Abstract
Background
Body fat and/or muscle composition influences prognosis in several cancer types. For advanced gastric and gastroesophageal junction cancer, we investigated which body composition parameters carry prognostic information beyond well‐established clinical parameters using robust model selection strategy such that parameters identified can be expected to generalize and to be reproducible beyond our particular data set. Then we modelled how differences in these parameters translate into survival outcomes.
Methods
Fat and muscle parameters were measured on baseline computed tomography scans in 761 patients with advanced gastric or gastroesophageal junction cancer from the phase III EXPAND trial, undergoing first‐line chemotherapy. Cox regression analysis for overall survival (OS) and progression‐free survival (PFS) included body composition parameters and clinical prognostic factors. All continuous variables were entered linearly into the model as there was no evidence of non‐linear prognostic impact. For transferability, the final model included only parameters that were picked by Bayesian information criterion model selection followed by bootstrap analysis to identify the most robust model.
Results
Muscle and fat parameters formed correlation clusters without relevant between‐cluster correlation. Mean muscle attenuation (MA) clusters with the fat parameters. In multivariate analysis, MA was prognostic for OS (P < 0.0001) but not for PFS, while skeletal muscle index was prognostic for PFS (P = 0.02) but not for OS. Worse performance status Eastern Cooperative Oncology Group (ECOG 1/0), younger age (on a linear scale), and the number of metastatic sites were strong negative clinical prognostic factors for both OS and PFS. MA remained in the model for OS (P = 0.0001) following Bayesian information criterion model selection in contrast to skeletal muscle index that remained prognostic for PFS (P = 0.009). Applying stricter criteria for transferability, MA represented the only prognostic body composition parameter for OS, selected in >80% of bootstrap replicates. Finally, Cox model‐derived survival curves indicated that large differences in MA translate into only moderate differences in expected OS in this cohort.
Conclusions
Among body composition parameters, only MA has robust prognostic impact for OS. Data suggest that treatment approaches targeting muscle quality are unlikely to prolong OS noticeably on their own in advanced gastric cancer patients, indicating that multimodal approaches should be pursued in the future.
Keywords: Sarcopenia, Computed tomography, Mean muscle attenuation, Smooth muscle index, Gastric cancer, Gastroesophageal junction cancer, Prognosis
Introduction
The impact of body composition on cancer prognosis and treatment‐related adverse events is increasingly recognized.1 Analysis of computed tomography (CT) scans at the level of the third lumbar spine is considered a standard approach to measure muscle and adipose tissue body composition parameters.2 Sarcopenia characterized by low muscle mass and/or quality was associated both with a decrease in overall survival (OS) and an increase in the frequency and severity of toxicities in cancer patients undergoing chemotherapy, molecular targeted therapy, or immunotherapy3 while the prognostic role of adipose tissue parameters is controversial.4, 5, 6, 7
Retrospective studies identified skeletal muscle index (SMI), representing a marker for muscle mass and mean muscle attenuation (MA), representing a marker for muscle quality, as prognostic factors in cancer patients, analysing large cohorts including different cancer entities and/or cancer patients at different clinical stages.8, 9, 10 Prognostic cut‐off values were used, optimized11 for the individual data set in question. This results in difficulties to transfer such cut‐off values between cohorts. More importantly, using cut‐values when prognosis varies linearly with the measurement precludes quantification of the potential impact of inducing specific differences in individual body composition parameters on survival. Extensive research efforts are ongoing to identify novel pharmacological targets12 as well as applying nutritional13 and/or exercise‐based interventions14 to improve muscle mass and/or quality in cancer patients and to improve tolerability of cancer treatment and survival outcomes. As cachexia/sarcopenia15 is common in locally advanced or metastatic gastric cancer and adenocarcinomas of the gastroesophageal junction (GEJ), gaining information on the amount of improvement in a body composition parameter needed to translate into clinically meaningful survival benefit is important for assessing the prospect of an intervention aiming to improve outcome.
Here, we analysed the prognostic impact of muscle and adipose tissue body composition parameters in patients with locally advanced or metastatic gastric cancer or adenocarcinomas of the GEJ from the phase 3 EXPAND trial.16 Our objectives were to understand the correlation between distinct muscle and fat parameters as well as body mass index (BMI), to determine which body composition parameters robustly correlate with OS or progression‐free survival (PFS) in conjunction with clinical parameters, and to provide a parsimonious model incorporating muscle and fat parameters continuously such that it can be used to assess the potential prospect of interventions aiming at improving body composition parameters.
