Purpose of review
Hyperkalemia is increasingly prevalent in the heart failure population as more people live with heart failure and comorbid conditions such as diabetes and chronic kidney disease. Furthermore, renin–angiotensin–aldosterone (RAAS) inhibitors are a key component of clinical therapy in these populations. Until now, we have not had any reliable or tolerable therapies for treatment of hyperkalemia resulting in inability to implement or achieve target doses of RAAS inhibition. This review will focus on two new therapies for hyperkalemia: patiromer and sodium zirconium cyclosilicate (SZC).
Recent findings
Patiromer and SZC have been studied in heart failure and both agents have demonstrated the ability to maintain normokalemia for extended periods of time with improved side effect profiles than existing potassium binders such as sodium polystyrene sulfate, though no direct comparisons have occurred. SZC has also shown promise in the treatment of acute hyperkalemia with its quick onset of action.
Summary
Patiromer and SZC will be useful adjuncts in the clinical care of heart failure patients with hyperkalemia. These agents will allow clinicians to maintain patients on RAAS inhibitors and uptitrate their guideline directed medical therapy to target doses without the additional concern for recurrent hyperkalemia and its untoward effects.
Keywords: chronic kidney disease, heart failure, hyperkalemia, patiromer, sodium zirconium cyclosilicate
INTRODUCTION
Hyperkalemia is a not infrequent clinical issue seen in the general population. The true incidence is unknown, but is estimated to be in the 1–10% range [1]. One reason for this is that there are varying definitions of hyperkalemia, with prior studies using a potassium cut-off of more than 5.5 mEq/l or as high as 6 mEq/l to define hyperkalemia. In general, hyperkalemia should be defined as a serum potassium level of more than 5 mEq/l (or mmol/l) and can be further subclassified as: mild (K+ 5–5.5 mEq/l), moderate (K+ 5.5–6 mEq/l) or severe (K+ ≥ 6 mEq/l) [2].
Hyperkalemia can be life-threatening. In a retrospective cohort study involving nearly 39 000 patients post myocardial infarction, potassium levels were found to have a nonlinear relationship where potassium levels less than 3.5 and more than 4.5 mEq/l were associated with higher rates of mortality [3,4]. Similar findings were seen in patients with chronic kidney disease (CKD) and diabetes [5,6].
Hyperkalemia decreases the concentration gradient across membranes which shortens the duration of the action potential. This is manifest on an ECG by prolongation of the PR segment, QRS complex and peaking of T waves. With worsening hyperkalemia, the ECG can adopt a sine-wave appearance, progressing to severe bradycardia leading to asystole. It can also interfere with the normal functioning of implantable cardiac devices possibly leading to higher pacing thresholds, inappropriate shocks due to T-wave oversensing or lack of morphology match (from a widened QRS) [7]. Noncardiac manifestations include paresthesias, muscle cramping, twitching and can extend to weakness and paralysis [8].
Box 1.
no caption available
MECHANISMS OF HYPERKALEMIA
In the setting of hyperkalemia, the accuracy of the measurement needs to be confirmed first. Pseudohyperkalemia, a falsely elevated serum potassium, can occur due to haemolysis, phlebotomy technique (even forceful fist clenching) or laboratory error [7]. If deemed to be accurate, then the presumptive mechanisms are increased intake or decreased excretion.
Inhibitors of the renin–angiotensin–aldosterone pathway (RAAS) are a common cause of hyperkalemia in the cardiac population. Angiotensin-converting enzyme (ACE) inhibitors and AT1 blockers (ARBs) cause hyperkalemia by inhibiting angiotensin-II-mediated aldosterone secretion by the adrenal gland. They also alter renal blood flow by causing efferent arteriolar vasodilation leading to lower glomerular filtration rates. Mineralocorticoid receptor antagonists (MRAs) directly block aldosterone secretion leading to hyperkalemia by reduced renal excretion of potassium [9]. A review of 39 studies found RAAS inhibitor use for hypertension was associated with a 2% or less occurrence of hyperkalemia (defined as K ≥ 5.5) with single agent RAAS blockade. However, in patients with heart failure or CKD or those on dual RAAS blockade, the incidence of hyperkalemia was in the 5–10% range [9].
