response to the ingestion of 15 g protein following resistance exercise in older men. Am J Physiol Endocrinol Metab 2019; 317:E473–E482. [DOI] [PubMed] [Google Scholar]
- 47.Wilkinson DJ, Hossain T, Limb MC, et al. Impact of the calcium form of beta-hydroxy-beta-methylbutyrate upon human skeletal muscle protein metabolism. Clin Nutr 2018; 37 (6 Pt A):2068–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomsen HH, Rittig N, Johannsen M, et al. Effects of 3-hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS-induced inflammation in humans: anticatabolic impact of ketone bodies. Am J Clin Nutr 2018; 108:857–867. [DOI] [PubMed] [Google Scholar]
- 49.Castela Forte J, Perner A, van der Horst ICC. The use of clustering algorithms in critical care research to unravel patient heterogeneity. Intensive Care Med 2019; 45:1025–1028. [DOI] [PubMed] [Google Scholar]
- 50.Seymour CW, Kennedy JN, Wang S, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA 2019; 321:2003–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wernerman J, Christopher KB, Annane D, et al. Metabolic support in the critically ill: a consensus of 19. Crit Care 2019; 23:318. [DOI] [PMC free article] [PubMed] [Google Scholar]