Methods
Patients
Patients with unresectable, locally advanced, or metastatic gastric or GEJ cancer from the EXPAND trial, which failed to demonstrate an improvement in PFS and OS with the addition of the anti‐epidermal growth factor receptor antibody cetuximab to standard chemotherapy (capecitabine/cisplatin),16 were studied. In this open‐label, randomized, controlled, phase 3 study, adults with histologically confirmed adenocarcinoma of the stomach or GEJ with locally advanced unresectable (M0) or metastatic (M1) disease were enrolled at 164 sites in 25 countries worldwide.16 Inclusion and exclusion criteria are provided in detail in the Supporting Information. A total of 904 patients were randomized for treatment. Of these, 802 baseline CT scans (89%) were evaluable for our analysis of body composition parameters. The reason for this difference is related to the fact that in EXPAND, there was an independent radiological review of CT scans as quality control for PFS. In rare cases, magnetic resonance imaging was used. The first tumour assessment imaging was scheduled after 6 weeks corresponding to two cycles of treatment. Patients without available baseline CT consist of those few imaged with magnetic resonance imaging and those who progressed or died before the first tumour assessment. In the later cases, there was no point in providing the baseline images to the independent reviewer. Thus, our study population excludes de facto early treatment failures before 6 weeks. Therefore, the analysis population is slightly favourably selected with a median OS of 10.8 months as compared with 10.2 months in the intent‐to treat population. The core analysis population comprises n = 761 patients with complete measurements and key clinical data (CONSORT diagram, Figure S1). The study was performed according to the principles of the Declaration of Helsinki, and translational research was approved by local/national ethics committee.
Measurement of body composition parameters
Two adjacent cross‐sectional CT images at the level of the third lumbar vertebra were analysed by two readers (K. L. and L. J.) who were blinded to clinical data. Segmentation of CT data was performed using a custom made and recently published software tool.17 Briefly, the tool starts by standardizing the thickness of CT slices to 1 mm. Next, density ranges −190 to −30 Hounsfield units (HU) and −29 to 150 HU for adipose and muscle tissue, respectively, are defined.8, 9, 18 During segmentation, the individual regions of interest are selected manually yielding areas of total adipose tissue (TAT) and visceral adipose tissue (VAT) as well as areas of ventral abdominal muscle (Mven), spinal muscle (Mspi), and psoas muscle (Mpso), as illustrated in Figure S2. Area of subcutaneous adipose tissue (SAT) was calculated by the difference of TAT and VAT. Total muscle area was determined as sum of Mven, Mspi, and Mpso. Additionally, intermuscular adipose tissue (IMF) was identified between muscle groups and beneath the muscle fascia of the Mpso and the dorsal spine musculature. Mean muscle attenuation (MA) was measured in HU based on the total muscle area at the third lumbar vertebra of the same CT images. All parameters were defined in the two adjacent CT slices, and mean values were used for further analysis. Reproducibility of results between the two readers was assessed by analysing CT data sets from 50 subjects randomly selected from the EXPAND cohort. An intra‐observer coefficient of variation of <1.3% was required, consistent with other reports in the literature.8, 9
Statistical analysis
Because no statistically significant differences in PFS or OS between treatment arms were found in EXPAND, all patients (n = 904) irrespective of the treatment arm were included if baseline CT scans were available. Analysis of normalized muscle/adipose tissue areas and MA was carried out on the original scale. We used Pearson's correlation coefficients to analyse correlation among parameters. For survival analysis (PFS and OS), standard Cox regression analysis relying on the proportional hazard assumption was used. Adipose tissue parameters and the well‐established muscle parameters MA and SMI together with key clinical parameters were studied in order to investigate whether the measurements carry additional information beyond standard clinical information. With the exception of BMI, all metric parameters including age (lower age was associated with unfavourable prognosis) could be modelled linearly as we carefully checked martingale plots for indication of non‐linearity and used modelling with fractional polynomials to investigate whether using a non‐linear transformation significantly improved the model (data not shown). In particular, there was no evidence of natural cut‐off values.
In a separate set of analysis, anatomic muscle areas normalized for body size were used to replace SMI in the models (i.e. Mven area, Mspi area, and Mpso area) to determine if they carry a prognostic role superior to SMI as a parameter of muscle mass on an exploratory basis.
We started the variable selection process with univariate analyses and a full multivariate model including all parameters considered. Next, Bayesian information criterion (BIC) was used to select a parsimonious model. Finally, robustness of the model selection and the transportability of results was assessed by generating n = 1000 bootstrap replicates of the data (sampling randomly with replacement from the original data set). Covariates selected in at least 80% of the bootstrap samples19 were included in the final model. For purpose of illustration, Cox model‐derived survival curves were generated for covariate constellations of interest: ECOG (0/1), and MA and age fixed at their 20% and 80% quintiles, respectively. Finally, interaction between clinical prognostic parameters from the final prognostic model and MA with respect to OS were analyzed.
Results
Patient characteristics
Characteristics of the 761 patients are given in Table 1. Four percent of patients had locally advanced unresectable disease; all other patients had metastatic disease. Median age was 59 years (range 18–84). At baseline, 60% of patients were normal weight, 23% overweight, 8% obese, and 9% underweight according to BMI categories. The majority of patients (75%) was male. Eighty‐three per cent of patients suffered from gastric cancer, while 17% were diagnosed with GEJ cancer. Forty percent of patients were from Asia, and 60% from non‐Asian regions in the world (Table 1).
Table 1.