Clinical trials in heart failure patients have reflected variable rates of hyperkalemia. Hyperkalemia (defined as >5.5 mEq/l) in the SOLVD trial occurred in 7.8% of patients in the enalapril treatment arm [10]. Clinically important hyperkalemia occurred significantly less often with the use of candesartan in CHARM, at 5.2% [11]. However, in the RALES trial, where combination RAAS blockade with spironolactone was employed, potassium levels of more than 5.5 mEq/l occurred in 19% of patients (and 51% of patients when a potassium >5 mEq/l was used as a cut-off) [12]. After the publication of RALES, a study out of Ontario showed a 4.5 fold increase in hyperkalemia hospitalizations (from 2.4 up to 11 per 1000 patients) and a resultant six-fold increase in mortality (0.3 up to two per 1000 patients) [13]. In PARADIGM-HF, there were similar rates of hyperkalemia (K ≥ 5.5 mEq/l) comparing enalapril with the combination drug sacubitril/valsartan at roughly 16–17%. Severe hyperkalemia (K > 6 mEq/l), was significantly higher in the enalapril arm in comparison with sacubitril/valsartan (5.6 vs. 4.3%) [14]. Other studies have also shown lower rates of hyperkalemia with ARBs compared with ACE inhibitors including in the CKD population. The mechanism may be related to less inhibition of aldosterone secretion by ARBs [15].
A contemporary analysis of the PROTECT cohort found incident hyperkalemia (K ≥ 5.5) in patients hospitalized for acute decompensated heart failure was not associated with poor outcomes. However, hyperkalemia was associated with dose reduction of MRAs in 15% of patients but did not impact dosing of other RAAS inhibitors. Patients who required dose reduction or discontinuation of RAAS inhibition had increased mortality at 180 days with an adjusted hazard ratio (HR) of 1.97 [16▪▪].
Worsening CKD also plays a significant role in the development of hyperkalemia. Under normal conditions, the kidneys excrete 90% of all potassium. Only 10% of all potassium will reach the distal tubule where excess potassium is excreted into the urine by the principal cells in the renal collecting duct. This is done with the aid of the Na+/K+ ATPase and luminal sodium channels. This process is regulated by both sodium concentration and serum aldosterone levels at the level of the distal tubule. In heart failure, renin is secreted by the juxtaglomerular cells in response to decreased renal blood flow and perfusion pressure initiating the RAAS cascade. In healthy patients, elevated aldosterone levels lead to potassium excretion. However, in patients with heart failure, elevated aldosterone promotes sodium reabsorption, thus less sodium is delivered to the distal nephron resulting in impaired excretion of potassium [17].
With underlying CKD and the use of RAAS inhibitors, aldosterone resistance may also develop. CKD can also cause impaired renin release leading to a hyporeninemic hypoaldosteronism [17]. Lastly, type IV renal tubular acidosis, a hypoaldosterone state also results in hyperkalemia. This can be inherited, but is usually acquired and can be present in people with diabetes and with certain medications including ACE inhibitors, MRAs, NSAIDs, heparin, beta blockers, digitalis, trimethoprim and cyclosporine [18].
Dietary intake of potassium also needs to be considered in patients with hyperkalemia. Potassium supplements as well as sodium supplements that can often be high in potassium need to be taken into account [19]. Finally, other causes of hyperkalemia in the general population should be considered such as adrenal insufficiency, metabolic acidosis, and trauma [19].