Baseline characteristics and muscle/fat parameters
| n (%) | VATa | SMI | Mspia | MA | |
|---|---|---|---|---|---|
| ECOG PS | |||||
| 1 | 360 (47) | 34.24 | 60.36 | 18.39 | 45.77 |
| 0 | 401 (53) | 37.59 | 62.17 | 19.06 | 45.91 |
| ns | P = 0.00872 | P = 0.00024 | ns | ||
| Age | |||||
| <65 | 571 (75) | 32.81 | 61.12 | 19.06 | 47.31 |
| ≥65 | 190 (25) | 45.62 | 61.94 | 18.22 | 41.27 |
| P < 0.0001 | ns | P = 0.0012 | P < 0.0001 | ||
| BMI | |||||
| <18.5 | 69 (9) | 7.3 | 53.68 | 16.30 | 50.36 |
| 18.5–25 | 458 (60) | 25.71 | 60.12 | 18.74 | 47.64 |
| 25–30 | 171 (23) | 64.61 | 65.94 | 19.82 | 41 |
| >30 | 63 (8) | 83.99 | 67.57 | 19.63 | 36.88 |
| P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.0001 | ||
| Sex | |||||
| Female | 189 (25) | 31.83 | 54.44 | 16.91 | 43.30 |
| Male | 572 (75) | 37.38 | 63.60 | 19.49 | 46.68 |
| P = 0.011 | P < 0.0001 | P < 0.0001 | P < 0.0001 | ||
| Metastatic sites | |||||
| ≥2 | 295 (39) | 39.38 | 61.65 | 18.81 | 44.05 |
| 1 | 466 (61) | 33.87 | 61.12 | 18.87 | 46.98 |
| P = 0.00837 | ns | ns | P < 0.0001 | ||
| Site | |||||
| Gastric | 621 (81) | 34.43 | 61.09 | 18.84 | 46.53 |
| GEJ | 127 (17) | 43.70 | 62.73 | 19.03 | 42.92 |
| Unknown | 13 (2) | 36.19 | 58.87 | 17.79 | 41.70 |
| P = 0.0149 | ns | ns | P < 0.00063 | ||
| Liver metastasis | |||||
| Yes | 351 (46) | 41.26 | 62.18 | 19.10 | 44.95 |
| No | 410 (44) | 31.51 | 60.60 | 18.64 | 46.61 |
| P < 0.0001 | P = 0.0188 | P = 0.051 | P = 0.0137 | ||
| Ethnicity | |||||
| Asian | 307 (40) | 29.07 | 59.50 | 18.83 | 49.40 |
| Non‐Asian | 454 (60) | 40.69 | 62.56 | 18.87 | 43.44 |
| P < 0.0001 | P < 0.0001 | ns | P < 0.0001 | ||
BMI, body mass index; GEJ, gastroesophageal junction; Mspi, spinal muscle; ns, not significant; SMI, skeletal muscle index; VAT, visceral adipose tissue.
Normalized for body size: area (cm2)/size (m2).
Body composition parameters
Body composition parameters at baseline by clinically defined subgroups are also summarized in Table 1. VAT significantly increased with age and with increasing BMI categories. Female patients showed slightly lower VAT values compared with male patients. Asian patients were characterized by significantly lower VAT values compared with non‐Asian patients. Patients with an ECOG performance status 0 compared with 1 were characterized by significantly higher SMI and Mspi muscle parameters. SMI and Mspi increased with higher BMI, while MA decreased significantly. There was no correlation between MA and ECOG performance status (PS) (data not shown). Asian patients showed higher MA values compared with non‐Asian patients, and the same was true for male compared with female patients.
Correlations between body composition parameters
Correlation between muscle parameters and BMI was weak. In contrast, adipose tissue‐related parameters (TAT, SAT, and VAT) showed strong positive correlations between each other and with BMI. MA was negatively correlated with adipose tissue parameters TAT, SAT, VAT, and IMF (Figure 1 and Table S1).
Figure 1.
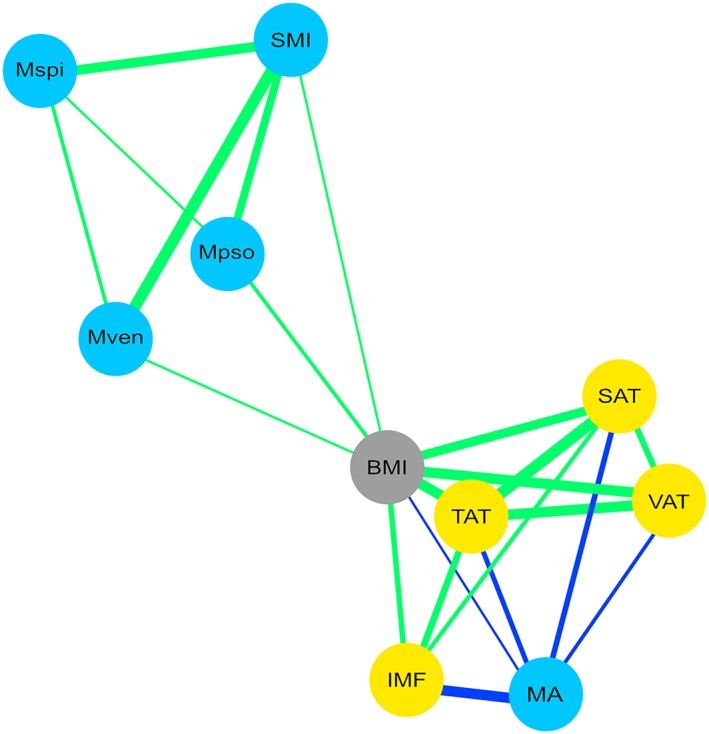
Graphical correlation matrix of body mass index (BMI) and fat/muscle‐related body composition parameters. Green lines indicate positive correlations; blue lines indicate negative correlations. Thickness of line indicates strength of the correlation (corresponding numerical values: see Table S1). Mpso, psoas muscle; Mspi, spinal muscle; Mven, ventral abdominal muscle; SAT, subcutaneous adipose tissue; SMI, skeletal muscle index; TAT, total adipose tissue; VAT, visceral adipose tissue.
Prognostic role of body mass index
Expectedly, there was evidence of non‐linearity in BMI when looking at survival differences between BMI categories: Patients with a BMI <18.5 kg/m2 and BMI >30 kg/m2 showed decreased survival rates at 12 months (25.6% and 22.5%, respectively) as compared with the BMI groups 18.5–25 and 25–30 kg/m2 (44% and 48.4%, respectively), log rank test, P = 0.0264 (Figure S3). In the Cox model‐based univariate and multivariate analyses, BMI (on any scale) was not prognostic for OS or PFS (Tables 2 and 3).
Table 2.
Univariate analysis overall survival and progression‐free survival
| Covariates | Overall survival | Progression‐free survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| ECOG (0/1) | 1.523 | 1.3–1.784 | <0.0001 | 1.416 | 1.185–1.691 | <0.0001 |
| Age | 0.991 | 0.984–0.999 | 0.022 | 0.994 | 0.985–1.002 | 0.142 |
| Male versus female | 0.799 | 0.666–0.958 | 0.016 | 0.821 | 0.67–1.007 | 0.059 |
| Asian versus non‐Asian | 1.223 | 1.039–1.223 | 0.016 | 0.976 | 0.815–1.169 | 0.795 |
| Number met. sites (1 vs. ≥2) | 1.258 | 1.07–1.479 | 0.005 | 1.405 | 1.173–1.682 | <0.0001 |
| BMI | 1.006 | 0.986–1.026 | 0.569 | 1.006 | 0.984–1.028 | 0.605 |
| MA | 0.984 | 0.975–0.992 | <0.0001 | 0.988 | 0.978–0.998 | 0.016 |
| SMI | 0.99 | 0.981–0.999 | 0.026 | 0.987 | 0.977–0.997 | 0.01 |
| VATa | 1.001 | 0.998–1.004 | 0.389 | 1.002 | 0.999–1.005 | 0.175 |
| SATa | 1.002 | 0.999–1.004 | 0.204 | 1.001 | 1.185–1.691 | 0.454 |
BMI, body mass index; CI, confidence interval; HR, hazard ratio; SAT, subcutaneous adipose tissue; SMI, skeletal muscle index; VAT, visceral adipose tissue.
Normalized for body size: area (cm2)/size (m2).
Table 3.
Multivariate analysis overall survival and progression‐free survival
| Covariates | Overall survival | Progression‐free survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| ECOG (0/1) | 1.551 | 1.319–1.823 | <0.0001 | 1.403 | 1.17–1.682 | <0.0001 |
| Age | 0.983 | 0.975–0.002 | <0.0001 | 0.987 | 0.978–0.997 | 0.007 |
| Male versus female | 0.929 | 0.736–1.174 | 0.539 | 0.879 | 0.675–1.145 | 0.34 |
| Asian versus non‐Asian | 1.169 | 0.978–1.398 | 0.086 | 0.919 | 0.755–1.118 | 0.397 |
| Number met. sites (1 vs. ≥2) | 1.269 | 1.076–1.496 | 0.005 | 1.451 | 1.206–1.745 | <0.0001 |
| BMI | 1.009 | 0.96–1.06 | 0.732 | 1.028 | 0.967–1.092 | 0.379 |
| MA | 0.979 | 0.968–0.991 | <0.0001 | 0.987 | 0.975–1 | 0.046 |
| SMI | 0.99 | 0.978–1.003 | 0.129 | 0.983 | 0.969–0.997 | 0.02 |
| VATa | 1.001 | 0.996–1.007 | 0.566 | 1.004 | 0.999–1.01 | 0.133 |
| SATa | 0.997 | 0.992–1.003 | 0.309 | 0.995 | 0.989–1.001 | 0.092 |
BMI, body mass index; CI, confidence interval; HR, hazard ratio; SAT, subcutaneous adipose tissue; SMI, skeletal muscle index; VAT, visceral adipose tissue.
Normalized for body size: area (cm2)/size (m2).
Univariate analysis of body composition and clinical parameters
Adipose tissue‐related parameters lacked prognostic impact. SMI as a measure of muscle mass was only weakly prognostic for OS (P = 0.026), while MA was highly prognostic (P < 0.0001; Table 2). For PFS, both MA and MSI were prognostic (P = 0.016 and P = 0.010, respectively; Table 2). ECOG PS was strongly prognostic in the log rank test, P < 0.0001, and represented the strongest clinical prognostic parameter for both PFS and OS (P < 0.0001, each) together with the number of metastatic sites (P < 0.0001 and P = 0.005, respectively) according to the Cox model‐based univariate analysis (Table 2).