ACUTE MANAGEMENT
Given the cardiotoxic effects of hyperkalemia, membrane stabilization with calcium is essential. Calcium is indicated when there is evidence of ECG changes including peaked T waves, widened QRS interval, ventricular arrhythmias or cardiac arrest. Intravenous calcium can be given in a chloride (0.5–1 g over 2–5 min) or gluconate formulation (1–3 g over 2–5 min). The effects should be seen within 5 min and calcium can be repeated at this interval if life-threatening ECG changes persist. Calcium does not lower the potassium level and needs to be combined with other therapies that act in that manner. Side effects of calcium include hypotension due to peripheral vasodilation as well as bradycardia [20▪]. To acutely lower serum potassium, agents that shift potassium intracellularly are utilized. These include insulin, salbutamol and sodium bicarbonate (Fig. 1).
FIGURE 1.
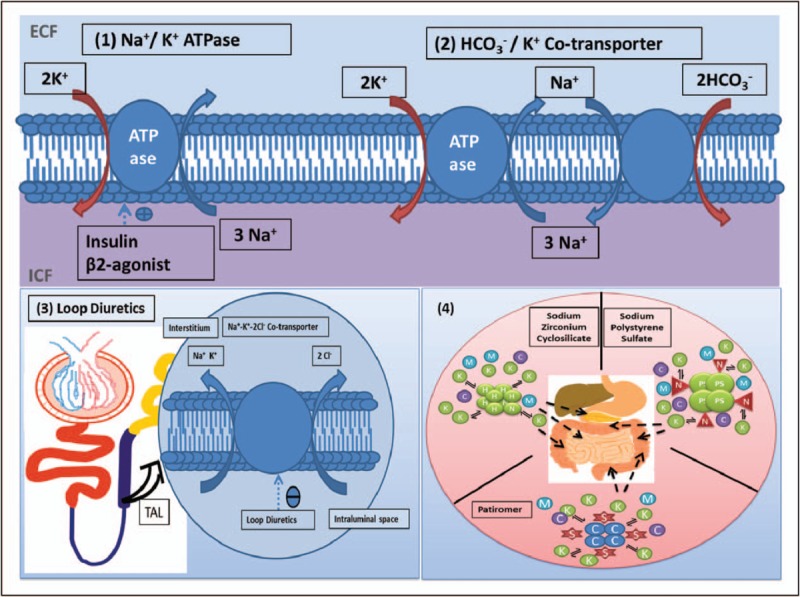
Mechanism of action of drugs for hyperkalemia. C, calcium; H, hydrogen; K, potassium; M, magnesium; N, sodium; S, sorbitol; TAL, thick ascending limb. (a) Shift K+ into cells. (1) Insulin and β2 agonists both stimulate the Na+/K+ ATPase facilitating extracellular potassium exchange for intracellular sodium. (2) Sodium bicarbonate stimulates the HCO3−/K+ cotransporter facilitating HCO3− and K+ cotransport in exchange for intracellular sodium. (b) Enhance K+ removal. (3) Via urine: Loop diuretics act on the thick ascending limb of the loop of Henle inhibiting the Na+–K+–2Cl− cotransporter resulting in decreased sodium and potassium reabsorption. (4) Via gastrointestinal lumen: sodium zirconium cyclosilicate, patiromer and sodium polystyrene sulfate work by binding K+ in exchange for hydrogen, calcium and sodium (respectively) in the gastrointestinal lumen, allowing more potassium excretion.
Insulin acts on the glucose transporter type 4 present in skeletal muscle and promotes intracellular movement of potassium via the Na+/K+ ATPase pump. It is typically dosed as a 10 unit bolus of regular insulin in addition to 1 ampule of dextrose 50 to prevent the hypoglycemic effects. Insulin has an onset of action of roughly 15 min [20▪].
Salbutamol is a beta-2 agonist that also activates the Na+/K+ ATPase transporter on both muscle and liver cells and directly promotes endogenous insulin release. A dose of 10 mg of salbutamol is as effective as 10 units of IV insulin – both interventions drop serum potassium by ∼1 mEq/l. Salbutamol via its beta agonist properties can lead to palpitations, anxiety and headaches. Salbutamol has an onset of action of 15–30 min [20▪].
Lastly, sodium bicarbonate is often given, but recent studies suggest that its role may be limited to patients who also have metabolic acidosis or in the setting of cardiac arrest. The dose is 50 mEq as an IV push. Side effects may include hypernatremia and associated volume overload [20▪].
After stabilization, the next step is to eliminate potassium via urinary excretion or excretion in the feces. Loop diuretics work by inhibiting the Na+/K+/2Cl− cotransporter in the loop of Henle and blocking sodium and potassium reabsorption (Fig. 1). Greater sodium delivery to the distal nephron stimulates potassium excretion. Care should be used to avoid diuretics in patients who are hypovolemic to prevent acute kidney injury which would further worsen the hyperkalemia. In rare situations, haemodialysis may be required [20▪].
The use of resins to bind potassium in the intestine is the final method by which potassium can be excreted and this will be discussed in the next section (Fig. 1).
CHRONIC MANAGEMENT
Sodium polystyrene sulfate
Sodium polystyrene sulfate (SPS) is the oldest of the potassium binding resins and has been in use for the past 60 years. SPS binds to potassium ions in the intestine in exchange for sodium ions. However, the binding is nonspecific, and calcium and magnesium may also be bound. Ultimately, SPS and its bound potassium transit through the colon and are eliminated in the feces [8].
SPS can be administered orally (15 g 1–4 times/day) or rectally (30–50 g every 6 h) and was often combined with sorbitol in the past to alleviate its constipating effects. Potassium lowering is dose dependent and one study showed a range of potassium lowering of between 0.82 and 1.4 mEq/l with the 15–60 g doses, respectively. The 30 g dose lowered potassium by roughly 1 mEq/l. The onset of action is variable at 2–6 h [21].
The use of SPS is mainly limited by side effects which can include nausea, vomiting, constipation or diarrhea. A recent observational analysis of the Swedish Renal registry revealed a higher incidence of minor gastrointestinal (GI) side effects (HR 1.11) as well as severe adverse events (HR 1.25) such as ulcers and perforations in patients with Stage 4+ CKD who had been newly started on SPS [22▪]. Cases of intestinal necrosis were also reported with the combination of SPS and sorbitol which led the Food and Drug Administration to release a black box warning in 2009, as such the combination is no longer recommended; however, SPS continues to be used without sorbitol [8].
Aggressive treatment with SPS can lead to hypokalemia and as such, serum potassium should be monitored frequently. Abrupt cessation could also lead to rebound hyperkalemia. Lastly, SPS does provide an increased sodium load (1500 mg of sodium in 15 g) which may lead to worsening hypertension or heart failure symptoms [8,23]. Given the affinity of SPS for other ions, its administration needs to occur in the absence of cation-containing antacids and laxatives. SPS can also reduce the absorption of levothyroxine and lithium [8].
Patiromer
Patiromer was designed to address the limitations of the early generation binding resins. It is entirely nonabsorbable (given a bead size of 100 μm), binds more potassium than SPS and exchanges potassium for calcium and therefore, is not an issue in patients with heart failure. In the setting of CKD, colonic big potassium channels are upregulated two to eight fold. As patiromer binds potassium, a concentration gradient develops that allows further potassium secretion by the big potassium channels [24].
This was illustrated in AMETHYST-DN, a phase II, open label study of patients with diabetic nephropathy that examined potassium reduction over 52 weeks. There was a greater reduction at 8 weeks in potassium in those who had moderate hyperkalemia (−0.97 mEq/l) vs. those whose who had mild hyperkalemia (−0.55 mEq/l) despite receiving the same dose of patiromer. In a year of follow-up, normokalemia was maintained in 77–95% of patients despite 95% of them remaining on RAAS blockade [25].