Multivariate analysis of body composition and clinical parameters
ECOG PS (0/1) and age (on a linear scale) were strongly prognostic (P < 0.0001, each) for OS and for PFS (P < 0.0001 and P = 0.0007, respectively). The number of metastatic sites was the only additional clinical parameter of prognostic influence for OS (P = 0.005) and PFS (P < 0.0001), while ethnic group (Asian vs. non‐Asian) was not prognostic (Table 3). SMI (P = 0.02) was prognostic for PFS, while MA (P = 0.046) almost lacked prognostic impact. In contrast, MA again was strongly prognostic for OS (P < 0.0001), while SMI was not (Table 3).
Bayesian information criterion model selection and bootstrap analysis
Following BIC selection in order to select a parsimonious model, SMI dropped out as a prognostic marker for OS, while MA was confirmed (P < 0.0001) in this model together with ECOG, age, and the number of metastatic sites (Table 4). No interaction was found between clinical parameters and MA in this model. For PFS, BIC selection resulted in a model composing of SMI, ECOG, and the number of metastatic sites (Table 3). Finally, only three prognostic parameters were reproduced for OS in more than 80% of n = 1000 bootstrap replicates: ECOG, age, and MA (P < 0.0001, each; Table 4). For PFS, only ECOG and the number of metastatic sites were reproduced. An analysis using anatomical muscle areas (Mpso, Mven, and Mspi) instead of MSI indicated that this did not lead to a more differentiated model. Interestingly, Mpso was selected in the BIC model for PFS but like SMI, failed to be selected in more than 80% of n = 1000 bootstrap replicates (Tables S2 and S3).
Table 4.
Bayesian information criterion selected multivariate Cox model for overall survival and progression‐free survival and number of bootstrap replicates
| Overall survival | ||||
|---|---|---|---|---|
| Covariates | HR | 95% confidence interval | P‐value | Bootstrap selected (%) |
| ECOG (0 vs. 1) | 1.523 | 1.300–1.784 | <0.0001 | 99.6 |
| Age | 0.984 | 0.984–0.999 | <0.0001 | 93.6 |
| MA | 0.975 | 0.975–0.992 | <0.0001 | 92 |
| Number met. sites (1 vs. ≥2) | 1.258 | 1.070–1.497 | 0.005 | 56.5 |
| Progression‐free survival | ||||
|---|---|---|---|---|
| ECOG (0 vs. 1) | 1.421 | 1.188–1.7 | <0.0001 | 92.4 |
| Number met. sites (1 vs. ≥2) | 1.476 | 1.231–1.77 | <0.0001 | 91.3 |
| SMI | 0.987 | 0.977–0.997 | 0.009 | 62.4 |
HR, hazard ratio; SMI, skeletal muscle index.
Cox model‐based illustration of the prognostic impact of parameters
Survival curves derived from the final robust Cox model for OS (Table 4) modelled for individual constellations of prognostic factor values, representing the 20% and 80% value of quintiles for MA, age, and ECOG (0/1), indicated a median OS of 7.5 months for age 46 years, MA 38.6 HU, and ECOG 1 as compared with 15.5 months for age 67 years, MA 53.5 HU, and ECOG 0 (Figure 2).
Figure 2.

Cox model‐derived survival curves generated for covariate constellations of interest: ECOG PS (0/1), MA, and age fixed at their 20% and 80% quintiles, respectively. OS, overall survival.
Model‐derived survival curve of the parameter MA on its own, again representing 20% and 80% value of quintiles, indicates a median OS of 9.7 months for MA 36.8 HU compared with 11.4 months for MA 53.3 HU (Figure S4).
Discussion
Several studies have demonstrated the influence of low muscle mass (sarcopenia) and/or poor muscle quality (low radiodensity) on cancer prognosis,8, 9, 10 reporting prognostic cut‐off values based on the statistical method of optimal stratification11 for respective parameters. Optimizing a cut‐value by minimizing the P‐value, however, can lead to bias, namely, an overestimation of the prognostic impact as the cut‐offs so determined heavily dependent on the case mix in the respective study populations, for example, according to age, gender, ethnicity, cancer type, and stage, and consequently cannot be applied uniformly in different cohorts as exemplified by considerable different cut‐off values reported in an Asian cohort4 compared with North American cohorts.8, 9, 10 In addition, defining a low‐risk group and a high‐risk group based on a cut‐value is conceptually misleading if the prognostic impact is in fact linear: It falsely suggests that there are two qualitatively different subgroups, although in reality, the hazard just varies proportional to the measurement. Accordingly, and as all body composition parameters as well as age were best modelled linearly in our data set, we used Cox regression analysis to investigate whether body composition parameters carry prognostic information beyond well‐established clinical parameters.
We furthermore aimed to identify which parameters can be expected to generalize and to be reproducible beyond our particular data set, as such parameters need to be considered when building conceptual models with respect to interventions aiming at improving body composition parameters to improve cancer care. Consequently, we choose to apply a robust model selection strategy (BIC model selection followed by bootstrap analysis) for transferability beyond multivariate analysis.