PEARL-HF was a 4 week, double-blind, placebo-controlled, multicenter trial designed to evaluate the role of patiromer in heart failure patients receiving spironolactone. The study population included patients with NYHA II or III heart failure, ejection fraction (EF) ∼ 40% and roughly half had CKD. Patiromer 15 mg BID compared with placebo lowered potassium by a mean of 0.45 mEq/l. As a result, fewer patients had hyperkalemia (7.3 vs. 24.5% with placebo) and virtually all patients were able to increase their spironolactone dosing to a target dose of 50 mg. A subgroup analysis of patients with heart failure and CKD revealed rates of hyperkalemia of 38.5% in the placebo arm vs. 6.7% in the patiromer arm [26]. Another heart failure trial (DIAMOND) will follow patients with reduced EF on RAAS inhibitors for 12 weeks (NCT03888066).
OPAL HK, was a 12 week, phase III study in Stage III and IV CKD patients on one or more RAAS inhibitors who had hyperkalemia (K ≥ 5.5) at baseline. Patiromer dosing was based on severity of baseline hyperkalemia and adjusted to maintain a potassium between 3.8 and 5.1 mEq/l. By week 4, 76% of patients in the treatment arm had potassium values within target range and the mean potassium reduction was 1 mEq/l. Doses of between 12.8 and 21.4 mg/day were required. In the subsequent withdrawal phase, 60% of patients in the placebo arm had recurrent hyperkalemia vs. 15% in the patiromer arm. This required discontinuation of RAAS inhibitors in more than 50% of the placebo group vs. ongoing therapy in 94% of the patiromer group [27].
Patiromer is available in 4.2-g increments, with a recommended dose of 8.4 g once daily to start. Given its slow onset of action (∼7 h), it is not approved for the acute management of hyperkalemia. Side effects with patiromer are often related to the sorbitol in the formulation (∼4 g in an 8.4-g dose) and include nausea, flatulence, constipation and diarrhea. No intestinal necrosis has been noted with patiromer. Hypomagnesemia occurs in ∼7% of patients.
All medications should be spaced apart by 3 h from patiromer [28].
Sodium zirconium cyclosilicate
Sodium zirconium cyclosilicate (SZC), also known as ZS-9, is an insoluble compound that works throughout the GI tract by binding potassium and exchanging it for sodium and hydrogen ions. Potassium ions are trapped in the cation binding pore (which is composed of zirconium, silicon and oxygen). Given the size of the pore, it preferentially binds potassium vs. smaller or larger cations. The crystal structure entraps the potassium and prevents enteric recirculation. In vitro, 9.3× more potassium was bound to SZC compared with magnesium or calcium. It is also 25× more selective for potassium than the binding resins discussed earlier [29,30].
HARMONIZE was a phase 3, randomized, double-blind, placebo-controlled study with an open label, run-in phase lasting 2 days; those patients who achieved normokalemia, progressed to the randomized phase lasting 28 days. Potassium levels rapidly corrected in 84% of patients within ∼2 h and 98% at 48 h. On average, the reduction in potassium at 48 h was 1 mEq/l. All three doses of SZC (5, 10 and 15 g) lowered potassium to the normal range compared with placebo [30].
Another Phase 3 study examined SZC over a 12-month period. The study design was similar with an initial correction phase where patients received 10 g TID for 1–3 days until K less than 5 mEq/l. Patients then entered a maintenance phase where 5 g daily was initiated and titrated to maintain normokalemia. In this study, 3/4 had CKD and 2/3 were on RAAS blockers at baseline. In 82% of patients, normokalemia was achieved within 24 h. The mean dose required over the maintenance phase was 7.2 g. Overall, 74% of patients maintained their RAAS blockade and 14% actually increased the dose [31▪▪]. Similar results were seen in a subgroup analysis looking at the heart failure patients in HARMONIZE [32].
A Phase 2, randomized, double-blind study in heart failure (NYHA II–IV) patients of SZC vs. placebo is currently underway with the intent to optimize RAAS inhibition over a 3-month period. The estimated study completion date of PRIORITIZE-HF is October 2020 (NCT03532009).
SZC is dosed 10 mg TID in the acute phase and dropped to 5–10 mg daily thereafter. Given that SZC is insoluble, is not systemically absorbed and does not expand on contact with water, its tolerability is high. GI-related side effects occurred less often than with patiromer and were comparable with placebo. Hypertension and peripheral edema (with the 15 g dose) occurred less than 1% of the time. Other adverse effects included urinary tract infections [28]. Given its rapid onset of action, SZC can be used for the acute treatment of hyperkalemia as well as chronic maintenance therapy [30]. SZC can transiently increase gastric pH and as such, medications that rely on an acidic environment (i.e. azole antifungals, antiretrovirals) should be administered 2 h apart [33].
CONCLUSION
Hyperkalemia is a life-threatening problem that is frequently encountered in heart failure patients. SPS has been the only option for chronic management of hyperkalemia and has its limitations. In recent years, there are two new therapeutic options: patiromer and SZC. SZC also adds to the armamentarium for the treatment of acute hyperkalemia.
Both agents are now available in North America and Europe. SZC has just received a notice of compliance from Health Canada. Cost may be a barrier – in the United States, a 30 day supply of patiromer 8.4 g is $850. SZC 10 mg for 30 days is slightly cheaper at $656 USD. These two agents have not been compared directly, but both have a role in managing chronic hyperkalemia and have allowed for successful continuation and even optimization of RAAS inhibitors in patients with heart failure, DM and CKD.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
S.Z. received consulting fees and speaker honoraria from Astra-Zeneca. Other authors have no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Ingelfinger J. A new era for the treatment of hyperkalemia. N Engl J Med 2015; 372:275–277. [DOI] [PubMed] [Google Scholar]
- 2.Roger V. Epidemiology of heart failure. Circ Res 2013; 113:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal A, Spertus J, Gosch K, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA 2012; 307:157–164. [DOI] [PubMed] [Google Scholar]
- 4.Núñez J, Bayés-Genís A, Zannad F, et al. Long-term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation 2018; 137:1320–1330. [DOI] [PubMed] [Google Scholar]
- 5.Luo J, Brunelli SM, Jensen DE, Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol 2016; 11:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins AJ, Pitt B, Reaven N, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol 2017; 46:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterns RH, Grieff M, Bernstein PL. Treatment of hyperkalemia: something old, something new. Kidney Int 2016; 89:546–554. [DOI] [PubMed] [Google Scholar]
- 8.Chaitman M, Dixit D, Bridgeman MB. Potassium-binding agents for the clinical management of hyperkalemia. P T 2016; 41:43–50. [PMC free article] [PubMed] [Google Scholar]
- 9.Weir MR, Rolfe M. Potassium homeostasis and renin–angiotensin–aldosterone system inhibitors. Clin J Am Soc Nephrol 2010; 5:531–548. [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S, Pitt B, Davis C, et al. SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325:293–302. [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-overall programme. Lancet 2003; 362:759–766. [DOI] [PubMed] [Google Scholar]
- 12.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717. [DOI] [PubMed] [Google Scholar]
- 13.Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the randomized aldactone evaluation study. N Engl J Med 2004; 351:543–551. [DOI] [PubMed] [Google Scholar]
- 14.McMurray JJV, Packer M, Desai AS, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004. [DOI] [PubMed] [Google Scholar]
- 15.Bakris GL, Siomos M, Richardson D, et al. ACE inhibition or angiotensin receptor blockade: impact on potassium in renal failure. Kidney Int 2000; 58:2084–2092. [DOI] [PubMed] [Google Scholar]
- 16▪▪.Beusekamp JC, Tromp J, Cleland JGF, et al. Hyperkalemia and treatment with RAAS-inhibitors during acute heart failure hospitalizations and their association with mortality. JACC Hear Fail 2019; 7:970–979. [DOI] [PubMed] [Google Scholar]; An important study that shows that being reactive to inpatient hyperkalemia leads to poorer outcomes.