Muscle and adipose tissue parameters formed distinct clusters showing more or less stringent correlations with BMI (Figure 1 and Table S1), supporting the view, that individual body composition parameters may allow a more differentiated modelling of prognostic impact compared with the global parameter BMI. As BMI does not distinguish muscle from adipose tissue or describes adipose tissue distribution, it was suggested that measures of body composition parameters beyond BMI represent a superior approach (reviewed in Caan et al.20). In our analysis, BMI was only weakly prognostic in the log rank test and lacked prognostic impact in the Cox regression analyses (Figure S3 and Tables 2 and 3). This is in accordance with recently published data from a smaller cohort of metastatic gastric or GEJ cancer patients where BMI also lacked prognostic impact.21
As expected, gender, age, and ethnic background were significantly related to differences in muscle and adipose tissue parameters (Table 1).22, 23, 24 Specifically, Asian compared with non‐Asian patients were characterized by markedly lower VAT and markedly higher SMI and MA values (Table 1). Because sarcopenia‐related parameters SMI and MA have been identified as promising prognostic markers in cancer patients (reviewed in Bozzetti3), the better overall prognosis reported for Asian gastric cancer patients compared with non‐Asian patients25 may at least in part be related to a more favourable body composition in Asian patients. In support of this hypothesis, ethnicity in contrast to MA was not prognostic for OS or PFS in multivariate analysis (Table 2).
On univariate and multivariate analyses for PFS and OS, all adipose tissue parameters lacked prognostic power. This adds to the controversial data published on the role of adipose tissue parameters for cancer prognosis.4, 5, 6, 7 SMI as a global parameter for muscle mass was prognostic for PFS in univariate and multivariate analyses (P = 0.01 and P = 0.02, respectively), but not for OS (P = 0.026 and P = 0.129, respectively). In contrast, MA as a parameter of muscle quality was strongly prognostic for OS in univariate und multivariate analyses (both P < 0.0001) but only very weakly for PFS (P = 0.016 and 0,046, respectively; Tables 2 and 3). It is still under debate whether parameters of muscle quantity (SMI) or quality (MA) are better suited as prognostic factors.3 Specifically, in a non‐small cell lung cancer cohort, MA was prognostic for OS, while SMI was not.26 The same was true in a cohort of pancreatic cancer patients undergoing surgery.27
Furthermore, in a very recent study in n = 88 advanced gastric and GEJ cancer patients, neither baseline SMI nor MA was prognostic for PFS or OS.21 As there are significant differences in patient characteristics, compared with our data set, they may most likely account for the differences. Overall, a growing body of evidence exists, demonstrating that muscle mass, as represented by SMI, influences the efficacy of chemotherapy in different cancer entities, which may very well explain the prognostic role for PFS found in our cohort. On the other hand, MA is increasingly recognized as a prognostic factor, in line with our findings.3
Additionally, we analysed muscle parameters according to distinct anatomical regions (Mspi, Mpso, and Mven) for their prognostic role. Overall, these parameters were not superior compared with SMI (Table S2). Interestingly, Mpso was the anatomic muscle parameter remaining in the model following BIC selection and thus seems to perform similar to SMI as a prognostic marker for PFS. While the Mpso was proposed as a sentinel for sarcopenia, this assumption, however, has recently been questioned.28, 29
With respect to the clinical parameters, ECOG PS (0/1), the number of metastatic sites, and age (on a linear scale) were prominent prognostic factors both for OS and PFS in multivariate analysis (Table 3). Interestingly, younger age was related to worse OS. This is in contrast to large epidemiological studies in advanced gastric cancer,30, 31 reporting improved survival outcomes in younger patients. These data sets, however, are less homogeneous, and no studies are available, entering age linearly into prognostic models. With respect to ECOG PS, a similar trend has recently been reported in a cohort of advanced gastric cancer patients (ECOG 0 and 1).32
Next, BIC was used to select a parsimonious model consistent with our exploratory approach. This resulted in a model composing of the clinical parameters ECOG, age (P < 0.0001 each), and the number of metastatic sites (P = 0.005) together with MA (P < 0.0001) as prognostic factors for OS (Table 4). Finally, in the bootstrap analysis, ECOG, age, and MA were selected in >80% of 1000 replicates further confirming the robustness of these parameters (Table 4). No interaction between clinical prognostic parameters and MA was found in this model, underscoring the independent prognostic role of MA in this model.
Interestingly, following this strategy, for PFS, only clinical parameters ECOG and the number of metastatic sites were selected in >80% (Table 4).