- 17.Sarwar CMS, Papadimitriou L, Pitt B, et al. Hyperkalemia in heart failure. J Am Coll Cardiol 2016; 68:1575–1589. [DOI] [PubMed] [Google Scholar]
- 18.Yaxley J, Pirrone C. Review of the diagnostic evaluation of renal tubular acidosis. Ochsner J 2016; 16:525–530. [PMC free article] [PubMed] [Google Scholar]
- 19.Llubani R, Vukadinović D, Werner C, et al. Hyperkalaemia in heart failure – pathophysiology, implications and therapeutic perspectives. Curr Heart Fail Rep 2018; 15:390–397. [DOI] [PubMed] [Google Scholar]
- 20▪.Liu M, Rafique Z. Acute management of hyperkalemia. Curr Heart Fail Rep 2019; 16:67–74. [DOI] [PubMed] [Google Scholar]; Great review on acute hyperkalemia treatment.
- 21.Mistry M, Shea A, Giguere P, Nguyen M-L. Evaluation of sodium polystyrene sulfonate dosing strategies in the inpatient management of hyperkalemia. Ann Pharmacother 2016; 50:455–462. [DOI] [PubMed] [Google Scholar]
- 22▪.Laureati P, Xu Y, Trevisan M, et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant 2019; doi: 10.1093/ndt/gfz150. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; Large renal registry showing poor outcomes with sodium polystyrene sulfate in chronic kidney disease.
- 23.Beccari MV, Meaney CJ. Clinical utility of patiromer, sodium zirconium cyclosilicate, and sodium polystyrene sulfonate for the treatment of hyperkalemia: an evidence-based review. Core Evid 2017; 12:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Harrison SD, Cope MJ, et al. Mechanism of action and pharmacology of patiromer, a nonabsorbed cross-linked polymer that lowers serum potassium concentration in patients with hyperkalemia. J Cardiovasc Pharmacol Ther 2016; 21:456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakris GL, Pitt B, Weir MR, et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease the AMETHYST-DN randomized clinical trial. JAMA 2015; 314:151–161. [DOI] [PubMed] [Google Scholar]
- 26.Weir M, Bakris G, Bushnisky D, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015; 372:211–221. [DOI] [PubMed] [Google Scholar]
- 27.Pitt B, Bakris GL, Bushinsky DA, et al. Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. Eur J Heart Fail 2015; 17:1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy 2017; 37:401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linder KE, Krawczynski MA, Laskey D. Sodium zirconium cyclosilicate (ZS-9): a novel agent for the treatment of hyperkalemia. Pharmacotherapy 2016; 36:923–933. [DOI] [PubMed] [Google Scholar]
- 30.Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia the HARMONIZE randomized clinical trial. JAMA 2015; 64111:2223–2233. [DOI] [PubMed] [Google Scholar]
- 31▪▪.Spinowitz BS, Fishbane S, Pergola PE, et al. Sodium zirconium cyclosilicate among individuals with hyperkalemia: a 12-month phase 3 study. Clin J Am Soc Nephrol 2019; 14:798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]; Long-term study of sodium zirconium cyclosilicate showing acute lowering of potassium and sustained response over a year with good tolerability.
- 32.Anker SD, Kosiborod M, Zannad F, et al. Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur J Heart Fail 2015; 17:1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.[No authors listed]. Sodium zirconium cyclosilicate (Lokelma) for hyperkalemia. Med Lett Drugs Ther 2018; 60:197–199. [PubMed] [Google Scholar]