Cox model‐based simulation of survival curves representing 20% and 80% value of quintiles for age and MA and including ECOG resulted in combined prognostic groups with a median OS ranging from 7.5 months for the worst prognostic group to 15.5 months for the best prognostic group (Figure 2). This robust model demonstrates that MA has a prognostic role in conjunction with age (on a linear scale) and ECOG PS (0/1) in a homogeneous cohort of fit advanced or metastatic gastric cancer or GEJ cancer patients undergoing first‐line chemotherapy. The model‐derived survival curves for MA alone representing 20% and 80% value of quintiles show that a difference of 14.7 HU (38.6 vs. 53.3 HU) translates into a median OS difference of only 1.7 month (9.7 vs. 11.4 months, Figure S4). Of course, our data do not prove a causal relation between MA and survival. However, because of the large improvements in MA needed to translate into survival benefits, they suggest that single pharmacological, nutritional, or exercise‐based treatment approaches targeting muscle quality are unlikely to prolong OS noticeably on their own in advanced stage gastric cancer patients. For example, 12 weeks of resistance exercise training in healthy elderly resulted in only a moderate increase in MA of 5.5 ± 1.8%.33 It can be speculated that this finding may also account for other tumour entities associated with rapidly developing cachexia related to aggressive tumour characteristics and overall short survival times. Consequently, multimodal approaches should be pursued in future clinical trials as an adjunct to effective cancer treatment in advanced stage cancer patients. It is important to add that in early stage cancers with an overall good prognosis like in breast cancer, modification of body composition by exercise training represents a very promising approach to improve clinical outcomes.10, 34
Because distinct cancer treatments were shown to negatively affect muscle parameters35, 36 while others37 even improved muscle mass or quality, studying the influence of cancer treatments on muscle mass and quality during treatment represents another important field of future research,1 which could easily be integrated into clinical trials, and MA represents a robust, promising parameter in this respect.
Conclusions
Among body composition parameters, MA has a robust prognostic role in conjunction with ECOG (0/1) and age for OS contributing to an easy to assess prognostic model for previously untreated advanced gastric and GEJ cancer patients in good PS. SMI has a prognostic role for PFS in conjunction with ECOG (0/1) and the number of metastatic sites; however, this parameter is less robust. Single treatment approaches targeting muscle quality are unlikely to prolong OS noticeably on their own in advanced gastric cancer. Findings support the development of multimodal approaches to target sarcopenia/muscle quality in advanced stage cancer patients.
Conflict of interest
None declared.
Supporting information
Data S1. Supporting Information.
Figure S1. CONSORT‐Diagram.
Figure S2. CT Segmentation software: screenshot from the results of the manual segmentation process using the custom made software tool. Methodological details are given in the methods section.
Figure S3. Kaplan‐Meier curves for OS according to BMI category.
Figure S4. Survival curve based on the multivariate Cox model, validated by bootstrap analysis, modeled for MA.
Table S1. Correlation matrix
Table S2. Multivariate analysis (anatomic muscle areas) for OS and PFS
Table S3. BIC selected multivariate Cox model (anatomic muscle areas) for OS and PFS and number of bootstrap replicates
Acknowledgement
The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017.38
Hacker U. T., Hasenclever D., Linder N., Stocker G., Chung H.‐C., Kang Y.‐K., Moehler M., Busse H., and Lordick F. (2020) Prognostic role of body composition parameters in gastric/gastroesophageal junction cancer patients from the EXPAND trial, Journal of Cachexia, Sarcopenia and Muscle, 11: 135–144. 10.1002/jcsm.12484.
References
- 1. Brown JC, Cespedes Feliciano EM, Caan BJ. The evolution of body composition in oncology‐epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle 2018;9:1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shen W, Punyanitya M, Wang Z, Gallagher D, St.‐Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol (1985) 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 3. Bozzetti F. Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol 2017;28:2107–2118. [DOI] [PubMed] [Google Scholar]
- 4. Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 2015;63:131–140. [DOI] [PubMed] [Google Scholar]
- 5. Harada K, Baba Y, Ishimoto T, Kosumi K, Tokunaga R, Izumi D, et al. Low visceral fat content is associated with poor prognosis in a database of 507 upper gastrointestinal cancers. Ann Surg Oncol 2015;22:3946–3953. [DOI] [PubMed] [Google Scholar]
- 6. Ladoire S, Bonnetain F, Gauthier M, Zanetta S, Petit JM, Guiu S, et al. Visceral fat area as a new independent predictive factor of survival in patients with metastatic renal cell carcinoma treated with antiangiogenic agents. Oncologist 2011;16:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee HW, Jeong BC, Seo SI, Jeon SS, Lee HM, Choi HY, et al. Prognostic significance of visceral obesity in patients with advanced renal cell carcinoma undergoing nephrectomy. Int J Urol 2015;22:455–461. [DOI] [PubMed] [Google Scholar]
- 8. Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 9. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 10. Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol 2018;4:798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams BA, Mandarekar JN, Mandrekar SJ, Mandrekar SJ, Cha SS, Furth AF. Finding optimal cutpoints for continuous covariates with binary and time‐to‐event outcomes. Majo Clinic Technical Report Series *79 2006.
- 12. Aversa Z, Costelli P, Muscaritoli M. Cancer‐induced muscle wasting: latest findings in prevention and treatment. Ther Adv Med Oncol 2017;9:369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11–48. [DOI] [PubMed] [Google Scholar]
- 14. Yoh K, Nishikawa H, Enomoto H, Ishii N, Iwata Y, Ishii A, et al. Effect of exercise therapy on sarcopenia in pancreatic cancer: a study protocol for a randomised controlled trial. BMJ Open Gastroenterol 2018;5:e000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Wys WD. Weight loss and nutritional abnormalities in cancer patients: Incidence, severity and significance In Clinics in Oncology. London: Saunders; 1986. p 251–261. [Google Scholar]
- 16. Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open‐label phase 3 trial. Lancet Oncol 2013;14:490–499. [DOI] [PubMed] [Google Scholar]
- 17. Linder N, Schaudinn A, Langenhan K, Krenzien F, Hau HM, Benzing C, et al. Power of computed‐tomography‐defined sarcopenia for prediction of morbidity after pancreaticoduodenectomy. BMC Med Imaging 2019;19:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115–122. [DOI] [PubMed] [Google Scholar]
- 19. Sauerbrei W, Royston P. Building multivariable prognostic and diagnostic models: transformation of the predictors by using fractional polynomials. J R Stat Soc 1999;162:71–94. [Google Scholar]
- 20. Caan BJ, Cespedes Feliciano EM, Kroenke CH. The importance of body composition in explaining the overweight paradox in cancer. Cancer Res 2018;78:1906–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dijksterhuis WPM, Pruijt MJ, van der Woude SO, Klaassen R, Kurk SA, van Oijen MG, et al. Association between body composition, survival, and toxicity in advanced esophagogastric cancer patients receiving palliative chemotherapy. J Cachexia Sarcopenia Muscle 2019;10:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Woo J, Arai H, Ng TP, Sayer AA, Wong M, Syddall H, et al. Ethnic and geographic variations in muscle mass, muscle strength and physical performance measures. Eur Geriatr Med 2014;5:155–164. [Google Scholar]
- 23. Deurenberg P, Deurenberg‐Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 2002;3:141–146. [DOI] [PubMed] [Google Scholar]
- 24. van der Werf A, Langius JAE, De Van Der Schueren MA, Nurmohamed SA, van der Pant KA, Blauwhoff‐Buskermolen S, et al. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur J Clin Nutr 2018;72:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gill S, Shah A, Le N, Cook EF, Yoshida EM. Asian ethnicity‐related differences in gastric cancer presentation and outcome among patients treated at a canadian cancer center. J Clin Oncol 2003;21:2070–2076. [DOI] [PubMed] [Google Scholar]
- 26. Sjoblom B, Gronberg BH, Wentzel‐Larsen T, Baracos VE, Hjermstad MJ, Aass N, et al. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non‐small cell lung cancer. Clin Nutr 2016;35:1386–1393. [DOI] [PubMed] [Google Scholar]
- 27. van Dijk DP, Bakens MJ, Coolsen MM, Rensen SS, van Dam RM, Bours MJ, et al. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle 2017;8:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baracos VE. Psoas as a sentinel muscle for sarcopenia: a flawed premise. J Cachexia Sarcopenia Muscle 2017;8:527–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rutten IJG, Ubachs J, Kruitwagen RF, Beets‐Tan RG, Olde Damink SW, Van Gorp T. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J Cachexia Sarcopenia Muscle 2017;8:630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dixon M, Mahar AL, Helyer LK, Vasilevska‐Ristovska J, Law C, Coburn NG. Prognostic factors in metastatic gastric cancer: results of a population‐based, retrospective cohort study in Ontario. Gastric Cancer 2016;19:150–159. [DOI] [PubMed] [Google Scholar]
- 31. Li X, Wang W, Ruan C, Wang Y, Wang H, Liang X, et al. Age‐specific impact on the survival of gastric cancer patients with distant metastasis: an analysis of SEER database. Oncotarget 2016;8:97090–97100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang J, Qu J, Li Z, Che X, Zhang J, Liu J, et al. A prognostic model in metastatic or recurrent gastric cancer patients with good performance status who received first‐line chemotherapy. Transl Oncol 2016;9:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taaffe DR, Henwood TR, Nalls MA, Walker DG, Lang TF, Harris TB. Alterations in muscle attenuation following detraining and retraining in resistance‐trained older adults. Gerontology 2009;55:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spei ME, Samoli E, Bravi F, La Vecchia C, Bamia C, Benetou V. Physical activity in breast cancer survivors: a systematic review and meta‐analysis on overall and breast cancer survival. Breast 2019;44:144–152. [DOI] [PubMed] [Google Scholar]
- 35. Poterucha T, Burnette B, Jatoi A. A decline in weight and attrition of muscle in colorectal cancer patients receiving chemotherapy with bevacizumab. Med Oncol 2012;29:1005–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blauwhoff‐Buskermolen S, Versteeg KS, de van der Schueren MA, Den Braver NR, Berkhof J, Langius JA, et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol 2016;34:1339–1344. [DOI] [PubMed] [Google Scholar]
- 37. Prado CM, Bekaii‐Saab T, Doyle LA, Shrestha S, Ghosh S, Baracos VE, et al. Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. Br J Cancer 2012;106:1583–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Figure S1. CONSORT‐Diagram.
Figure S2. CT Segmentation software: screenshot from the results of the manual segmentation process using the custom made software tool. Methodological details are given in the methods section.
Figure S3. Kaplan‐Meier curves for OS according to BMI category.
Figure S4. Survival curve based on the multivariate Cox model, validated by bootstrap analysis, modeled for MA.
Table S1. Correlation matrix
Table S2. Multivariate analysis (anatomic muscle areas) for OS and PFS
Table S3. BIC selected multivariate Cox model (anatomic muscle areas) for OS and PFS and number of bootstrap replicates


